1. Introduction
Individuals living with kidney failure experience a high symptom burden related to both disease and treatment. The extensive burden of symptoms in this condition is comparable to that of other terminal illnesses including cancer, heart failure and lung disease [
1]. Fatigue, pruritus, decreased appetite and muscle cramps are amongst the most prevalent symptoms associated with kidney failure and in addition to these symptoms, pain has also been documented as a common problem [
1].
Pain is defined as an unpleasant sensory and emotional experience associated with, or resembling actual or potential harm [
2]. Pain is experienced in a multidimensional capacity by patients with kidney failure and encompasses nociceptive, neuropathic and non-neuropathic modalities [
3]. The underlying aetiology of pain includes comorbidities such as diabetic or uremic neuropathy or peripheral vascular disease, conditions specific to kidney failure such as mineral bone disease or treatment specific causes such as pain during peritoneal dialysis (PD) exchanges, or headaches, muscle cramps or arteriovenous access pain experienced during haemodialysis (HD) [
4]. Despite the overwhelming occurrence of pain, recognition and management of pain remain inadequate. The Dialysis Outcomes and Practice Patterns Study (DOPPS) study showed a large proportion (74%) of patients with moderate to severe pain were not prescribed analgesics, and providers underreported and underestimated symptoms in over half of cases [
5,
6].
Most studies on pain in kidney failure have been conducted in those undertaking HD but many aspects of impact from pain remain unclear, including how pain impacts on food intake and eating, as well as the general quality of life in those living with kidney failure. Few studies have explored pain using a mixed methods approach. There is also a lack of qualitative data on pain, especially in patients undertaking dialysis in the Australian context.
The aims of this study were (i) to quantify the prevalence and severity of pain in patients with kidney failure in the Illawarra Shoalhaven Local Health District, (ii) to identify the characteristics of patients with kidney failure who are most impacted by pain and (iii) to explore the impact of pain on daily living in people with kidney failure undergoing dialysis.
3. Results
3.1. Characteristics of Survey Participants
A total of 131 surveys were completed by dialysis patients (111 in-centre HD, 17 PD and 3 home HD). Response rates differed between treatment groups, with a response rate of 81% of patients on in-centre HD, 47% of patients on PD and 19% of those on home HD.
Demographic details and clinical characteristics of participants are shown in
Table 2. The majority of the survey participants were undergoing in centre HD (87.2%). The median age of participants was 69 years (IQR 57–76) and for the HD group median age 58 years (IQR 29–77). Of the 17 PD patients, 12 (71%) also had vascular access in situ. The PD catheter was not reported as a source of pain in any PD patient.
For those undergoing dialysis treatment, the median (IQR) dialysis vintage was 4 (1–8) years. The most common chronic comorbid condition was diabetes mellitus (51.7%), followed by coronary artery disease (47.8%), peripheral vascular disease (37.15), chronic lung disease (19.6%) and cerebrovascular disease (16.0%).
3.2. Prevalence of Pain
Pain was reported in 121 out of the 131 participants (92.3%) as shown in
Table 3. Only male participants reported having no pain. Pain prevalence was similar in old (88.0%) and young (93.5%) participants. Thirty percent (
n = 35) patients on HD experienced arteriovenous fistula access pain. Approximately 65% (
n = 77) patients reported having control over their pain.
3.3. Pain by Treatment Group
Pain was highly prevalent in all three treatment groups. Every patient receiving home HD treatment reported pain. The prevalence of pain in the PD group was 94.1% and 91.9% in the HD group.
3.4. Pain Severity
Pain intensity ratings were obtained from 114 of 131 participants. Of these, over half of participants (62.3%) rated their pain as severe to excruciating. More females (70.0%) than males (56.3%) rated their pain as severe to excruciating (p = 0.004). With respect to age, there were no statistically significant differences between the age categories for pain severity when categorised as <40, 40–59, 60–74 and ≥75 years (p = 0.56, data not shown). Pain severity was reported as severe to excruciating by 60.6% of those aged 65 years and older and 65.9% of those aged <65 years, but this was also not statistically different (p = 0.57).
3.5. Characteristics of People Experiencing Pain
Results from binary logistic regression are shown in
Table 4. In the final model, age ≥ 75 years, peripheral vascular disease, and a longer time on dialysis were associated with greater odds of experiencing pain, however these were not statistically significant.
3.6. Type of Pain
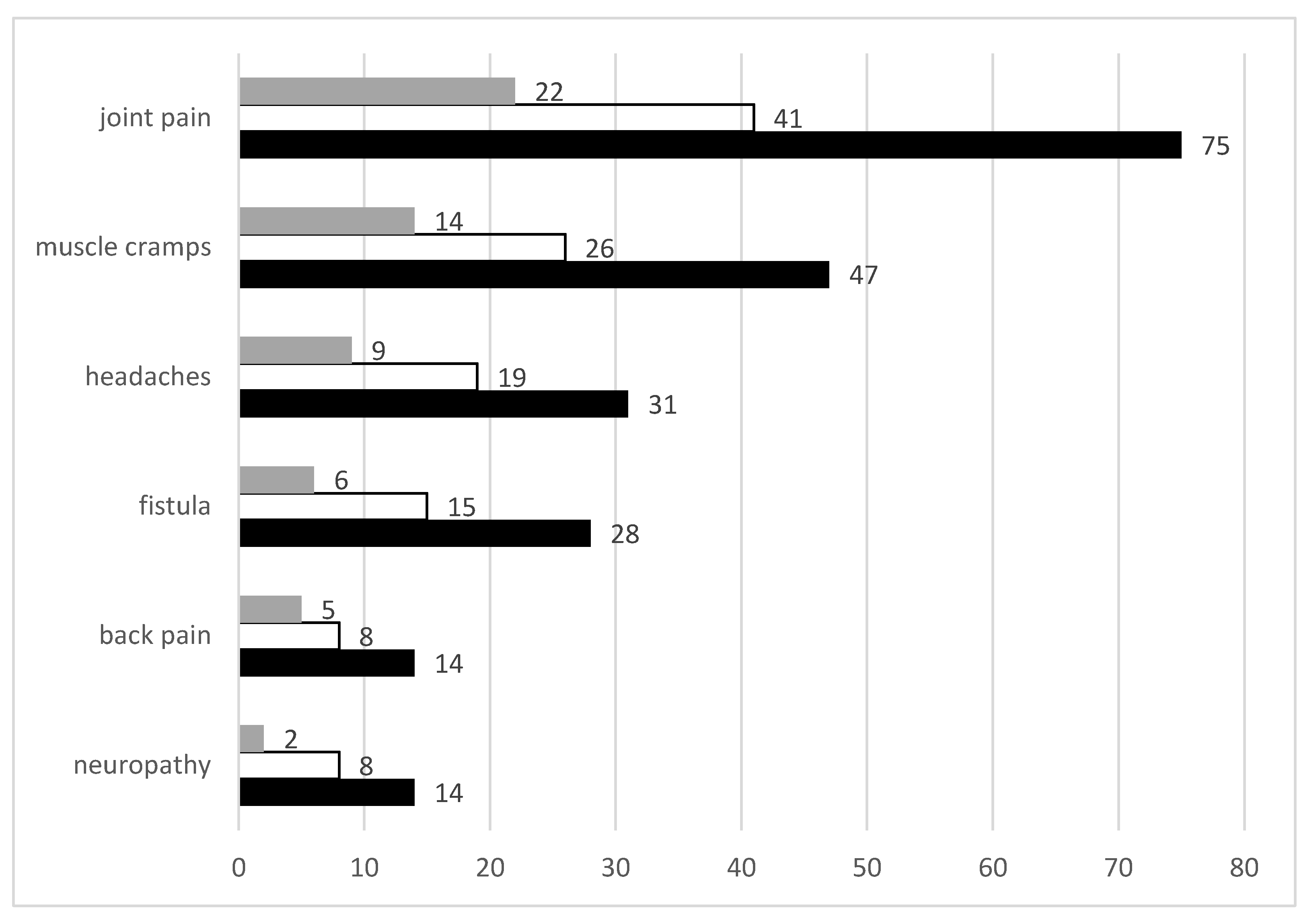
The most prevalent pain modalities reported in the study are shown in
Figure 1 below. Joint pain was the most common form of pain reported impacting 75 of 131 (57%) of participants. This was followed by muscle cramps (36%), headaches (24%), fistula pain (21%), back pain (11%) and neuropathy/nerve pain (11%). Pain due to amputations, liver trauma, haemorrhoids, migraine, steal syndrome and foot ulcers as well as stomach, chest, eye, foot, muscle and kidney pain were reported to a lesser extent, all in <5% of participants.
3.7. Arteriovenous Access Type
Of those who reported fistula pain (21.0%) the most common access type was arteriovenous fistula (AVF) (78.0%), followed by native vein grafts (12.5%) and prosthetic arteriovenous grafts (9.4%). The most common location of arteriovenous access amongst those experiencing fistula pain was the left forearm (50.0%), followed by the right forearm (21.9%), left upper arm (18.8%), right upper arm (6.25%) and left mid-forearm (3.1%).
3.8. Thematic Analysis of Open-Ended Questions
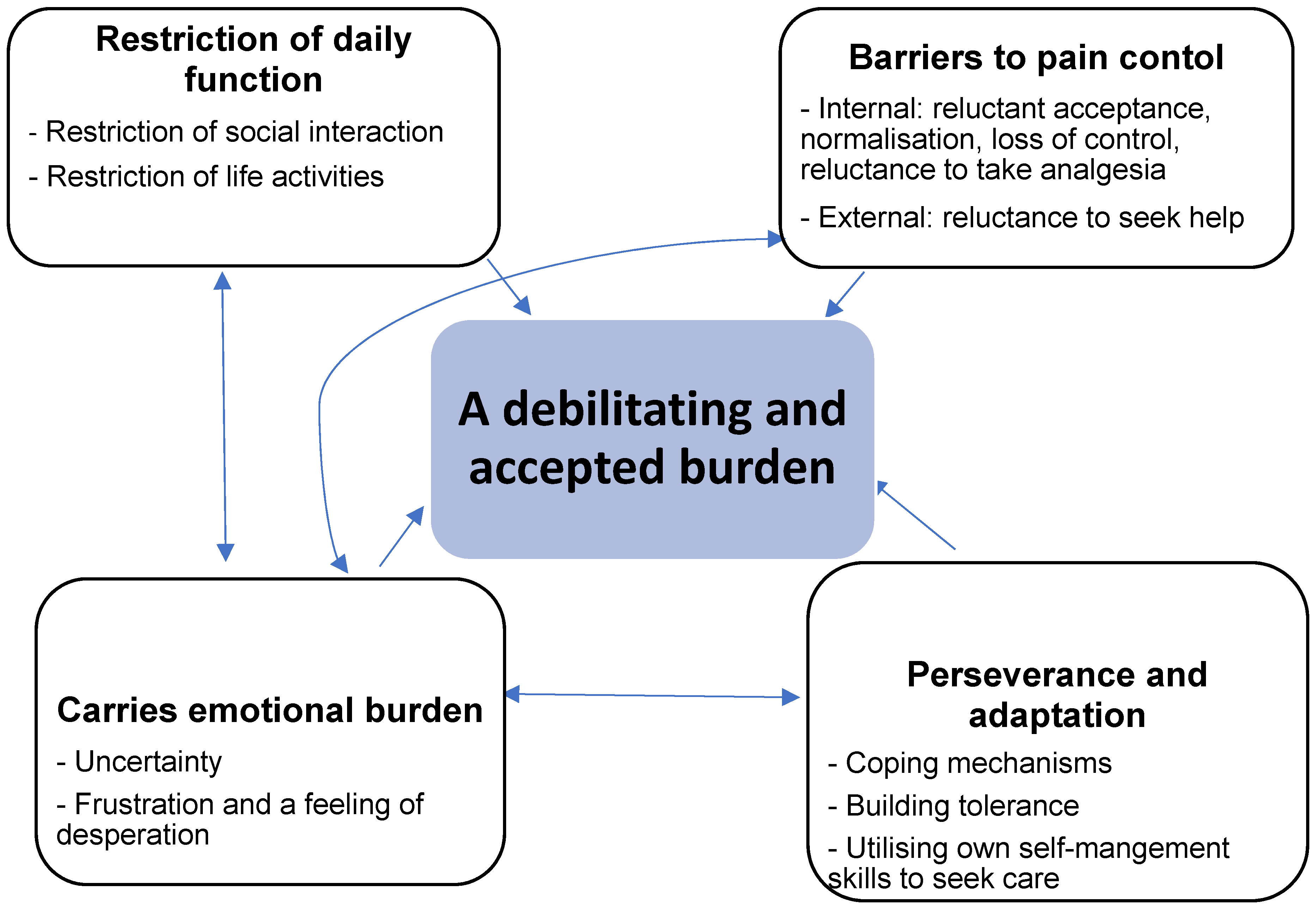
The overarching theme identified that characterised the lived experience of pain by people with kidney failure was ‘a debilitating and accepted burden’. The relationship between the overarching theme and subthemes is show in
Figure 2. The major subthemes were: restriction of daily function, extensive barriers to pain control; carrying an emotional burden and perseverance and adaptation.
3.8.1. Restriction of Daily Function
Restriction of Social Interaction
Participants felt unable to engage in outings: ‘if it (pain) was really bad I wouldn’t leave the house’ and social interaction through fear of the onset of pain interrupting; ‘I’d like to go and play bowls but I might be there when it gets me bad. I have to stop and it ruins it for everyone.’ Some felt pain impacted on their personality and created a barrier to experiencing meaningful and pleasant interactions with loved ones; ‘it is hard to maintain relationships, be positive and friendly when you’re in pain.’ ‘I’m aware it effects other people. My mood is shitty. I internalise it.’
Restriction of Life Activities
Participants felt pain led to disruption of their daily lives including sleep ‘my back will wake me up at three, four o’clock in the morning. I find it very hard to get back to sleep’ and eating ‘if it’s bad, I can’t eat but I usually take some medication to subside it,’ ‘pain… makes me want to eat and I have been putting on weight.’ Participants spoke about pain impacting not only their ability to engage in exercise ‘I can’t walk the dog anymore’, ‘had to give up netball because of arthritis’ but the burden it had on their activities of daily living ‘it makes me very uncomfortable to the point where I just want to lay down’, ‘it’s bad when I shop because I’m on my feet. I don’t do too much because if I stand on them for too long I pay for it afterwards.’ Some felt that pain influenced their physical capabilities ‘it imposes physical limitations on what I can and can’t do’, ‘pain in my fingers makes me frustrated, when I can’t do what I want to do.’
3.8.2. Barriers to Pain Control
Barriers to pain control were described and were related to internal and external factors.
Internal Barriers
Reluctant acceptance
Participants perceived pain as beyond their control ‘it is what it is. I can’t do much about it.’ They came to accept their pain through a belief that nothing could be done and their only option was to ‘just put up with it’, ‘I’ve grown used to it. I know there’s not much that can be done.’
Normalisation
Pain was viewed by participants as a normal part of the disease process and ‘part of the journey’. They perceived that the experience of pain was an expected part of dialysis treatment, ‘just par for the course of dialysis.’ Patients frequently compared their experience of pain to that of others who they perceived to be more severely impacted which normalised and minimised their own discomfort, ‘it’s important for me to know there are people worse than me- normalising it for me’, ‘I just think about the people that are worse off than me. I might be in a little bit of pain.’
Loss of control
Participants felt like there was nothing they could do to control their pain, ‘if it’s happening, it’s happening. That’s it,’ ‘I refer to it as an entity, something I can control. But it’s not.’ Pain contributed to a sense of helplessness, ‘you’re wandering around the house thinking when’s this gonna stop,’ ‘I control it but the pain is there. I don’t know, I just, I just pray.’ Another factor contributing to this sense of loss of control was a perceived lack of effectiveness of pain relief prescribed; ‘some days are very bad and pain relief does not help.’ It was also perceived that mechanisms suggested by staff to assist patients to cope were futile, ‘distraction techniques do not work.’
Reluctance to take analgesics
Participants reported an extensive rationale for avoiding or minimising analgesic use. Side effects were commonly described as barriers to effective pain control, ‘I have the side effects of constipation and so forth.’ Fear of opioid addiction also concerned participants, ‘I’ve been taking Endone. I only take it when I absolutely have to because I don’t like being on Opioids. They’re addictive.’ Use of strong analgesics led to feelings of fear and guilt, ‘I know I’m not allowed to but Panadol(paracetamol) doesn’t take the pain away’, ‘when it gets severe I take Endone but I try and avoid it. I try not to get hooked to it.’ Polypharmacy and pill burden arose frequently as a barrier to effective pain relief ‘I’m on eighteen tablets a day in the morning, plus ten at night, so it’s twenty-eight, plus insulin, plus nebulisers and god knows what else, so yep. It’s called chronic’.
External Barriers
Reluctance to seek help
Participants reported reluctance to seek help from health professionals predominantly due to confusing or unhelpful advice. Some also reported the pain management techniques suggested by health professionals were unrealistic, ‘I know what my doctor will say, you’ve got to lose weight, but I can’t exercise’. Others perceived their complaints about pain were not taken seriously, ‘I don’t tell them anything. They don’t believe me. I have to look after myself.’ A need for better communication between specialties and members of the multidisciplinary team was identified: ‘it is a tug of war between specialists, communication would be better between teams so you can understand what medications you can take. Not understanding my condition between each specialty is a concern.’
3.8.3. Carrying an Emotional Burden
The third subtheme identified regarding the lived experience of pain was the emotional burden.
Uncertainty
Participants frequently expressed uncertainty in regard to their pain. This included uncertainty about the onset of pain, pain intensity and time experienced ‘you just want to be stuck at home. You don’t know when the pain comes’; the aetiology of their pain, ‘my hips have been really bad and my knees lazy’; and the support services available to them, ‘not sure what they can do so all I can do is tell you’, ‘what are safe medications to take? Information would be good.’
Frustration and a Feeling of Desperation
Participants also described a range of negative emotions that contributed to the sense of uncertainty. These included desperation and depression; ‘Sometimes I think, what am I doing here’, ‘you become anxious, depressed. Definite connection between pain and depression’, denial; ‘For me it’s not a real thing, the pain that I experience’. Frustration was felt in regard to the experience of pain, ‘it makes me sit down, and I don’t want to be sat down. It makes me aggravated because I can’t do the things I want to do’. Frustration was also felt towards health professionals when seeking help ‘doctor comes, doesn’t help with pain’. Hopelessness was felt by participants also, ‘everything wakes me up at night, my neck wakes me up, I have to sit up… it is a pain in the bum. Really they should just stick a needle in my arm’, ‘bring back euthanasia, write that down’.
Perseverance and Adaptation
Despite the widespread nature of pain, participants described a number of strategies that indicted perseverance and adaptation to pain.
Coping mechanisms
Participants reported employing a variety of coping mechanisms to manage pain. Exercise was a common strategy used to control painful symptoms ‘I’ve been exercising, it does help’, ‘when I start walking it just releases and gets better.’ Use of distraction techniques was also reported ‘I sometimes get up and colour in, a distraction technique.’ Others reported taking a more passive approach to pain management, ‘I sit down when I need to, that will control the pain’, ‘I just sleep it off.’ Participants also spoke of seeking help from health professionals as a means of gaining control, ‘If I had a problem I would go and see a GP or consult a nurse.’
Building tolerance
Participants felt they had the ability to cope with their pain over time through the development of a high pain threshold ‘I’ve got a pretty high tolerance to pain, threshold I suppose’. Some felt they had even built immunity to the impact of their pain, ‘I think I’ve just become immune to whatever pain it is. I don’t know what pain is anymore.’ Participants reported persevering through the pain to do the things they wanted to do, ‘I still play bowls, grit my teeth and do what I want to do,’ ‘mind over matter, you don’t let the pain stop you.’
Utilising their own self-management skills to seek care
Not all participants reported a reluctance to access health care providers and services, ‘if I had a problem I would go and see a GP or consult a nurse here.’ A willingness to discuss pain was reported also by some, ‘I’m quite open about the pain.’ Participants appreciated when clinicians raised the topic of their pain during consultations, ‘it is helpful when they ask about pain as you go in focusing on the subject and forget to ask about anything else.’ Others felt that it was the patient’s responsibility to communicate their pain management needs, ‘You need to let people know if you are in pain and you just gotta be proactive in seeking treatment and I know there is no one treatment that works for all’.
4. Discussion
Our cross-sectional survey study provides insight into the prevalence and lived experience of pain in an Australian cohort of people living with kidney failure and undertaking dialysis. Pain was experienced by an extensive proportion of the surveyed population and most participants reported their pain to be severe to excruciating in nature. Whilst evidence exists from previous studies on the prevalence of pain in those living with kidney failure, this study is novel in its qualitative exploration of pain in this population. Pain impacted many aspects of participants daily lives including eating, sleeping, socialising and engaging in physical activity. It also impacted heavily on the participant’s emotional state which created barriers and influenced coping mechanisms and willingness to seek support as well as acceptance of analgesics and health care services.
This study adds to the existing literature indicating a high prevalence of pain observed in this population. Findings were consistent with those reported by Lowe and Robinson [
3] who found 60–90% of patients receiving kidney replacement therapy experienced pain. A systematic review [
10] found the prevalence of chronic pain in the HD population ranged between 33–82%, and acute pain during the past four weeks ranged between 21–92%. A recent metanalysis of pain in the CKD population reporting on 40,678 individuals with CKD [
11] found that the pooled prevalence of pain for dialysis patients was 63% (95% CI: 57–68) and in those with non-dialysis CKD 63% (95% CI: 55–70). Davison et al. [
12] reported more detail in their exploration of pain in people with CKD, and found that 49% of patients experienced pain that was moderate to severe in intensity. This was similar to the proportion of participants who described their pain as severe to excruciating in the present study, whilst the findings of Brkovic, Burilovic and Puljak [
10] concluded a large range of pain severity exists in the HD population, with studies included in their systemic review reporting the prevalence of severe/intensive pain was anywhere from 0–76%.By comparison, studies of chronic pain in the general population suggest a pooled prevalence of 10.6 to 11.8% [
13], 16.2% in those with diabetic neuropathy [
14] and 18% in those with type 2 diabetes [
15] indicating the large disparity in the burden of pain for people with kidney failure.
Unique to this study was the inclusion of assessment of the participants perceived level of control over their pain. Whilst most participants reported they did have control over their pain, free text responses suggested otherwise with many factors acting as barriers to adequate pain management. This was consistent with the findings of Lowe and Robinson [
3] who reported that three-quarters of patients described their pain management regime as inadequate. The heavy emotional burden from pain, including feelings of loss of control, uncertainty, frustration and desperation were key themes identified in this study, along with a sense of social isolation due to the restrictions imposed by pain. The emotionally debilitating experience of pain may contribute to the high rates of mental health conditions, such as depression and anxiety, experienced at nearly three times greater rates in those with kidney failure than the general population [
16]. Pain itself has been found to be a risk factor for depression. Means-Christensen et al. [
16] also found those with chronic pain had increased odds of having a major depressive disorder. These findings, along with those observed in the present study suggest a need for pain and mental health to be assessed collaboratively in those with kidney failure.
This study found a significant association between female gender and the presence of pain. This is consistent with reports from the International Association for the Study of Pain [
2] that a higher proportion of women experience chronic pain than men, and that this pain is frequently more severe, recurrent and persistent. The mechanisms responsible for these differences remain unclear [
17]. Factors likely to contribute include the impact of sex hormones and genotype, sex-linked variation in the cortical processing of pain-stimuli, differences in coping mechanisms between genders, as well as sociocultural beliefs leading to biased reporting from males due to concerns surrounding masculinity and pain acceptance.
One of the key aims of this study was to explore the lived experience of pain. The themes identified were comparable to those described by Tong et al. [
18] where patient experiences and perspectives of living with CKD were explored in focus groups. Sub-themes of physical constraints, emotional struggle and coping, managing medicines and managing and monitoring health drew similarity to themes identified in the present study. More recent metasyntheses of pain in CKD [
19] also identified that suffering in silence was apparent. The impact of pain on eating in any of these qualitative studies was specifically not explored.
In the present study, the impact of pain on eating was variable. Many participants did not report that pain impacted eating. A small subset of patients did feel that pain had hindered their intake, however, and others felt they consumed more when in pain as a comfort mechanism. Janke et al. [
20] reported that acute and chronic stress are associated with both increased and decreased caloric intake, with pain being identified as a common stressor. It was however reported in this study that pain is most associated with preference for high-energy foods and overconsumption and that intake of foods high in fat and sugar can increase pain tolerance. Alternatively, pain has been identified as a nutrition impact symptom increasing malnutrition risk due to decreased intake in cancer patients [
21], a population where pain prevalence has been identified as comparable to those with kidney failure [
1]. Given the extensive prevalence and complexity of responses to pain, it is not surprising to find there is variation in the way individuals describe the impact of pain on eating.
Acceptance and normalisation of pain and a sense of loss of control impacted pain management and the ability to proactively seek care. Participants interpreted pain as an experience they simply had to accept, propagating feelings of hopelessness, adding to the emotional burden and decreasing the overall quality of life. These findings complement those of [
22] who found pain was an independent predictor of both mental and physical health-related quality of life. The reluctance to take analgesics reported by participants was similarly reported by Tong et al. [
18] where participants spoke of confusion around medication regimes and the onset of unpleasant side effects. Compliance to pharmacological treatments was maintained to avoid guilt and blame, out of a sense of duty to their care providers and to survive. In contrast to the sense of duty participants felt to their hospital and physician, individuals involved in the current study were reluctant to seek help as previous advice provided had been inappropriate and not individualised to meet their needs or due to their belief that a solution did not exist.
High response rates in the HD population could be attributed to the face-to-face recruitment method employed in this treatment group, as opposed to via a mail out or over the phone. Heerman et al. [
23] similarly found the face-to-face method fostered the highest response rate for large-scale recruitment in a range of populations. This population were also inconvenienced to a lesser extent as they were surveyed during dialysis. Characteristics of age and gender of those who did not participate in the HD group were similar to those of the studied population.
There are several strengths to this study, one of which being the high recruitment rate among dialysis patients. We also have explored specifically the topic of access site pain. This is of great interest and concern to patients and health professionals [
24,
25]. The mixed method approach also extends our understanding of pain to a greater extent than surveys alone. In addition to generating insights into the Australian experience, the novelty of the current study is that we have extended the limited evidence base on the study of pain in patients undertaking peritoneal dialysis. Zhang et al. [
19] in their metasynthesis of qualitative studies of pain in CKD found only six Australian qualitative studies on pain which comprised of 189 haemodialysis and 19 peritoneal dialysis patients. In addition, previous studies published in the Australian context were all published more than a decade ago. Therefore, the experiences of the 111 HD, 3 home HD and 17 PD patients in this study provide important contemporary insights into this topic. However, this study was not without limitations. Firstly, pain in this population varies in intensity and frequency over time. Findings from this study report only a snapshot of the dynamic nature of pain. As the research took place in one health district, the results may not be generalizable to a wider population. We do not have a true control group without kidney failure for comparison, so it is unclear how this may compare. Response bias may have also occurred with those who experience pain potentially more likely to agree to participate in the research. Conversely, those who experience pain may have been less likely to participate as the research poses no immediate benefit to their condition. This may lead to an overestimation of the prevalence of pain. Those who were experiencing severe pain may have been unable to participate due to the impact of their symptoms. We also did not explore in depth whether the burden of pain is accepted because undertaking dialysis is already a severe burden to the individual. In terms of thematic analysis, whilst a rigid analysis framework was utilised, the deduction of codes and themes was subject to the interpretation of researchers. The final themes and subthemes included in this study were by nature, subjective.