Myocardial Remodeling in Early Chronic Kidney Disease—Mineral and Bone Disorder Model with Low Bone Turnover
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Laboratory Measurements
2.3. Inductively Coupled Plasma Atomic Emission Spectroscopy
2.4. Real-Time Polymerase Chain Reaction
2.5. Histology
2.6. Quantitative Morphometry
2.7. Statistical Analyses
3. Results
3.1. Animal Models of Chronic Kidney Disease—Mineral and Bone Disorder with Low Bone Turnover
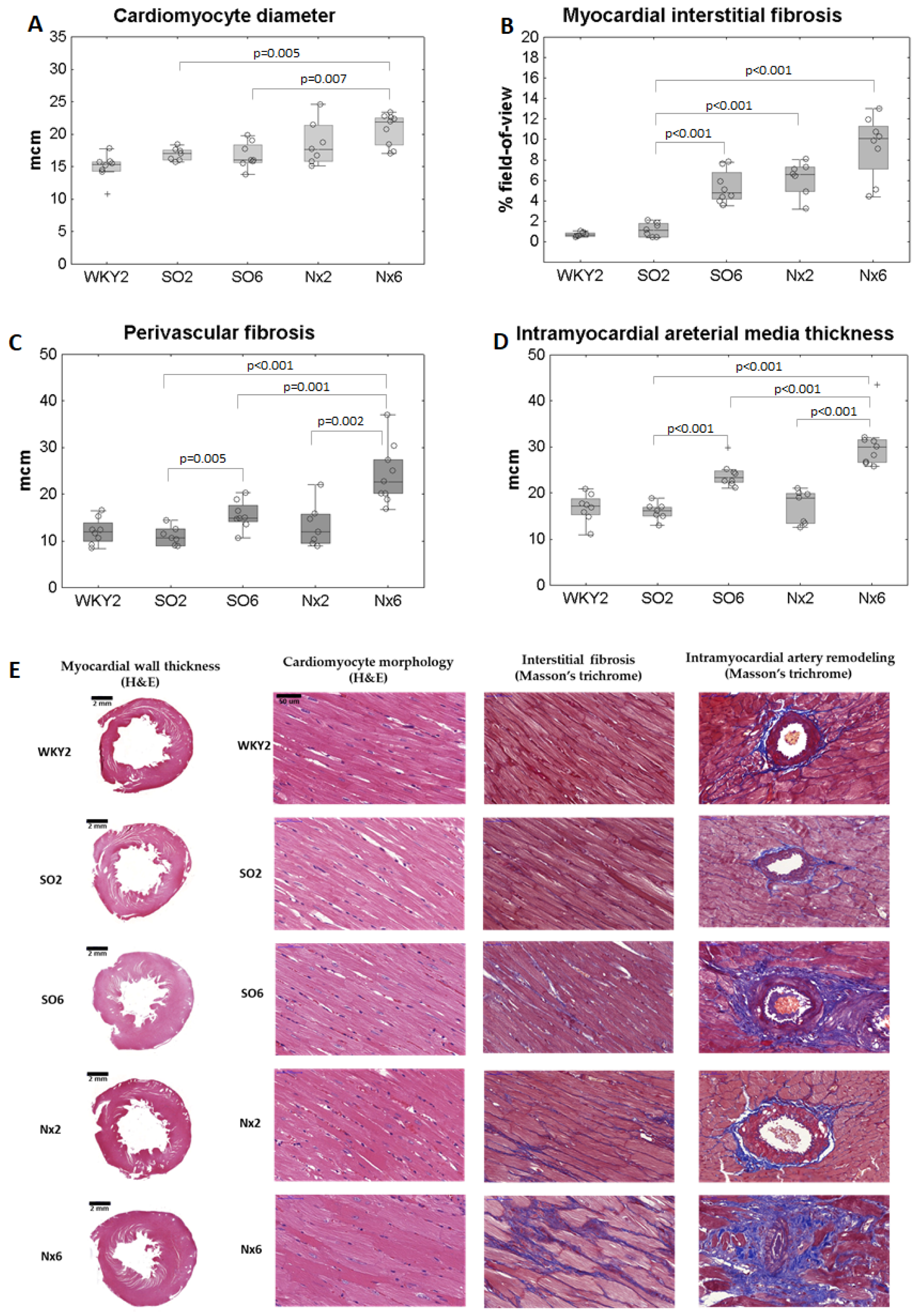
3.2. Myocardial Remodeling in Applied CKD-MBD Models
3.3. Myocardial Histology and Phosphate Indexes
3.4. Gene Expression Profile Related to Myocardial Hypertrophy and Fibrosis in Mild CKD-MBD
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Go, A.S.; Chertow, G.M.; Fan, D.; McCulloch, C.E.; Hsu, C.Y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N. Engl. J. Med. 2004, 351, 1296–1305. [Google Scholar] [CrossRef] [PubMed]
- Jankowski, J.; Floege, J.; Fliser, D.; Böhm, M.; Marx, N. Cardiovascular Disease in Chronic Kidney Disease: Pathophysiological Insights and Therapeutic Options. Circulation 2021, 143, 1157–1172. [Google Scholar] [CrossRef] [PubMed]
- Blecker, S.; Matsushita, K.; Köttgen, A.; Loehr, L.R.; Bertoni, A.G.; Boulware, L.E.; Coresh, J. High-normal albuminuria and risk of heart failure in the community. Am. J. Kidney Dis. 2011, 58, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Parfrey, P.S.; Harnett, J.D.; Griffiths, S.M.; Taylor, R.; Hand, J.; King, A.; Barre, P.E. The clinical course of left ventricular hypertrophy in dialysis patients. Nephron 1990, 55, 114–120. [Google Scholar] [CrossRef]
- Levin, A.; Singer, J.; Thompson, C.R.; Ross, H.; Lewis, M. Prevalent left ventricular hypertrophy in the predialysis population: Identifying opportunities for intervention. Am. J. Kidney Dis. 1996, 27, 347–354. [Google Scholar] [CrossRef]
- Xie, J.; Yoon, J.; An, S.W.; Kuro-o, M.; Huang, C.L. Soluble Klotho Protects against Uremic Cardiomyopathy Independently of Fibroblast Growth Factor 23 and Phosphate. J. Am. Soc. Nephrol. 2015, 26, 1150–1160. [Google Scholar] [CrossRef]
- Buckalew, V.M., Jr.; Berg, R.L.; Wang, S.R.; Porush, J.G.; Rauch, S.; Schulman, G. Prevalence of hypertension in 1795 subjects with chronic renal disease: The modification of diet in renal disease study baseline cohort. Modification of Diet in Renal Disease Study Group. Am. J. Kidney Dis. 1996, 28, 811–821. [Google Scholar] [CrossRef]
- Nadruz, W. Myocardial remodeling in hypertension. J. Hum. Hypertens. 2015, 29, 1–6. [Google Scholar] [CrossRef]
- Silberberg, J.S.; Barre, P.E.; Prichard, S.S.; Sniderman, A.D. Impact of left ventricular hypertrophy on survival in end-stage renal disease. Kidney Int. 1989, 36, 286–290. [Google Scholar] [CrossRef]
- London, G.M. Left ventricular alterations and end-stage renal disease. Nephrol. Dial. Transplant. 2002, 17 (Suppl. S1), 29–36. [Google Scholar] [CrossRef]
- Williams, M.J.; White, S.C.; Joseph, Z.; Hruska, K.A. Updates in the chronic kidney disease-mineral bone disorder show the role of osteocytic proteins, a potential mechanism of the bone-Vascular paradox, a therapeutic target, and a biomarker. Front. Physiol. 2023, 14, 1120308. [Google Scholar] [CrossRef] [PubMed]
- Drüeke, T.B.; Massy, Z.A. Changing bone patterns with progression of chronic kidney disease. Kidney Int. 2016, 89, 289–302. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.C.; Ferrari, G.O.; Neves, K.R.; Cavallari, R.T.; Dominguez, W.V.; dos Reis, L.M.; Graciolli, F.G.; Oliveira, E.C.; Liu, S.; Sabbagh, Y.; et al. Effects of dietary phosphate on adynamic bone disease in rats with chronic kidney disease—Role of sclerostin? PLoS ONE 2013, 8, e79721. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki-Ishizuka, Y.; Yamato, H.; Nii-Kono, T.; Kurokawa, K.; Fukagawa, M. Downregulation of parathyroid hormone receptor gene expression and osteoblastic dysfunction associated with skeletal resistance to parathyroid hormone in a rat model of renal failure with low turnover bone. Nephrol. Dial. Transplant. 2005, 20, 1904–1911. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Ginsberg, C.; Seifert, M.; Agapova, O.; Sugatani, T.; Register, T.C.; Freedman, B.I.; Monier-Faugere, M.-C.; Malluche, H.; Hruska, K.A. CKD-induced wingless/integration1 inhibitors and phosphorus cause the CKD-mineral and bone disorder. J. Am. Soc. Nephrol. 2014, 25, 1760–1773. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, P.; Sharp, C.A.; Magnusson, M.; Risteli, J.; Davie, M.W.; Larsson, L. Effect of chronic renal failure on bone turnover and bone alkaline phosphatase isoforms. Kidney Int. 2001, 60, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Nickolas, T.L.; Stein, E.M.; Dworakowski, E.; Nishiyama, K.K.; Komandah-Kosseh, M.; Zhang, C.A.; McMahon, D.J.; Liu, X.S.; Boutroy, S.; Cremers, S.; et al. Rapid cortical bone loss in patients with chronic kidney disease. J. Bone Miner. Res. 2013, 28, 1811–1820. [Google Scholar] [CrossRef]
- Tasnim, N.; Dutta, P.; Nayeem, J.; Masud, P.; Ferdousi, A.; Ghosh, A.S.; Hossain, M.; Rajia, S.; Kubra, K.T.; Sakibuzzaman, M.; et al. Osteoporosis, an Inevitable Circumstance of Chronic Kidney Disease: A Systematic Review. Cureus 2021, 13, e18488. [Google Scholar] [CrossRef]
- Malluche, H.H.; Ritz, E.; Lange, H.P.; Kutschera, J.; Hodgson, M.; Seiffert, U.; Schoeppe, W. Bone histology in incipient and advanced renal failure. Kidney Int. 1976, 9, 355–362. [Google Scholar] [CrossRef]
- Raggi, P.; Bellasi, A.; Bushinsky, D.; Bover, J.; Rodriguez, M.; Ketteler, M.; Sinha, S.; Salcedo, C.; Gillotti, K.; Padgett, C.; et al. Slowing Progression of Cardiovascular Calcification with SNF472 in Patients on Hemodialysis: Results of a Randomized Phase 2b Study. Circulation 2020, 141, 728–739. [Google Scholar] [CrossRef]
- Ogata, H.; Fukagawa, M.; Hirakata, H.; Kagimura, T.; Fukushima, M.; Akizawa, T.; LANDMARK Investigators and Committees. Effect of Treating Hyperphosphatemia with Lanthanum Carbonate vs Calcium Carbonate on Cardiovascular Events in Patients with Chronic Kidney Disease Undergoing Hemodialysis: The LANDMARK Randomized Clinical Trial. JAMA 2021, 325, 1946–1954. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, J.P.; Zhou, A.; Hyppönen, E. Vitamin D Deficiency Increases Mortality Risk in the UK Biobank: A Nonlinear Mendelian Randomization Study. Ann. Intern. Med. 2022, 175, 1552–1559. [Google Scholar] [CrossRef] [PubMed]
- Malluche, H.H.; Mawad, H.W.; Monier-Faugere, M.C. Renal osteodystrophy in the first decade of the new millennium: Analysis of 630 bone biopsies in black and white patients. J. Bone Miner. Res. 2011, 26, 1368–1376. [Google Scholar] [CrossRef] [PubMed]
- Sprague, S.M.; Bellorin-Font, E.; Jorgetti, V.; Carvalho, A.B.; Malluche, H.H.; Ferreira, A.; D’Haese, P.C.; Drüeke, T.B.; Du, H.; Manley, T. Diagnostic accuracy of bone turnover markers and bone histology in patients with CKD treated by dialysis. Am. J. Kidney Dis. 2016, 67, 559–566. [Google Scholar] [CrossRef]
- El-Husseini, A.; Abdalbary, M.; Lima, F.; Issa, M.; Ahmed, M.-T.; Winkler, M.; Srour, H.; Davenport, D.; Wang, G.; Faugere, M.-C. Low turnover renal osteodystrophy with abnormal bone quality and vascular calcification in patients with mild-to-moderate CKD. Kidney Int. Rep. 2022, 7, 1016–1026. [Google Scholar] [CrossRef] [PubMed]
- Smogorzewski, M.; Zayed, M.; Zhang, Y.B.; Roe, J.; Massry, S.G. Parathyroid hormone increases cytosolic calcium concentration in adult rat cardiac myocytes. Am. J. Physiol. 1993, 264 Pt 2, H1998–H2006. [Google Scholar] [CrossRef]
- Maulik, S.K.; Mishra, S. Hypertrophy to failure: What goes wrong with the fibers of the heart? Indian Heart J. 2015, 67, 66–69. [Google Scholar] [CrossRef]
- Intengan, H.D.; Schiffrin, E.L. Vascular remodeling in hypertension: Roles of apoptosis, inflammation, and fibrosis. Hypertension 2001, 38 Pt 2, 581–587. [Google Scholar] [CrossRef]
- Korsgaard, N.; Mulvany, M.J. Cellular hypertrophy in mesenteric resistance vessels from renal hypertensive rats. Hypertension 1988, 12, 162–167. [Google Scholar] [CrossRef]
- Molkentin, J.D.; Lu, J.R.; Antos, C.L.; Markham, B.; Richardson, J.; Robbins, J.; Grant, S.R.; Olson, E.N. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell 1998, 93, 215–228. [Google Scholar] [CrossRef]
- Bacmeister, L.; Schwarzl, M.; Warnke, S.; Stoffers, B.; Blankenberg, S.; Westermann, D.; Lindner, D. Inflammation and fibrosis in murine models of heart failure. Basic Res. Cardiol. 2019, 114, 19. [Google Scholar] [CrossRef]
- Shimizu, I.; Minamino, T. Physiological and pathological cardiac hypertrophy. J. Mol. Cell Cardiol. 2016, 97, 245–262. [Google Scholar] [CrossRef]
- Liu, Y.-L.; Huang, C.-C.; Chang, C.-C.; Chou, C.-Y.; Lin, S.-Y.; Wang, I.-K.; Hsieh, D.J.-Y.; Jong, G.-P.; Huang, C.-Y.; Wang, C.-M. Hyperphosphate-Induced Myocardial Hypertrophy through the GATA-4/NFAT-3 Signaling Pathway Is Attenuated by ERK Inhibitor Treatment. CardioRenal Med. 2015, 5, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Bogdanova, E.; Beresneva, O.; Galkina, O.; Zubina, I.; Ivanova, G.; Parastaeva, M.; Semenova, N.; Dobronravov, V. Myocardial Hypertrophy and Fibrosis Are Associated with Cardiomyocyte Beta-Catenin and TRPC6/Calcineurin/NFAT Signaling in Spontaneously Hypertensive Rats with 5/6 Nephrectomy. Int. J. Mol. Sci. 2021, 22, 4645. [Google Scholar] [CrossRef] [PubMed]
- Gross, M.L.; Ritz, E. Hypertrophy and fibrosis in the cardiomyopathy of uremia--beyond coronary heart disease. Semin. Dial. 2008, 21, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Creemers, E.E.; Pinto, Y.M. Molecular mechanisms that control interstitial fibrosis in the pressure-overloaded heart. Cardiovasc. Res. 2011, 89, 265–272. [Google Scholar] [CrossRef]
- Weber, K.T.; Brilla, C.G. Pathological hypertrophy and cardiac interstitium. Fibrosis and renin-angiotensin-aldosterone system. Circulation 1991, 83, 1849–1865. [Google Scholar] [CrossRef]
- Code of Practice for the Housing and Care of Animals Bred, Supplied or Used for Scientific Purposes Presented to Parliament Pursuant to Section 21 (5) of the Animals (Scientific Procedures) ACT 1986; Home Office: London, UK, 2014.
- Directive 2010/63/EU of the European Parliament and of the Council; Official Journal of the European Union, Publications Office of the European Unio: Luxembourg, 2020.
- Guide for the Care and Use of Laboratory Animals, 8th edition National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals; National Academies Press (US): Washington, DC, USA, 2011.
- Lee, H.B.; Blaufox, M.D. Blood volume in the rat. J. Nucl. Med. 1985, 26, 72–76. [Google Scholar]
- Fleischer, H.; Vorberg, E.; Thurow, K.; Warkentin, M.; Behrend, D. Determination of Calcium and Phosphor in Bones Using Microwave Digestion and ICP-MS. In Imeko Tc19 Symp, 5th ed.; International Measurement Confederation (IMEKO): Lecce, Italy, 2014; ISBN 978-92-990073-6-5. [Google Scholar]
- Erben, R.G.; Glösmann, M. Histomorphometry in Rodents. In Bone Research Protocols. Methods in Molecular Biology; Idris, A., Ed.; Humana Press: New York, NY, USA, 2019; Volume 1914. [Google Scholar]
- Dempster, D.W.; Compston, J.E.; Drezner, M.K.; Glorieux, F.H.; Kanis, J.A.; Malluche, H.; Meunier, P.J.; Ott, S.M.; Recker, R.R.; Parfitt, A.M. Standardized nomenclature, symbols, and units for bone histomorphometry: A 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J. Bone Miner. Res. 2013, 28, 2–17. [Google Scholar] [CrossRef]
- Aoki, J.; Ikari, Y.; Nakajima, H.; Mori, M.; Sugimoto, T.; Hatori, M.; Tanimoto, S.; Amiya, E.; Hara, K. Clinical and pathologic characteristics of dilated cardiomyopathy in hemodialysis patients. Kidney Int. 2005, 67, 333–340. [Google Scholar] [CrossRef]
- Law, J.P.; Pickup, L.; Pavlovic, D.; Townend, J.N.; Ferro, C.J. Hypertension and cardiomyopathy associated with chronic kidney disease: Epidemiology, pathogenesis and treatment considerations. J. Hum. Hypertens. 2023, 37, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Mark, P.B.; Johnston, N.; Groenning, B.A.; Foster, J.E.; Blyth, K.G.; Martin, T.N.; Steedman, T.; Dargie, H.J.; Jardine, A.G. Redefinition of uremic cardiomyopathy by contrast-enhanced cardiac magnetic resonance imaging. Kidney Int. 2006, 69, 1839–1845. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhang, J.; Kalantar-Zadeh, K.; Chen, J. Focusing on Phosphorus Loads: From Healthy People to Chronic Kidney Disease. Nutrients 2023, 15, 1236. [Google Scholar] [CrossRef] [PubMed]
- Bevington, A.; Mundy, K.I.; Yates, A.J.; Kanis, J.A.; Russell, R.G.; Taylor, D.J.; Rajagopalan, B.; Radda, G.K. A study of intracellular orthophosphate concentration in human muscle and erythrocytes by 31P nuclear magnetic resonance spectroscopy and selective chemical assay. Clin. Sci. 1986, 71, 729–735. [Google Scholar] [CrossRef]
- Chazot, G.; Lemoine, S.; Kocevar, G.; Kalbacher, E.; Sappey-Marinier, D.; Rouvière, O.; Juillard, L. Intracellular Phosphate and ATP Depletion Measured by Magnetic Resonance Spectroscopy in Patients Receiving Maintenance Hemodialysis. J. Am. Soc. Nephrol. 2021, 32, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Polovlkova, O.G.; Makeeva, O.A.; Lezhnev, A.A.; Goncharova, I.A.; Kulish, E.V.; Shipulin, V.M.; Puzyrev, V.P. Expression profile of calcineurin pathway genes in myocardium tissues in relation to ischemic heart remodeling in humans. Mol. Biol. 2013, 47, 433–440. (In Russian) [Google Scholar]
- Heineke, J.; Wollert, K.C.; Osinska, H.; Sargent, M.A.; York, A.J.; Robbins, J.; Molkentin, J.D. Calcineurin protects the heart in a murine model of dilated cardiomyopathy. J. Mol. Cell Cardiol. 2010, 48, 1080–1087. [Google Scholar] [CrossRef][Green Version]
- Ha, S.W.; Park, J.; Habib, M.M.; Beck, G.R., Jr. Nano-Hydroxyapatite Stimulation of Gene Expression Requires Fgf Receptor, Phosphate Transporter, and Erk1/2 Signaling. ACS Appl. Mater. Interfaces 2017, 9, 39185–39196. [Google Scholar] [CrossRef]
- Bon, N.; Couasnay, G.; Bourgine, A.; Sourice, S.; Beck-Cormier, S.; Guicheux, J.; Beck, L. Phosphate (Pi)-regulated heterodimerization of the high-affinity sodium-dependent Pi transporters PiT1/Slc20a1 and PiT2/Slc20a2 underlies extracellular Pi sensing independently of Pi uptake. J. Biol. Chem. 2018, 293, 2102–2114. [Google Scholar] [CrossRef]
- Szeri, F.; Niaziorimi, F.; Donnelly, S.; Fariha, N.; Tertyshnaia, M.; Patel, D.; Lundkvist, S.; Wetering, K. The Mineralization Regulator ANKH Mediates Cellular Efflux of ATP, Not Pyrophosphate. J. Bone Miner. Res. 2022, 37, 1024–1031. [Google Scholar] [CrossRef]
- Huang, C.K.; Dai, D.; Xie, H.; Zhu, Z.; Hu, J.; Su, M.; Liu, M.; Lu, L.; Shen, W.; Ning, G.; et al. Lgr4 Governs a Pro-Inflammatory Program in Macrophages to Antagonize Post-Infarction Cardiac Repair. Circ. Res. 2020, 127, 953–973. [Google Scholar] [CrossRef] [PubMed]
- Brayden, D.J.; Alonso, M.J. Oral delivery of peptides: Opportunities and issues for translation. Adv. Drug Deliv. Rev. 2016, 106, 193–195. [Google Scholar] [CrossRef]
- Shao, J.S.; Cai, J.; Towler, D.A. Molecular mechanisms of vascular calcification: Lessons learned from the aorta. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 1423–1430. [Google Scholar] [CrossRef] [PubMed]
- Rathinavel, A.; Sankar, J.; Mohammed Sadullah, S.S.; Niranjali Devaraj, S. Oligomeric proanthocyanidins protect myocardium by mitigating left ventricular remodeling in isoproterenol-induced postmyocardial infarction. Fundam. Clin. Pharmacol. 2018, 32, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Huo, R.; Sheng, Y.; Li, Y.; Xie, X.; Chen, C.; Liu, H.B.; Li, N.; Li, C.B.; Guo, W.T.; et al. Bone morphogenetic protein-4 mediates cardiac hypertrophy, apoptosis, and fibrosis in experimentally pathological cardiac hypertrophy. Hypertension 2013, 61, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Dave, R.K.; Ellis, T.; Toumpas, M.C.; Robson, J.P.; Julian, E.; Adolphe, C.; Bartlett, P.F.; Cooper, H.M.; Reynolds, B.A.; Wainwright, B.J. Sonic hedgehog and notch signaling can cooperate to regulate neurogenic divisions of neocortical progenitors. PLoS ONE 2011, 6, e14680. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, Y.Q.; Ai, W.B.; Hu, Q.T.; Zhang, Q.J.; Wan, L.Y.; Wang, X.L.; Liu, C.B.; Wu, J.F. Hes1, an important gene for activation of hepatic stellate cells, is regulated by Notch1 and TGF-β/BMP signaling. World J. Gastroenterol. 2015, 21, 878–887. [Google Scholar] [CrossRef]
- Ingram, W.J.; McCue, K.I.; Tran, T.H.; Hallahan, A.R.; Wainwright, B.J. Sonic Hedgehog regulates Hes1 through a novel mechanism that is independent of canonical Notch pathway signalling. Oncogene 2008, 27, 1489–1500. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, Z.; Xu, Z.; Yin, H.; Bai, L.; Ma, Z.; Decoster, M.A.; Qian, G.; Wu, G. Activation of the sonic hedgehog signaling controls human pulmonary arterial smooth muscle cell proliferation in response to hypoxia. Biochim. Biophys. Acta 2010, 1803, 1359–1367. [Google Scholar] [CrossRef]
- Katoh, M.; Katoh, M. NUMB is a break of WNT-Notch signaling cycle. Int. J. Mol. Med. 2006, 18, 517–521. [Google Scholar] [CrossRef]
- Ortega-Campos, S.M.; García-Heredia, J.M. The Multitasker Protein: A Look at the Multiple Capabilities of NUMB. Cells 2023, 12, 333. [Google Scholar] [CrossRef] [PubMed]

| Cardiomyocyte Diameter, Mcm | Media Thickness, Mcm | Perivascular Fibrosis, Mcm | Myocardial Interstitial Fibrosis, % | |
|---|---|---|---|---|
| Myocardial P, | −0.34 | 0.52 | −0.41 | 0.13 |
| mg/kg | p = 0.17 | p = 0.029 | p = 0.09 | p = 0.60 |
| Serum phosphate, | 0.58 | 0.68 | 0.65 | 0.50 |
| mmol/L | p = 0.012 | p = 0.002 | p = 0.003 | p = 0.026 |
| Fractional phosphate | 0.21 | 0.14 | 0.38 | 0.35 |
| excretion, % | p = 0.38 | p = 0.59 | p = 0.041 | p = 0.16 |
| PTH, pg/mL | 0.60 | −0.20 | 0.27 | 0.11 |
| p = 0.008 | p = 0.42 | p = 0.27 | p = 0.65 | |
| FGF23, pg/mL | 0.21 | −0.37 | −0.05 | −0.18 |
| p = 0.39 | p = 0.13 | p = 0.83 | p = 0.48 | |
| Serum Klotho, | −0.33 | −0.22 | 0.12 | −0.22 |
| pg/mL | p = 0.12 | p = 0.37 | p = 0.64 | p = 0.19 |
| Left Ventricular | Myocardial | Vascular | Perivascular | |
|---|---|---|---|---|
| Wall Thickness | Interstitial Fibrosis | Media Thickness | Fibrosis | |
| Mapk1 | 0.43 * | 0.33 | 0.39 * | 0.33 |
| Mapk3 | −0.27 | −0.23 | −0.33 | −0.32 |
| Notch1 | −0.35 * | 0.24 | −0.34 | −0.33 |
| Numb | −0.45 * | −0.49 * | −0.51 * | −0.54 * |
| Ppp3ca | 0.44 * | 0.02 | 0.14 | 0.05 |
| Slc20a1 | −0.32 | −0.34 | −0.34 | −0.34 |
| Slc20a2 | −0.05 | −0.07 | 0.14 | 0.01 |
| Ctnnb1 | −0.22 | 0.26 | −0.32 | −0.23 |
| Jag1 | −0.46 * | −0.71 * | −0.70 * | −0.72 * |
| Hes1 | −0.43 * | −0.34 | −0.45 * | −0.42 * |
| Ptch1 | 0.20 | −0.41 | 0.14 | −0.04 |
| Tgfb1 | −0.12 | −0.22 | −0.34 | −0.19 |
| Lgr4 | −0.12 | 0.18 | 0.71 * | 0.52 * |
| Bmp4 | −0.36 * | −0.34 | −0.69 * | −0.57 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bogdanova, E.; Sadykov, A.; Ivanova, G.; Zubina, I.; Beresneva, O.; Galkina, O.; Parastaeva, M.; Sharoyko, V.; Dobronravov, V. Myocardial Remodeling in Early Chronic Kidney Disease—Mineral and Bone Disorder Model with Low Bone Turnover. Kidney Dial. 2023, 3, 322-334. https://doi.org/10.3390/kidneydial3040028
Bogdanova E, Sadykov A, Ivanova G, Zubina I, Beresneva O, Galkina O, Parastaeva M, Sharoyko V, Dobronravov V. Myocardial Remodeling in Early Chronic Kidney Disease—Mineral and Bone Disorder Model with Low Bone Turnover. Kidney and Dialysis. 2023; 3(4):322-334. https://doi.org/10.3390/kidneydial3040028
Chicago/Turabian StyleBogdanova, Evdokia, Airat Sadykov, Galina Ivanova, Irina Zubina, Olga Beresneva, Olga Galkina, Marina Parastaeva, Vladimir Sharoyko, and Vladimir Dobronravov. 2023. "Myocardial Remodeling in Early Chronic Kidney Disease—Mineral and Bone Disorder Model with Low Bone Turnover" Kidney and Dialysis 3, no. 4: 322-334. https://doi.org/10.3390/kidneydial3040028
APA StyleBogdanova, E., Sadykov, A., Ivanova, G., Zubina, I., Beresneva, O., Galkina, O., Parastaeva, M., Sharoyko, V., & Dobronravov, V. (2023). Myocardial Remodeling in Early Chronic Kidney Disease—Mineral and Bone Disorder Model with Low Bone Turnover. Kidney and Dialysis, 3(4), 322-334. https://doi.org/10.3390/kidneydial3040028






