1. Introduction
Citrate-anticoagulation is increasingly used for continuous venovenous hemofiltration (CVVH) in ICU patients due to its lower risk of bleeding [
1] as well as prolonged filter survival time [
2]. We recently reported that citrate anticoagulated CVVH may lead to loss of calcium in the ultrafiltrate, resulting in a substantially negative calcium balance [
3] despite serum ionized calcium levels well above the generally recommended target of at least 1 mmol/L [
4,
5]. Plasma calcium concentrations may remain normal despite calcium losses due to the body’s homeostatic mechanisms, most importantly the production of parathormone (PTH). Loss of calcium–citrate complexes in the CVVH ultrafiltrate will lead to increased PTH secretion, normalizing plasma ionized calcium concentration at the cost of calcium loss from the skeleton [
6]. Critical illness is associated with increased bone loss and osteoporosis and fractures are more frequent after ICU treatments [
7,
8,
9]. Additional losses of calcium by CVVH may increase these complications even more. To avoid net calcium losses during CVVH, calcium loss in the ultrafiltrate can be measured directly or calculated using a mathematical equation based on ultrafiltrate volume, plasma total calcium concentration, and blood pump flow rate [
3]. As an alternative, it has been suggested that increased PTH plasma levels could indicate net calcium losses and therefore be used as a trigger for more calcium supplementation [
6].
This study aimed to describe the association between the CVVH calcium balance and PTH plasma levels in critically ill patients on citrate-anticoagulated CVVH. We hypothesized that plasma PTH levels are increased in patients if they have a negative CVVH calcium balance and that measurement of plasma PTH could help identify patients who could benefit from additional calcium supplementation.
2. Materials and Methods
This is a retrospective cohort study of all patients, 18 years or older, treated with CVVH and citrate anticoagulation in the ICU of the Leiden University Medical Center (LUMC) from 1 June 2021 to 27 January 2023. The LUMC has a 24-bed mixed medical and surgical ICU. Patients were included if at least one plasma PTH measurement was performed. The relation between PTH and CVVH calcium balance was studied for all PTH measurements in patients treated with CVVH for at least 36 h in the 72 h preceding a PTH measurement.
All data used in this study were routinely collected. According to our local protocol, since June 2021, PTH was measured with 7-day intervals in all patients from the start of citrate CVVH. A generation second-generation intact PTH (normal range 0.7–8.0 pmol/L) Elecsys assay of Roche Diagnostics was used on the Cobas e602 Immunoanalyser (Cat nr: 11972103122). Long-term analytic variation was less than 5% within the reference range. The assay has a 99% cross-reactivity to the PTH fragment 7–84 compared to the intact 1–84 full-length PTH.
Free calcium (normal range 1.15–1.33 mmol/L) was measured four times daily.
CVVH parameters such as replacement flow, blood flow, fluid withdrawal by CVVH, calcium supplementation, and citrate dose were registered automatically every hour in the Patient Data Management System. Citrate CVVH was performed using Prismaflex® or PrisMax® equipment (Baxter International Inc., Deerfield, IL, USA) and either the HF1400 hemofilter (polyarylethysulfone (PAES) membrane, 1.4 m2) or ST150 hemofilter (AN 69 ST membrane, 1.5 m2) (Baxter International Inc., Deerfield, IL, USA). Citrate was administered pre-filter as Prismocitrate 18/0®, containing 18 mmol of citrate per liter as well as sodium (140 meq/L) and chloride (86 meq/L). At the initiation of CVVH, citrate was started at a rate of 2.4 mmol/L blood (8 mL prismocitrate 18/0 per mL/h blood flow). The rate of citrate administration could be lowered according to our local protocol if the ratio of free calcium to total calcium decreased, or the citrate dose could be increased in case of frequent clotting of the circuit. As a substitution fluid, we used Biphozyl® (Baxter Holding B.V., Utrecht, The Netherlands) containing no calcium, Phoxilium® (Baxter Holding B.V., Utrecht, The Netherlands) containing 1.25 mmol/L calcium, or Prismasol® (Baxter Holding B.V., Utrecht, The Netherlands) containing 1.75 mmol calcium per liter, depending on serum bicarbonate and potassium values. Extracorporeal calcium losses were managed by using the Prismaflex®/PrisMax® closed-loop algorithm for post-filter calcium/magnesium infusion (Ca-chloride 540 mmol/L and magnesium 240 mmol/L). Initially, calcium compensation was set at 100% and adjusted to maintain a target ionized calcium concentration within the range of 1.15–1.30 mmol/L. Free calcium was measured four times daily.
CVVH calcium balance was measured as the difference between calcium intake by CVVH and calcium excretion by CVVH. Calcium intake by CVVH was calculated as the sum of calcium in the replacement fluid and calcium given as post-filter Ca/Mg infusion. Calcium excretion in CVVH ultrafiltrate was calculated as described earlier [
3] using the equation: calcium excretion (mmol/24 h) = −1.2877 + 0.646 × [Ca]
blood,total × ultrafiltrate (l/24 h) + 0.107 × blood flow (mL/h). This equation was developed in our ICU and validated in an independent dataset of patients treated with the same CVVH protocol as in the present study [
3]. The mean error of the estimation was −1.0 ± 6.7 mmol/24 h. Calcium loss in the stools and calcium intake by enteral feeding was not included in the calculated CVVH calcium balance. According to our protocols, patients received Nutrison Protein Plus enteral feeding (Nutricia, Zoetermeer, The Netherlands), 1 mL/kg body weight/h containing 22 mmol calcium and 17 µg vitamin D per liter, or 1 mL/kg/h parenteral feeding (SmofKabiven, Fresenius Kabi, Huis ter Heide, The Netherlands) containing 2.53 mmol calcium per liter.
Statistical analyses were performed in SPSS (IBM Corp. Released 2017. IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY, USA: IBM Corp.). Concomitantly measured ionized calcium [Ca++] was the measured plasma [Ca++] at the exact time of PTH measurement or a maximum of 1 h earlier or later. Differences in categorical data were tested by the Chi-Square test. Continuous data were tested by Mann–Whitney U test or t-test depending on the distribution of the data. Normality of data was assessed using the Shapiro–Wilk test. One-way ANOVA was used for comparisons with more than two independent variables.
3. Results
From 1 June 2021 to 27 January 2023, 274 PTH-measurements were performed in 111 patients who were all treated with citrate CVVH at any time prior to the PTH measurement during ICU admission. Characteristics of the patients and measurements are shown in
Table 1. Median PTH was 2.4 pmol/L (IQR 1.6–6.5). Values higher than the upper limit of normal (8 pmol/L) were found in 61 (22%) measurements in 45 patients. In patients with increased PTH, the median PTH was 17.2 (IQR 12.7–30.1) pmol/L.
Based on the first available PTH value, patients with PTH higher than the upper limit of normal (8 pmol/L) were more severely ill at ICU admission as reflected by a higher APACHE IV score. The mean plasma ionized calcium was lower in patients with PTH measurements above 8 pmol/L (1.06 ± 0.14 mmol/L) compared to those with PTH < 8 pmol/L (1.21 ± 0.09 mmol/L, p = 0.001).
The time interval between a PTH measurement and the previous CVVH treatment was significantly longer for measurements with high PTH plasma concentrations (PTH > 8 pmol/L) at a median (IQR) of 0.8 (0–49.4) hours, compared to patients with PTH < 8 pmol/L, who had a median (IQR) of 0.4 (0–1.5) hours (p = 0.001).
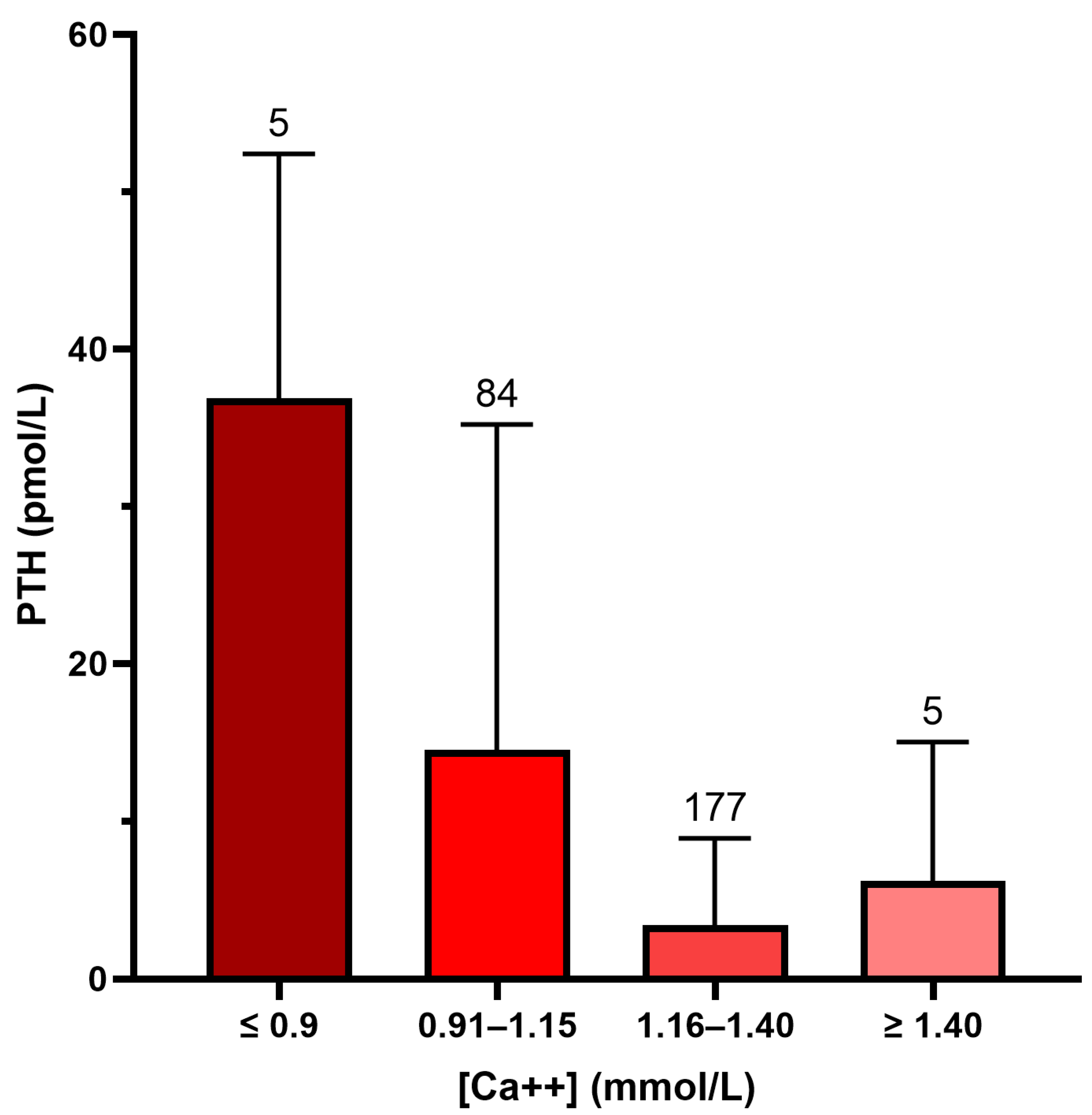
Figure 1 shows that PTH values were highest in patients with a low plasma ionized calcium (PTH 36.9 ± 15.5 pmol/L if [Ca++] < 0.9 mmol/L, PTH 14.5 ± 20.7 if [Ca++] 0.91–1.15, PTH 3.4 ± 5.5 if [Ca++] 1.16–1.40, and PTH 6.2 ± 8.7 if [Ca++] > 1.40;
p < 0.001 by One-way Anova).
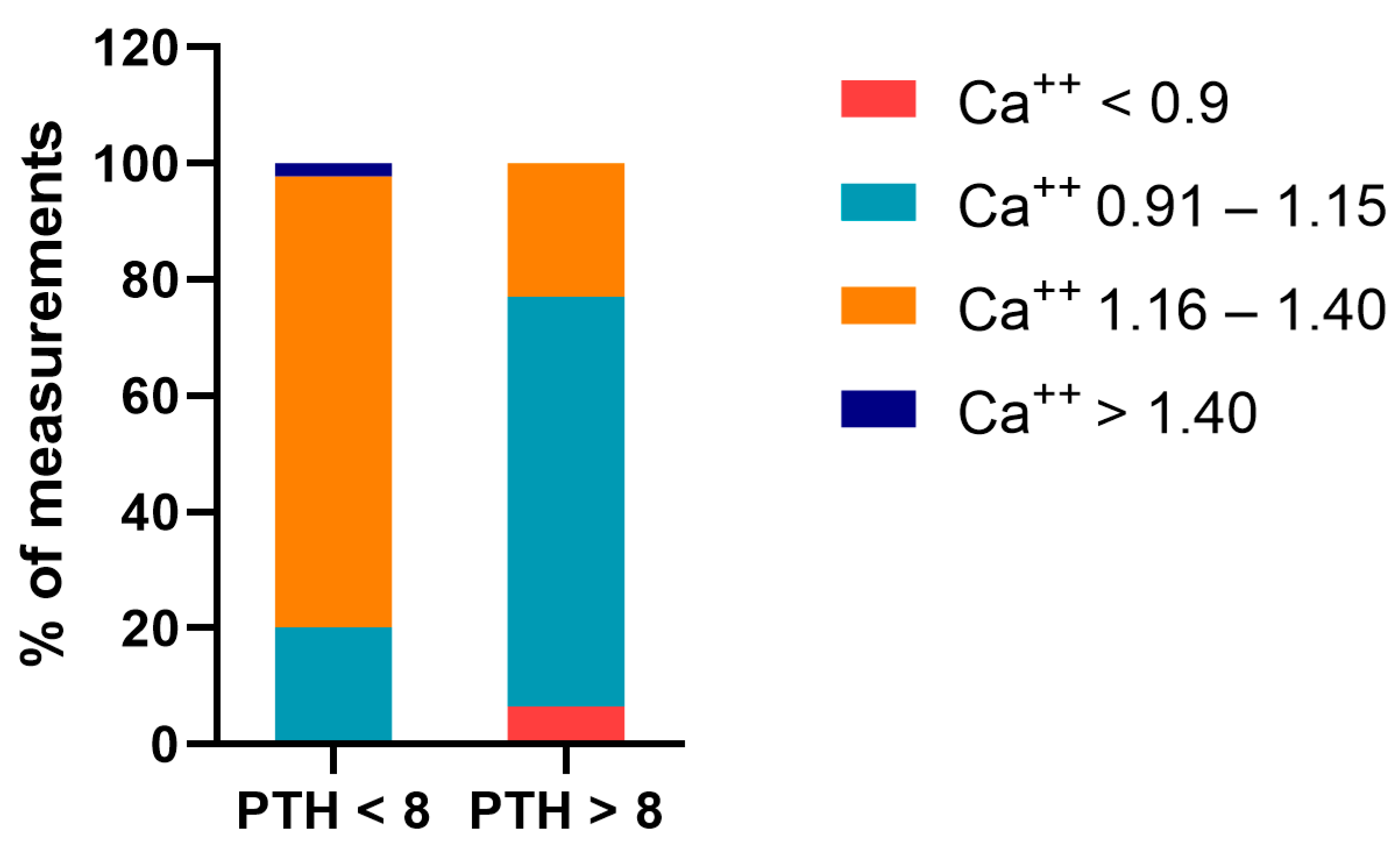
If the measurement of PTH was higher than the upper limit of normal (n = 61), plasma ionized calcium was less than 1.16 mmol/L in 77.1% of measurements. In contrast, this was only the case in 20.2% of measurements when the PTH was normal (
Figure 2).
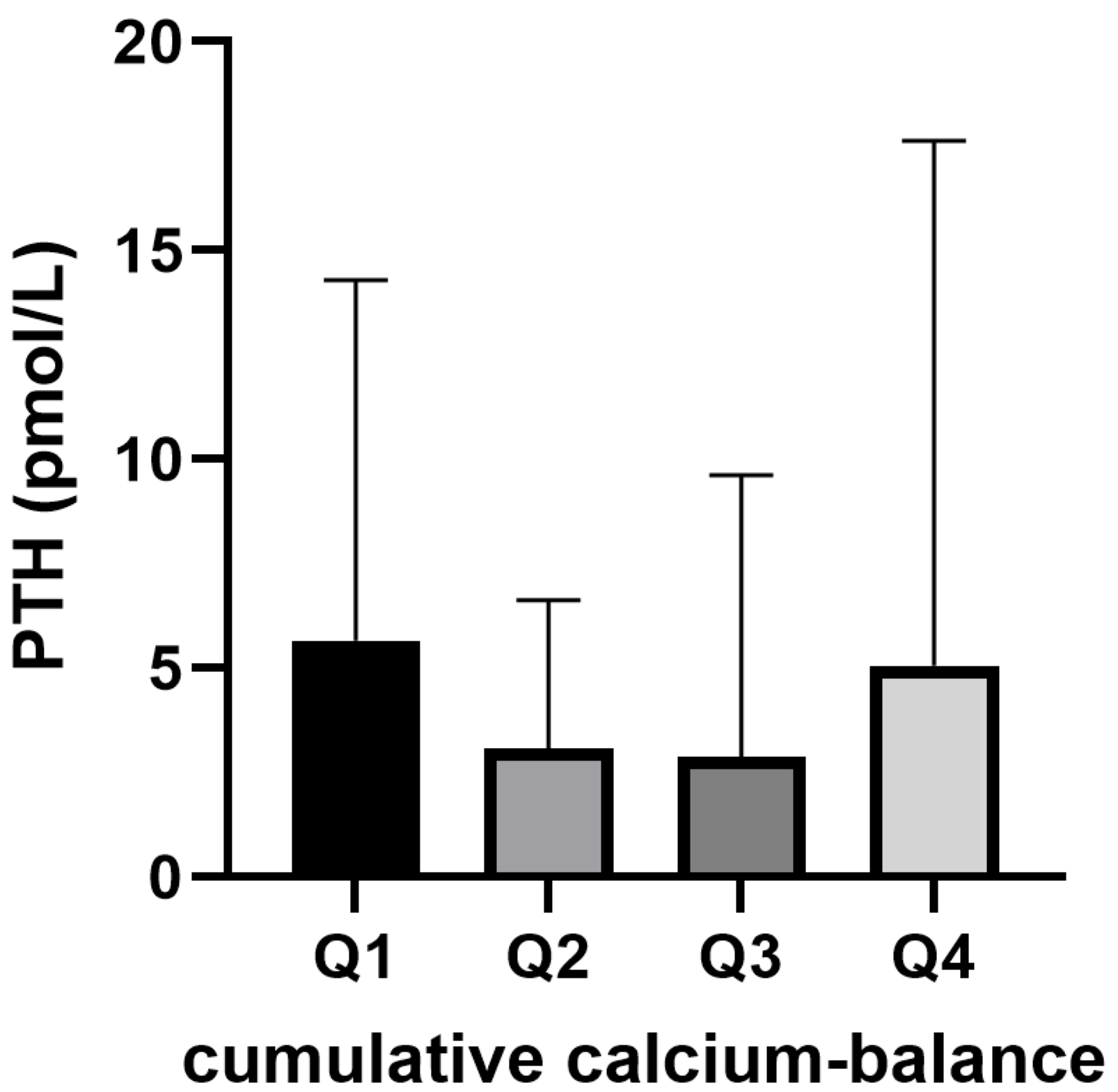
In total, 181 PTH-measurements were performed in 87 patients who were treated with citrate CVVH for at least 36 h within the three days preceding the PTH measurement. In this sub-population, the median PTH was 2.0 (IQR 1.3–2.9) pmol/L. PTH was higher than the upper limit of normal (8 pmol/L) in 16 samples (9%) in 11 patients. PTH levels did not differ between quartiles of the cumulative CVVH calcium balance over the last 72 h (
Figure 3).
The diagnostic accuracy of using the upper limit of normal PTH (8 pmol/L) as the cutoff for having a negative CVVH calcium balance is given in
Table 2.
4. Discussion
This study was designed to test the hypothesis that measuring plasma PTH levels could help identify patients with a negative CVVH calcium balance as being at risk of bone loss. In a population of ICU patients treated with citrate anticoagulated CVVH with a target plasma concentration of ionized calcium between 1.15 and 1.30 mmol/L, PTH values were frequently higher than the upper limit of normal but no association between PTH values and cumulative CVVH calcium balance over the preceding 72 h was identified.
We here showed that PTH values higher than normal were often identified (22% of measurements) and that in most cases these were secondary to low plasma ionized calcium levels. Primary hyperparathyroidism with plasma ionized calcium higher than 1.4 mmol/L was not present in any of our patients.
In this study, no correlation was found between PTH levels and cumulative CVVH calcium balance in the three days preceding the blood sampling. Importantly, a negative CVVH calcium balance was present in only 20% of the studied patients. PTH levels may be more often increased in populations of patients in whom calcium balances are more negative. In an earlier study from our ICU, we reported markedly negative calcium balances during citrate CVVH in the period in which we used another CVVH protocol aiming at lower plasma ionized calcium levels between 0.9 and 1.1 mmol/L, changing to positive calcium balances after increasing target ionized calcium to 1.15–1.3 mmol/L [
3]. Unfortunately, we did not measure plasma PTH levels during the initial period, so we are unable to compare both periods adequately.
Our findings differ from earlier publications. Van der Voort and others studied 27 ICU patients treated with CVVH with either citrate or nadroparin anticoagulation and 15 ICU patients not treated with CVVH (controls). They found plasma PTH concentrations higher than normal in almost all patients on citrate CVVH. In these patients, PTH levels were much higher than in patients with nadroparin CVVH and controls. Mean CVVH calcium balance was negative in patients on citrate CVVH, while positive in patients on nadroparin anticoagulated CVVH. A likely explanation for these different findings is the target plasma ionized calcium concentrations being 1.15–1.3 mmol/L in our study compared to 0.8–1.0 mmol/L in the earlier study [
6].
In the past, low target ionized calcium levels (much lower than normal physiologic values) were advocated in guidelines and position papers [
4,
5]. These low plasma ionized calcium targets were based on the observation that plasma ionized calcium is often decreased in critically ill patients and the assumption that this might be a protective mechanism [
4] based on occasional animal experiments [
5,
10]. Given the fact that low ionized calcium targets during CVVH lead to more negative calcium balances [
3] in ICU patients who are already prone to bone loss and fractures [
7,
8,
9], we recommend adhering to higher ionized calcium levels during citrate CVVH.
Interestingly, PTH levels exceeding the upper limit of normal were associated with a longer interval between PTH blood sampling and the last CVVH session. In 35% of such cases, CVVH was interrupted for at least 24 h at the time of PTH sampling. In the absence of CVVH, when there is no calcium loss in the ultrafiltrate, the elevated levels of PTH in the plasma should therefore be explained by an alternative mechanism. Although speculative, we suggest that this association could result from decreased clearance of PTH by the hemofilter after discontinuation of CVVH [
6,
11,
12]. In accordance, previous studies have demonstrated that both polysulfone and AN-69 membranes have the capacity to substantially absorb PTH (MW 9500 daltons) with limited losses in the ultrafiltrate [
13]. Furthermore, with the discontinuation of CVVH, supplementation of calcium was also stopped, potentially leading to lower ionized calcium and higher PTH levels. Indeed, in our cohort, calcium supplementation during CVVH was more than losses of calcium.
Some limitations of this study should be discussed. First, in patients with renal failure and also in patients on CVVH in the ICU, a substantial proportion of circulating PTH may be in the oxidized form [
14,
15]. Our assay can make no distinction between biologically inactive oxidized PTH and active non-oxidized PTH. Furthermore, findings may be much different in patients treated with CVVH protocols aiming at lower plasma ionized calcium levels. In these circumstances, PTH levels will be much higher than in our patients. Still, we do not recommend measuring PTH levels in those situations, but rather we advocate to increase the target plasma concentrations of ionized calcium during citrate CVVH to at least 1.15 mmol/L. Furthermore, we only studied the calcium balance as influenced by CVVH. We did not measure calcium excretion by the kidneys (likely negligible due to the frequent absence of diuresis) or in the stool and we did not include calcium intake by enteral or parenteral nutrition. Our recommendation to increase target plasma ionized calcium concentrations is based on its effect on calcium balance and bone loss. We cannot rule out the possibility that a higher target could have effects on other biological processes. It has been reported that lower calcium levels inhibit inflammatory pathways [
16] and, theoretically, this could improve outcomes other than bone loss in ICU patients. We could, however, find no evidence in the literature that any relevant clinical outcome is improved by hypocalcemia. Therefore, for now, the evidence is strongly in favor of avoiding marked hypocalcemia during citrate CVVH.
In conclusion, although increased PTH levels are frequently present, it is not necessary to measure PTH plasma levels to avoid bone loss during citrate CVVH. Instead, we recommend to adhere to higher plasma ionized calcium targets between 1.15 and 1.30 mmol/L. Earlier, we showed that this will prevent negative CVVH calcium balances in the vast majority of patients [
3]. Moreover, it will avoid hypocalcemia, which is closely associated with increased PTH levels. Theoretically, an increase in PTH levels leading to bone loss can still occur even with plasma ionized calcium levels within the target range if the body’s own homeostatic setpoint for PTH regulation is at a higher than normal plasma calcium concentration [
17]. Therefore, to be certain that the calcium balance during CVVH will not be negative, it is necessary to measure or calculate calcium excretion in the ultrafiltrate. Calculation of calcium excretion by CVVH is reliably possible using only plasma total calcium concentration, ultrafiltration rate, and CVVH blood flow [
3].
Author Contributions
Conceptualization, C.V.E.K., N.M.A.-D., B.E.P.B.B., N.A.d.F., D.J.v.W. and E.d.J.; Methodology, C.V.E.K. and E.d.J. Formal Analysis, C.V.E.K. and E.d.J.; Writing—Original Draft Preparation, C.V.E.K. and E.d.J.; Writing—Review and Editing, C.V.E.K., N.M.A.-D., B.E.P.B.B., N.A.d.F., D.J.v.W. and E.d.J. All authors have contributed significantly to the work. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The medical ethics committee of the Leiden University Medical Center (Medisch-Ethische Toetsingscommissie Leiden-Den Haag-Delft) waived the need for approval for this study (MEC waiver G21.169 and G21.191).
Informed Consent Statement
Patient consent was waived because this concerned a retrospective study on anonymized routinely collected data.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, E.d.J., upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gao, J.; Wang, F.; Wang, Y.; Jin, D.; Tang, L.; Pan, K. A mode of CVVH with regional citrate anticoagulation compared to no anticoagulation for acute kidney injury patients at high risk of bleeding. Sci. Rep. 2019, 9, 6607. [Google Scholar] [CrossRef]
- Joannidis, M.; Oudemans-van Straaten, H.M. Clinical review: Patency of the circuit in continuous renal replacement therapy. Crit. Care 2007, 11, 218. [Google Scholar] [CrossRef] [PubMed]
- de Jonge, E.; van der Vooren, M.; Gillis, J.; Del Prado, M.R.; Wigbers, J.; Bakhshi-Raiez, F.; Elzo Kraemer, C.V. Negative calcium balance despite normal plasma ionized calcium concentrations during citrate anticoagulated continuous venovenous hemofiltration (CVVH) in ICU patients. J. Nephrol. 2023, 36, 1019–1026. [Google Scholar] [CrossRef] [PubMed]
- Honore, P.M.; De Bels, D.; Preseau, T.; Redant, S.; Spapen, H.D. Citrate: How to Get Started and What, When, and How to Monitor? J. Transl. Int. Med. 2018, 6, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Oudemans-van Straaten, H.M.; Ostermann, M. Bench-to-bedside review: Citrate for continuous renal replacement therapy, from science to practice. Crit. Care 2012, 16, 249. [Google Scholar] [CrossRef] [PubMed]
- van der Voort, P.H.; Postma, S.R.; Kingma, W.P.; Boerma, E.C.; de Heide, L.J.; Bakker, A.J. An observational study on the effects of nadroparin-based and citrate-based continuous venovenous hemofiltration on calcium metabolism. Blood Purif. 2007, 25, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Orford, N.R.; Bailey, M.; Bellomo, R.; Pasco, J.A.; Cattigan, C.; Elderkin, T.; Brennan-Olsen, S.L.; Cooper, D.J.; Kotowicz, M.A. The association of time and medications with changes in bone mineral density in the 2 years after critical illness. Crit. Care 2017, 21, 69. [Google Scholar] [CrossRef] [PubMed]
- Orford, N.R.; Lane, S.E.; Bailey, M.; Pasco, J.A.; Cattigan, C.; Elderkin, T.; Brennan-Olsen, S.L.; Bellomo, R.; Cooper, D.J.; Kotowicz, M.A. Changes in Bone Mineral Density in the Year after Critical Illness. Am. J. Respir. Crit. Care Med. 2016, 193, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, A.F.; Kerschan-Schindl, K.; Scherkl, M.; Amrein, K. Bone metabolism and fracture risk during and after critical illness. Curr. Opin. Crit. Care 2020, 26, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Zaloga, G.P.; Sager, A.; Black, K.W.; Prielipp, R. Low dose calcium administration increases mortality during septic peritonitis in rats. Circ. Shock 1992, 37, 226–229. [Google Scholar] [PubMed]
- Czarnik, A.; Gawda, R.; Piwoda, M.; Marszalski, M.; Molsa, M.; Pietka, M.; Bolanowski, M.; Czarnik, T. Parathyroid hormone serum concentration kinetic profile in critically ill patients undergoing continuous renal replacement therapies: A prospective observational study. Endokrynol. Pol. 2021, 72, 329–335. [Google Scholar] [PubMed]
- De Francisco, A.L.; Amado, J.A.; Prieto, M.; Alcalde, G.; Sanz de Castro, S.; Ruiz, J.C.; Morales, P.; Arias, M. Dialysis membranes and PTH changes during hemodialysis in patients with secondary hyperparathyroidism. Nephron 1994, 66, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Balducci, A.; Coen, G.; Manni, M.; Perruzza, I.; Fassino, V.; Sardella, D.; Grandi, F. In vivo assessment of intact parathyroid hormone adsorption by different dialysis membranes during hemodialysis. Artif. Organs 2004, 28, 1067–1075. [Google Scholar] [CrossRef] [PubMed]
- Hocher, B.; Armbruster, F.P.; Stoeva, S.; Reichetzeder, C.; Grön, H.J.; Lieker, I.; Khadzhynov, D.; Slowinski, T.; Roth, H.J. Measuring parathyroid hormone (PTH) in patients with oxidative stress—Do we need a fourth generation parathyroid hormone assay? PLoS ONE 2012, 7, e40242. [Google Scholar] [CrossRef]
- Boer, W.; Fivez, T.; Vander Laenen, M.; Bruckers, L.; Grön, H.J.; Schetz, M.; Oudemans-van Straaten, H. Citrate dose for continuous hemofiltration: Effect on calcium and magnesium balance, parathormone and vitamin D status, a randomized controlled trial. BMC Nephrol. 2021, 22, 409. [Google Scholar] [CrossRef]
- Dong, Z.; Saikumar, P.; Weinberg, J.M.; Venkatachalam, M.A. Calcium in cell injury and death. Annu. Rev. Pathol. 2006, 1, 405–434. [Google Scholar] [CrossRef] [PubMed]
- Malberti, F.; Farina, M.; Imbasciati, E. The PTH-calcium curve and the set point of calcium in primary and secondary hyperparathyroidism. Nephrol. Dial. Transplant. 1999, 14, 2398–2406. [Google Scholar] [CrossRef] [PubMed][Green Version]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).