Abstract
This study reviews the renal aspects of diuretic resistance occurring in diuretic treatment, mostly with loop diuretics of congestive heart failure. A short discussion on the different classes of diuretics, including the recently introduced sodium-glucose transporter 2 inhibitors, and their mechanism of action in the nephron is provided, followed by a summary of recent data discussing the different causes and pathophysiological mechanisms of diuretic resistance. The major cause of diuretic resistance appears to be localized within the distal tubule. Traditionally, the concept of compensatory post-diuretic sodium reabsorption (CPDSR) was considered the major cause of diuretic resistance; however, recent studies have disputed this traditional concept and demonstrated that patients with congestive heart failure are in constant sodium-avid state. Finally, the different options of therapeutic strategies, combining different classes of diuretics are summarized.
1. Introduction
Diuretic resistance (DR) can be defined as a failure to increase fluid and sodium output sufficiently to relieve generalized edema, volume overload or congestion despite treatment with a full dose of a loop diuretic [1,2,3].
Diuretic resistance is a frequent problem in severe congestive heart failure (CHF), and extensive literature is published in the cardiology literature. This contribution summarizes selected and recent insights in diuretic resistance for a nephrology readership.
Treatment of generalized edema primarily relies on the administration of natriuretic agents which diminish sodium reabsorption at different sites in the nephron, thereby increasing urinary sodium and water losses. The terms “diuretics” and the more exact term of “natriuretics” are often used interchangeably.
“Aquaretic” agents are vasopressin 2 anta-agonists (VRAs), acting at the collecting duct, which increase free water excretion, and are, strictly speaking, not “diuretics”.
The molecular targets of diuretic drugs are predominantly sodium (Na+) transport pathways at the apical (luminal) surface of the kidney tubule cells. When coupled with the basolateral cell membrane Na+/K+-ATPase, these pathways permit the vectorial transport of sodium.
Traditionally, diuretics are classified according to their predominant sites of action in the tubular segments of the nephron and by the mechanism by which they inhibit transport [4].
Figure 1 modified from [1] illustrates the sites of action in the nephron of different diuretics in relation to the percentage of filtrate reabsorption at the different nephron segments.
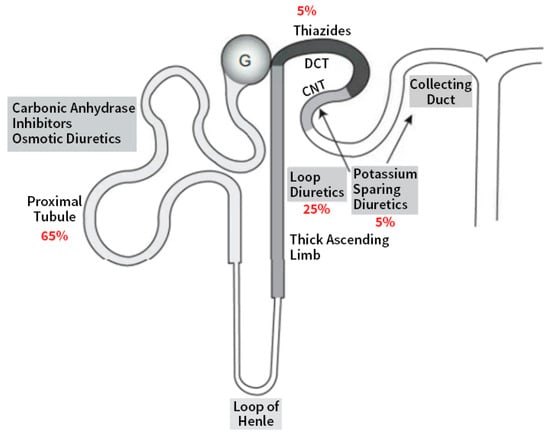
Figure 1.
Schematic of a nephron shows sites of action of diuretics along the various segments. Adapted with permission from Ref. [1]. 2022, Elsevier. (DCT: distal convoluted tubule; CNT: connecting tubule).
Table 1 adapted from [5] summarizes the classification of commonly used diuretics and aquaretics based on the site and mechanism of action.

Table 1.
Diuretics classified according to their localization and their mechanisms of action on the sodium and vasopressin transporters in the nephron. Adapted with permission from Ref. [5] 2022, Oxford Publishing Limited.
The discussion on osmotic diuretics (i.e., mannitol) is beyond the scope of this paper.
Diuretics block the function of sodium transport proteins in the apical plasma membrane of kidney epithelial cells and, except for mineralocorticoid receptor blockers, secretion into the tubular fluid is required for all “traditional” diuretics [4,6].
The SGLT2 inhibitors are believed to be freely filtered, where they bind to SGLT2s in the luminal membrane of the epithelial cells in the proximal tubule [7].
The mineralocorticoid blocker, spironolactone, and the new nonsteroidal mineralocorticoid blocker, finerenone, act within cells and do not require secretion into the tubule lumen [4].
Detailed pharmacokinetic and pharmacodynamic aspects of the different classes of diuretics are available in [1,4,5,8,9].
2. Diuretics Acting on the Proximal Tubule (PT)
The proximal tubules reabsorb around 70% of the filtrate. Despite this high absorptive capacity, large losses of sodium and water by diuretics acting in the PT, including acetazolamide and SGLT2 inhibitors, cannot be expected because almost all of the excess fluid delivered out of the PT is reabsorbed in the loop of Henle and the distal tubule.
2.1. Acetazolamide
Acetazolamide inhibits the activity of carbonic anhydrases, a ubiquitous superfamily of enzymes that catalyze the hydration of CO2 to bicarbonate and protons which plays an important role in proximal bicarbonate, sodium, and chloride reabsorption. As a result, this agent results in the loss of both NaCl and NaHCO3 [5,8].
2.2. SGLT2 Inhibitors (SGLT2i)
SGLT2 inhibitors decrease proximal tubular sodium and chloride reabsorption and increase macula densa sodium and chloride delivery. This occurs via the tubulo-glomerular feedback (TGF) mechanism adenosine-induced vasoconstriction of the afferent arteriole and increased prostaglandin secretion with dilatation of the efferent arteriole, resulting in a decline in intraglomerular filtration pressure and in glomerular filtration rate (GFR) [10]. SGLT2 inhibitors further decrease proximal tubular sodium reabsorption via inhibition of the sodium–hydrogen transporter (NHE3) [11].
Recent reviews have summarized the cardiovascular and renal benefits of SGLT2 inhibitors in diabetic as well as nondiabetic patients with heart failure with reduced and preserved ejection fraction and patients with renal dysfunction [12,13,14,15,16].
One of the potential mechanisms of the benefits in cardiovascular protection of the SGLT2 inhibitors may be related to their diuretic effects.
However, the diuretic effects of SGLT2 inhibitors are limited and the natriuresis disappears after 4 days of treatment [17].
Several mechanisms besides the increased diuresis and natriuresis have been suggested to mediate the cardiovascular benefits with SGLT2 inhibitors (for review see [18]).
Despite the high capacity for sodium reabsorption in the PT, relatively large losses of sodium and water by diuretics acting in the PT, including acetazolamide and SGLT2 inhibitors cannot be expected because almost all of the excess fluid delivered out of the PT is reabsorbed more distally, in both the loop of Henle and the distal tubule.
The role of acetazolamide and SGLT2 inhibitors in the management of DR will be discussed below.
3. Diuretics Acting in the Loop of Henle
Filtered sodium chloride enters the cells in the thick ascending limb of the loop of Henle via cotransporter Na-K-2Cl carrier isoform 2 (NKCC2) at the apical membrane of the tubular cells and the macula densa [8,19]. The macula densa cells are affected by luminal chloride concentration.
Loop diuretics (bumetanide, ethacrynic acid, furosemide, and torsemide) act principally by blocking the luminal NKCC2 transporter. This inhibition leads to increased renin secretion and increase in intraglomerular pressures by preventing feedback (TGF) [5,8]. The blockade of the sodium chloride reabsorption at the macula densa disrupts TGF [20] and maintains the GFR, despite ongoing diuresis, and causes the release of volume-independent renin from the juxtaglomerular apparatus and subsequent renin-angiotensin-aldosterone system (RAS) activation.
By inhibiting the NaCl reabsorption in the water-impermeable parts of Henle’s loop, loop diuretics decrease the tonicity in the renal interstitium resulting in increased free water excretion. Because the thick ascending limb reabsorbs 20–25% of the filtered sodium load, these drugs are among the strongest diuretics available [8,19,21].
Though loop diuretics are effective in natriuresis in the acute setting, chronic use can lead to a compensatory increase in sodium reabsorption, which distally mitigates their natriuretic effects [22] (see below).
Loop diuretics circulate bound to proteins (>90%), limiting their volumes of distribution.
The oral absorption of furosemide is highly variable, with bioavailability ranging between 10% and 100% (on average, 50%). In congested patients, bowel edema can reduce its absorption, causing lower concentrations of peak plasma.
Bumetanide and torsemide have higher and more consistent oral bioavailability than furosemide (>90%), and do not exhibit absorption-limited kinetics, making oral and intravenous doses similar [19].
Doses of different loop diuretics can be converted to furosemide equivalents with 1 mg bumetanide = 20 mg torsemide = 80 mg furosemide for oral, and 1 mg bumetanide = 20 mg torsemide = 40 mg furosemide for intravenous diuretics [23,24,25].
4. The Distal Nephron Acting Diuretics
The sodium chloride co-transporter (NCC), also known as the “thiazide-sensitive” transporter, is mainly located at the distal convoluted tubule (DCT). Thiazides and thiazide-likes (indapamide (INDA) and chlorthalidone (CLTD) achieve their diuretic action via inhibition of the NCC, which then prevents the co-transport of sodium.
The majority of thiazides may also interact with multiple sodium transport pathways such as carbonic anhydrase, pendrin, and the sodium-dependent chloride/bicarbonate exchanger (NDCBE). The distal convoluted tubule is part of the diluting nephron segment, and blocking salt reabsorption with thiazides impairs urinary dilution, potentially resulting in hyponatremia.
Because the distal convoluted tubule is responsible for only around 5% of sodium reabsorption, thiazide diuretics only have a limited capacity to change sodium balance.
5. Diuretics Mainly Acting on the Collecting Duct
The collecting duct contributes to up to 2% of absorption of the filtered salt load.
The potassium-sparing diuretics include two drug classes, namely, epithelial sodium channel (ENaC) blockers and mineralocorticoid receptor antagonists (MRAs).
5.1. The Potassium-Sparing Diuretics
ENaC is the primary channel for sodium reabsorption in the principal cells of the connecting tubules and collecting ducts [26,27]. Amiloride and triamterene directly but reversibly block luminal ENaCs without affecting the mineralocorticoid receptor.
The inhibition of ENaC disrupts the normal lumen-negative potential in the collecting duct, inhibiting potassium and hydrogen ion secretion and promoting calcium and magnesium reabsorption.
Pendrin is a Cl−/HCO3− exchanger located in type B intercalated cells and non-type A/non-type B intercalated cells, as well as in thyroid, inner ear, and the adrenal medulla. In people, suffering from Pendred syndrome, and in mice, pendrin gene ablation can lead to hypothyroidism, deafness, volume contraction, and reduced blood pressure (for review [28]).
Angiotensin II and aldosterone stimulate pendrin, which increases renal chloride absorption, thereby contributing to the pressor response seen in response to these hormones. In kidney, pendrin increases renal ENaC activity and abundance, thereby stimulating the renal absorption of both Na+ and Cl−. Metabolic alkalosis stimulates pendrin, thereby increasing the secretion of HCO3− into the pro-urine, which mitigates the alkalosis.
Of physiological and clinical significance is the role of these pendrin-positive intercalated cells in blood pressure regulation, which occurs, at least in part, through pendrin-mediated renal Cl− absorption, as well as their effect, on the epithelial Na+ channel, ENaC. Aldosterone stimulates ENaC directly through principal cell mineralocorticoid hormone receptor (ligand) binding and also indirectly through its effect on pendrin expression and function. Thus, pendrin contributes to the aldosterone pressor response.
Pendrin may also modulate blood pressure in part through its action in the adrenal medulla, where it modulates the release of catecholamines, or through an indirect effect on vascular contractile force [28].
Recent findings suggest pendrin inhibition as a future novel approach to potentiate the action of loop diuretics for treatment of hypertension and edema, including diuretic-resistant edema [29].
5.2. Aldosterone Antagonists
Aldosterone is a mineralocorticoid hormone produced in the zona glomerulosa of the adrenal cortex and acts on principal-like cells in the late DCT, the connecting tubule (CNT), and the cortical collecting duct (CCD), favoring sodium and water reabsorption and potassium excretion while also contributing to the acid–base balance. As with all steroid hormones, aldosterone passes through cell membranes to bind to cytoplasmic receptors which translocate to the nucleus to influence mRNA transcription and subsequently protein synthesis. Aldosterone stimulates ENaC directly through principal cell mineralocorticoid hormone receptor (ligand) binding and also indirectly through its effect on pendrin expression and function [30].
The mineralocorticoid receptor antagonists (MRAS) include, in order of increasing mineralocorticoid receptor selectivity, spironolactone, eplerenone, and finerenone.
In the kidney, spironolactone and its metabolites bind to cytosolic mineralocorticoid receptors, and act as competitive inhibitors of the endogenous hormone. Spironolactone induces a mild increase in sodium excretion (1–2%) and a decrease in potassium and hydrogen ion excretion [5]. Eplerenone is more selective for mineralocorticoid receptors, and therefore less likely to cause side effects [31,32].
Finerenone, is the first of a new class of nonsteroidal MRAs with stronger mineralocorticoid receptor binding compared with eplerenone and spironolactone [33,34]. The FDA recently approved the use of finenerone to decrease the risk of CKD progression and cardiovascular events in patients with diabetic kidney disease [9,35,36].
6. Diuretic Resistance (DR) in Congestive Heart Failure
Congestion is the major cause for hospital admission in acute decompensated heart failure (ADHF) and DR leading to refractory congestion is associated with worse outcomes, increased length of stay, and other intermediate complications [22,24,37,38].
The small increases in serum creatinine (SCr) or Cystatin C, often observed in ADHF patients during decongestion therapy with either diuretics or ultrafiltration, are frequently labelled as worsening renal function (WRF). These fluctuations in GFR are intrinsic to the intrarenal hemodynamics of decongestion and do not reflect tubular injury as observed in acute kidney injury (AKI). Increasing levels of SCr (up to 0.5 mg/dL) and cystatin C reflecting adequate decongestion) are associated with better chances of 6-month survival [39]. However, if WRF occurs in the setting of DR and persistent congestion, prognosis is exceedingly poor [40,41]. Therefore, WRF by itself should not be a reason to withdraw decongestive therapies and should not limit achieving euvolemia [39,42].
Quantitative definitions of DR include failure of oral furosemide (160 mg twice daily or equivalent) to increase sodium excretion by at least 90 mmol over 3 days. Alternatively, a spot urine sample with a sodium output <50 mmol, obtained 1 to 2 h after a loop diuretic, can predict sodium output and possible DR [3].
Several pathways contribute to DR in patients with heart failure [43].
Major mechanisms of DR can be localized either before or after the distal/collecting tubule nephron segments.
7. Pre- Distal/Tubular Mechanisms of Diuretic Resistance
Acidosis, Non-steroidal anti-inflammatory drugs (NSAIDs), and uremic anions compete with loop diuretics for secretion by the organic ion transporters, whereas reduced GFR in CKD patients can further modify loop diuretic pharmacokinetics and dynamics [4].
It is doubtful that hypoalbuminemia and albuminuria with binding of diuretics in the loop of Henle play an important role in diuretic resistance, with the exception of heavy albuminuria in severe nephrotic syndrome.
Decreased renal perfusion due to low mean arterial pressure or high central venous pressure in advanced CHF or cardiogenic shock with low cardiac output limit the secretion of loop diuretics into the tubule and decrease GFR. In these circumstances diuretic agents are ineffective and renal perfusion must first be restored [44].
Excessive abdominal congestion with increased intra-abdominal pressure and poor renal perfusion can be reversed by ultrafiltration or the removal of ascites [45,46].
The well-known loop DR observed in acute kidney disease (AKD), including acute kidney injury (AKI), and chronic kidney disease (CKD) is probably the combined effect of lower functioning nephron mass, associated metabolic acidosis and the interference at the proximal tubular secretory sites by drugs and/or uremic toxic anions. Defects in glomerular filtration are less important mediators of diuretic resistance than tubular mechanisms [47].
Recent epidemiological studies identified hypochloremia, rather than hyponatremia, as the primary ion driving the poor survival in heart failure [48,49,50,51]. Chloride, and not sodium, has a predominant role in renal salt-sensing mechanisms, and TGF and renin release are primarily driven by chloride [52,53,54]. Chloride is a critical regulator of sodium transporter pathways through phosphorylation of a family of serine–threonine kinases [55,56].
Hanberg et al. [57] found that hypochloremia is associated with neurohormonal activation and diuretic resistance; sodium-free chloride supplementation was associated with increases in serum chloride and beneficial changes in several cardiorenal parameters. For a recent review on hypochloremia in heart failure, see [58].
Recent opinions consider that high dietary sodium intake does not directly reduce diuretic response and, if anything, increases it [47].
8. Contribution of Proximal versus Distal Tubular Mechanisms of Diuretic Resistance (DR)
A recent study [59] revealed that distal tubular mechanisms explained >70% of the variability in diuretic response, indicating that natriuresis is determined primarily by distal tubular mechanisms.
The contributions of proximal and distal nephron’s sodium reabsorption after IV furosemide were explored by comparing the differences in the fractional urinary excretion of Lithium (FELi) and fractional urinary excretion of sodium (FENa) between patients in CHF and controls [60]. The FELi reflects an in vivo assessment of proximal tubular and loop of Henle sodium handling and assesses sodium loop exit after loop diuretic administration. FENa assesses the net sodium excreted into the urine. Lithium is not reabsorbed in the distal nephron; thus, a disproportionate increase in FELi after furosemide administration suggests that distal sodium reabsorption is at play. The prediuretic FELi was 16.2%, similar to that in a control cohort without HF not receiving diuretics. IV furosemide increased FELi by 12.6%, but increased FENa by only 4.8%, meaning that only 34% of the estimated diuretic-induced sodium release did not undergo distal reabsorption. These data suggest that a high-dose loop diuretic in patients with CHF yields meaningful increases in sodium exit from the proximal tubule/loop of Henle, indicating adequate drug delivery; however, little of this sodium seems to reach the urine, indicating that the nephron distal to the loop reabsorbs most of that sodium.
The majority of diuretic resistance in CHF is thus not from impaired drug delivery, but from a change in the nephron’s response [61].
9. Distal Tubular Mechanisms in DR
One rather exceptional mechanism of diuretic resistance at the level of the distal/collecting tubule may play a role in diuretic-resistant edema in nephrotic syndrome. This is attributed to a direct activation of the sodium transporter ENaC by plasmin, present in nephrotic urine [1,62]. In agreement, nephrotic urine contains soluble plasmin that proteolytically activates ENaC in vitro [63]. Blocking ENaC by amiloride [61] or triamterene [1,64,65] showed a therapeutic effect in cases with resistant nephrotic edema.
As reviewed and explained in several recent papers, compensatory post-diuretic sodium reabsorption (CPDSR) was, until recently, considered to be the main cause of diuretic resistance in ADHF [3,9,22,37,38,44,60,66].
Two mechanisms with a major effect on the diuretic response in the acute and chronic post diuretic period can play an important role in the pathogenesis of DR and are illustrated in Figure 2 (taken from [67]).
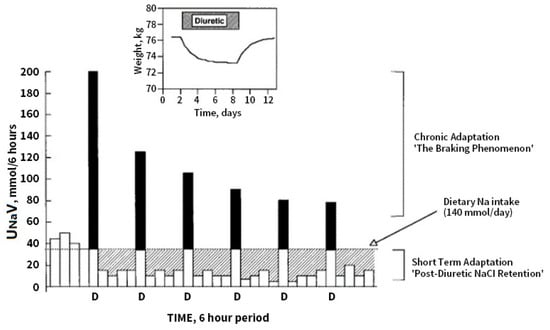
Figure 2.
Effects of diuretics on urinary Na excretion and extracellular fluid (ECF) volume. Inset: effect of a diuretic on body weight, taken as an index of ECF volume. Bars represent 6-hour periods before (in Na balance) and after doses of loop diuretic (D). The dotted line indicates dietary Na intake. The solid portion of the bars indicates the amount by which Na excretion exceeds intake during natriuresis. The hatched areas indicate the amount of positive Na balance after the diuretic effect has worn off. Net Na balance during 24 h is the difference between the hatched area (postdiuretic NaCl retention) and the solid area (diuretic-induced natriuresis). After the first dose of the diuretic the excess natriuresis triggers a compensatory post-diuretic sodium reabsorption of equal magnitude, resulting in a net even sodium balance (compensatory post diuretic sodium reabsorption). With chronic administration of the same dose of diuretic, chronic adaptation is indicated by progressively smaller peak natriuretic effects (the braking phenomenon) and is mirrored by a return to neutral balance, as indicated in the inset, where the solid and hatched areas are equal. Note that steady state is reached within 6–8 days, despite continued diuretic administration. Reprinted with permission from Ref. [67]. 2022, Karger.
The post diuretic effect is observed within hours, and sodium loss is thought to play a role in the upregulation of proximal and distal sodium transporters and distal tubular hypertrophy in the long term. High doses of loop diuretics, when used chronically, induce distal nephron remodeling, with hypertrophy and hyperplasia of the distal convoluted tubule, connecting tubule, and collecting duct, which increase the number of apical NaCl symporters as well as the combined physiologic effects of pendrin and the sodium-dependent chloride/bicarbonate exchanger available for sodium reabsorption [68,69]. CPDSR is a mechanism by which, despite an adequate acute diuretic response, patients fail to achieve a negative sodium/fluid balance secondary to an equal amount of compensatory/rebound sodium reabsorption in the post-diuretic period [19,22,70].
The braking phenomenon occurring after repetitive administration of the diuretic acts as a protective mechanism triggered by acute sodium and water loss to avoid over-diuresis. The initial fluid loss leads to activation of the renin–angiotensin–aldosterone (RAAS), norepinephrine and sympathetic nervous systems can all promote tubular sodium reabsorption (for review [8]).
Figure 3, taken from [67], shows that in CHF, the diuretic concentration–Na+ excretion curve is displaced downward and to the right, so that the threshold concentration of drug required to achieve any diuretic effect rises, and the maximal diuresis that can be achieved declines.
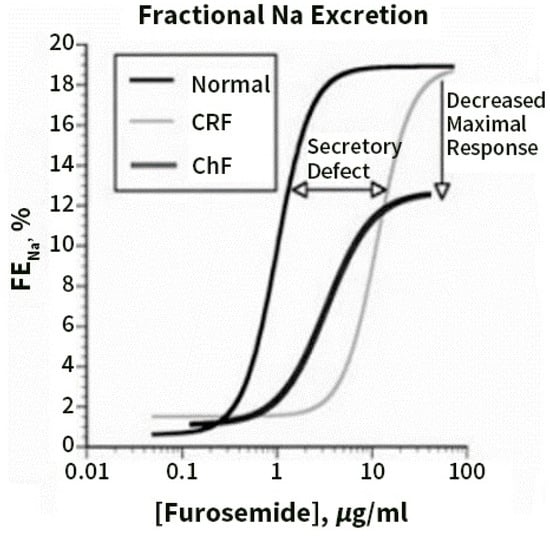
Figure 3.
Dose–response curves for loop diuretics illustrate the fractional Na excretion (FENa) as a function of loop diuretic urine concentration. Compared with normal patients, patients with chronic renal failure (CRF) show a rightward shift in the curve, owing to impaired diuretic secretion. The maximal response is preserved when expressed as FENa, but not when expressed as absolute Na excretion. Patients with congestive heart failure (CHF) demonstrate a rightward and downward shift, even when the response is expressed as FENa, and thus are relatively diuretic resistant. Reprinted with permission from Ref. [67]. 2022, Karger.
The “ceiling dose” refers to the single dose amount that is required to reach the ceiling of diuretic efficiency; doses above this level do not meaningfully increase diuresis. While 80 mg of oral furosemide is a typical ceiling dose for “diuretic-naïve” individuals, increased doses of oral diuretics can be required in the setting of impaired kidney drug delivery. For example, in CKD, ceiling doses can be as high as 400 mg furosemide per dose. Once the diuretic ceiling dose has been identified for an individual, this dose can be given up to every 6 h to maximize the total daily urine output. The ceiling dose can change in an individual over time, pending the factors that impact loop diuretic drug delivery and efficacy discussed in the previous section.
The idea that compensatory post-diuretic sodium reabsorption (CPDSR) is a major cause of diuretic resistance in HF patients has been derived from observations in euvolemic, healthy volunteers, and has “silently” been extrapolated to patients with acute HF. A recent landmark mechanistic paper [71] established that on a population level, CPDSR following loop-diuretic-induced natriuresis, as observed in healthy volunteers, was not an important driver of diuretic resistance in hypervolemic ACHF patients. On the contrary, in that study the opposite results were observed; increasing diuretic-induced natriuresis with loop diuretics was followed by greater spontaneous sodium excretion in the post-diuretic periods. Importantly, the intensification of diuretic therapies in poor diuretic responders was shown to increase natriuresis; however, the levels of post-diuretic sodium excretion remained unchanged, indicating that patients with hypervolemic acute heart failure do not have CPDSR. It thus appears that the strongest driver of both diuretic-induced and post-diuretic sodium excretion was basal kidney sodium avidity. Patients with advanced HF and DR seem to be in a continuous sodium-avid state [47,71].
10. Diagnosing Diuretic Resistance
Diagnosing DR and identifying the driving cause is difficult in practice. A spot 2 h urine sodium concentration after administering a loop diuretic can help predict whether or not natriuresis will be adequate and can facilitate early recognition of an impaired diuretic response. In patients with congestion, an hourly urine output of <100–150 mL during the first 6 h and/or spot urinary sodium content of <50–70 mmol 2 h after loop diuretic administration generally indicates an inadequate response [72]; determining whether this is due to insufficient loop diuretic delivery or distal sodium reabsorption is more challenging. One method used in research studies but not yet in clinical practice is to measure the relative changes in the FeNa and endogenous lithium FeLi, as described above [60].
Testani et al. [73] published preliminary observations on a natriuretic response prediction equation (NRPE). With a spot urine sample obtained 2 h after loop diuretic administration, the 6 h cumulative total sodium output was accurately predicted and outperformed clinical parameters such as net fluid output and weight loss. Using a nurse-driven automated diuretic titration protocol, including the NRPE, resulted in a rapid and well-tolerated decongestion [74]. Additionally, a metric of diuretic efficiency, calculated as the urine output in ml per 40 mg of furosemide equivalents administered was developed [24].
A low diuretic efficiency during decongestive therapy portends poorer long-term outcomes above and beyond traditional prognostic factors in patients hospitalized with decompensated HF.
The most relevant causes of insufficient diuretic delivery, i.e., the pre-distal tubular causes of DR, as described in Ref [43], should be excluded in individual patients.
11. Diagnosis of Congestion/Decongestion
The cardiologic assessment of hypervolemia is outside the scope of this review and has been extensively reviewed [72,75].
12. Role of Diuretics in the Treatments of Diuretic Resistance
Figure 4, taken from ref [22] is an algorithm for titrating diuretic strategies in patients with persistent congestion and DR, on the basis of urine sodium measurement and urine output.
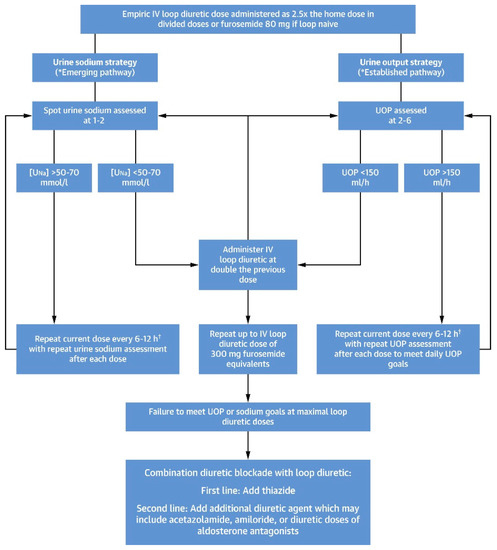
Figure 4.
A proposed algorithm for titrating diuretic strategies in patients with persistent congestion and diuretic resistance, on the basis of urine sodium measurement and urine output, is shown. For more details, see [22]. Reprinted with permission from Ref. [22] 2022, Elsevier. †: loop diuretic doses exceeding >1000 mg of furosemide equivalents/day have limited safety data and should be used cautiously. Bumetanide may confer less risk at higher doses because it has demonstrated reduced ototoxicity risk in animal models.
Please note that in current clinical practice as the last step where combination diuretic blockades are recommended, SGLT2 inhibitors should also be included.
13. Co-Administration of Diuretics with Albumin
Severe hypoalbuminemia might impair diuretic effectiveness, owing to impaired delivery to the kidney, and albumin administration might enhance natriuresis. However, studies have produced mixed results. A meta-analysis concluded that this policy suggests transient effects of modest clinical significance [76]. Some guidelines continue to suggest that albumin infusion should be used as an adjunct to diuretics when nephrotic patients appear to have vascular volume depletion (or appear to be “underfilled”) [77].
14. Continuous Infusion of Loop Diuretics
Compared with bolus therapy, continuous infusion of diuretics provides more sustained and uniform drug delivery and prevents post-diuretic sodium retention.
The Diuretic Optimization Strategies Evaluation (DOSE) trial compared the efficacy and safety of continuous vs. bolus furosemide therapy in 308 patients with acute HF [78]. There was no difference in symptom control or net fluid loss at 72 h in either group. Other studies have shown more diuresis with continuous infusion than with a similarly dosed bolus regimen [79]. However, at this point, definitive clinical evidence to support routine use of continuous loop diuretic therapy is lacking.
15. Hypertonic Saline Infusion (HSS)
HSS along with diuretics induces fluid flow from interstitial spaces to the intravascular space which leads to congestion relief by decreasing interstitial volume [80].
A meta-analysis demonstrated a significant decrease in all-cause mortality rate, reduced length of hospital stay, increased weight loss, and fewer re-hospitalizations [81]. Additionally, no electrolyte abnormalities requiring a change in dose or HSS discontinuation were observed.
A recent study reported that hypertonic saline administration was associated with increased diuretic efficiency, as well as fluid and weight loss, without adverse respiratory or neurological signals [82].
16. Combination Diuretic Therapy
Sequential nephron blockade with combination diuretic therapy is an important therapeutic strategy against diuretic resistance and is the next step when the desired diuretic response is not obtained with high doses of loop diuretic monotherapy.
16.1. Thiazides
Thiazide diuretics as monotherapy is not successful in DHF [83]; however, the addition of 25–100 mg thiazide to a high dose of i.v. furosemide in 20 NYHA (The New York Heart Association) class 3 and 4 HF patients with diuretic resistance increased body weight reduction and urine output [84].
The most popular combination is a loop diuretic plus a thiazide, although no large-scale placebo-controlled trials have been performed [85]. Metolazone (a thiazide-like diuretic) is typically used due to its low cost and availability [86]. Metolazone also blocks sodium reabsorption at the proximal tubule, which may contribute to its synergistic effect.
Chlorothiazide is available in an intravenous formulation and has a faster onset of action than metolazone. However, studies have failed to detect any benefit of one over the other [87].
A recent propensity analysis, however, found that in DR HF patients, up-titration of loop diuretics may be a preferred strategy over routine early addition of a thiazide type diuretics when diuresis is inadequate [88].
16.2. Aldosterone Antagonists
A number of studies explored the potential advantages and safety of adding spironolactone, mostly to loop diuretics.
The first major double-blind RALES study where spironolactone was added to an ACE inhibitor, a loop diuretic, and in most cases digoxin, was discontinued early, because an interim analysis showed lower mortality, a lower frequency of hospitalization for worsening HF and a significant improvement in HF symptoms with spironolactone. Importantly, the incidence of serious hyperkalemia was minimal in both groups of patients [89].
After reporting the RALES results, early single center [90] and later, a large epidemiological survey [91] reported a clear rise in hospitalization for hyperkalemia associated with a dramatic increase in mortality in the aldosterone-treated group [91].
In the ATHENA-HF trial, spironolactone in high doses (100 mg/d) was added to usual therapy [83]. The primary end point was the change in NT-proBNP levels from baseline to 96 h. Secondary end points included the clinical congestion score, dyspnea assessment, net urine output, and net weight change. The result showed that adding spironolactone to usual care was well tolerated, but did not improve the primary or secondary efficacy end points.
16.3. Acetazolamide
A meta-analysis including nine studies up to mid-2017 in 229 patients with HF concluded that, when compared with the placebo, acetazolamide significantly increased natriuresis and decreased the apnea-hypopnea index (and central apnea index) among HF patients [92].
Results from an observational study [93] and a small, prospective, randomized trial [94] suggest that the addition of acetazolamide (at a dose of 500 mg administered intravenously once daily) to IV loop-diuretic therapy increased urinary sodium excretion in patients with ADHF.
Mullens et al. [95] recently published the Acetazolamide in Decompensated Heart Failure With Volume OveRload (ADVOR) study, a multicenter, placebo-controlled Belgian trial where 519 patients with HF received standardized loop diuretics and were randomized towards once daily IV acetazolamide (500 mg) versus placebo. The acetazolamide-loop diuretic combination resulted in a more complete clinical decongestion than placebo at 3 days (42% vs. 31%), as well as the benefits associated with a shorter hospital stay. The incidence of worsening kidney function, hypokalemia, hypotension, and adverse events was similar in the two groups.
As underlined in an accompanying editorial [96], a limitation of this trial was the exclusion in the trial of patients receiving SGLT2 inhibitors, which in contemporary clinical care would probably be added in Acute Decompensated Heart Disease Failure (ADHDF) therapy.
16.4. Multi-Nephron Segment Diuretic Therapy (MSDT)
MSDT is defined as the simultaneous use of 4 diuretic classes with actions along the whole nephron is a potential method to overcome severe diuretic resistance in Acute Heart Failure (AHF).
A retrospective analysis [97] of a study in 167 patients hospitalized with AHF and diuretic resistance showed that MSDT was associated with increased median 24 h urine output in the first day of therapy compared with the previous day (2.16 L to 3.08 L) in the total cohort and in the severe diuretic resistance cohort (0.91 L to 2.08 L). The median cumulative weight loss at day 7 or discharge was −7.4 kg (−15.3 to −3.4 kg). There were no changes in serum chemistries or kidney function. More prospective studies of MSDT in AHF and diuretic resistance are clearly warranted.
16.4.1. SGLT2 Inhibitors
As outlined above, the major mechanism of action of SGLT2 inhibitors occurs in the proximal renal tubule (the same location as acetazolamide), and SGLT2 inhibitors themselves have (weak) diuretic effects.
According to a pilot study, randomized, double-blind, placebo-controlled, multi-center study on the effects of empagliflozin on clinical outcomes in patients with ADHF (EMPARESPONSE-AHF), the use of empagliflozin did not affect the response to diuretics. However, it was safe and reduced both mortality and the frequency of rehospitalization 60 days post discharge [98].
Wilcox et al. [99] investigated the interaction between a loop diuretic (bumetanide) and dapagliflozin in normal subjects. There was no first-dose synergy between the two diuretics; however, dapagliflozin administration for 1 week enhanced the sodium excretion with bumetanide, and vice versa. Thus, there was significant two-way adaptive natriuretic synergy. Griffin et al. [100] retrospectively analyzed patients who received adjuvant SGLT-2i in 31 patients, 58% of whom had type 2 diabetes. Compared with the measurements taken 24 h prior to SGLT-2i initiation, SGLT-2i’s improved weight loss, urine output, and diuretic efficiency without worsening of creatinine, potassium, or blood pressure. The role of SGLT2 inhibitors as adjuvant therapy in the diuretic treatment of resistance is still not yet established; however, the mechanism of their diuretic action and the preliminary results as described above make further clinical investigations necessary. When diuretic resistance limits the efficient treatment of the congestion in SGLT2 inhibitor naïve patients, adding these drugs to loop diuretics is recommended.
In patients treated with background SGLT2 inhibitors, additional dosages of acetazolamide could further increase the efficacy of decongestion management.
16.4.2. Aquaretics
In hyponatremic patients with CHF, tolvaptan acutely increases serum sodium and decreases body weight but does not improve long-term CHF morbidity or mortality [101]. In the TACTICS-HF trial, the addition of tolvaptan to a standardized furosemide regimen did not improve the number of responders at 24 h despite greater weight loss [102]. Similarly, the Short-Term Effects of Tolvaptan (SECRET) of CHF trial did not show significant improvement in dyspnea in patients with AHF who were selected for greater potential benefit from tolvaptan [103].
16.4.3. Ultrafiltration
This topic is beyond the topic of this contribution. It is sufficient to state that ultrafiltration has not demonstrated decongestive superiority over diuretic therapy in randomized AHF trials and was associated with more complications [104,105].
17. Discussion
This review focuses on selected renal aspects of diuretic treatment in congestive heart failure. The different classes of diuretics and their mechanism of action in the nephron are discussed. Several mechanisms of loop diuretic resistance have traditionally been proposed; however, recent evidence indicates that a constant sodium-avid state is the main driver of diuretic resistance patients in congestive heart failure. Different therapeutic options including combinations of diuretics within so-called nephron blockage strategies are discussed.
18. Conclusions
Despite an impressive knowledge on the fascinating topic of congestive heart failure exists, ongoing research to optimally evaluate full decongestion (eu-volemia) and to determine the ideal diuretic strategy is necessary.
Parenteral loop diuretic agents are the mainstay in the treatment of congestive heart failure. Diuretic resistance in loop diuretic monotherapy may however develop, attenuating the maximal diuretic effect and thereby preventing full decongestion. The mechanisms behind diuretic resistance are diverse; however, contrary to traditional opinion, basal sodium avidity, rather than diuretic-induced compensatory post-diuretic sodium reabsorption (CPDSR), appears to be the predominant mechanism of diuretic resistance. A mechanism-based classification can guide medical strategies to restore diuretic efficacy. Optimization of loop diuretic regimens based upon diuretic response should be the primary strategy, followed by combination nephron blockage with thiazides and an eventual low dose of aldosterone blockers. Recent studies show an emerging role for sodium- glucose transporter 2 inhibitors, possibly in combination with acetazolamide for the future management of diuretic resistance.
Future research into the specific kidney transporters causing diuretic resistance in heart failure is crucial to guiding future therapeutic treatment strategies.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The author declares no conflict of interest.
References
- Hoorn, E.J.; Ellison, D.H. Diuretic Resistance. Am. J. Kidney Dis. 2017, 69, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Mullens, W.; Martens, P.; Testani, J.M.; Tang, W.H.W.; Skouri, H.; Verbrugge, F.H.; Fudim, M.; Iacoviello, M.; Franke, J.; Flammer, A.J.; et al. Renal effects of guideline-directed medical therapies in heart failure: A consensus document from the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2022, 24, 603–619. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, C.S.; Testani, J.M.; Pitt, B. Pathophysiology of Diuretic Resistance and Its Implications for the Management of Chronic Heart Failure. Hypertension 2020, 76, 1045–1054. [Google Scholar] [CrossRef]
- Ellison, D.H. Clinical Pharmacology in Diuretic Use. Clin. J. Am. Soc. Nephrol. 2019, 14, 1248–1257. [Google Scholar] [CrossRef]
- Ellison, D.H.; Subramanya, A.R. Clinical Use of Diuretics. In Oxford Textbook of Clinical Nephrology, 4th ed.; Turner, N., Lameire, N., Goldsmith, D.J., Winearls, C.G., Himmelfarb, J., Remuzzi, G., Eds.; Oxford University Press: Oxford, UK, 2016; pp. 299–322. [Google Scholar]
- Wu, W.; Bush, K.T.; Nigam, S.K. Key Role for the Organic Anion Transporters, OAT1 and OAT3, in the in vivo Handling of Uremic Toxins and Solutes. Sci. Rep. 2017, 7, 4939. [Google Scholar] [CrossRef] [PubMed]
- Wright, E.M. SGLT2 Inhibitors: Physiology and Pharmacology. Kidney360 2021, 2, 2027–2037. [Google Scholar] [CrossRef]
- Brater, D.C.; Ellison, D.H. Mechanism of Action of Diuretics; Post, T.W., Ed.; UpToDate: Waltham, MA, USA, 2020; Volume 2020. [Google Scholar]
- Novak, J.E.; Ellison, D.H. Diuretics in States of Volume Overload: Core Curriculum 2022. Am. J. Kidney Dis. 2022, 80, 264–276. [Google Scholar] [CrossRef]
- Vallon, V.; Thomson, S.C. The tubular hypothesis of nephron filtration and diabetic kidney disease. Nat. Rev. Nephrol. 2020, 16, 317–336. [Google Scholar] [CrossRef]
- Onishi, A.; Fu, Y.; Patel, R.; Darshi, M.; Crespo-Masip, M.; Huang, W.; Song, P.; Freeman, B.; Kim, Y.C.; Soleimani, M.; et al. A role for tubular Na(+)/H(+) exchanger NHE3 in the natriuretic effect of the SGLT2 inhibitor empagliflozin. Am. J. Physiol. Renal. Physiol. 2020, 319, F712–F728. [Google Scholar] [CrossRef]
- Aguilar-Gallardo, J.S.; Correa, A.; Contreras, J.P. Cardio-renal benefits of sodium-glucose co-transporter 2 inhibitors in heart failure with reduced ejection fraction: Mechanisms and clinical evidence. Eur. Heart J. Cardiovasc. Pharmacother. 2022, 8, 311–321. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Szarek, M.; Steg, P.G.; Cannon, C.P.; Leiter, L.A.; McGuire, D.K.; Lewis, J.B.; Riddle, M.C.; Voors, A.A.; Metra, M.; et al. Sotagliflozin in Patients with Diabetes and Recent Worsening Heart Failure. N. Engl. J. Med. 2021, 384, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Maddox, T.M.; Januzzi, J.L., Jr.; Allen, L.A.; Breathett, K.; Butler, J.; Davis, L.L.; Fonarow, G.C.; Ibrahim, N.E.; Lindenfeld, J.; Masoudi, F.A.; et al. 2021 Update to the 2017 ACC Expert Consensus Decision Pathway for Optimization of Heart Failure Treatment: Answers to 10 Pivotal Issues About Heart Failure with Reduced Ejection Fraction: A Report of the American College of Cardiology Solution Set Oversight Committee. J. Am. Coll. Cardiol. 2021, 77, 772–810. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef] [PubMed]
- Mordi, N.A.; Mordi, I.R.; Singh, J.S.; McCrimmon, R.J.; Struthers, A.D.; Lang, C.C. Renal and Cardiovascular Effects of SGLT2 Inhibition in Combination with Loop Diuretics in Patients with Type 2 Diabetes and Chronic Heart Failure: The RECEDE-CHF Trial. Circulation 2020, 142, 1713–1724. [Google Scholar] [CrossRef] [PubMed]
- Vallon, V.; Verma, S. Effects of SGLT2 Inhibitors on Kidney and Cardiovascular Function. Annu. Rev. Physiol. 2021, 83, 503–528. [Google Scholar] [CrossRef] [PubMed]
- Ellison, D.H.; Felker, G.M. Diuretic Treatment in Heart Failure. N. Engl. J. Med. 2017, 377, 1964–1975. [Google Scholar] [CrossRef]
- Schnermann, J.; Briggs, J.P. Synthesis and secretion of renin in mice with induced genetic mutations. Kidney Int. 2012, 81, 529–538. [Google Scholar] [CrossRef]
- Ellison, D.H. Mechanistic Insights into Loop Diuretic Responsiveness in Heart Failure. Clin. J. Am. Soc. Nephrol. 2019, 14, 650–652. [Google Scholar] [CrossRef]
- Felker, G.M.; Ellison, D.H.; Mullens, W.; Cox, Z.L.; Testani, J.M. Diuretic Therapy for Patients with Heart Failure. JACC State Art Rev. 2019, 75, 1178–1195. [Google Scholar] [CrossRef]
- Brater, D.C.; Day, B.; Burdette, A.; Anderson, S. Bumetanide and furosemide in heart failure. Kidney Int. 1984, 26, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Testani, J.M.; Brisco, M.A.; Turner, J.M.; Spatz, E.S.; Bellumkonda, L.; Parikh, C.R.; Tang, W.H. Loop diuretic efficiency: A metric of diuretic responsiveness with prognostic importance in acute decompensated heart failure. Circ. Heart Fail. 2014, 7, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Vargo, D.L.; Kramer, W.G.; Black, P.K.; Smith, W.B.; Serpas, T.; Brater, D.C. Bioavailability, pharmacokinetics, and pharmacodynamics of torsemide and furosemide in patients with congestive heart failure. Clin. Pharmacol. Ther. 1995, 57, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Pearce, D.; Manis, A.D.; Nesterov, V.; Korbmacher, C. Regulation of distal tubule sodium transport: Mechanisms and roles in homeostasis and pathophysiology. Pflugers Arch. 2022, 474, 869–884. [Google Scholar] [CrossRef] [PubMed]
- Pearce, D.; Soundararajan, R.; Trimpert, C.; Kashlan, O.B.; Deen, P.M.; Kohan, D.E. Collecting duct principal cell transport processes and their regulation. Clin. J. Am. Soc. Nephrol. 2015, 10, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Wall, S.M.; Verlander, J.W.; Romero, C.A. The Renal Physiology of Pendrin-Positive Intercalated Cells. Physiol. Rev. 2020, 100, 1119–1147. [Google Scholar] [CrossRef]
- Cil, O.; Haggie, P.M.; Phuan, P.W.; Tan, J.A.; Verkman, A.S. Small-Molecule Inhibitors of Pendrin Potentiate the Diuretic Action of Furosemide. J. Am. Soc. Nephrol. JASN 2016, 27, 3706–3714. [Google Scholar] [CrossRef]
- Soleimani, M. The multiple roles of pendrin in the kidney. Nephrol. Dial. Transplant. 2015, 30, 1257–1266. [Google Scholar] [CrossRef]
- Brown, R.; Quirk, J.; Kirkpatrick, P. Eplerenone. Nat. Rev. Drug Discov. 2003, 2, 177–178. [Google Scholar] [CrossRef]
- Weinberger, M.H.; Roniker, B.; Krause, S.L.; Weiss, R.J. Eplerenone, a selective aldosterone blocker, in mild-to-moderate hypertension. Am. J. Hypertens. 2002, 15, 709–716. [Google Scholar] [CrossRef]
- Agarwal, R.; Kolkhof, P.; Bakris, G.; Bauersachs, J.; Haller, H.; Wada, T.; Zannad, F. Steroidal and non-steroidal mineralocorticoid receptor antagonists in cardiorenal medicine. Eur. Heart J. 2021, 42, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Rico-Mesa, J.S.; White, A.; Ahmadian-Tehrani, A.; Anderson, A.S. Mineralocorticoid Receptor Antagonists: A Comprehensive Review of Finerenone. Curr. Cardiol. Rep. 2020, 22, 140. [Google Scholar] [CrossRef] [PubMed]
- Bakris, G.L.; Agarwal, R.; Anker, S.D.; Pitt, B.; Ruilope, L.M.; Rossing, P.; Kolkhof, P.; Nowack, C.; Schloemer, P.; Joseph, A.; et al. Effect of Finerenone on Chronic Kidney Disease Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2020, 383, 2219–2229. [Google Scholar] [CrossRef] [PubMed]
- Pitt, B.; Filippatos, G.; Agarwal, R.; Anker, S.D.; Bakris, G.L.; Rossing, P.; Joseph, A.; Kolkhof, P.; Nowack, C.; Schloemer, P.; et al. Cardiovascular Events with Finerenone in Kidney Disease and Type 2 Diabetes. N. Engl. J. Med. 2021, 385, 2252–2263. [Google Scholar] [CrossRef] [PubMed]
- Cox, Z.L.; Testani, J.M. Loop diuretic resistance complicating acute heart failure. Heart Fail. Rev. 2020, 25, 133–145. [Google Scholar] [CrossRef]
- Ellison, D.H. Diuretic resistance: Physiology and therapeutics. Semin. Nephrol. 1999, 19, 581–597. [Google Scholar]
- Ahmad, T.; Jackson, K.; Rao, V.S.; Tang, W.H.W.; Brisco-Bacik, M.A.; Chen, H.H.; Felker, G.M.; Hernandez, A.F.; O’Connor, C.M.; Sabbisetti, V.S.; et al. Worsening Renal Function in Patients with Acute Heart Failure Undergoing Aggressive Diuresis Is Not Associated with Tubular Injury. Circulation 2018, 137, 2016–2028. [Google Scholar] [CrossRef]
- Fudim, M.; Loungani, R.; Doerfler, S.M.; Coles, A.; Greene, S.J.; Cooper, L.B.; Fiuzat, M.; O’Connor, C.M.; Rogers, J.G.; Mentz, R.J. Worsening renal function during decongestion among patients hospitalized for heart failure: Findings from the Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness (ESCAPE) trial. Am. Heart J. 2018, 204, 163–173. [Google Scholar] [CrossRef]
- Metra, M.; Davison, B.; Bettari, L.; Sun, H.; Edwards, C.; Lazzarini, V.; Piovanelli, B.; Carubelli, V.; Bugatti, S.; Lombardi, C.; et al. Is worsening renal function an ominous prognostic sign in patients with acute heart failure? The role of congestion and its interaction with renal function. Circ. Heart Fail. 2012, 5, 54–62. [Google Scholar] [CrossRef]
- Rao, V.S.; Ahmad, T.; Brisco-Bacik, M.A.; Bonventre, J.V.; Wilson, F.P.; Siew, E.D.; Felker, G.M.; Anstrom, K.K.; Mahoney, D.D.; Bart, B.A.; et al. Renal Effects of Intensive Volume Removal in Heart Failure Patients with Preexisting Worsening Renal Function. Circ. Heart Fail. 2019, 12, e005552. [Google Scholar] [CrossRef]
- Lo, K.B.; Rangaswami, J. Mechanistic Insights in Cardiorenal Syndrome. NEJM Evid. 2022, 1, EVIDra2200053. [Google Scholar] [CrossRef]
- Verbrugge, F.H. Editor’s Choice-Diuretic resistance in acute heart failure. Eur. Heart J. Acute Cardiovasc. Care 2018, 7, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Mullens, W.; Abrahams, Z.; Francis, G.S.; Sokos, G.; Taylor, D.O.; Starling, R.C.; Young, J.B.; Tang, W.H.W. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J. Am. Coll. Cardiol. 2009, 53, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Verbrugge, F.H.; Dupont, M.; Steels, P.; Grieten, L.; Malbrain, M.; Tang, W.H.; Mullens, W. Abdominal contributions to cardiorenal dysfunction in congestive heart failure. J. Am. Coll. Cardiol. 2013, 62, 485–495. [Google Scholar] [CrossRef]
- Cox, Z.L.; Rao, V.S.; Testani, J.M. Classic and Novel Mechanisms of Diuretic Resistance in Cardiorenal Syndrome. Kidney360 2022, 3, 954–967. [Google Scholar]
- Grodin, J.L.; Simon, J.; Hachamovitch, R.; Wu, Y.; Jackson, G.; Halkar, M.; Starling, R.C.; Testani, J.M.; Tang, W.H. Prognostic Role of Serum Chloride Levels in Acute Decompensated Heart Failure. J. Am. Coll. Cardiol. 2015, 66, 659–666. [Google Scholar] [CrossRef]
- Grodin, J.L.; Testani, J.M.; Pandey, A.; Sambandam, K.; Drazner, M.H.; Fang, J.C.; Tang, W.H.W. Perturbations in serum chloride homeostasis in heart failure with preserved ejection fraction: Insights from TOPCAT. Eur. J. Heart Fail. 2018, 20, 1436–1443. [Google Scholar] [CrossRef]
- Grodin, J.L.; Verbrugge, F.H.; Ellis, S.G.; Mullens, W.; Testani, J.M.; Tang, W.H. Importance of Abnormal Chloride Homeostasis in Stable Chronic Heart Failure. Circ. Heart Fail. 2016, 9, e002453. [Google Scholar] [CrossRef]
- Testani, J.M.; Hanberg, J.S.; Arroyo, J.P.; Brisco, M.A.; Ter Maaten, J.M.; Wilson, F.P.; Bellumkonda, L.; Jacoby, D.; Tang, W.H.; Parikh, C.R. Hypochloraemia is strongly and independently associated with mortality in patients with chronic heart failure. Eur. J. Heart Fail. 2016, 18, 660–668. [Google Scholar] [CrossRef]
- Briggs, J. The macula densa sensing mechanism for tubuloglomerular feedback. Fed. Proc. 1981, 40, 99–103. [Google Scholar]
- Kotchen, T.A.; Luke, R.G.; Ott, C.E.; Galla, J.H.; Whitescarver, S. Effect of chloride on renin and blood pressure responses to sodium chloride. Ann. Intern. Med. 1983, 98 Pt 2, 817–822. [Google Scholar] [CrossRef] [PubMed]
- Wesson, D.E. Glomerular filtration effects of acute volume expansion: Importance of chloride. Kidney Int. 1987, 32, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Piala, A.T.; Moon, T.M.; Akella, R.; He, H.; Cobb, M.H.; Goldsmith, E.J. Chloride sensing by WNK1 involves inhibition of autophosphorylation. Sci. Signal. 2014, 7, ra41. [Google Scholar] [CrossRef] [PubMed]
- Ponce-Coria, J.; San-Cristobal, P.; Kahle, K.T.; Vazquez, N.; Pacheco-Alvarez, D.; de Los Heros, P.; Juárez, P.; Muñoz, E.; Michel, G.; Bobadilla, N.A.; et al. Regulation of NKCC2 by a chloride-sensing mechanism involving the WNK3 and SPAK kinases. Proc. Natl. Acad. Sci. USA 2008, 105, 8458–8463. [Google Scholar] [CrossRef]
- Hanberg, J.S.; Rao, V.; Ter Maaten, J.M.; Laur, O.; Brisco, M.A.; Perry Wilson, F.; Grodin, J.L.; Assefa, M.; Samuel Broughton, J.; Planavsky, N.J.; et al. Hypochloremia and Diuretic Resistance in Heart Failure: Mechanistic Insights. Circ. Heart Fail. 2016, 9, e003180. [Google Scholar] [CrossRef]
- Kataoka, H. Chloride in Heart Failure Syndrome: Its Pathophysiologic Role and Therapeutic Implication. Cardiol. Ther. 2021, 10, 407–428. [Google Scholar] [CrossRef]
- Ter Maaten, J.M.; Rao, V.S.; Hanberg, J.S.; Perry Wilson, F.; Bellumkonda, L.; Assefa, M.; Sam Broughton, J.; D’Ambrosi, J.; Wilson Tang, W.H.; Damman, K.; et al. Renal tubular resistance is the primary driver for loop diuretic resistance in acute heart failure. Eur. J. Heart Fail. 2017, 19, 1014–1022. [Google Scholar] [CrossRef] [PubMed]
- Rao, V.S.; Planavsky, N.; Hanberg, J.S.; Ahmad, T.; Brisco-Bacik, M.A.; Wilson, F.P.; Jacoby, D.; Chen, M.; Tang, W.H.W.; Cherney, D.Z.I.; et al. Compensatory Distal Reabsorption Drives Diuretic Resistance in Human Heart Failure. J. Am. Soc. Nephrol. JASN 2017, 28, 3414–3424. [Google Scholar] [CrossRef]
- Brater, D.C.; Seiwell, R.; Anderson, S.; Burdette, A.; Dehmer, G.J.; Chennavasin, P. Absorption and disposition of furosemide in congestive heart failure. Kidney Int. 1982, 22, 171–176. [Google Scholar] [CrossRef]
- Bockenhauer, D. Over- or underfill: Not all nephrotic states are created equal. Pediatr. Nephrol. 2013, 28, 1153–1156. [Google Scholar] [CrossRef]
- Svenningsen, P.; Bistrup, C.; Friis, U.G.; Bertog, M.; Haerteis, S.; Krueger, B.; Stubbe, J.; Jensen, O.N.; Thiesson, H.C.; Uhrenholt, T.R.; et al. Plasmin in nephrotic urine activates the epithelial sodium channel. J. Am. Soc. Nephrol. JASN 2009, 20, 299–310. [Google Scholar] [CrossRef]
- Hinrichs, G.R.; Mortensen, L.A.; Jensen, B.L.; Bistrup, C. Amiloride resolves resistant edema and hypertension in a patient with nephrotic syndrome; a case report. Physiol. Rep. 2018, 6, e13743. [Google Scholar] [CrossRef]
- Yamaguchi, E.; Yoshikawa, K.; Nakaya, I.; Kato, K.; Miyasato, Y.; Nakagawa, T.; Kakizoe, Y.; Mukoyama, M.; Soma, J. Liddle’s-like syndrome associated with nephrotic syndrome secondary to membranous nephropathy: The first case report. BMC Nephrol. 2018, 19, 122. [Google Scholar] [CrossRef] [PubMed]
- Cox, Z.L.; Fleming, J.; Ivey-Miranda, J.; Griffin, M.; Mahoney, D.; Jackson, K.; Hodson, D.Z.; Thomas, D., Jr.; Gomez, N.; Rao, V.S.; et al. Mechanisms of Diuretic Resistance Study: Design and rationale. ESC Heart Fail. 2020, 7, 4458–4464. [Google Scholar] [CrossRef] [PubMed]
- Ellison, D.H. Diuretic therapy and resistance in congestive heart failure. Cardiology 2001, 96, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Ellison, D.H.; Velázquez, H.; Wright, F.S. Adaptation of the distal convoluted tubule of the rat. Structural and functional effects of dietary salt intake and chronic diuretic infusion. J. Clin. Investig. 1989, 83, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Stanton, B.A.; Kaissling, B. Regulation of renal ion transport and cell growth by sodium. Am. J. Physiol. 1989, 257 Pt 2, F1–F10. [Google Scholar] [CrossRef]
- Wilcox, C.S.; Mitch, W.E.; Kelly, R.A.; Skorecki, K.; Meyer, T.W.; Friedman, P.A.; Souney, P.F. Response of the kidney to furosemide. I. Effects of salt intake and renal compensation. J. Lab. Clin. Med. 1983, 102, 450–458. [Google Scholar]
- Cox, Z.L.; Rao, V.S.; Ivey-Miranda, J.B.; Moreno-Villagomez, J.; Mahoney, D.; Ponikowski, P.; Biegus, J.; Turner, J.M.; Maulion, C.; Bellumkonda, L.; et al. Compensatory post-diuretic renal sodium reabsorption is not a dominant mechanism of diuretic resistance in acute heart failure. Eur. Heart J. 2021, 42, 4468–4477. [Google Scholar] [CrossRef]
- Mullens, W.; Damman, K.; Harjola, V.P.; Mebazaa, A.; Brunner-La Rocca, H.P.; Martens, P.; Testani, J.M.; Tang, W.H.W.; Orso, F.; Rossignol, P.; et al. The use of diuretics in heart failure with congestion—A position statement from the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2019, 21, 137–155. [Google Scholar] [CrossRef]
- Testani, J.M.; Hanberg, J.S.; Cheng, S.; Rao, V.; Onyebeke, C.; Laur, O.; Kula, A.; Chen, M.; Wilson, F.P.; Darlington, A.; et al. Rapid and Highly Accurate Prediction of Poor Loop Diuretic Natriuretic Response in Patients with Heart Failure. Circ. Heart Fail. 2016, 9, e002370. [Google Scholar] [CrossRef] [PubMed]
- Rao, V.S.; Ivey-Miranda, J.B.; Cox, Z.L.; Riello, R.; Griffin, M.; Fleming, J.; Soucier, R.; Sangkachand, P.; O’Brien, M.; LoRusso, F.; et al. Natriuretic Equation to Predict Loop Diuretic Response in Patients with Heart Failure. J. Am. Coll. Cardiol. 2021, 77, 695–708. [Google Scholar] [CrossRef] [PubMed]
- Girerd, N.; Seronde, M.F.; Coiro, S.; Chouihed, T.; Bilbault, P.; Braun, F.; Kenizou, D.; Maillier, B.; Nazeyrollas, P.; Roul, G.; et al. Integrative Assessment of Congestion in Heart Failure Throughout the Patient Journey. JACC Heart Fail. 2018, 6, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Kitsios, G.D.; Mascari, P.; Ettunsi, R.; Gray, A.W. Co-administration of furosemide with albumin for overcoming diuretic resistance in patients with hypoalbuminemia: A meta-analysis. J. Crit. Care 2014, 29, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Pasini, A.; Benetti, E.; Conti, G.; Ghio, L.; Lepore, M.; Massella, L.; Molino, D.; Peruzzi, L.; Emma, F.; Fede, C.; et al. The Italian Society for Pediatric Nephrology (SINePe) consensus document on the management of nephrotic syndrome in children: Part I—Diagnosis and treatment of the first episode and the first relapse. Ital. J. Pediatr. 2017, 43, 41. [Google Scholar] [CrossRef]
- Felker, G.M.; Lee, K.L.; Bull, D.A.; Redfield, M.M.; Stevenson, L.W.; Goldsmith, S.R.; LeWinter, M.M.; Deswal, A.; Rouleau, J.L.; Ofili, E.O.; et al. Diuretic strategies in patients with acute decompensated heart failure. N. Engl. J. Med. 2011, 364, 797–805. [Google Scholar] [CrossRef]
- Thomson, M.R.; Nappi, J.M.; Dunn, S.P.; Hollis, I.B.; Rodgers, J.E.; Van Bakel, A.B. Continuous versus intermittent infusion of furosemide in acute decompensated heart failure. J. Card. Fail. 2010, 16, 188–193. [Google Scholar] [CrossRef]
- Monteiro Pacheco, A., Jr.; Martins Coimbra, R.S.; Kreimeier, U.; Frey, L.; Messmer, K. Hypertonic volume therapy: Feasibility in the prevention and treatment of multiple organ failure and sepsis. Sao Paulo Med. J. 1995, 113, 1053–1060. [Google Scholar] [CrossRef][Green Version]
- De Vecchis, R.; Esposito, C.; Ariano, C.; Cantatrione, S. Hypertonic saline plus i.v. furosemide improve renal safety profile and clinical outcomes in acute decompensated heart failure: A meta-analysis of the literature. Herz 2015, 40, 423–435. [Google Scholar] [CrossRef]
- Griffin, M.; Soufer, A.; Goljo, E.; Colna, M.; Rao, V.S.; Jeon, S.; Raghavendra, P.; D’Ambrosi, J.; Riello, R.; Coca, S.G.; et al. Real World Use of Hypertonic Saline in Refractory Acute Decompensated Heart Failure: A U.S. Center’s Experience. JACC Heart Fail. 2020, 8, 199–208. [Google Scholar] [CrossRef]
- Butler, J.; Anstrom, K.J.; Felker, G.M.; Givertz, M.M.; Kalogeropoulos, A.P.; Konstam, M.A.; Mann, D.L.; Margulies, K.B.; McNulty, S.E.; Mentz, R.J.; et al. Efficacy and Safety of Spironolactone in Acute Heart Failure: The ATHENA-HF Randomized Clinical Trial. JAMA Cardiol. 2017, 2, 950–958. [Google Scholar] [CrossRef] [PubMed]
- Dormans, T.P.; Gerlag, P.G. Combination of high-dose furosemide and hydrochlorothiazide in the treatment of refractory congestive heart failure. Eur. Heart J. 1996, 17, 1867–1874. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.M.; Konopka, E.; Hyderi, A.F.; Hshieh, S.; Tsuji, Y.; Kim, B.J.; Han, S.Y.; Phan, D.H.; Jeng, A.I.; Lou, M.; et al. Comparison of bumetanide- and metolazone-based diuretic regimens to furosemide in acute heart failure. J. Cardiovasc. Pharmacol. Ther. 2013, 18, 345–353. [Google Scholar] [CrossRef]
- Sica, D.A. Metolazone and its role in edema management. Congest. Heart Fail. 2003, 9, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Moranville, M.P.; Choi, S.; Hogg, J.; Anderson, A.S.; Rich, J.D. Comparison of metolazone versus chlorothiazide in acute decompensated heart failure with diuretic resistance. Cardiovasc. Ther. 2015, 33, 42–49. [Google Scholar] [CrossRef]
- Brisco-Bacik, M.A.; Ter Maaten, J.M.; Houser, S.R.; Vedage, N.A.; Rao, V.; Ahmad, T.; Wilson, F.P.; Testani, J.M. Outcomes Associated with a Strategy of Adjuvant Metolazone or High-Dose Loop Diuretics in Acute Decompensated Heart Failure: A Propensity Analysis. J. Am. Heart Assoc. 2018, 7, e009149. [Google Scholar] [CrossRef] [PubMed]
- Pitt, B.; Zannad, F.; Remme, W.J.; Cody, R.; Castaigne, A.; Perez, A.; Palensky, J.; Wittes, J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N. Engl. J. Med. 1999, 341, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Schepkens, H.; Vanholder, R.; Billiouw, J.M.; Lameire, N. Life-threatening hyperkalemia during combined therapy with angiotensin-converting enzyme inhibitors and spironolactone: An analysis of 25 cases. Am. J. Med. 2001, 110, 438–441. [Google Scholar] [CrossRef] [PubMed]
- Juurlink, D.N.; Mamdani, M.M.; Lee, D.S.; Kopp, A.; Austin, P.C.; Laupacis, A.; Redelmeier, D.A. Rates of hyperkalemia after publication of the Randomized Aldactone Evaluation Study. N. Engl. J. Med. 2004, 351, 543–551. [Google Scholar] [CrossRef]
- Wongboonsin, J.; Thongprayoon, C.; Bathini, T.; Ungprasert, P.; Aeddula, N.R.; Mao, M.A.; Cheungpasitporn, W. Acetazolamide Therapy in Patients with Heart Failure: A Meta-Analysis. J. Clin. Med. 2019, 8, 349. [Google Scholar] [CrossRef]
- Verbrugge, F.H.; Dupont, M.; Bertrand, P.B.; Nijst, P.; Penders, J.; Dens, J.; Verhaert, D.; Vandervoort, P.; Tang, W.H.; Mullens, W. Determinants and impact of the natriuretic response to diuretic therapy in heart failure with reduced ejection fraction and volume overload. Acta Cardiol. 2015, 70, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Verbrugge, F.H.; Martens, P.; Ameloot, K.; Haemels, V.; Penders, J.; Dupont, M.; Tang, W.H.W.; Droogné, W.; Mullens, W. Acetazolamide to increase natriuresis in congestive heart failure at high risk for diuretic resistance. Eur. J. Heart Fail. 2019, 21, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Mullens, W.; Dauw, J.; Martens, P.; Verbrugge, F.H.; Nijst, P.; Meekers, E.; Tartaglia, K.; Chenot, F.; Moubayed, S.; Dierckx, R.; et al. Acetazolamide in Acute Decompensated Heart Failure with Volume Overload. N. Engl. J. Med. 2022, 387, 1185–1195. [Google Scholar] [CrossRef] [PubMed]
- Felker, G.M. New Decongestion Strategies in an Evolving Heart Failure Landscape. N. Engl. J. Med. 2022, 387, 1231–1233. [Google Scholar] [CrossRef] [PubMed]
- Cox, Z.L.; Sarrell, B.A.; Cella, M.K.; Tucker, B.; Arroyo, J.P.; Umanath, K.; Tidwell, W.; Guide, A.; Testani, J.M.; Lewis, J.B.; et al. Multinephron Segment Diuretic Therapy to Overcome Diuretic Resistance in Acute Heart Failure: A Single-Center Experience. J. Card. Fail. 2022, 28, 21–31. [Google Scholar] [CrossRef]
- Damman, K.; Beusekamp, J.C.; Boorsma, E.M.; Swart, H.P.; Smilde, T.D.J.; Elvan, A.; van Eck, J.W.M.; Heerspink, H.J.L.; Voors, A.A. Randomized, double-blind, placebo-controlled, multicentre pilot study on the effects of empagliflozin on clinical outcomes in patients with acute decompensated heart failure (EMPA-RESPONSE-AHF). Eur. J. Heart Fail. 2020, 22, 713–722. [Google Scholar] [CrossRef]
- Wilcox, C.S.; Shen, W.; Boulton, D.W.; Leslie, B.R.; Griffen, S.C. Interaction Between the Sodium-Glucose-Linked Transporter 2 Inhibitor Dapagliflozin and the Loop Diuretic Bumetanide in Normal Human Subjects. J. Am. Heart Assoc. 2018, 7, e007046. [Google Scholar] [CrossRef]
- Griffin, M.; Riello, R.; Rao, V.S.; Ivey-Miranda, J.; Fleming, J.; Maulion, C.; McCallum, W.; Sarnak, M.; Collins, S.; Inzucchi, S.E.; et al. Sodium glucose cotransporter 2 inhibitors as diuretic adjuvants in acute decompensated heart failure: A case series. ESC Heart Fail. 2020, 7, 1966–1971. [Google Scholar] [CrossRef]
- Konstam, M.A.; Gheorghiade, M.; Burnett, J.C., Jr.; Grinfeld, L.; Maggioni, A.P.; Swedberg, K.; Udelson, J.E.; Zannad, F.; Cook, T.; Ouyang, J.; et al. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: The EVEREST Outcome Trial. JAMA 2007, 297, 1319–1331. [Google Scholar] [CrossRef]
- Felker, G.M.; Mentz, R.J.; Cole, R.T.; Adams, K.F.; Egnaczyk, G.F.; Fiuzat, M.; Patel, C.B.; Echols, M.; Khouri, M.G.; Tauras, J.M.; et al. Efficacy and Safety of Tolvaptan in Patients Hospitalized with Acute Heart Failure. J. Am. Coll. Cardiol. 2017, 69, 1399–1406. [Google Scholar] [CrossRef]
- Konstam, M.A.; Kiernan, M.; Chandler, A.; Dhingra, R.; Mody, F.V.; Eisen, H.; Haught, W.H.; Wagoner, L.; Gupta, D.; Patten, R.; et al. Short-Term Effects of Tolvaptan in Patients with Acute Heart Failure and Volume Overload. J. Am. Coll. Cardiol. 2017, 69, 1409–1419. [Google Scholar] [CrossRef] [PubMed]
- Bart, B.A.; Goldsmith, S.R.; Lee, K.L.; Givertz, M.M.; O’Connor, C.M.; Bull, D.A.; Redfield, M.M.; Deswal, A.; Rouleau, J.L.; LeWinter, M.M.; et al. Ultrafiltration in decompensated heart failure with cardiorenal syndrome. N. Engl. J. Med. 2012, 367, 2296–2304. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, M.R.; Negoianu, D.; Jaski, B.E.; Bart, B.A.; Heywood, J.T.; Anand, I.S.; Smelser, J.M.; Kaneshige, A.M.; Chomsky, D.B.; Adler, E.D.; et al. Aquapheresis versus Intravenous Diuretics and Hospitalizations for Heart Failure. JACC Heart Fail. 2016, 4, 95–105. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).