Alterations in the Cellular Metabolic Footprint Induced by Mayaro Virus
Abstract
1. Introduction
2. Materials and Methods
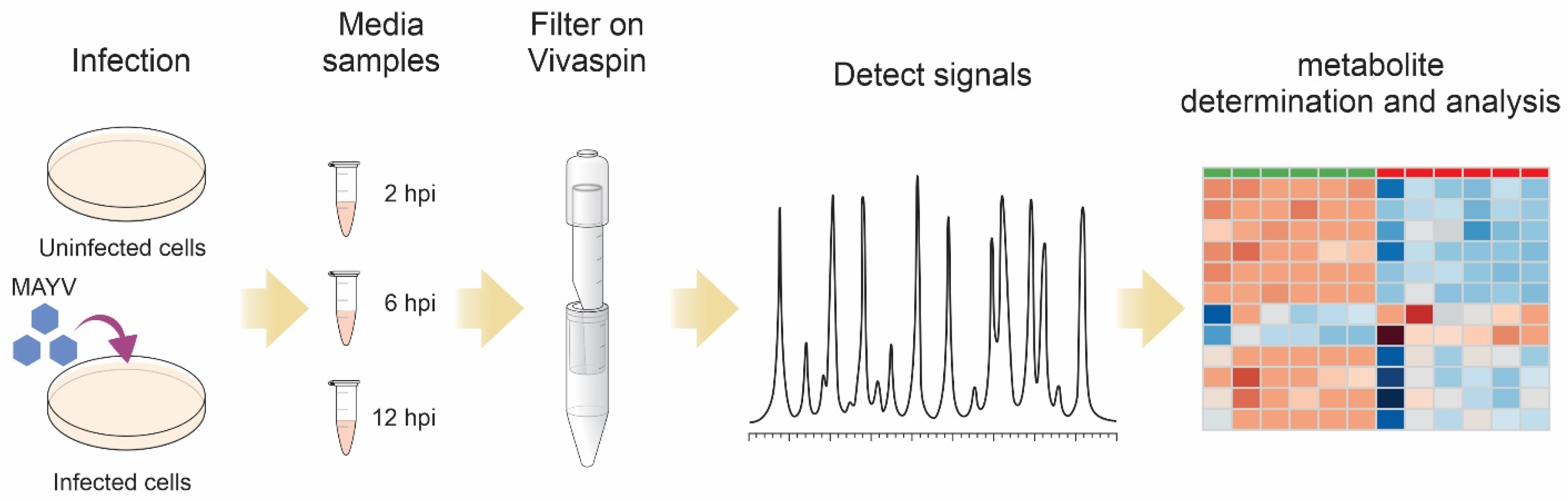
2.1. Cell Cultures and Virus
2.2. Preparations of Samples for NMR
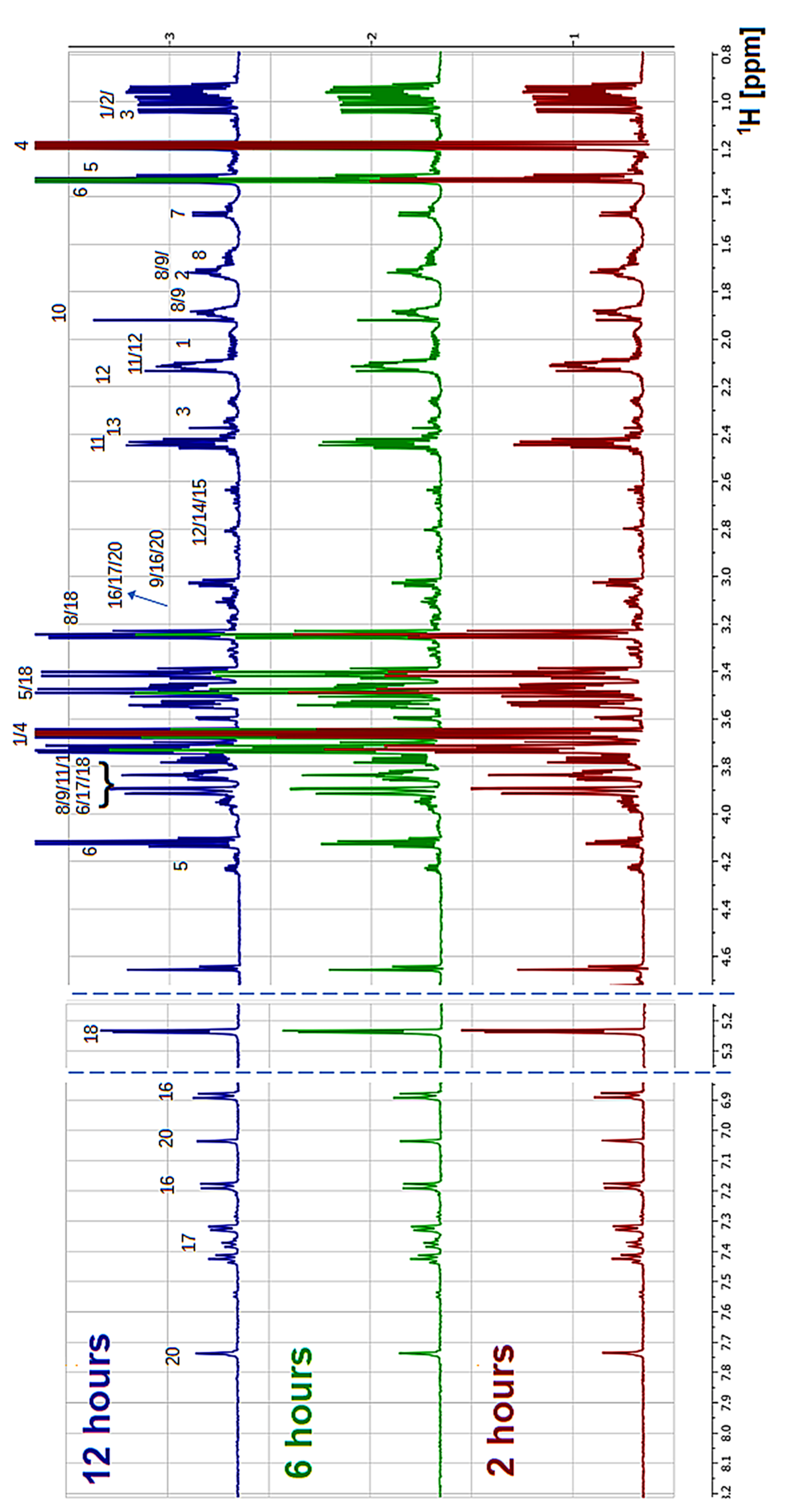
2.3. Proton Nuclear Magnetic Resonance (NMR) Spectroscopy
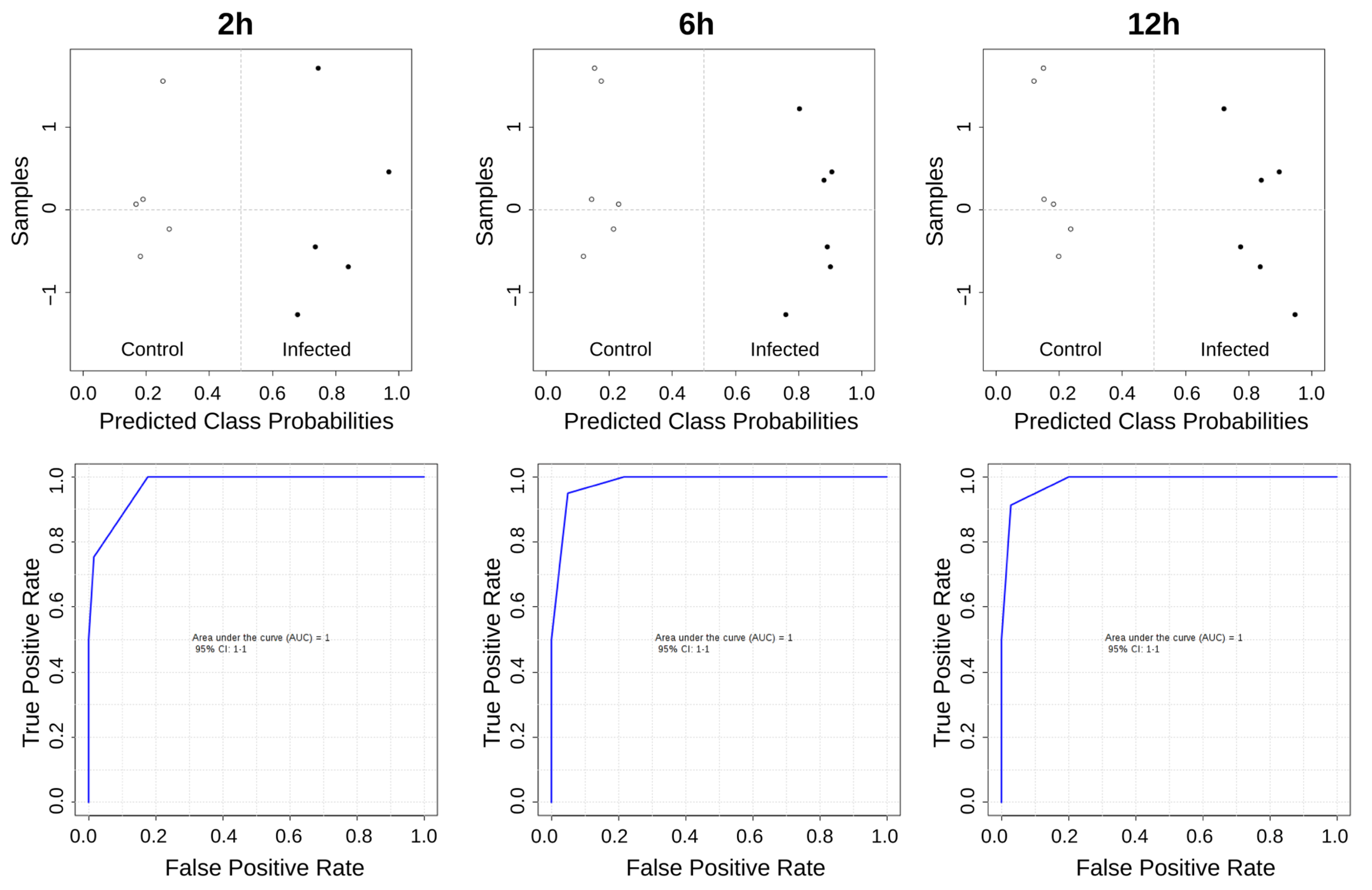
2.4. Chemometric and Statistical Analyses
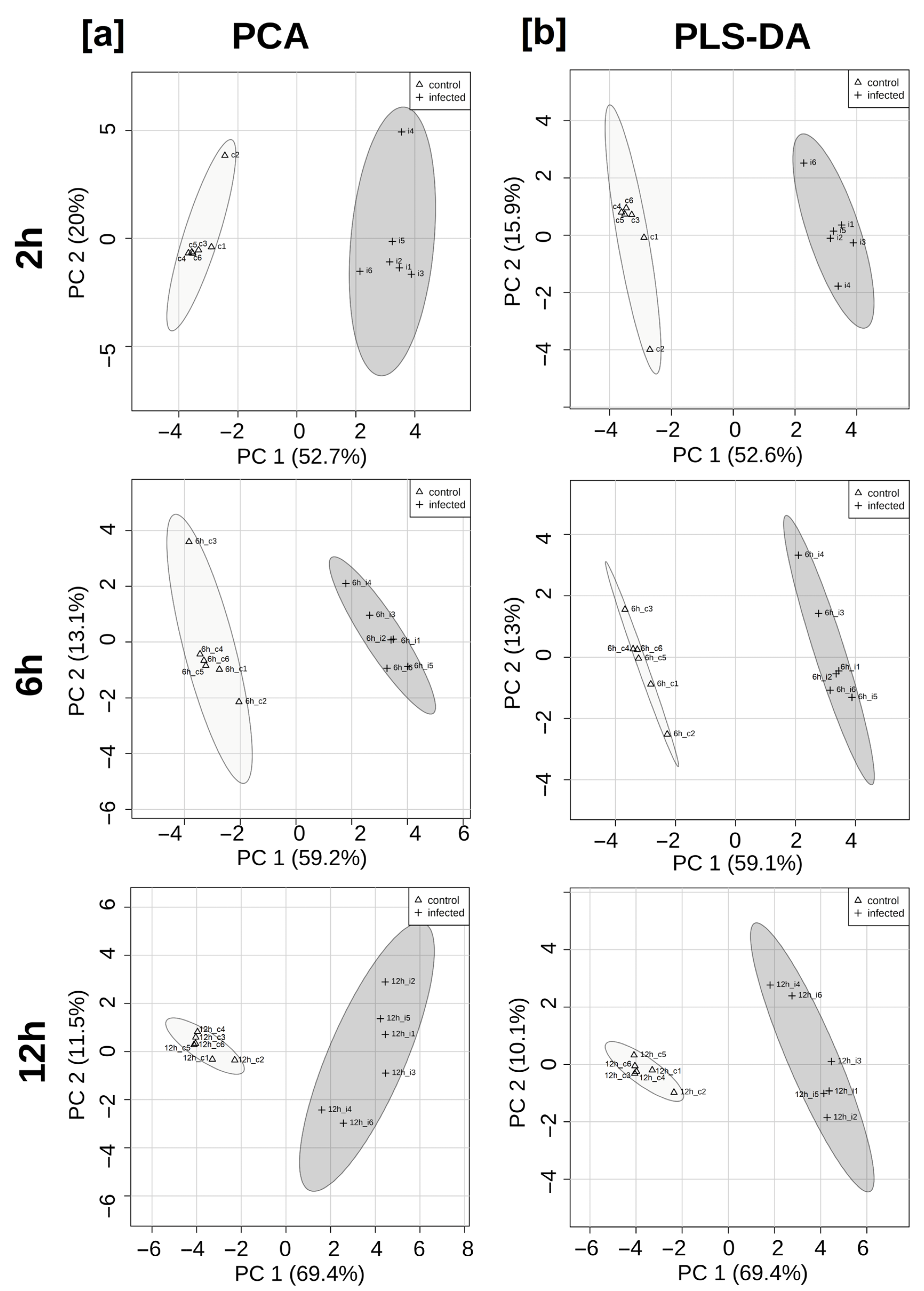
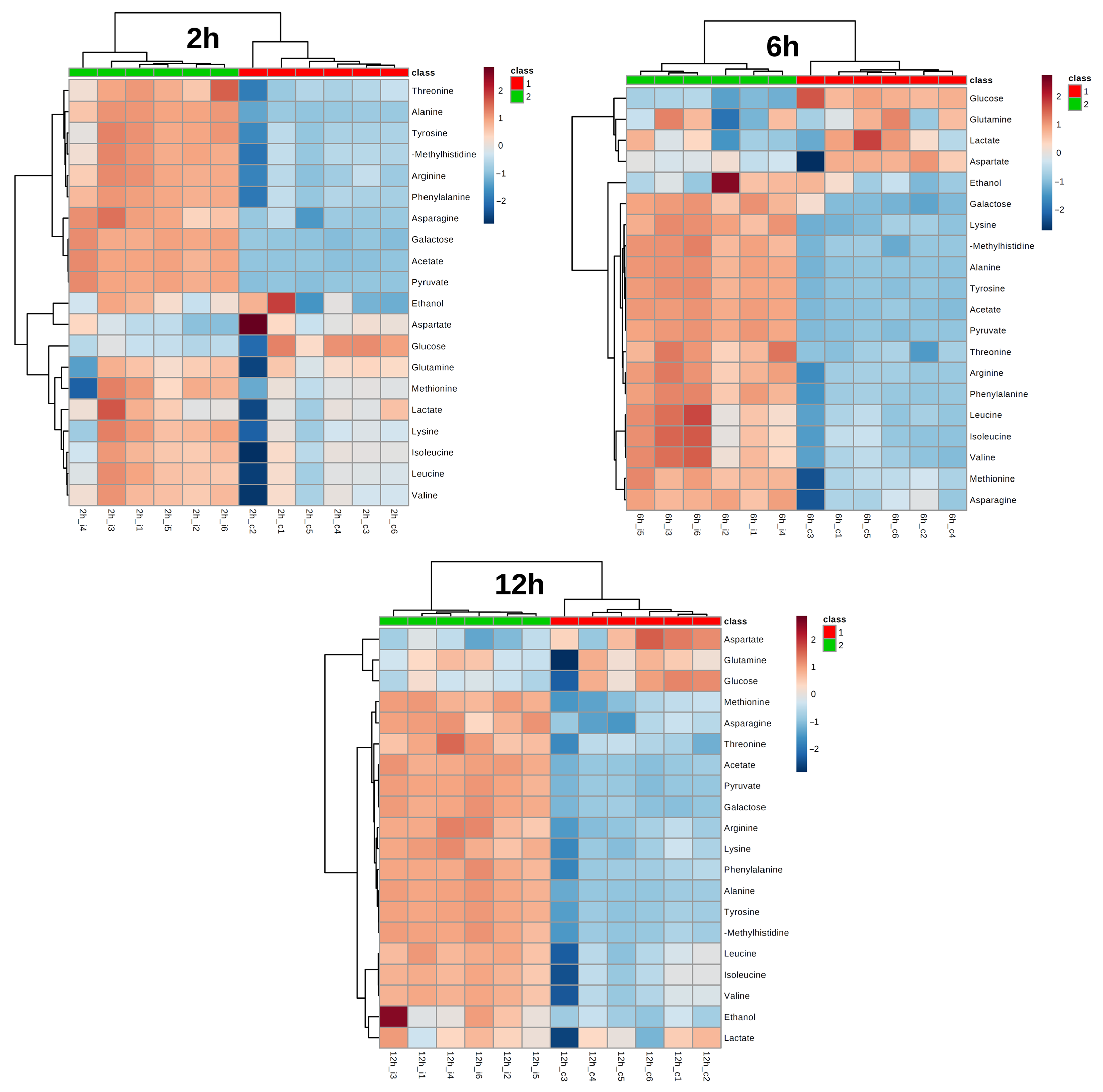
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Diagne, C.T.; Bengue, M.; Choumet, V.; Hamel, R.; Pompon, J.; Missé, D. Mayaro Virus Pathogenesis and Transmission Mechanisms. Pathogens 2020, 9, 738. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro-Filho, H.V.; Coimbra, L.D.; Cassago, A.; Rocha, R.P.F.; Guerra, J.; de Felicio, R.; Carnieli, C.M.; Leme, L.; Padilha, A.C.; Paes Leme, A.F.; et al. Cryo-EM structure of the mature and infective Mayaro virus at 4.4 A resolution reveals features of arthritogenic alphaviruses. Nat. Commun. 2021, 12, 3038. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.R.; Wattley, G.H.; Ahin, N.W.; Downs, W.G.; Reese, A.A. Mayaro Virus: A New Human Disease Agent. II. Isolation from Blood of Patients in Trinidad, B.W.I. Am. J. Trop. Med. Hyg. 1957, 6, 1012–1016. [Google Scholar] [CrossRef]
- Vieira, C.J.D.S.P.; da Silva, D.J.F.; Barreto, E.S.; Siqueira, C.E.H.; Colombo, T.E.; Ozanic, K.; Schmidt, D.J.; Drumond, B.P.; Mondini, A.; Nogueira, M.L.; et al. Detection of Mayaro virus infections during a dengue outbreak in Mato Grosso, Brazil. Acta Trop. 2015, 147, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Pauvolid-Corrêa, A.; Juliano, R.S.; Campos, Z.; Velez, J.; Nogueira, R.M.R.; Komar, N. Neutralising antibodies for Mayaro virus in Pantanal, Brazil. Memórias Inst. Oswaldo Cruz 2015, 110, 125–133. [Google Scholar] [CrossRef]
- Mavian, C.; Rife, B.D.; Dollar, J.J.; Cella, E.; Ciccozzi, M.; Prosperi, M.C.F.; Lednicky, J.; Morris, J.G.; Capua, I.; Salemi, M. Emergence of recombinant Mayaro virus strains from the Amazon basin. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- da Costa, V.G.; Féres, V.C.D.R.; Saivish, M.V.; Gimaque, J.B.D.L.; Moreli, M.L. Silent emergence of Mayaro and Oropouche viruses in humans in Central Brazil. Int. J. Infect. Dis. 2017, 62, 84–85. [Google Scholar] [CrossRef]
- Brunini, S.; França, D.D.S.; Silva, J.B.; Silva, L.N.; Silva, F.P.A.; Spadoni, M.; Rezza, G. High Frequency of Mayaro Virus IgM among Febrile Patients, Central Brazil. Emerg. Infect. Dis. 2017, 23, 1025–1026. [Google Scholar] [CrossRef]
- Mota, M.T.D.O.; Ribeiro, M.R.; Vedovello, D.; Nogueira, M.L. Mayaro virus: A neglected arbovirus of the Americas. Futur. Virol. 2015, 10, 1109–1122. [Google Scholar] [CrossRef]
- Mackay, I.M.; Arden, K. Mayaro virus: A forest virus primed for a trip to the city? Microbes Infect. 2016, 18, 724–734. [Google Scholar] [CrossRef]
- De Figueiredo, M.L.G.; Figueiredo, L.T.M. Emerging alphaviruses in the Americas: Chikungunya and Mayaro. Rev. Soc. Bras. Med. Trop. 2014, 47, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, F.P.; Travassos da Rosa, A.P. Arboviral Zoonoses of Central and South America. In Handbook of Zoonoses: Viral Zoonoses; Beran, G.W., Ed.; Taylor & Francis: Abingdon, UK, 1994. [Google Scholar]
- American Society of Tropical Medicine and Hygiene. International Catalogue of Arboviruses, Including Certain Other Viruses of Vertebrates; American Society of Tropical Medicine and Hygiene: Arlington, VA, USA, 1985. [Google Scholar]
- Forshey, B.M.; Guevara, C.; Laguna-Torres, V.A.; Cespedes, M.; Vargas, J.; Gianella, A.; Vallejo, E.; Madrid, C.; Aguayo, N.; Gotuzzo, E.; et al. Arboviral Etiologies of Acute Febrile Illnesses in Western South America, 2000–2007. PLOS Negl. Trop. Dis. 2010, 4, e787. [Google Scholar] [CrossRef]
- Weaver, S.C.; Reisen, W.K. Present and future arboviral threats. Antivir. Res. 2010, 85, 328–345. [Google Scholar] [CrossRef] [PubMed]
- Long, K.C.; Ziegler, S.A.; Thangamani, S.; Hausser, N.L.; Kochel, T.J.; Higgs, S.; Tesh, R.B. Experimental Transmission of Mayaro Virus by Aedes aegypti. Am. J. Trop. Med. Hyg. 2011, 85, 750–757. [Google Scholar] [CrossRef]
- Smith, G.C.; Francy, D.B. Laboratory studies of a Brazilian strain of Aedes albopictus as a potential vector of Mayaro and Oropouche viruses. J. Am. Mosq. Control. Assoc. 1991, 7, 89–93. [Google Scholar]
- Abad-Franch, F.; Grimmer, G.H.; de Paula, V.; Figueiredo, L.T.M.; Braga, W.S.M.; Luz, S.L.B. Mayaro Virus Infection in Amazonia: A Multimodel Inference Approach to Risk Factor Assessment. PLOS Negl. Trop. Dis. 2012, 6, e1846. [Google Scholar] [CrossRef]
- Estofolete, C.F.; Mota, M.T.O.; Vedovello, D.; De Góngora, D.V.N.; Maia, I.L.; Nogueira, M.L. Mayaro fever in an HIV-infected patient suspected of having Chikungunya fever. Rev. Soc. Bras. Med. Trop. 2016, 49, 648–652. [Google Scholar] [CrossRef]
- Auguste, A.J.; Liria, J.; Forrester, N.L.; Giambalvo, D.; Moncada, M.; Long, K.C.; Morón, D.; de Manzione, N.; Tesh, R.B.; Halsey, E.S.; et al. Evolutionary and Ecological Characterization of Mayaro Virus Strains Isolated during an Outbreak, Venezuela, 2010. Emerg. Infect. Dis. 2015, 21, 1742–1750. [Google Scholar] [CrossRef] [PubMed]
- Mourão, M.P.G.; Bastos, M.D.S.; De Figueiredo, R.P.; Gimaque, J.B.L.; Galusso, E.D.S.; Kramer, V.M.; De Oliveira, C.M.C.; Naveca, F.; Figueiredo, L.T.M. Mayaro Fever in the City of Manaus, Brazil, 2007–2008. Vector-Borne Zoonotic Dis. 2012, 12, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Ganjian, N.; Riviere-Cinnamond, A. Mayaro virus in Latin America and the Caribbean. Rev. Panam. Salud Pública 2020, 44, e14. [Google Scholar] [CrossRef]
- Lorenz, C.; Ribeiro, A.F.; Chiaravalloti-Neto, F. Mayaro virus distribution in South America. Acta Trop. 2019, 198, 105093. [Google Scholar] [CrossRef]
- McSweegan, E.; Weaver, S.C.; Lecuit, M.; Frieman, M.; Morrison, T.E.; Hrynkow, S. The Global Virus Network: Challenging chikungunya. Antivir. Res. 2015, 120, 147–152. [Google Scholar] [CrossRef]
- Zuchi, N.; Heinen, L.B.D.S.; Dos Santos, M.A.M.; Pereira, F.C.; Slhessarenko, R.D. Molecular detection of Mayaro virus during a dengue outbreak in the state of Mato Grosso, Central-West Brazil. Memórias Inst. Oswaldo Cruz 2014, 109, 820–823. [Google Scholar] [CrossRef]
- Serra, O.P.; Cardoso, B.F.; Ribeiro, A.L.M.; Santos, F.; Slhessarenko, R.D. Mayaro virus and dengue virus 1 and 4 natural infection in culicids from Cuiabá, state of Mato Grosso, Brazil. Memórias Inst. Oswaldo Cruz 2016, 111, 20–29. [Google Scholar] [CrossRef]
- De Curcio, J.S.; Salem-Izacc, S.M.; Neto, L.M.P.; Nunes, E.B.; Anunciação, C.E.; Silveira-Lacerda, E.D.P. Detection of Mayaro virus in Aedes aegypti mosquitoes circulating in Goiânia-Goiás-Brazil. Microbes Infect. 2022, 24, 104948. [Google Scholar] [CrossRef]
- Neves, N.A.D.S.; Ferreira, R.D.S.; Morais, D.O.; Pavon, J.A.R.; de Pinho, J.B.; Slhessarenko, R.D. Chikungunya, Zika, Mayaro, and Equine Encephalitis virus detection in adult Culicinae from South Central Mato Grosso, Brazil, during the rainy season of 2018. Braz. J. Microbiol. 2021, 53, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Silveira-Lacerda, E.D.P.; Herlinger, A.L.; Tanuri, A.; Rezza, G.; Anunciação, C.E.; Ribeiro, J.P.; Tannous, I.P.; Abrantes, G.R.; da Silva, E.G.; Arruda, K.F.; et al. Molecular epidemiological investigation of Mayaro virus in febrile patients from Goiania City, 2017–2018. Infect. Genet. Evol. 2021, 95, 104981. [Google Scholar] [CrossRef]
- Romeiro, M.F.; Fumagalli, M.J.; Dos Anjos, A.B.; Figueiredo, L.T.M. Serological evidence of Mayaro virus infection in blood donors from São Carlos, São Paulo, Brazil. Trans. R. Soc. Trop. Med. Hyg. 2020, 114, 693–696. [Google Scholar] [CrossRef]
- Pezzi, L.; Rodriguez-Morales, A.; Reusken, C.; Ribeiro, G.; LaBeaud, A.; Lourenço-De-Oliveira, R.; Brasil, P.; Lecuit, M.; Failloux, A.; Gallian, P.; et al. GloPID-R report on chikungunya, o’nyong-nyong and Mayaro virus, part 3: Epidemiological distribution of Mayaro virus. Antivir. Res. 2019, 172, 104610. [Google Scholar] [CrossRef]
- Lednicky, J.; De Rochars, V.M.B.; ElBadry, M.; Loeb, J.; Telisma, T.; Chavannes, S.; Anilis, G.; Cella, E.; Ciccozzi, M.; Okech, B.; et al. Mayaro Virus in Child with Acute Febrile Illness, Haiti, 2015. Emerg. Infect. Dis. 2016, 22, 2000–2002. [Google Scholar] [CrossRef]
- Caetano, C.C.D.S.; Camini, F.C.; Almeida, L.T.; Ferraz, A.C.; da Silva, T.F.; Lima, R.L.S.; Carvalho, M.M.D.F.; Castro, T.D.F.; Carneiro, C.M.; Silva, B.D.M.; et al. Mayaro Virus Induction of Oxidative Stress is Associated with Liver Pathology in a Non-Lethal Mouse Model. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- De Castro-Jorge, L.A.; de Carvalho, R.; Klein, T.M.; Hiroki, C.H.; Lopes, A.H.; Guimarães, R.M.; Fumagalli, M.J.; Floriano, V.G.; Agostinho, M.R.; Slhessarenko, R.D.; et al. The NLRP3 inflammasome is involved with the pathogenesis of Mayaro virus. PLOS Pathog. 2019, 15, e1007934. [Google Scholar] [CrossRef]
- Ferraz, A.C.; Almeida, L.T.; Caetano, C.C.D.S.; Menegatto, M.B.D.S.; Lima, R.L.S.; de Senna, J.P.N.; Cardoso, J.M.D.O.; Perucci, L.O.; Talvani, A.; de Lima, W.G.; et al. Hepatoprotective, antioxidant, anti-inflammatory, and antiviral activities of silymarin against mayaro virus infection. Antivir. Res. 2021, 194, 105168. [Google Scholar] [CrossRef] [PubMed]
- Chuong, C.; Bates, T.A.; Weger-Lucarelli, J. Infectious cDNA clones of two strains of Mayaro virus for studies on viral pathogenesis and vaccine development. Virology 2019, 535, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, H.; Zhang, Y.; Li, J.; Wang, Z.; Deng, C.; Jardim, A.C.G.; Terzian, A.C.B.; Nogueira, M.; Zhang, B. Development of a rapid antiviral screening assay based on eGFP reporter virus of Mayaro virus. Antivir. Res. 2019, 168, 82–90. [Google Scholar] [CrossRef]
- Mota, M.T.D.O.; Costa, V.V.; Sugimoto, M.A.; Guimarães, G.D.F.; Queiroz-Junior, C.M.; Moreira, T.P.; de Sousa, C.D.; Santos, F.M.; Queiroz, V.F.; Passos, I.; et al. In-depth characterization of a novel live-attenuated Mayaro virus vaccine candidate using an immunocompetent mouse model of Mayaro disease. Sci. Rep. 2020, 10, 1–16. [Google Scholar] [CrossRef]
- Behrends, V.; Williams, H.D.; Bundy, J.G. Metabolic footprinting: Extracellular metabolomic analysis. In Pseudomonas Methods and Protocols; Filloux, A., Ramos, J.-L., Eds.; Springer: New York, NY, USA, 2014; pp. 281–292. [Google Scholar]
- Kell, D.B.; Brown, M.; Davey, H.M.; Dunn, W.B.; Spasic, I.; Oliver, S.G. Metabolic footprinting and systems biology: The medium is the message. Nat. Rev. Microbiol. 2005, 3, 557–565. [Google Scholar] [CrossRef]
- Mo, M.L.; Palsson, B.Ø.; Herrgård, M.J. Connecting extracellular metabolomic measurements to intracellular flux states in yeast. BMC Syst. Biol. 2009, 3, 37. [Google Scholar] [CrossRef]
- Sanchez, E.L.; Lagunoff, M. Viral activation of cellular metabolism. Virology 2015, 479–480, 609–618. [Google Scholar] [CrossRef]
- Xia, J.; Sinelnikov, I.V.; Han, B.; Wishart, D.S. MetaboAnalyst 3.0—Making metabolomics more meaningful. Nucleic Acids Res. 2015, 43, W251–W257. [Google Scholar] [CrossRef]
- Xia, J.; Wishart, D.S. Web-based inference of biological patterns, functions and pathways from metabolomic data using MetaboAnalyst. Nat. Protoc. 2011, 6, 743–760. [Google Scholar] [CrossRef]
- Fawcett, T. An Introduction to ROC analysis. Pattern Recogn. Lett. 2006, 27, 861–874. [Google Scholar] [CrossRef]
- Nelson, D.L.; Cox, M.M. Biosynthesis of Amino Acids, Nucletides, and Related Molecules. In Lehninger Principles of Biochemistry; Nelson, D.L., Cox, M.M., Eds.; W.H. Freeman: New York, NY, USA, 2008; pp. 833–880. [Google Scholar]
- Nelson, D.L.; Cox, M.M. Amino Acid Oxidation and the Production of Urea. In Lehninger Principles of Biochemistry; Nelson, D.L., Cox, M.M., Eds.; W.H. Freeman: New York, NY, USA, 2008; pp. 656–689. [Google Scholar]
- Martínez, Y.; Li, X.; Liu, G.; Bin, P.; Yan, W.; Más, D.; Valdivié, M.; Hu, C.-A.A.; Ren, W.; Yin, Y. The role of methionine on metabolism, oxidative stress, and diseases. Amino Acids 2017, 49, 2091–2098. [Google Scholar] [CrossRef]
- Cynober, L.; Le Boucher, J.; Vasson, M.-P. Arginine metabolism in mammals. J. Nutr. Biochem. 1995, 6, 402–413. [Google Scholar] [CrossRef]
- El-Bacha, T.; Menezes, M.M.; Silva, M.C.A.E.; Sola-Penna, M.; Da Poian, A. Mayaro virus infection alters glucose metabolism in cultured cells through activation of the enzyme 6-phosphofructo 1-kinase. Mol. Cell. Biochem. 2004, 266, 191–198. [Google Scholar] [CrossRef]
- Findlay, J.; Ulaeto, D. Semliki Forest virus and Sindbis virus, but not vaccinia virus, require glycolysis for optimal replication. J. Gen. Virol. 2015, 96, 2693–2696. [Google Scholar] [CrossRef]
- Frolova, E.I.; Gorchakov, R.; Pereboeva, L.; Atasheva, S.; Frolov, I. Functional Sindbis Virus Replicative Complexes Are Formed at the Plasma Membrane. J. Virol. 2010, 84, 11679–11695. [Google Scholar] [CrossRef]
- Ahola, T.; Lampio, A.; Auvinen, P.; Kääriäinen, L. Semliki Forest virus mRNA capping enzyme requires association with anionic membrane phospholipids for activity. EMBO J. 1999, 18, 3164–3172. [Google Scholar] [CrossRef]
- Kielian, M.; Chatterjee, P.K.; Gibbons, D.L.; Lu, Y.E. Specific Roles for Lipids in Virus Fusion and Exit Examples from the Alphaviruses. In Fusion of Biological Membranes and Related Problems; Springer: Berlin/Heidelberg, Germany, 2002; Volume 34, pp. 409–455. [Google Scholar] [CrossRef]
- Ng, C.G.; Coppens, I.; Govindarajan, D.; Pisciotta, J.; Shulaev, V.; Griffin, D.E. Effect of host cell lipid metabolism on alphavirus replication, virion morphogenesis, and infectivity. Proc. Natl. Acad. Sci. USA 2008, 105, 16326–16331. [Google Scholar] [CrossRef]
- Bose, S.; Ramesh, V.; Locasale, J.W. Acetate Metabolism in Physiology, Cancer, and Beyond. Trends Cell Biol. 2019, 29, 695–703. [Google Scholar] [CrossRef]
- Nelson, D.L.; Cox, M.M. The Citric Acid Cycle. In Lehninger Principles of Biochemistry; Nelson, D.L., Cox, M.M., Eds.; W.H. Freeman: New York, NY, USA, 2008; pp. 601–630. [Google Scholar]
- Nelson, D.L.; Cox, M.M. Lipids. In Lehninger Principles of Biochemistry; Nelson, D.L., Cox, M.M., Eds.; W.H. Freeman: New York, NY, USA, 2008; pp. 343–368. [Google Scholar]
- Nelson, D.L.; Cox, M.M. Bioenergetics and Metabolism. In Lehninger Principles of Biochemistry; Nelson, D.L., Cox, M.M., Eds.; W. H. Freeman: New York, NY, USA, 2008; pp. 481–488. [Google Scholar]
- Kempf, C.; Kohler, U.; Michel, M.R.; Koblet, H. Semliki Forest virus-induced polykaryocyte formation is an ATP-dependent event. Arch. Virol. 1987, 95, 111–122. [Google Scholar] [CrossRef]
| Metabolite | 2 hpi | 6 hpi | 12 hpi | |||
|---|---|---|---|---|---|---|
| Control | Infected | Control | Infected | Control | Infected | |
| Leucine | 9.48 ± 1.66 | 11.41 ± 0.71 | 10.64 ± 0.17 | 11.60 ± 0.40 | 6.45 ± 0.72 | 7.87 ± 0.15 |
| Isoleucine | 6.71 ± 1.30 | 7.93 ± 0.53 | 7.47 ± 0.15 | 8.11 ± 0.25 | 6.79 ± 0.82 | 8.21 ± 0.13 |
| Valine | 6.76 ± 1.17 | 8.12 ± 0.34 | 7.41 ± 0.15 | 8.23 ± 0.28 | 6.74 ± 0.84 | 8.38 ± 0.13 |
| Ethanol | 102.90 ± 23.81 | 110.75 ± 9.51 | 103.40 ± 18.91 | 123.10 ± 33.30 | 75.54 ± 9.87 | 129.52 ± 40.60 |
| Threonine | 3.78 ± 0.76 | 6.20 ± 0.69 | 4.31 ± 0.30 | 6.26 ± 0.41 | 4.78 ± 0.53 | 6.68 ± 0.38 |
| Lactate | 8.13 ± 1.07 | 9.08 ± 0.65 | 18.39 ± 1.02 | 17.74 ± 0.76 | 31.58 ± 4.43 | 34.15 ± 1.66 |
| Alanine | 3.48 ± 0.71 | 10.72 ± 0.74 | 4.82 ± 0.27 | 10.94 ± 0.48 | 4.81 ± 0.59 | 11.21 ± 0.33 |
| Arginine | 0.37 ± 0.09 | 0.70 ± 0.05 | 0.42 ± 0.06 | 0.73 ± 0.05 | 0.38 ± 0.06 | 0.71 ± 0.05 |
| Acetate | 1.42 ± 0.09 | 7.32 ± 0.41 | 2.67 ± 0.25 | 8.91 ± 0.29 | 4.06 ± 0.56 | 12.45 ± 0.46 |
| Pyruvate | 0.50 ± 0.10 | 6.01 ± 0.35 | 0.92 ± 0.10 | 4.09 ± 0.16 | 1.44 ± 0.10 | 3.22 ± 0.10 |
| Glutamine | 3.05 ± 0.80 | 3.35 ± 0.55 | 3.51 ± 0.10 | 3.47 ± 0.14 | 3.05 ± 0.34 | 3.09 ± 0.12 |
| Methionine | 0.97 ± 0.21 | 1.25 ± 0.55 | 0.93 ± 0.26 | 1.48 ± 0.07 | 0.94 ± 0.16 | 1.55 ± 0.05 |
| Aspartate | 0.34 ± 0.22 | 0.15 ± 0.10 | 0.15 ± 0.25 | 0.08 ± 0.03 | 0.22 ± 0.09 | 0.07 ± 0.04 |
| Asparagine | 0.35 ± 0.07 | 0.76 ± 0.08 | 0.22 ± 0.23 | 0.70 ± 0.05 | 0.28 ± 0.14 | 0.79 ± 0.08 |
| Lysine | 1.24 ± 0.30 | 1.70 ± 0.27 | 1.42 ± 0.04 | 1.78 ± 0.04 | 1.34 ± 0.10 | 1.73 ± 0.04 |
| Glucose | 10.13 ± 1.53 | 9.10 ± 0.22 | 10.20 ± 0.34 | 8.40 ± 0.32 | 7.70 ± 1.19 | 7.10 ± 0.28 |
| Galactose | 0.04 ± 0.03 | 1.10 ± 0.06 | 0.22 ± 0.23 | 1.00 ± 0.09 | 0.10 ± 0.05 | 0.93 ± 0.04 |
| Tyrosine | 3.02 ± 0.66 | 5.71 ± 0.72 | 3.42 ± 0.10 | 5.94 ± 0.19 | 3.17 ± 0.38 | 5.97 ± 0.12 |
| Phenylalanine | 2.68 ± 0.40 | 4.03 ± 0.10 | 2.92 ± 0.16 | 3.99 ± 0.16 | 2.68 ± 0.32 | 4.01 ± 0.11 |
| τ-Methylhistidine | 2.07 ± 0.50 | 3.56 ± 0.33 | 2.34 ± 0.13 | 3.69 ± 0.17 | 2.19 ± 0.26 | 3.81 ± 0.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro, C.M.O.; Mota, M.T.O.; Vidotto, A.; Caruso, Í.P.; Ribeiro, M.R.; Moraes, F.R.; Souza, F.P.; Nogueira, M.L. Alterations in the Cellular Metabolic Footprint Induced by Mayaro Virus. BioMed 2023, 3, 152-165. https://doi.org/10.3390/biomed3010013
Castro CMO, Mota MTO, Vidotto A, Caruso ÍP, Ribeiro MR, Moraes FR, Souza FP, Nogueira ML. Alterations in the Cellular Metabolic Footprint Induced by Mayaro Virus. BioMed. 2023; 3(1):152-165. https://doi.org/10.3390/biomed3010013
Chicago/Turabian StyleCastro, Ceyla M. O., Mânlio T. O. Mota, Alessandra Vidotto, Ícaro P. Caruso, Milene R. Ribeiro, Fábio R. Moraes, Fátima P. Souza, and Mauricio L. Nogueira. 2023. "Alterations in the Cellular Metabolic Footprint Induced by Mayaro Virus" BioMed 3, no. 1: 152-165. https://doi.org/10.3390/biomed3010013
APA StyleCastro, C. M. O., Mota, M. T. O., Vidotto, A., Caruso, Í. P., Ribeiro, M. R., Moraes, F. R., Souza, F. P., & Nogueira, M. L. (2023). Alterations in the Cellular Metabolic Footprint Induced by Mayaro Virus. BioMed, 3(1), 152-165. https://doi.org/10.3390/biomed3010013






