Advances in Kidney Transplant, Machine Perfusion, and Viability Markers
Abstract
1. Introduction
2. Kidney Perfusion
2.1. Hypothermic Machine Perfusion
2.2. Hypothermic Oxygenated Machine Perfusion
2.3. Normothermic Machine Perfusion
2.4. Current Perfusion Devices
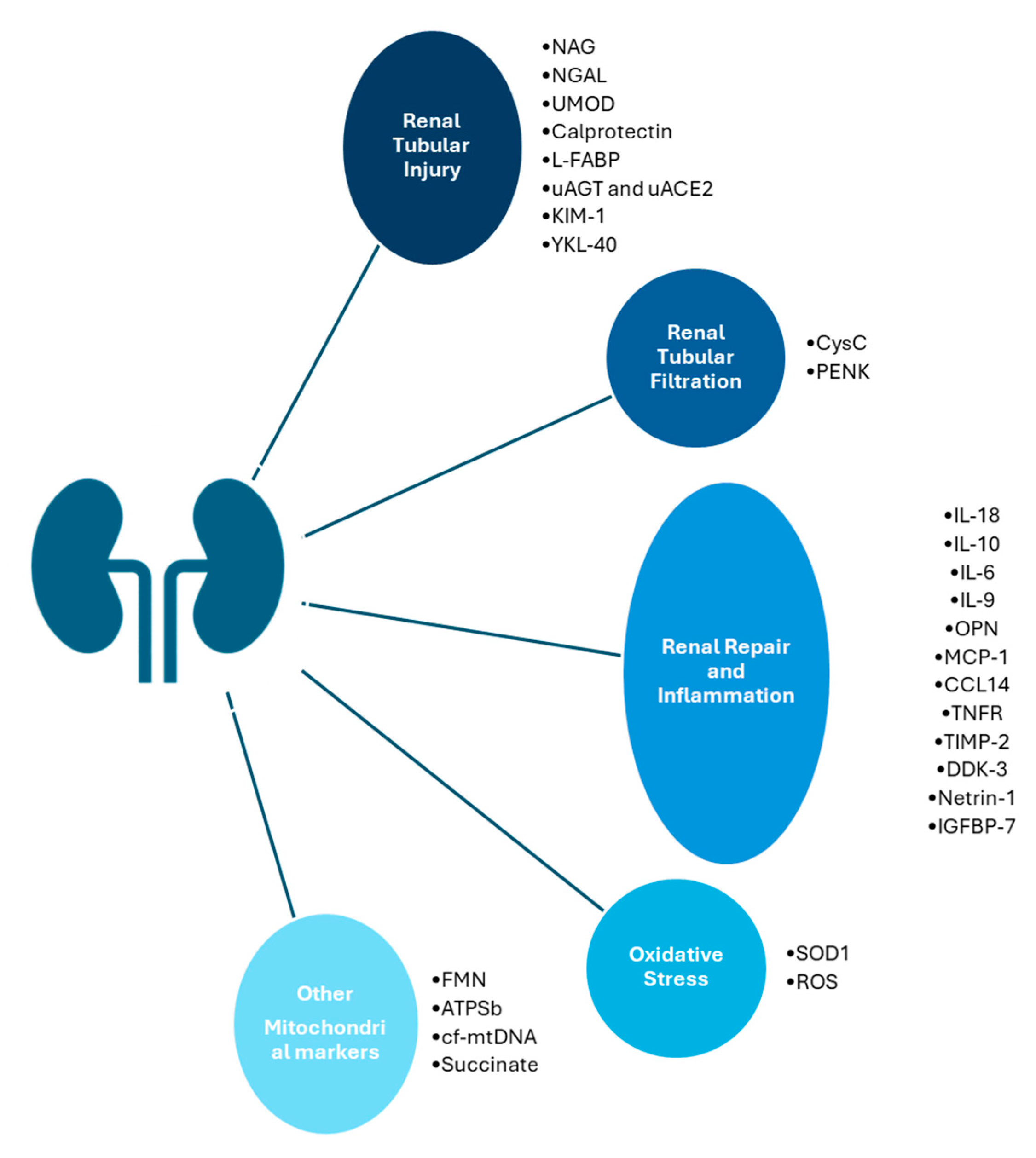
3. Viability Markers
3.1. Perfusion Technology and Biomarkers
3.2. Kidney Injury Biomarkers
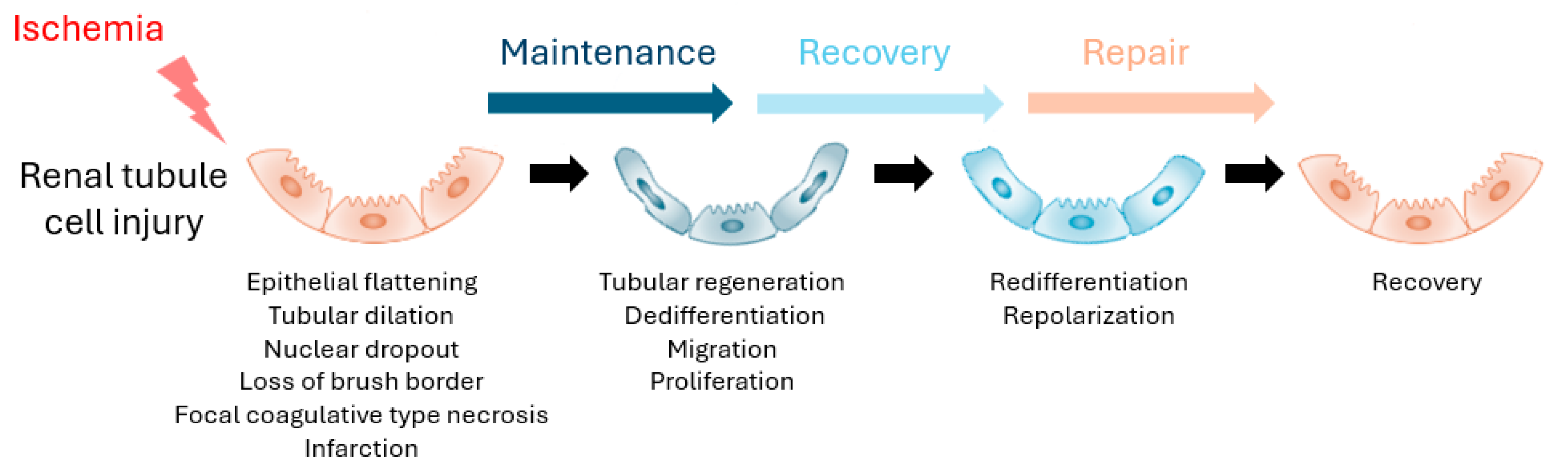
3.3. Kidney Repair Biomarkers
4. Summary
Funding
Conflicts of Interest
Abbreviations
| AKI | acute kidney injury |
| ATP | adenosine triphosphate |
| ATPSb | ATP synthase subunit b |
| cf-mtDNA | cell-free mitochondrial DNA |
| CIT | cold ischemia time |
| DAMPs | damage-associated molecular pattern |
| DGF | delayed graft function |
| DCD | donation after circulatory death |
| ECD | expanded criteria donor |
| eGFR | estimated glomerular filtration rate |
| FMN | flavin mononucleotide |
| HMP | hypothermic machine perfusion |
| HTK | histidine-tryptophan-ketoglutarate |
| HOPE | hypothermic oxygenated machine perfusion |
| IRI | ischemic reperfusion injury |
| KIM-1 | kidney injury molecule-1 |
| L-FABP | liver-type fatty acid binding protein |
| NGAL | neutrophil gelatinase–associated lipocalin |
| NMP | normothermic machine perfusion |
| OPN | osteopontin |
| PNF | primary non-function |
| SCD | standard criteria donors |
| SCS | static cold storage |
| UMOD | uromodulin |
| UW | University of Wisconsin |
References
- Lentine, K.L.; Smith, J.M.; Lyden, G.R.; Miller, J.M.; Dolan, T.G.; Bradbrook, K.; Larkin, L.; Temple, K.; Handarova, D.K.; Weiss, S.; et al. OPTN/SRTR 2022 Annual Data Report: Kidney. Am. J. Transplant. 2024, 24, S19–S118. [Google Scholar] [CrossRef]
- Jadlowiec, C.C.; Hanna, W.A.; Ninan, J.; Ryan, M.S.; Das, D.M.; Smith, M.; Khamash, H.; Mathur, A.K.; Singer, A.; Moss, A.; et al. Transplant outcomes using kidneys from high KDPI acute kidney injury donors. Clin. Transplant. 2021, 35, e14279. [Google Scholar] [CrossRef] [PubMed]
- Jadlowiec, C.C.; Heilman, R.L.; Smith, M.L.; Khamash, H.A.; Huskey, J.L.; Harbell, J.; Reddy, K.S.; Moss, A.A. Transplanting kidneys from donation after cardiac death donors with acute kidney injury. Am. J. Transplant. 2020, 20, 864–869. [Google Scholar] [CrossRef]
- Jadlowiec, C.C.; Ohara, S.Y.; Punukollu, R.; Wagler, J.; Ruch, B.; Kumm, K.; Budhiraja, P.; Me, H.M.; Mathur, A.K.; Reddy, K.S.; et al. Outcomes with transplanting kidneys offered through expedited allocation. Clin. Transplant. 2023, 37, e15094. [Google Scholar] [CrossRef]
- Hosgood, S.A.; Brown, R.J.; Nicholson, M.L. Advances in Kidney Preservation Techniques and Their Application in Clinical Practice. Transplantation 2021, 105, e202–e214. [Google Scholar] [CrossRef]
- Yemaneberhan, K.H.; Kang, M.; Jang, J.H.; Kim, J.H.; Kim, K.S.; Park, H.B.; Choi, D. Beyond the icebox: Modern strategies in organ preservation for transplantation. Clin. Transplant. Res. 2024, 38, 377–403. [Google Scholar] [CrossRef]
- Chen, Y.; Shi, J.; Xia, T.C.; Xu, R.; He, X.; Xia, Y. Preservation Solutions for Kidney Transplantation: History, Advances and Mechanisms. Cell Transplant. 2019, 28, 1472–1489. [Google Scholar] [CrossRef]
- Hamed, M.O.; Chen, Y.; Pasea, L.; Watson, C.J.; Torpey, N.; Bradley, J.A.; Pettigrew, G.; Saeb-Parsy, K. Early graft loss after kidney transplantation: Risk factors and consequences. Am. J. Transplant. 2015, 15, 1632–1643. [Google Scholar] [CrossRef]
- Heylen, L.; Jochmans, I.; Samuel, U.; Tieken, I.; Naesens, M.; Pirenne, J.; Sprangers, B. The duration of asystolic ischemia determines the risk of graft failure after circulatory-dead donor kidney transplantation: A Eurotransplant cohort study. Am. J. Transplant. 2018, 18, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Calva Lopez, A.; Robles Garcia, J.E.; Yanez Ruiz, C.A.; Tapia Tapia, M.D.; Talavera Cobo, V.; Munoz Bastidas, C.A.; Sanchez Zalabardo, D.; Minana Lopez, B. The Evolution of Kidney Graft Preservation Through the Years. Life 2024, 14, 1647. [Google Scholar] [CrossRef] [PubMed]
- Hosgood, S.A.; Yang, B.; Bagul, A.; Mohamed, I.H.; Nicholson, M.L. A comparison of hypothermic machine perfusion versus static cold storage in an experimental model of renal ischemia reperfusion injury. Transplantation 2010, 89, 830–837. [Google Scholar] [CrossRef]
- Moers, C.; Smits, J.M.; Maathuis, M.H.; Treckmann, J.; van Gelder, F.; Napieralski, B.P.; van Kasterop-Kutz, M.; van der Heide, J.J.; Squifflet, J.P.; van Heurn, E.; et al. Machine perfusion or cold storage in deceased-donor kidney transplantation. N. Engl. J. Med. 2009, 360, 7–19. [Google Scholar] [CrossRef]
- Treckmann, J.; Moers, C.; Smits, J.M.; Gallinat, A.; Maathuis, M.H.; van Kasterop-Kutz, M.; Jochmans, I.; Homan van der Heide, J.J.; Squifflet, J.P.; van Heurn, E.; et al. Machine perfusion versus cold storage for preservation of kidneys from expanded criteria donors after brain death. Transpl. Int. 2011, 24, 548–554. [Google Scholar] [CrossRef]
- Jochmans, I.; Moers, C.; Smits, J.M.; Leuvenink, H.G.; Treckmann, J.; Paul, A.; Rahmel, A.; Squifflet, J.P.; van Heurn, E.; Monbaliu, D.; et al. Machine perfusion versus cold storage for the preservation of kidneys donated after cardiac death: A multicenter, randomized, controlled trial. Ann. Surg. 2010, 252, 756–764. [Google Scholar] [CrossRef] [PubMed]
- Gill, J.; Dong, J.; Eng, M.; Landsberg, D.; Gill, J.S. Pulsatile perfusion reduces the risk of delayed graft function in deceased donor kidney transplants, irrespective of donor type and cold ischemic time. Transplantation 2014, 97, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Foguenne, M.; MacMillan, S.; Kron, P.; Nath, J.; Devresse, A.; De Meyer, M.; Michel, M.; Hosgood, S.; Darius, T. Current Evidence and Future Perspectives to Implement Continuous and End-Ischemic Use of Normothermic and Oxygenated Hypothermic Machine Perfusion in Clinical Practice. J. Clin. Med. 2023, 12, 3207. [Google Scholar] [CrossRef]
- Jochmans, I.; Brat, A.; Davies, L.; Hofker, H.S.; van de Leemkolk, F.E.M.; Leuvenink, H.G.D.; Knight, S.R.; Pirenne, J.; Ploeg, R.J.; Collaboration, C.T.; et al. Oxygenated versus standard cold perfusion preservation in kidney transplantation (COMPARE): A randomised, double-blind, paired, phase 3 trial. Lancet 2020, 396, 1653–1662. [Google Scholar] [CrossRef] [PubMed]
- Pravisani, R.; Baccarani, U.; Molinari, E.; Cherchi, V.; Bacchetti, S.; Terrosu, G.; Avital, I.; Ekser, B.; Adani, G.L. PO(2) 21% oxygenated hypothermic machine perfusion in kidney transplantation: Any clinical benefit? Int. J. Artif. Organs 2022, 45, 666–671. [Google Scholar] [CrossRef]
- Husen, P.; Boffa, C.; Jochmans, I.; Krikke, C.; Davies, L.; Mazilescu, L.; Brat, A.; Knight, S.; Wettstein, D.; Cseprekal, O.; et al. Oxygenated End-Hypothermic Machine Perfusion in Expanded Criteria Donor Kidney Transplant: A Randomized Clinical Trial. JAMA Surg. 2021, 156, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chang, Y.H.; Ohara, S.Y.; Reddy, K.S.; Jadlowiec, C.C.; Mathur, A.K.; Nguyen, M.C. Normothermic Machine Perfusion Improves Outcomes for Donation After Cardiac Death Allografts with Extended Donor Warm Ischemia Time. Clin. Transplant. 2025, 39, e70133. [Google Scholar] [CrossRef]
- Nguyen, M.C.; Zhang, C.; Chang, Y.H.; Li, X.; Ohara, S.Y.; Kumm, K.R.; Cosentino, C.P.; Aqel, B.A.; Lizaola-Mayo, B.C.; Frasco, P.E.; et al. Improved Outcomes and Resource Use with Normothermic Machine Perfusion in Liver Transplantation. JAMA Surg. 2025, 160, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Hosgood, S.A.; Patel, M.; Nicholson, M.L. The conditioning effect of ex vivo normothermic perfusion in an experimental kidney model. J. Surg. Res. 2013, 182, 153–160. [Google Scholar] [CrossRef]
- Nicholson, M.L.; Hosgood, S.A. Renal transplantation after ex vivo normothermic perfusion: The first clinical study. Am. J. Transplant. 2013, 13, 1246–1252. [Google Scholar] [CrossRef]
- Hosgood, S.A.; Callaghan, C.J.; Wilson, C.H.; Smith, L.; Mullings, J.; Mehew, J.; Oniscu, G.C.; Phillips, B.L.; Bates, L.; Nicholson, M.L. Normothermic machine perfusion versus static cold storage in donation after circulatory death kidney transplantation: A randomized controlled trial. Nat. Med. 2023, 29, 1511–1519. [Google Scholar] [CrossRef]
- Mazilescu, L.I.; Urbanellis, P.; Kim, S.J.; Goto, T.; Noguchi, Y.; Konvalinka, A.; Reichman, T.W.; Sayed, B.A.; Mucsi, I.; Lee, J.Y.; et al. Normothermic Ex Vivo Kidney Perfusion for Human Kidney Transplantation: First North American Results. Transplantation 2022, 106, 1852–1859. [Google Scholar] [CrossRef] [PubMed]
- Dumbill, R.; Knight, S.; Hunter, J.; Fallon, J.; Voyce, D.; Barrett, J.; Ellen, M.; Conroy, E.; Roberts, I.S.; James, T.; et al. Prolonged normothermic perfusion of the kidney prior to transplantation: A historically controlled, phase 1 cohort study. Nat. Commun. 2025, 16, 4584. [Google Scholar] [CrossRef] [PubMed]
- Weissenbacher, A.; Lo Faro, L.; Boubriak, O.; Soares, M.F.; Roberts, I.S.; Hunter, J.P.; Voyce, D.; Mikov, N.; Cook, A.; Ploeg, R.J.; et al. Twenty-four-hour normothermic perfusion of discarded human kidneys with urine recirculation. Am. J. Transplant. 2019, 19, 178–192. [Google Scholar] [CrossRef] [PubMed]
- Hosgood, S.A.; Saeb-Parsy, K.; Wilson, C.; Callaghan, C.; Collett, D.; Nicholson, M.L. Protocol of a randomised controlled, open-label trial of ex vivo normothermic perfusion versus static cold storage in donation after circulatory death renal transplantation. BMJ Open 2017, 7, e012237. [Google Scholar] [CrossRef]
- Minor, T.; von Horn, C.; Paul, A. Role of erythrocytes in short-term rewarming kidney perfusion after cold storage. Artif. Organs 2019, 43, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Rijkse, E.; de Jonge, J.; Kimenai, H.J.A.N.; Hoogduijn, M.J.; de Bruin, R.W.F.; van den Hoogen, M.W.F.; IJzermans, J.N.M.; Minnee, R.C. Safety and feasibility of 2 h of normothermic machine perfusion of donor kidneys in the Eurotransplant Senior Program. BJS Open 2021, 5, zraa024. [Google Scholar] [CrossRef]
- Puehringer, M.; Messner, F.; Schneeberger, S. Normothermic Machine Perfusion of Kidney Grafts: Devices, Endpoints, and Clinical Implementation. Eur. Surg. 2025, 57, 88–99. [Google Scholar] [CrossRef]
- Guarrera, J.V.; Goldstein, M.J.; Samstein, B.; Henry, S.; Reverte, C.; Arrington, B.; Brown, T.; Coleman, T.K.; Mattei, G.; Mendez, N.; et al. ‘When good kidneys pump badly’: Outcomes of deceased donor renal allografts with poor pulsatile perfusion characteristics. Transpl. Int. 2010, 23, 444–446. [Google Scholar] [CrossRef]
- Sonnenday, C.J.; Cooper, M.; Kraus, E.; Gage, F.; Handley, C.; Montgomery, R.A. The hazards of basing acceptance of cadaveric renal allografts on pulsatile perfusion parameters alone. Transplantation 2003, 75, 2029–2033. [Google Scholar] [CrossRef]
- Werenski, H.; Stratta, R.J.; Sharda, B.; Garner, M.; Farney, A.C.; Orlando, G.; McCracken, E.; Jay, C.L. Knowing When to Ignore the Numbers: Single-Center Experience Transplanting Deceased Donor Kidneys with Poor Perfusion Parameters. J. Am. Coll. Surg. 2023, 236, 848–857. [Google Scholar] [CrossRef]
- Hoetzenecker, K.; Ali, A.; Campo-Canaveral de la Cruz, J.; Schwarz, S.; Crowley Carrasco, S.; Romero Roman, A.; Aladaileh, M.; Benazzo, A.; Jaksch, P.; Wakeam, E.; et al. Prolonged Preservation of up to 24 Hours at 10 degrees C Does Not Impact Outcomes after Lung Transplantation. Ann. Surg. 2025, 281, 664–670. [Google Scholar] [CrossRef]
- Trahanas, J.M.; Harris, T.; Petrovic, M.; Dreher, A.; Pasrija, C.; DeVries, S.A.; Bommareddi, S.; Lima, B.; Wang, C.C.; Cortelli, M.; et al. Out of the ice age: Preservation of cardiac allografts with a reusable 10 degrees C cooler. JTCVS Open 2024, 21, 197–209. [Google Scholar] [CrossRef]
- Sousa Da Silva, R.X.; Darius, T.; Mancina, L.; Eden, J.; Wernle, K.; Ghoneima, A.S.; Barlow, A.D.; Clavien, P.A.; Dutkowski, P.; Kron, P. Real-time assessment of kidney allografts during HOPE using flavin mononucleotide (FMN)—A preclinical study. Front. Transplant. 2023, 2, 1132673. [Google Scholar] [CrossRef]
- van de Leemkolk, F.E.M.; Lo Faro, M.L.; Shaheed, S.; Mulvey, J.F.; Huurman, V.A.L.; Alwayn, I.P.J.; Putter, H.; Jochmans, I.; Lindeman, J.H.N.; Ploeg, R.J.; et al. The role of flavin mononucleotide (FMN) as a potentially clinically relevant biomarker to predict the quality of kidney grafts during hypothermic (oxygenated) machine perfusion. PLoS ONE 2023, 18, e0287713. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Thompson, E.; Bates, L.; Pither, T.L.; Hosgood, S.A.; Nicholson, M.L.; Watson, C.J.E.; Wilson, C.; Fisher, A.J.; Ali, S.; et al. Flavin Mononucleotide as a Biomarker of Organ Quality—A Pilot Study. Transplant. Direct 2020, 6, e600. [Google Scholar] [CrossRef] [PubMed]
- Siew, E.D.; Ware, L.B.; Ikizler, T.A. Biological markers of acute kidney injury. J. Am. Soc. Nephrol. 2011, 22, 810–820. [Google Scholar] [CrossRef] [PubMed]
- Haase, M.; Bellomo, R.; Devarajan, P.; Schlattmann, P.; Haase-Fielitz, A.; Group, N.M.-a.I. Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: A systematic review and meta-analysis. Am. J. Kidney Dis. 2009, 54, 1012–1024. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, Z.; Zhang, H.; Chen, H.; Hao, J.; Liu, H.; Li, X. Mitochondrial metabolism and targeted treatment strategies in ischemic-induced acute kidney injury. Cell Death Discov. 2024, 10, 69. [Google Scholar] [CrossRef] [PubMed]
- Reese, P.P.; Hall, I.E.; Weng, F.L.; Schroppel, B.; Doshi, M.D.; Hasz, R.D.; Thiessen-Philbrook, H.; Ficek, J.; Rao, V.; Murray, P.; et al. Associations between Deceased-Donor Urine Injury Biomarkers and Kidney Transplant Outcomes. J. Am. Soc. Nephrol. 2016, 27, 1534–1543. [Google Scholar] [CrossRef]
- Andrianova, N.V.; Buyan, M.I.; Zorova, L.D.; Pevzner, I.B.; Popkov, V.A.; Babenko, V.A.; Silachev, D.N.; Plotnikov, E.Y.; Zorov, D.B. Kidney Cells Regeneration: Dedifferentiation of Tubular Epithelium, Resident Stem Cells and Possible Niches for Renal Progenitors. Int. J. Mol. Sci. 2019, 20, 6326. [Google Scholar] [CrossRef]
- LaFavers, K.A.; Micanovic, R.; Sabo, A.R.; Maghak, L.A.; El-Achkar, T.M. Evolving Concepts in Uromodulin Biology, Physiology, and Its Role in Disease: A Tale of Two Forms. Hypertension 2022, 79, 2409–2418. [Google Scholar] [CrossRef]
- Mansour, S.G.; Liu, C.; Jia, Y.; Reese, P.P.; Hall, I.E.; El-Achkar, T.M.; LaFavers, K.A.; Obeid, W.; Rosenberg, A.Z.; Daneshpajouhnejad, P.; et al. Uromodulin to Osteopontin Ratio in Deceased Donor Urine Is Associated with Kidney Graft Outcomes. Transplantation 2021, 105, 876–885. [Google Scholar] [CrossRef] [PubMed]
| Preservation Temperature (°C) | Oxygenation | Preservation Time (Hours) | Key Benefits | Limitations | |
|---|---|---|---|---|---|
| SCS | 0–4 | No | Up to 24 (ideally) | Low cost Simple to operate Easy to transport | Higher risk of IRI |
| HMP | 1–10 | No | 24–36 | Reduces IRI Reduces rates of DGF | High cost Logistics Technical difficulties Need for trained personnel |
| HOPE | 1–10 | Yes | Variable | Reduces IRI ATP restoration | Mixed outcomes High cost Logistics Technical difficulties Need for trained personnel |
| NMP | 12–37 | Yes | 1–4 | Reduces IRI Physiologic function Viability assessment Upregulation of repair pathways | Ongoing clinical trial Upregulation of inflammatory pathways Very high cost Logistics Technical difficulties Need for trained personnel |
| Type of Study | Comparison | Study Groups | Type of Donors | Key Outcomes | Results | |
|---|---|---|---|---|---|---|
| Moers et al. 2009 [12] | Randomized controlled trial | SCS vs. HMP | SCS, n = 336 HMP, n = 336 | Deceased donor | DGF PNF Graft and patient survival | HMP decreased rate of DGF (adjusted odds ratio 0.57, p = 0.01) HMP improved 1-year allograft survival (94% vs. 90%, p = 0.04) |
| Treckmann et al. 2011 [13] | Randomized controlled trial | SCS vs. HMP | SCS, n = 91 HMP, n = 91 | ECD | DGF Non-function Graft survival | HMP decreased rate of DGF (odds ratio 0.46, p = 0.047) HMP decreased rate of non-function (3% vs. 12%, p = 0.04) HMP improved 1-year graft survival (92.3% vs. 80.2%, p = 0.02) |
| Jochmans et al. 2010 [14] | Randomized controlled trial | SCS vs. HMP | SCS, n = 82 HMP, n = 82 | Deceased donor | DGF | HMP decreased rate of DGF (adjusted odds ratio 0.43, p = 0.025) |
| Gil et al. 2014 [15] | Retrospective analysis | SCS vs. HMP | Standard criteria donors, n = 7192 ECD, n = 15,122 DCD, n = 8395 CIT groups: Increments of 6 h from 0 to 36 h | Standard criteria donors ECD DCD | DGF within each donor group by CIT categories | Standard criteria donor group: adjusted odds of DGF lower with HMP across all CIT groups ECD group: adjusted odds of DGF lower with HMP with CIT > 6 h DCD group: adjusted odds of DGF lower with HMP with CIT between 6 and 24 h |
| Jochmans et al. 2020 [17] | Randomized controlled trial | HMP vs. HOPE | HMP, n = 106 HOPE, n = 106 | DCD | One-year eGFR Post-operative complications Acute rejection Graft and patient survival | There were no differences in 1-year eGFR or 1-year patient survival in the HOPE group compared to the HMP group HOPE decreased rate of severe post-operative complications (11% vs. 16%, p = 0.032) HOPE decreased rate of acute rejection (14% vs. 26%, p = 0.04) HOPE decreased rate of 1-year graft loss (3% vs. 10%, p = 0.028) |
| Pravisani et al. 2022 [18] | Retrospective analysis | HMP vs. HOPE | HMP, n = 52 HOPE, n = 51 | DBD ECD | DGF Vascular and urologic complications Graft rejection One year creatinine serum levels | There were no differences in rates of DGF, vascular complications, urologic complications, episodes of graft rejection or 1-year serum creatinine levels between the HOPE group and the HMP group |
| Husen et al. 2021 [19] | Randomized controlled trial | SCS vs. HOPE | SCS, n = 135 HOPE, n = 127 | ECD | DGF PNF Acute rejection Graft survival | There were no differences in rates of DGF, PNF, acute rejection or graft survival between the HOPE group and the HMP group |
| Nicholson et al. 2013 [23] | Prospective study | SCS vs. NMP | SCS, n = 47 NMP, n = 18 | ECD | DGF Graft and patient survival | There was no difference in graft or patient survival between the NMP and SCS groups NMP decreased rate of DGF (5.6% vs. 36.2%, p = 0.014) |
| Hosgood et al. 2023 [24] | Randomized controlled trial | SCS vs. NMP | SCS, n = 168 NMP, n = 170 | DCD | DGF | There was no difference in rate of DGF between the NMP and SCS groups (58.5% vs. 60.7%, p = 0.624) |
| Mazilescu et al. 2022 [25] | Prospective study | HMP vs. HMP + NMP | HMP, n = 26 HMP + NMP, n = 13 | DBD DCD | DGF Graft and patient survival | There were no differences in rate of DGF or one-year graft and patient survival between the HMP and HMP + NMP groups |
| LifePort | Kidney Assist Transport (XVIVO) | RM4 (IGL) | Paragonix Kidney Vault | Kidney Assist (XVIVO) | ARK Kidney (Ebers—Undergoing Clinical Trial) | |
|---|---|---|---|---|---|---|
| Flow | Pulsatile | Pulsatile | Pulsatile | Pulsatile | Pulsatile | Pulsatile |
| Number of grafts | 1 kidney | 1 kidney | 1–2 kidneys | 1 kidney | 1 kidney | 1 kidney |
| Hypothermic or Normothermic | Hypothermic | Hypothermic | Hypothermic | Hypothermic | Normothermic | Normothermic |
| Oxygen | Optional | Yes | Yes | No | Yes | Yes |
| Temperature range (° C) | 1–8 | 2–10 | 2–8 | 4–8 | 12–37 | 15–30 |
| Key variables measured | Pressure, Flow rate, Resistance, Temperature | Pressure, Flow rate, Resistance, Temperature | Pressure, Flow rate, Resistance, Temperature | Pressure, Flow rate, Resistance, Temperature, Location tracking | Pressure, Flow rate, Resistance, Temperature | Not reported |
| Adjustable variables | Pressure | Pressure | Pressure | None | Pressure, Temperature, Oxygen | Not reported |
| Weight (kg) (fully loaded) | 20.4 | 28.4 | 20.0 | 11.3 | 68.0 | Not reported |
| Dimension (cm) | 61.96 × 36.83 × 36.2 | 61.5 × 39.5 × 34 | 51 × 37.8 × 21.9 | 45.72 × 40.64 × 43.18 | 112 × 92.5 × 62.5 | 54.0 × 90.0 × 52.5 |
| Regulatory status | FDA cleared | FDA cleared | FDA cleared | FDA cleared | CE marked * | CE marked * |
| Source | Function | Correlation with Outcomes | Strengths | Limitations | |
|---|---|---|---|---|---|
| FMN | Perfusate | Mitochondrial injury | Increased levels linked to increased rates of DGF and PNF | Non-invasive | Limited data |
| NGAL | Urine | Tubular injury | Increased levels linked to increased rate of DGF | Widely studied | Poor specificity |
| ATPSb | Perfusate | Mitochondrial health | Decreases IRI | Novel target | Limited clinical validation |
| UMOD | Urine | Tubular repair | Decreased levels linked to increased risk of AKI Increased levels linked to increased risk of graft failure | Potential repair marker | Not perfusate tested |
| OPN | Urine | Inflammation repair | Increased levels linked to increased risk of AKI Increased levels linked to lower risk of graft failure | Complementary to UMOD | Not perfusate tested |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ohara, S.Y.; Chavez-Villa, M.; Mao, S.; Clendenon, J.; Heimbach, J.; Ryan, R.; Kodali, L.; Nguyen, M.C.; Nateras-Nunez, R.; Jadlowiec, C.C. Advances in Kidney Transplant, Machine Perfusion, and Viability Markers. Kidney Dial. 2025, 5, 37. https://doi.org/10.3390/kidneydial5030037
Ohara SY, Chavez-Villa M, Mao S, Clendenon J, Heimbach J, Ryan R, Kodali L, Nguyen MC, Nateras-Nunez R, Jadlowiec CC. Advances in Kidney Transplant, Machine Perfusion, and Viability Markers. Kidney and Dialysis. 2025; 5(3):37. https://doi.org/10.3390/kidneydial5030037
Chicago/Turabian StyleOhara, Stephanie Y., Mariana Chavez-Villa, Shennen Mao, Jacob Clendenon, Julie Heimbach, Randi Ryan, Lavanya Kodali, Michelle C. Nguyen, Rafael Nateras-Nunez, and Caroline C. Jadlowiec. 2025. "Advances in Kidney Transplant, Machine Perfusion, and Viability Markers" Kidney and Dialysis 5, no. 3: 37. https://doi.org/10.3390/kidneydial5030037
APA StyleOhara, S. Y., Chavez-Villa, M., Mao, S., Clendenon, J., Heimbach, J., Ryan, R., Kodali, L., Nguyen, M. C., Nateras-Nunez, R., & Jadlowiec, C. C. (2025). Advances in Kidney Transplant, Machine Perfusion, and Viability Markers. Kidney and Dialysis, 5(3), 37. https://doi.org/10.3390/kidneydial5030037







