Asymptomatic Bacteriuria in Kidney Transplant Recipients: Always Not to Treat?
Abstract
1. Introduction
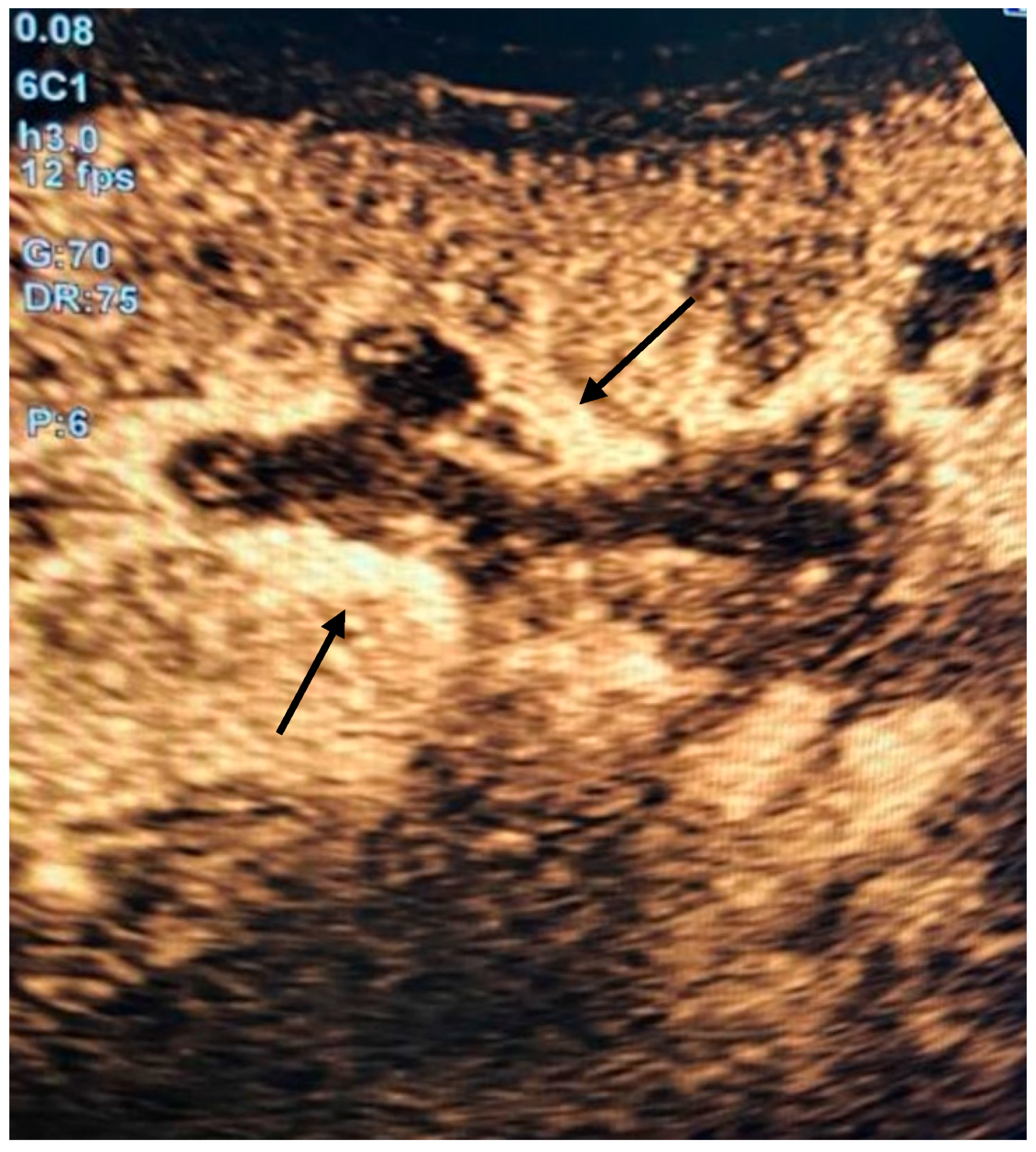
2. Detailed Case Description
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alangaden, G.J.; Thyagarajan, R.; Gruber, S.A.; Morawski, K.; Garnick, J.; El-Amm, J.M.; West, M.S.; Sillix, D.H.; Chandrasekar, P.H.; Haririan, A. Infectious complications after kidney transplantation: Current epidemiology and associated risk factors. Clin. Transplant. 2006, 20, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Gołębiewska, J.E.; Dębska-Ślizień, A.; Rutkowski, B. Urinary tract infections during the first year after renal transplantation: One center’s experience and a review of the literature. Clin. Transplant. 2014, 28, 1263–1270. [Google Scholar] [CrossRef] [PubMed]
- Mella, A.; Mariano, F.; Dolla, C.; Gallo, E.; Manzione, A.M.; Di Vico, M.C.; Cavallo, R.; De Rosa, F.G.; Costa, C.; Biancone, L. Bacterial and Viral Infection and Sepsis in Kidney Transplanted Patients. Biomedicines 2022, 10, 701. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.R.; Bang, H.; Dadhania, D.; Hartono, C.; Aull, M.J.; Satlin, M.; August, P.; Suthanthiran, M.; Muthukumar, T. Independent risk factors for urinary tract infection and for subsequent bacteremia or acute cellular rejection: A single-center report of 1166 kidney allograft recipients. Transplantation 2013, 96, 732–738. [Google Scholar] [CrossRef]
- Al-Hasan, M.N.; Razonable, R.R.; Kremers, W.K.; Baddour, L.M. Impact of Gram-negative bloodstream infection on long-term allograft survival after kidney transplantation. Transplantation 2011, 91, 1206–1210. [Google Scholar] [CrossRef]
- Ariza-Heredia, E.J.; Beam, E.N.; Lesnick, T.G.; Kremers, W.K.; Cosio, F.G.; Razonable, R.R. Urinary tract infections in kidney transplant recipients: Role of gender, urologic abnormalities, and antimicrobial prophylaxis. Ann. Transplant. 2013, 18, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Ariza-Heredia, E.J.; Beam, E.N.; Lesnick, T.G.; Cosio, F.G.; Kremers, W.K.; Razonable, R.R. Impact of urinary tract infection on allograft function after kidney transplantation. Clin. Transplant. 2014, 28, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Pellé, G.; Vimont, S.; Levy, P.P.; Hertig, A.; Ouali, N.; Chassin, C.; Arlet, G.; Rondeau, E.; Vandewalle, A. Acute pyelonephritis represents a risk factor impairing long-term kidney graft function. Am. J. Transplant. 2007, 7, 899–907. [Google Scholar] [CrossRef] [PubMed]
- Senger, S.S.; Arslan, H.; Azap, O.K.; Timurkaynak, F.; Cağir, U.; Haberal, M. Urinary tract infections in renal transplant recipients. Transplant. Proc. 2007, 39, 1016–1017. [Google Scholar] [CrossRef] [PubMed]
- Green, H.; Rahamimov, R.; Gafter, U.; Leibovitci, L.; Paul, M. Antibiotic prophylaxis for urinary tract infections in renal transplant recipients: A systematic review and meta-analysis. Transpl. Infect. Dis. 2011, 13, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Bemelman, F.J.; Hodiamont, C.J.; Idu, M.M.; Ten Berge, I.J.; Geerlings, S.E. The impact of trimethoprim-sulfamethoxazole as Pneumocystis jiroveci pneumonia prophylaxis on the occurrence of asymptomatic bacteriuria and urinary tract infections among renal allograft recipients: A retrospective before-after study. BMC Infect. Dis. 2016, 16, 90. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Thompson, E.R.; Hosgood, S.A.; Nicholson, M.L.; Wilson, C.H. Early versus late ureteric stent removal after kidney transplantation. Cochrane Database Syst. Rev. 2018, 1, CD011455. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Goodfellow, M.; Thompson, E.R.; Tingle, S.J.; Wilson, C. Early versus late removal of urinary catheter after kidney transplantation. Cochrane Database Syst. Rev. 2023, 7, CD013788. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- López-Medrano, F.; García-Bravo, M.; Morales, J.M.; Andrés, A.; San Juan, R.; Lizasoain, M.; Aguado, J.M. Urinary tract infection due to Corynebacterium urealyticum in kidney transplant recipients: An underdiagnosed etiology for obstructive uropathy and graft dysfunction-results of a prospective cohort study. Clin. Infect. Dis. 2008, 46, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Soriano, F.; Tauch, A. Microbiological and clinical features of Corynebacterium urealyticum: Urinary tract stones and genomics as the Rosetta Stone. Clin. Microbiol. Infect. 2008, 14, 632–643. [Google Scholar] [CrossRef] [PubMed]
- Heytens, S.; De Sutter, A.; Coorevits, L.; Cools, P.; Boelens, J.; Van Simaey, L.; Christiaens, T.; Vaneechoutte, M.; Claeys, G. Women with symptoms of a urinary tract infection but a negative urine culture: PCR-based quantification of Escherichia coli suggests infection in most cases. Clin. Microbiol. Infect. 2017, 23, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Goldman, J.D.; Julian, K. Urinary tract infections in solid organ transplant recipients: Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin. Transplant. 2019, 33, e13507. [Google Scholar] [CrossRef] [PubMed]
- Avni-Nachman, S.; Yahav, D.; Nesher, E.; Rozen-Zvi, B.; Rahamimov, R.; Mor, E.; Ben-Zvi, H.; Milo, Y.; Atamna, A.; Green, H. Short versus prolonged antibiotic treatment for complicated urinary tract infection after kidney transplantation. Transpl. Int. 2021, 34, 2686–2695. [Google Scholar] [CrossRef] [PubMed]
- Nicolle, L.E.; Bradley, S.; Colgan, R.; Rice, J.C.; Schaeffer, A.; Hooton, T.M. Infectious Diseases Society of America; American Society of Nephrology; American Geriatric Society. Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin. Infect. Dis. 2005, 40, 643–654, Erratum in Clin. Infect. Dis. 2005, 40, 1556. [Google Scholar] [CrossRef] [PubMed]
- Nicolle, L.E.; Gupta, K.; Bradley, S.F.; Colgan, R.; DeMuri, G.P.; Drekonja, D.; Eckert, L.O.; Geerlings, S.E.; Köves, B.; Hooton, T.M.; et al. Clinical Practice Guideline for the Management of Asymptomatic Bacteriuria: 2019 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2019, 68, 1611–1615. [Google Scholar] [CrossRef]
- Kelley, D.; Aaronson, P.; Poon, E.; McCarter, Y.S.; Bato, B.; Jankowski, C.A. Evaluation of an antimicrobial stewardship approach to minimize overuse of antibiotics in patients with asymptomatic bacteriuria. Infect. Control. Hosp. Epidemiol. 2014, 35, 193–195. [Google Scholar] [CrossRef] [PubMed]
- Fiorante, S.; López-Medrano, F.; Lizasoain, M.; Lalueza, A.; Juan, R.S.; Andrés, A.; Otero, J.R.; Morales, J.M.; Aguado, J.M. Systematic screening and treatment of asymptomatic bacteriuria in renal transplant recipients. Kidney Int. 2010, 78, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Coussement, J.; Maggiore, U.; Manuel, O.; Scemla, A.; López-Medrano, F.; Nagler, E.V.; Aguado, J.M.; Abramowicz, D.; European Renal Association-European Dialysis Transplant Association (ERA-EDTA) Developing Education Science and Care for Renal Transplantation in European States (DESCARTES) Working Group; European Study Group for Infections in Compromised Hosts (ESGICH) of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID). Diagnosis and management of asymptomatic bacteriuria in kidney transplant recipients: A survey of current practice in Europe. Nephrol. Dial. Transplant. 2018, 33, 1661–1668. [Google Scholar] [CrossRef] [PubMed]
- Coussement, J.; Scemla, A.; Abramowicz, D.; Nagler, E.V.; Webster, A.C. Antibiotics for asymptomatic bacteriuria in kidney transplant recipients. Cochrane Database Syst. Rev. 2018, 2, CD011357. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Medina-Polo, J.; Falkensammer, E.; Köves, B.; Kranz, J.; Tandogdu, Z.; Tapia, A.M.; Cai, T.; Wagenlehner, F.M.E.; Schneidewind, L.; Bjerklund Johansen, T.E.; et al. Systematic Review and Meta-Analysis Provide no Guidance on Management of Asymptomatic Bacteriuria within the First Year after Kidney Transplantation. Antibiotics 2024, 13, 442. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fiorentino, M.; Pesce, F.; Schena, A.; Simone, S.; Castellano, G.; Gesualdo, L. Updates on urinary tract infections in kidney transplantation. J. Nephrol. 2019, 32, 751–761. [Google Scholar] [CrossRef] [PubMed]
- Medani, S.; Dorais, M.; Poulin, A.; Tavares-Brum, A.; Mawad, H.; Duclos, A.; Barama, A.; Cardinal, H. Clinical Urinary Tract Infections in Kidney Transplant Recipients with Initially Asymptomatic Bacteriuria: A Single-Center Retrospective Cohort Study. Kidney Med. 2024, 7, 100946. [Google Scholar] [CrossRef]
- Salvadé, V.; Manuel, O.; Golshayan, D.; Obregon, C. Monocyte-derived dendritic cells can be detected in urine of kidney transplant recipients with pathogenic asymptomatic bacteriuria. Front. Transplant. 2024, 3, 1366104. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Langermann, S.; Palaszynski, S.; Barnhart, M.; Auguste, G.; Pinkner, J.S.; Burlein, J.; Barren, P.; Koenig, S.; Leath, S.; Jones, C.H.; et al. Prevention of mucosal Escherichia coli infection by FimH-adhesin-based systemic vaccination. Science 1997, 276, 607. [Google Scholar] [CrossRef]
- Langermann, S.; Möllby, R.; Burlein, J.E.; Palaszynski, S.R.; Auguste, C.G.; DeFusco, A.; Strouse, R.; Schenerman, M.A.; Hultgren, S.J.; Pinkner, J.S.; et al. Vaccination with FimH adhesin protects cynomolgus monkeys from colonization and infection by uropathogenic Escherichia coli. J. Infect. Dis. 2000, 181, 774–778. [Google Scholar] [CrossRef]
- Coussement, J.; Argudín, M.A.; Heinrichs, A.; Racapé, J.; de Mendonça, R.; Nienhaus, L.; Le Moine, A.; Roisin, S.; Dodémont, M.; Jacobs, F.; et al. Host and microbial factors in kidney transplant recipients with Escherichia coli acute pyelonephritis or asymptomatic bacteriuria: A prospective study using whole-genome sequencing. Nephrol. Dial. Transplant. 2019, 34, 878–885. [Google Scholar] [CrossRef] [PubMed]
- Nelson, Z.; Aslan, A.T.; Beahm, N.P.; Blyth, M.; Cappiello, M.; Casaus, D.; Dominguez, F.; Egbert, S.; Hanretty, A.; Khadem, T.; et al. Guidelines for the Prevention, Diagnosis, and Management of Urinary Tract Infections in Pediatrics and Adults: A WikiGuidelines Group Consensus Statement. JAMA Netw. Open 2024, 7, e2444495, Erratum in JAMA Netw. Open 2024, 7, e2453497. [Google Scholar] [CrossRef] [PubMed]
| Day −14 | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | Day 8 | |
|---|---|---|---|---|---|---|---|---|---|
| Creatinine (mg/dL) | 1.1 | 4.9 | 6.4 | 4.6 | 3.0 | 2.3 | 1.7 | 1.4 | 1.2 |
| Urea (mg/dL) | 37 | 96 | 209 | 225 | 184 | 126 | 84 | 59 | 48 |
| WBC (uL) | 5510 | 35,000 | 20,120 | 10,460 | 7750 | 8450 | 7590 | 9444 | 8280 |
| CRP (mg/dL) | 0.11 | 34 | 24 | 12.8 | 7.3 | 6.9 | 5.2 | 4.7 | 3.2 |
| Procalcitonin (ng/mL) | <0.5 | 130 | 71.6 | NA | NA | 3.7 | 1.5 | 0.8 | NA |
| Blood Pressure (mmHg) | 120/70 | 80/50 | 90/60 | 120/60 | 120/60 | 120/70 | 145/90 | 140/90 | 160/90 |
| Heart Ratio (bpm) | 76 | 90 | 82 | 63 | 77 | 77 | 97 | 70 | 95 |
| Body Temperature (°C) | 36.3 | 36.6 | 36.5 | 36.2 | 37.7 | 37.7 | 37.6 | 37.1 | 36.5 |
| Selected organism: | Escherichia coli | ||||
| Origin: | Blood culture | Collected on 18/AUG/2023 | |||
| ANTIBIOGRAM | |||||
| Antimicrobial | MIC | Interpretation | Antimicrobial | MIC | Interpretation |
| ESBL | NEG | - | |||
| Amoxicillin/clavulanic acid | >16 | R | Meropenem | ≤0.25 | S |
| Piperacillin/tazobactam | ≤4 | S | Imipenem | ≤0.25 | S |
| Cefotaxime | ≤0.25 | S | Amikacin | 4 | S |
| Ceftriaxone | ≤0.12 | S | Gentamicin | ≤1 | S |
| Ceftazidime | ≤0.12 | S | Tobramycin | ≤1 | S |
| Ceftazidime/avibactam | ≤0.12 | S | Ciprofloxacin | ≤0.06 | S |
| Ceftolozane/tazobactam | ≤0.25 | S | Colistin | ≤0.05 | S |
| Cefepime | ≤0.12 | S | Trimethoprim/Sulfamethoxazole | ≤20 | S |
| Selected organism: | Escherichia coli | ||||
| Origin: | Urine culture | Collected on 01/AUG/2023 | |||
| ANTIBIOGRAM | |||||
| Antimicrobial | MIC | Interpretation | Antimicrobial | MIC | Interpretation |
| ESBL | NEG | - | Meropenem | ≤0.25 | S |
| Amoxicillin/clavulanic acid | >16 | R | Ertapenem | ≤0.12 | S |
| Piperacillin/tazobactam | ≤4 | S | Imipenem | ≤0.25 | S |
| Cefotaxime | ≤0.25 | S | Amikacin | 4 | S |
| Ceftriaxone | ≤0.12 | S | Gentamicin | ≤1 | S |
| Ceftazidime | ≤0.12 | S | Ciprofloxacin | ≤0.06 | S |
| Cefepime | ≤0.12 | S | Nitrofurantoin | ≤16 | S |
| Fosfomycin | ≤16 | S | Trimethoprim/Sulfamethoxazole | ≤20 | S |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garofalo, C.; Ruotolo, C.; Nardelli, C.; Di Martino, L.; Cinone, F.; Prestano, R.; Fava, I.; Altruda, C.; Feliciano, M.F.; Russo, A.; et al. Asymptomatic Bacteriuria in Kidney Transplant Recipients: Always Not to Treat? Kidney Dial. 2025, 5, 28. https://doi.org/10.3390/kidneydial5030028
Garofalo C, Ruotolo C, Nardelli C, Di Martino L, Cinone F, Prestano R, Fava I, Altruda C, Feliciano MF, Russo A, et al. Asymptomatic Bacteriuria in Kidney Transplant Recipients: Always Not to Treat? Kidney and Dialysis. 2025; 5(3):28. https://doi.org/10.3390/kidneydial5030028
Chicago/Turabian StyleGarofalo, Carlo, Chiara Ruotolo, Christian Nardelli, Luigi Di Martino, Francesca Cinone, Raffaele Prestano, Ilaria Fava, Concetta Altruda, Maria Federica Feliciano, Antonio Russo, and et al. 2025. "Asymptomatic Bacteriuria in Kidney Transplant Recipients: Always Not to Treat?" Kidney and Dialysis 5, no. 3: 28. https://doi.org/10.3390/kidneydial5030028
APA StyleGarofalo, C., Ruotolo, C., Nardelli, C., Di Martino, L., Cinone, F., Prestano, R., Fava, I., Altruda, C., Feliciano, M. F., Russo, A., Borrelli, S., De Nicola, L., & Minutolo, R. (2025). Asymptomatic Bacteriuria in Kidney Transplant Recipients: Always Not to Treat? Kidney and Dialysis, 5(3), 28. https://doi.org/10.3390/kidneydial5030028







