Role of Mineralocorticoid Receptor Antagonists in Diabetic Kidney Disease
Abstract
:1. Introduction
2. Pleiotropic Effects of Aldosterone in Diabetic Kidney Disease
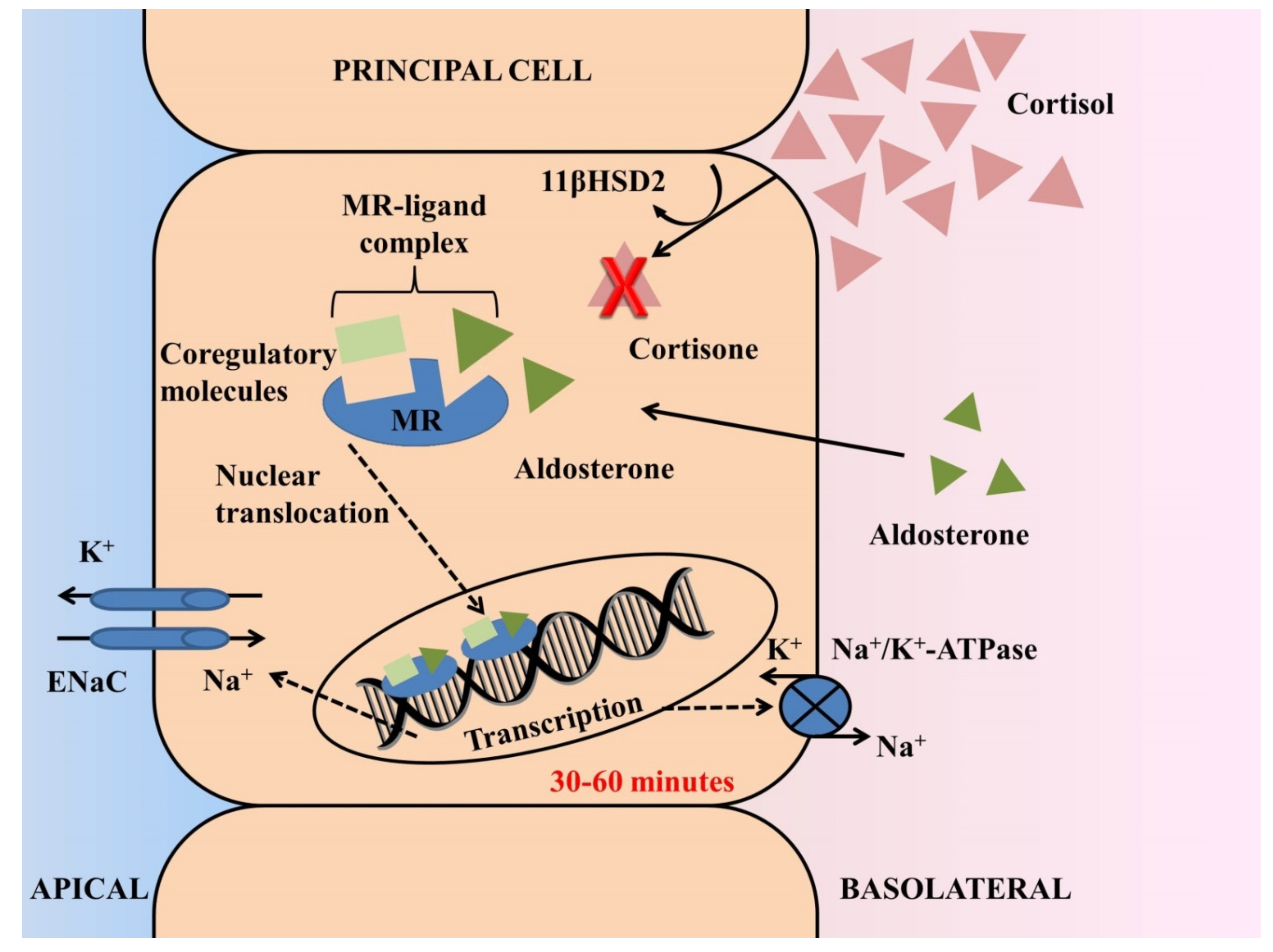
2.1. Physiological Role of Aldosterone and Mechanisms of Ligand-Specific Activation of Mineralocorticoid Receptors
2.2. Aldosterone Breakthrough and Potential Mechanisms of Aldosterone-Induced Diabetic Kidney Disease and Cardiac Damage
3. Clinical Studies of Mineralocorticoid Receptor Antagonism in Diabetic Kidney Disease
3.1. Effect of Mineralocorticoid Receptor Antagonists in Albuminuria/Proteinuria and Kidney-Related Outcomes
3.1.1. Spironolactone
3.1.2. Eplerenone
3.1.3. Finerenone
3.1.4. Canrenone
3.1.5. Esaxerenone
3.1.6. Apararenone
| Study | Study Design | Follow UP | Nr of Patients | Patient Characteristics | Active Treatment | Comparator | Back- Ground Treatment | Main Results |
|---|---|---|---|---|---|---|---|---|
| Fogari et al., 2014 [57] | Open-label, parallel- group RCT | 24 weeks | 120 | T2DM and UACR 60–300 mg/g | Canrenone | Hydrochlorothiazide | Valsartan | ↓ UACR −45.3% for canrenone; −20.3% for hydrochlorothiazide (p < 0.01) |
| Derosa et al., 2018 [90] | Double-blind, parallel-group RCT | 12 months | 182 | T2DM and hypertension | Canrenone | Hydrochlorothiazide | ARB | Significant ↓ K only for hydrochlorothiazide (p < 0.05); neutral effect for canrenone Significant ↓ eGFR for hydrochlorothiazide (p < 0.01) Significant ↑ eGFR for canrenone (p < 0.05) |
| Ito et al., 2019 [91] | Double-blind, parallel-group RCT | 12 weeks | 365 | T2DM, UACR 45–300 mg/g, eGFR ≥ 30 mL/min/1.73 m2 | Esaxerenone | Placebo | ACEi or ARB | ↓ UACR −38% for esaxerenone 1.25 mg; −50% for esaxerenone 2.5 mg; −56% for esaxerenone 5 mg; −7% for placebo (p < 0.001) Remission of albuminuria: 21% for esaxerenone groups 2.5 and 5.0 mg; 3% for placebo (p < 0.05 for both comparisons) HyperK leading to drug discontinuation: 3% for esaxerenone 1.25 and 2.5 mg; 10% esaxerenone 10 mg; 1% for placebo |
| Ito et al., 2020 (ESAX-DN) [92] | Double-blind, parallel-group RCT | 52 weeks | 455 | T2DM, UACR 45–300 mg/g, eGFR ≥ 30 mL/min/1.73 m2 | Esaxerenone | Placebo | ACEi or ARB | ↓ UACR −58% for esaxerenone; +8% for placebo (p < 0.001); eGFR −11% for esaxerenone; −1% for placebo Remission of albuminuria: 22% in esaxerenone; 4% in placebo (p < 0.001) Time to 1st transition to overt proteinuria: HR 0.23; 95%CI 0.11–0.48 for esaxerenone HyperK episodes (>6.0 mmol/L or ≥5.5 mmol/L at two consecutive occasions): 9% esaxerenone; 2% placebo (p = 0.002) HyperK leading to drug discontinuation: 4% for esaxerenone; 1% for placebo |
| Ito et al., 2021 [93] | Open-label, single-arm study | 28 weeks | 56 | T2DM, UACR ≥ 300 mg/g, eGFR ≥ 30 mL/min/1.73 m2 | Esaxerenone | - | ACEi or ARB | ↓ UACR −54.6% (p < 0.001); eGFR −8.3 mL/min /1.73 m2 for esaxerenone HyperK episodes (>6.0 mmol/L or ≥5.5 mmol/L at two consecutive occasions): 5.4% for esaxerenone |
| Wada et al., 2021 [62] | Double-blind, parallel-group RCT with open-label extension | 24 weeks and 28 weeks | 293 | T2DM, UACR 50–300 mg/g | Apararenone | Placebo | ACEi or ARB | ↓ UACR at 23 weeks −62.9% apararenone 2.5 mg; −50.8% apararenone 5 mg; −46.5% apararenone 10 mg; +113.7% placebo (p < 0.001 vs. placebo for all comparisons) % change in eGFR at 52 weeks: −5.3% (−22.0, +10.5) apararenone 2.5 mg; −10.2% (−34.5, +14.6) apararenone 5 mg; −10.80% (−36.8, +19.1) apararenone 10 mg ↑ K at 52 weeks: +0.14 mmol/L (0.006–0.22) apararenone 2.5 mg; +0.18 mmol/L (0.1–0.26) apararenone 5 mg; +0.25 mmol/L (0.16–0.33) apararenone 10 mg |
3.2. Meta-Analyses of Randomized Clinical Studies with Mineralocorticoid Receptor Antagonists in Diabetic Kidney Disease
4. Future Directions and Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and Regional Diabetes Prevalence Estimates for 2019 and Projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th Edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuttle, K.R.; Bakris, G.L.; Bilous, R.W.; Chiang, J.L.; de Boer, I.H.; Goldstein-Fuchs, J.; Hirsch, I.B.; Kalantar-Zadeh, K.; Narva, A.S.; Navaneethan, S.D.; et al. Diabetic Kidney Disease: A Report from an ADA Consensus Conference. Diabetes Care 2014, 37, 2864–2883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- United States Renal Data System. 2021 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States; National Institutes of Health; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2021. [Google Scholar]
- Afkarian, M.; Sachs, M.C.; Kestenbaum, B.; Hirsch, I.B.; Tuttle, K.R.; Himmelfarb, J.; de Boer, I.H. Kidney Disease and Increased Mortality Risk in Type 2 Diabetes. J. Am. Soc. Nephrol. 2013, 24, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Brenner, B.M.; Cooper, M.E.; de Zeeuw, D.; Keane, W.F.; Mitch, W.E.; Parving, H.H.; Remuzzi, G.; Snapinn, S.M.; Zhang, Z.; Shahinfar, S. Effects of Losartan on Renal and Cardiovascular Outcomes in Patients with Type 2 Diabetes and Nephropathy. N. Engl. J. Med. 2001, 345, 861–869. [Google Scholar] [CrossRef] [Green Version]
- Lewis, E.J.; Hunsicker, L.G.; Clarke, W.R.; Berl, T.; Pohl, M.A.; Lewis, J.B.; Ritz, E.; Atkins, R.C.; Rohde, R.; Raz, I. Renoprotective Effect of the Angiotensin-Receptor Antagonist Irbesartan in Patients with Nephropathy Due to Type 2 Diabetes. N. Engl. J. Med. 2001, 345, 851–860. [Google Scholar] [CrossRef] [Green Version]
- Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group. KDIGO 2020 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease. Kidney Int. 2020, 98, S1–S115. [Google Scholar] [CrossRef]
- Sarafidis, P.A.; Alexandrou, M.E.; Ruilope, L.M. A Review of Chemical Therapies for Treating Diabetic Hypertension. Expert Opin. Pharmacother. 2017, 18, 909–923. [Google Scholar] [CrossRef]
- Parving, H.H.; Brenner, B.M.; McMurray, J.J.; de Zeeuw, D.; Haffner, S.M.; Solomon, S.D.; Chaturvedi, N.; Persson, F.; Desai, A.S.; Nicolaides, M.; et al. Cardiorenal End Points in a Trial of Aliskiren for Type 2 Diabetes. N. Engl. J. Med. 2012, 367, 2204–2213. [Google Scholar] [CrossRef] [Green Version]
- Fried, L.F.; Emanuele, N.; Zhang, J.H.; Brophy, M.; Conner, T.A.; Duckworth, W.; Leehey, D.J.; McCullough, P.A.; O’Connor, T.; Palevsky, P.M.; et al. Combined Angiotensin Inhibition for the Treatment of Diabetic Nephropathy. N. Engl. J. Med. 2013, 369, 1892–1903. [Google Scholar] [CrossRef] [Green Version]
- Lim, P.O.; Young, W.F.; MacDonald, T.M. A Review of the Medical Treatment of Primary Aldosteronism. J. Hypertens. 2001, 19, 353–361. [Google Scholar] [CrossRef]
- Williams, B.; MacDonald, T.M.; Morant, S.; Webb, D.J.; Sever, P.; McInnes, G.; Ford, I.; Cruickshank, J.K.; Caulfield, M.J.; Salsbury, J.; et al. Spironolactone versus Placebo, Bisoprolol, and Doxazosin to Determine the Optimal Treatment for Drug-Resistant Hypertension (PATHWAY-2): A Randomised, Double-Blind, Crossover Trial. Lancet 2015, 386, 2059–2068. [Google Scholar] [CrossRef] [Green Version]
- Oxlund, C.S.; Henriksen, J.E.; Tarnow, L.; Schousboe, K.; Gram, J.; Jacobsen, I.A. Low Dose Spironolactone Reduces Blood Pressure in Patients with Resistant Hypertension and Type 2 Diabetes Mellitus: A Double Blind Randomized Clinical Trial. J. Hypertens. 2013, 31, 2094–2102. [Google Scholar] [CrossRef] [PubMed]
- Alexandrou, M.-E.; Papagianni, A.; Tsapas, A.; Loutradis, C.; Boutou, A.; Piperidou, A.; Papadopoulou, D.; Ruilope, L.; Bakris, G.; Sarafidis, P. Effects of Mineralocorticoid Receptor Antagonists in Proteinuric Kidney Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Hypertens. 2019, 37, 2307–2324. [Google Scholar] [CrossRef] [PubMed]
- Pitt, B.; Zannad, F.; Remme, W.J.; Cody, R.; Castaigne, A.; Perez, A.; Palensky, J.; Wittes, J. The Effect of Spironolactone on Morbidity and Mortality in Patients with Severe Heart Failure. Randomized Aldactone Evaluation Study Investigators. N. Engl. J. Med. 1999, 341, 709–717. [Google Scholar] [CrossRef] [Green Version]
- Pitt, B.; Remme, W.; Zannad, F.; Neaton, J.; Martinez, F.; Roniker, B.; Bittman, R.; Hurley, S.; Kleiman, J.; Gatlin, M. Eplerenone, a Selective Aldosterone Blocker, in Patients with Left Ventricular Dysfunction after Myocardial Infarction. N. Engl. J. Med. 2003, 348, 1309–1321. [Google Scholar] [CrossRef]
- Zannad, F.; McMurray, J.J.V.; Krum, H.; van Veldhuisen, D.J.; Swedberg, K.; Shi, H.; Vincent, J.; Pocock, S.J.; Pitt, B. EMPHASIS-HF Study Group Eplerenone in Patients with Systolic Heart Failure and Mild Symptoms. N. Engl. J. Med. 2011, 364, 11–21. [Google Scholar] [CrossRef] [Green Version]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.; Coats, A.J.; Falk, V.; Gonzalez-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC). Developed with the Special Contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2016, 18, 891–975. [Google Scholar]
- Ferreira, J.P.; Abreu, P.; McMurray, J.J.V.; van Veldhuisen, D.J.; Swedberg, K.; Pocock, S.J.; Vincent, J.; Lins, K.; Rossignol, P.; Pitt, B.; et al. Renal Function Stratified Dose Comparisons of Eplerenone versus Placebo in the EMPHASIS-HF Trial. Eur. J. Heart Fail. 2019, 21, 345–351. [Google Scholar] [CrossRef] [Green Version]
- Trevisan, M.; de Deco, P.; Xu, H.; Evans, M.; Lindholm, B.; Bellocco, R.; Barany, P.; Jernberg, T.; Lund, L.H.; Carrero, J.J. Incidence, Predictors and Clinical Management of Hyperkalaemia in New Users of Mineralocorticoid Receptor Antagonists. Eur. J. Heart Fail. 2018, 20, 1217–1226. [Google Scholar] [CrossRef] [Green Version]
- Mullens, W.; Damman, K.; Testani, J.M.; Martens, P.; Mueller, C.; Lassus, J.; Tang, W.H.W.; Skouri, H.; Verbrugge, F.H.; Orso, F.; et al. Evaluation of Kidney Function throughout the Heart Failure Trajectory—A Position Statement from the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2020, 22, 584–603. [Google Scholar] [CrossRef]
- Agarwal, R.; Kolkhof, P.; Bakris, G.; Bauersachs, J.; Haller, H.; Wada, T.; Zannad, F. Steroidal and Non-Steroidal Mineralocorticoid Receptor Antagonists in Cardiorenal Medicine. Eur. Heart J. 2021, 42, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Bakris, G.L.; Agarwal, R.; Anker, S.D.; Pitt, B.; Ruilope, L.M.; Rossing, P.; Kolkhof, P.; Nowack, C.; Schloemer, P.; Joseph, A.; et al. Effect of Finerenone on Chronic Kidney Disease Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2020, 383, 2219–2229. [Google Scholar] [CrossRef] [PubMed]
- Pitt, B.; Filippatos, G.; Agarwal, R.; Anker, S.D.; Bakris, G.L.; Rossing, P.; Joseph, A.; Kolkhof, P.; Nowack, C.; Schloemer, P.; et al. Cardiovascular Events with Finerenone in Kidney Disease and Type 2 Diabetes. N. Engl. J. Med. 2021, 385, 2252–2263. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Sanchez, E.; Gomez-Sanchez, C.E. The Multifaceted Mineralocorticoid Receptor. Compr. Physiol. 2014, 4, 965–994. [Google Scholar] [PubMed] [Green Version]
- Sarafidis, P.A.; Memmos, E.; Alexandrou, M.-E.; Papagianni, A. Mineralocorticoid Receptor Antagonists for Nephroprotection: Current Evidence and Future Perspectives. Curr. Pharm. Des. 2018, 24, 5528–5536. [Google Scholar] [CrossRef]
- Shibata, S.; Rinehart, J.; Zhang, J.; Moeckel, G.; Castañeda-Bueno, M.; Stiegler, A.L.; Boggon, T.J.; Gamba, G.; Lifton, R.P. Mineralocorticoid Receptor Phosphorylation Regulates Ligand Binding and Renal Response to Volume Depletion and Hyperkalemia. Cell Metab. 2013, 18, 660–671. [Google Scholar] [CrossRef] [Green Version]
- Brown, N.J. Contribution of Aldosterone to Cardiovascular and Renal Inflammation and Fibrosis. Nat. Rev. Nephrol. 2013, 9, 459–469. [Google Scholar] [CrossRef]
- Lavall, D.; Selzer, C.; Schuster, P.; Lenski, M.; Adam, O.; Schäfers, H.-J.; Böhm, M.; Laufs, U. The Mineralocorticoid Receptor Promotes Fibrotic Remodeling in Atrial Fibrillation. J. Biol. Chem. 2014, 289, 6656–6668. [Google Scholar] [CrossRef] [Green Version]
- Shibata, S.; Nagase, M.; Yoshida, S.; Kawachi, H.; Fujita, T. Podocyte as the Target for Aldosterone: Roles of Oxidative Stress and Sgk1. Hypertension 2007, 49, 355–364. [Google Scholar] [CrossRef] [Green Version]
- Calò, L.A.; Zaghetto, F.; Pagnin, E.; Davis, P.A.; De Mozzi, P.; Sartorato, P.; Martire, G.; Fiore, C.; Armanini, D. Effect of Aldosterone and Glycyrrhetinic Acid on the Protein Expression of PAI-1 and P22(Phox) in Human Mononuclear Leukocytes. J. Clin. Endocrinol. Metab. 2004, 89, 1973–1976. [Google Scholar] [CrossRef]
- Terada, Y.; Kobayashi, T.; Kuwana, H.; Tanaka, H.; Inoshita, S.; Kuwahara, M.; Sasaki, S. Aldosterone Stimulates Proliferation of Mesangial Cells by Activating Mitogen-Activated Protein Kinase 1/2, Cyclin D1, and Cyclin A. J. Am. Soc. Nephrol. 2005, 16, 2296–2305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrera-Chimal, J.; Girerd, S.; Jaisser, F. Mineralocorticoid Receptor Antagonists and Kidney Diseases: Pathophysiological Basis. Kidney Int. 2019, 96, 302–319. [Google Scholar] [CrossRef] [PubMed]
- Bledsoe, R.K.; Madauss, K.P.; Holt, J.A.; Apolito, C.J.; Lambert, M.H.; Pearce, K.H.; Stanley, T.B.; Stewart, E.L.; Trump, R.P.; Willson, T.M.; et al. A Ligand-Mediated Hydrogen Bond Network Required for the Activation of the Mineralocorticoid Receptor. J. Biol. Chem. 2005, 280, 31283–31293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seckl, J.R.; Walker, B.R. Minireview: 11beta-Hydroxysteroid Dehydrogenase Type 1- a Tissue-Specific Amplifier of Glucocorticoid Action. Endocrinology 2001, 142, 1371–1376. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Chang, C.; Safi, R.; Morgan, J.; McDonnell, D.P.; Fuller, P.J.; Clyne, C.D.; Young, M.J. Identification of Ligand-Selective Peptide Antagonists of the Mineralocorticoid Receptor Using Phage Display. Mol. Endocrinol. 2011, 25, 32–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viengchareun, S.; Le Menuet, D.; Martinerie, L.; Munier, M.; Pascual-Le Tallec, L.; Lombès, M. The Mineralocorticoid Receptor: Insights into Its Molecular and (Patho)Physiological Biology. Nucl. Recept. Signal. 2007, 5, e012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hermidorff, M.M.; de Assis, L.V.M.; Isoldi, M.C. Genomic and Rapid Effects of Aldosterone: What We Know and Do Not Know Thus Far. Heart Fail. Rev. 2017, 22, 65–89. [Google Scholar] [CrossRef]
- Ruhs, S.; Nolze, A.; Hübschmann, R.; Grossmann, C. 30 Years of the Mineralocorticoid Receptor: Nongenomic Effects via the Mineralocorticoid Receptor. J. Endocrinol. 2017, 234, T107–T124. [Google Scholar] [CrossRef] [Green Version]
- Bomback, A.S.; Klemmer, P.J. The Incidence and Implications of Aldosterone Breakthrough. Nat. Clin. Pract. Nephrol. 2007, 3, 486–492. [Google Scholar] [CrossRef]
- Sarafidis, P.A.; Ruilope, L.M. Cardiorenal Disease Development under Chronic Renin-Angiotensin-Aldosterone System Suppression. J. Renin Angiotensin Aldosterone Syst. 2012, 13, 217–219. [Google Scholar] [CrossRef]
- Rocha, R.; Rudolph, A.E.; Frierdich, G.E.; Nachowiak, D.A.; Kekec, B.K.; Blomme, E.A.G.; McMahon, E.G.; Delyani, J.A. Aldosterone Induces a Vascular Inflammatory Phenotype in the Rat Heart. Am. J. Physiol. Heart Circ. Physiol. 2002, 283, H1802–H1810. [Google Scholar] [CrossRef]
- Greene, E.L.; Kren, S.; Hostetter, T.H. Role of Aldosterone in the Remnant Kidney Model in the Rat. J. Clin. Investig. 1996, 98, 1063–1068. [Google Scholar] [CrossRef] [PubMed]
- Nagase, M.; Ayuzawa, N.; Kawarazaki, W.; Ishizawa, K.; Ueda, K.; Yoshida, S.; Fujita, T. Oxidative Stress Causes Mineralocorticoid Receptor Activation in Rat Cardiomyocytes: Role of Small GTPase Rac1. Hypertension 2012, 59, 500–506. [Google Scholar] [CrossRef] [Green Version]
- Köhler, E.; Bertschin, S.; Woodtli, T.; Resink, T.; Erne, P. Does Aldosterone-Induced Cardiac Fibrosis Involve Direct Effects on Cardiac Fibroblasts? J. Vasc. Res. 1996, 33, 315–326. [Google Scholar] [PubMed]
- Chai, W.; Garrelds, I.M.; de Vries, R.; Batenburg, W.W.; van Kats, J.P.; Danser, A.H.J. Nongenomic Effects of Aldosterone in the Human Heart: Interaction with Angiotensin II. Hypertension 2005, 46, 701–706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taira, M.; Toba, H.; Murakami, M.; Iga, I.; Serizawa, R.; Murata, S.; Kobara, M.; Nakata, T. Spironolactone Exhibits Direct Renoprotective Effects and Inhibits Renal Renin-Angiotensin-Aldosterone System in Diabetic Rats. Eur. J. Pharmacol. 2008, 589, 264–271. [Google Scholar] [CrossRef]
- Nishikawa, T.; Matsuzawa, Y.; Suematsu, S.; Saito, J.; Omura, M.; Kino, T. Effect of Atorvastatin on Aldosterone Production Induced by Glucose, LDL or Angiotensin II in Human Renal Mesangial Cells. Arzneimittelforschung 2010, 60, 445–451. [Google Scholar] [CrossRef]
- Fujisawa, G.; Okada, K.; Muto, S.; Fujita, N.; Itabashi, N.; Kusano, E.; Ishibashi, S. Spironolactone Prevents Early Renal Injury in Streptozotocin-Induced Diabetic Rats. Kidney Int. 2004, 66, 1493–1502. [Google Scholar] [CrossRef] [Green Version]
- Han, S.-Y.; Kim, C.-H.; Kim, H.-S.; Jee, Y.-H.; Song, H.-K.; Lee, M.-H.; Han, K.-H.; Kim, H.-K.; Kang, Y.-S.; Han, J.-Y.; et al. Spironolactone Prevents Diabetic Nephropathy through an Anti-Inflammatory Mechanism in Type 2 Diabetic Rats. J. Am. Soc. Nephrol. 2006, 17, 1362–1372. [Google Scholar] [CrossRef] [Green Version]
- Kang, Y.S.; Ko, G.J.; Lee, M.H.; Song, H.K.; Han, S.Y.; Han, K.H.; Kim, H.K.; Han, J.Y.; Cha, D.R. Effect of Eplerenone, Enalapril and Their Combination Treatment on Diabetic Nephropathy in Type II Diabetic Rats. Nephrol. Dial. Transplant. 2009, 24, 73–84. [Google Scholar] [CrossRef] [Green Version]
- Lachaux, M.; Barrera-Chimal, J.; Nicol, L.; Rémy-Jouet, I.; Renet, S.; Dumesnil, A.; Wecker, D.; Richard, V.; Kolkhof, P.; Jaisser, F.; et al. Short- and Long-Term Administration of the Non-Steroidal Mineralocorticoid Receptor Antagonist Finerenone Opposes Metabolic Syndrome-Related Cardio-Renal Dysfunction. Diabetes Obes. Metab. 2018, 20, 2399–2407. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.P.; Zhou, H.; Setia, O.; Dardik, A.; Fernandez-Hernando, C.; Goodwin, J. Podocyte Glucocorticoid Receptors Are Essential for Glomerular Endothelial Cell Homeostasis in Diabetes Mellitus. J. Am. Heart Assoc. 2021, 10, e019437. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.P.; Zhou, H.; Setia, O.; Liu, B.; Kanasaki, K.; Koya, D.; Dardik, A.; Fernandez-Hernando, C.; Goodwin, J. Loss of Endothelial Glucocorticoid Receptor Accelerates Diabetic Nephropathy. Nat. Commun. 2021, 12, 2368. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhou, H.; Zhang, T.; Gao, X.; Tao, B.; Xing, H.; Zhuang, Z.; Dardik, A.; Kyriakides, T.R.; Goodwin, J.E. Loss of Endothelial Glucocorticoid Receptor Promotes Angiogenesis via Upregulation of Wnt/β-Catenin Pathway. Angiogenesis 2021, 24, 631–645. [Google Scholar] [CrossRef] [PubMed]
- Rogerson, F.M.; Yao, Y.; Smith, B.J.; Fuller, P.J. Differences in the Determinants of Eplerenone, Spironolactone and Aldosterone Binding to the Mineralocorticoid Receptor. Clin. Exp. Pharmacol. Physiol. 2004, 31, 704–709. [Google Scholar] [CrossRef]
- Fogari, R.; Derosa, G.; Zoppi, A.; Lazzari, P.; D’Angelo, A.; Mugellini, A. Comparative Effect of Canrenone or Hydrochlorothiazide Addition to Valsartan/Amlodipine Combination on Urinary Albumin Excretion in Well-Controlled Type 2 Diabetic Hypertensive Patients with Microalbuminuria. Expert Opin. Pharmacother. 2014, 15, 453–459. [Google Scholar] [CrossRef]
- Gomez-Sanchez, E.P. Third-Generation Mineralocorticoid Receptor Antagonists: Why Do We Need a Fourth? J. Cardiovasc. Pharmacol. 2016, 67, 26–38. [Google Scholar] [CrossRef] [Green Version]
- Kolkhof, P.; Delbeck, M.; Kretschmer, A.; Steinke, W.; Hartmann, E.; Bärfacker, L.; Eitner, F.; Albrecht-Küpper, B.; Schäfer, S. Finerenone, a Novel Selective Nonsteroidal Mineralocorticoid Receptor Antagonist Protects from Rat Cardiorenal Injury. J. Cardiovasc. Pharmacol. 2014, 64, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Ubukata, O.; Homma, T.; Asoh, Y.; Honzumi, M.; Hayashi, N.; Saito, K.; Tsuruoka, H.; Aoki, K.; Hanzawa, H. Crystal Structure of the Mineralocorticoid Receptor Ligand-Binding Domain in Complex with a Potent and Selective Nonsteroidal Blocker, Esaxerenone (CS-3150). FEBS Lett. 2020, 594, 1615–1623. [Google Scholar] [CrossRef]
- Ito, S.; Itoh, H.; Rakugi, H.; Okuda, Y.; Yoshimura, M.; Yamakawa, S. Double-Blind Randomized Phase 3 Study Comparing Esaxerenone (CS-3150) and Eplerenone in Patients With Essential Hypertension (ESAX-HTN Study). Hypertension 2020, 75, 51–58. [Google Scholar] [CrossRef]
- Wada, T.; Inagaki, M.; Yoshinari, T.; Terata, R.; Totsuka, N.; Gotou, M.; Hashimoto, G. Apararenone in Patients with Diabetic Nephropathy: Results of a Randomized, Double-Blind, Placebo-Controlled Phase 2 Dose-Response Study and Open-Label Extension Study. Clin. Exp. Nephrol. 2021, 25, 120–130. [Google Scholar] [CrossRef]
- Sato, A.; Hayashi, K.; Naruse, M.; Saruta, T. Effectiveness of Aldosterone Blockade in Patients with Diabetic Nephropathy. Hypertension 2003, 41, 64–68. [Google Scholar] [CrossRef] [Green Version]
- Chrysostomou, A.; Pedagogos, E.; MacGregor, L.; Becker, G.J. Double-Blind, Placebo-Controlled Study on the Effect of the Aldosterone Receptor Antagonist Spironolactone in Patients Who Have Persistent Proteinuria and Are on Long-Term Angiotensin-Converting Enzyme Inhibitor Therapy, with or without an Angiotensin II Receptor Blocker. Clin. J. Am. Soc. Nephrol. 2006, 1, 256–262. [Google Scholar]
- Rossing, K.; Schjoedt, K.J.; Smidt, U.M.; Boomsma, F.; Parving, H.H. Beneficial Effects of Adding Spironolactone to Recommended Antihypertensive Treatment in Diabetic Nephropathy: A Randomized, Double-Masked, Cross-over Study. Diabetes Care 2005, 28, 2106–2112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schjoedt, K.J.; Rossing, K.; Juhl, T.R.; Boomsma, F.; Rossing, P.; Tarnow, L.; Parving, H.H. Beneficial Impact of Spironolactone in Diabetic Nephropathy. Kidney Int. 2005, 68, 2829–2836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schjoedt, K.J.; Rossing, K.; Juhl, T.R.; Boomsma, F.; Tarnow, L.; Rossing, P.; Parving, H.H. Beneficial Impact of Spironolactone on Nephrotic Range Albuminuria in Diabetic Nephropathy. Kidney Int. 2006, 70, 536–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van den Meiracker, A.H.; Baggen, R.G.; Pauli, S.; Lindemans, A.; Vulto, A.G.; Poldermans, D.; Boomsma, F. Spironolactone in Type 2 Diabetic Nephropathy: Effects on Proteinuria, Blood Pressure and Renal Function. J. Hypertens. 2006, 24, 2285–2292. [Google Scholar] [CrossRef]
- Saklayen, M.G.; Gyebi, L.K.; Tasosa, J.; Yap, J. Effects of Additive Therapy with Spironolactone on Proteinuria in Diabetic Patients Already on ACE Inhibitor or ARB Therapy: Results of a Randomized, Placebo-Controlled, Double-Blind, Crossover Trial. J. Investig. Med. 2008, 56, 714–719. [Google Scholar] [CrossRef] [PubMed]
- Mehdi, U.F.; Adams-Huet, B.; Raskin, P.; Vega, G.L.; Toto, R.D. Addition of Angiotensin Receptor Blockade or Mineralocorticoid Antagonism to Maximal Angiotensin-Converting Enzyme Inhibition in Diabetic Nephropathy. J. Am. Soc. Nephrol. 2009, 20, 2641–2650. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, S.E.; Persson, F.; Frandsen, E.; Sugaya, T.; Hess, G.; Zdunek, D.; Shjoedt, K.J.; Parving, H.H.; Rossing, P. Spironolactone Diminishes Urinary Albumin Excretion in Patients with Type 1 Diabetes and Microalbuminuria: A Randomized Placebo-Controlled Crossover Study. Diabet. Med. 2012, 29, e184–e190. [Google Scholar] [CrossRef]
- Ziaee, A.; Abbas Vaezi, A.; Oveisi, S.; Javadi, A.; Hashemipour, S.; Kazemifar, A.M. Effects of Additive Therapy with Spironolactone on Albuminuria in Diabetes Mellitus: A Pilot Randomized Clinical Trial. Casp. J. Intern. Med. 2013, 4, 648–653. [Google Scholar]
- Esteghamati, A.; Noshad, S.; Jarrah, S.; Mousavizadeh, M.; Khoee, S.H.; Nakhjavani, M. Long-Term Effects of Addition of Mineralocorticoid Receptor Antagonist to Angiotensin II Receptor Blocker in Patients with Diabetic Nephropathy: A Randomized Clinical Trial. Nephrol. Dial. Transpl. 2013, 28, 2823–2833. [Google Scholar] [CrossRef] [Green Version]
- Kato, S.; Maruyama, S.; Makino, H.; Wada, J.; Ogawa, D.; Uzu, T.; Araki, H.; Koya, D.; Kanasaki, K.; Oiso, Y.; et al. Anti-Albuminuric Effects of Spironolactone in Patients with Type 2 Diabetic Nephropathy: A Multicenter, Randomized Clinical Trial. Clin. Exp. Nephrol. 2015, 19, 1098–1106. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Liu, P.; Chen, X.; Li, Y.; Zhang, F.; Wang, Y. Effects of Different Doses of Irbesartan Combined With Spironolactone on Urinary Albumin Excretion Rate in Elderly Patients With Early Type 2 Diabetic Nephropathy. Am. J. Med. Sci. 2018, 355, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.E.; Schjoedt, K.J.; Rossing, K.; Persson, F.; Schalkwijk, C.G.; Stehouwer, C.D.A.; Parving, H.-H.; Rossing, P. Levels of NT-ProBNP, Markers of Low-Grade Inflammation, and Endothelial Dysfunction during Spironolactone Treatment in Patients with Diabetic Kidney Disease. J. Renin Angiotensin Aldosterone Syst. 2013, 14, 161–166. [Google Scholar] [CrossRef] [Green Version]
- Epstein, M.; Williams, G.H.; Weinberger, M.; Lewin, A.; Krause, S.; Mukherjee, R.; Patni, R.; Beckerman, B. Selective Aldosterone Blockade with Eplerenone Reduces Albuminuria in Patients with Type 2 Diabetes. Clin. J. Am. Soc. Nephrol. 2006, 1, 940–951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Mokadem, M.; Abd El Hady, Y.; Aziz, A. A Prospective Single-Blind Randomized Trial of Ramipril, Eplerenone and Their Combination in Type 2 Diabetic Nephropathy. Cardiorenal Med. 2020, 10, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Brandt-Jacobsen, N.H.; Johansen, M.L.; Rasmussen, J.; Forman, J.L.; Holm, M.R.; Faber, J.; Rossignol, P.; Schou, M.; Kistorp, C. Effect of High-Dose Mineralocorticoid Receptor Antagonist Eplerenone on Urinary Albumin Excretion in Patients with Type 2 Diabetes and High Cardiovascular Risk: Data from the MIRAD Trial. Diabetes Metab. 2021, 47, 101190. [Google Scholar] [CrossRef]
- Bakris, G.L.; Agarwal, R.; Chan, J.C.; Cooper, M.E.; Gansevoort, R.T.; Haller, H.; Remuzzi, G.; Rossing, P.; Schmieder, R.E.; Nowack, C.; et al. Effect of Finerenone on Albuminuria in Patients With Diabetic Nephropathy: A Randomized Clinical Trial. JAMA 2015, 314, 884–894. [Google Scholar] [CrossRef]
- Katayama, S.; Yamada, D.; Nakayama, M.; Yamada, T.; Myoishi, M.; Kato, M.; Nowack, C.; Kolkhof, P.; Yamasaki, Y. A Randomized Controlled Study of Finerenone versus Placebo in Japanese Patients with Type 2 Diabetes Mellitus and Diabetic Nephropathy. J. Diabetes Complicat. 2017, 31, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Carrero, J.J.; Grams, M.E.; Sang, Y.; Ärnlöv, J.; Gasparini, A.; Matsushita, K.; Qureshi, A.R.; Evans, M.; Barany, P.; Lindholm, B.; et al. Albuminuria Changes Are Associated with Subsequent Risk of End-Stage Renal Disease and Mortality. Kidney Int. 2017, 91, 244–251. [Google Scholar] [CrossRef] [Green Version]
- Heerspink, H.J.L.; Greene, T.; Tighiouart, H.; Gansevoort, R.T.; Coresh, J.; Simon, A.L.; Chan, T.M.; Hou, F.F.; Lewis, J.B.; Locatelli, F.; et al. Change in Albuminuria as a Surrogate Endpoint for Progression of Kidney Disease: A Meta-Analysis of Treatment Effects in Randomised Clinical Trials. Lancet Diabetes Endocrinol. 2019, 7, 128–139. [Google Scholar] [CrossRef]
- Agarwal, R.; Filippatos, G.; Pitt, B.; Anker, S.D.; Rossing, P.; Joseph, A.; Kolkhof, P.; Nowack, C.; Gebel, M.; Ruilope, L.M.; et al. Cardiovascular and Kidney Outcomes with Finerenone in Patients with Type 2 Diabetes and Chronic Kidney Disease: The FIDELITY Pooled Analysis. Eur. Heart J. 2021, 43, 474–484. [Google Scholar] [CrossRef] [PubMed]
- Filippatos, G.; Bakris, G.L.; Pitt, B.; Agarwal, R.; Rossing, P.; Ruilope, L.M.; Butler, J.; Lam, C.S.P.; Kolkhof, P.; Roberts, L.; et al. Finerenone Reduces New-Onset Atrial Fibrillation in Patients With Chronic Kidney Disease and Type 2 Diabetes. J. Am. Coll. Cardiol. 2021, 78, 142–152. [Google Scholar] [CrossRef]
- Filippatos, G.; Anker, S.D.; Agarwal, R.; Pitt, B.; Ruilope, L.M.; Rossing, P.; Kolkhof, P.; Schloemer, P.; Tornus, I.; Joseph, A.; et al. Finerenone and Cardiovascular Outcomes in Patients with Chronic Kidney Disease and Type 2 Diabetes. Circulation 2021, 143, 540–552. [Google Scholar] [CrossRef]
- Perkovic, V.; Jardine, M.J.; Neal, B.; Bompoint, S.; Heerspink, H.J.L.; Charytan, D.M.; Edwards, R.; Agarwal, R.; Bakris, G.; Bull, S.; et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N. Engl. J. Med. 2019, 380, 2295–2306. [Google Scholar] [CrossRef] [Green Version]
- Heerspink, H.J.L.; Stefánsson, B.V.; Correa-Rotter, R.; Chertow, G.M.; Greene, T.; Hou, F.-F.; Mann, J.F.E.; McMurray, J.J.V.; Lindberg, M.; Rossing, P.; et al. Dapagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2020, 383, 1436–1446. [Google Scholar] [CrossRef] [PubMed]
- Rossing, P.; Filippatos, G.; Agarwal, R.; Anker, S.D.; Pitt, B.; Ruilope, L.M.; Chan, J.C.N.; Kooy, A.; McCafferty, K.; Schernthaner, G.; et al. Finerenone in Predominantly Advanced CKD and Type 2 Diabetes With or Without Sodium-Glucose Cotransporter-2 Inhibitor Therapy. Kidney Int. Rep. 2022, 7, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Derosa, G.; Gaudio, G.; Pasini, G.; D’Angelo, A.; Maffioli, P. A Randomized, Double-Blind Clinical Trial of Canrenone vs Hydrochlorothiazide in Addition to Angiotensin II Receptor Blockers in Hypertensive Type 2 Diabetic Patients. Drug Des. Dev. Ther. 2018, 12, 2611–2616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, S.; Shikata, K.; Nangaku, M.; Okuda, Y.; Sawanobori, T. Efficacy and Safety of Esaxerenone (CS-3150) for the Treatment of Type 2 Diabetes with Microalbuminuria: A Randomized, Double-Blind, Placebo-Controlled, Phase II Trial. Clin. J. Am. Soc. Nephrol. 2019, 14, 1161–1172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, S.; Kashihara, N.; Shikata, K.; Nangaku, M.; Wada, T.; Okuda, Y.; Sawanobori, T. Esaxerenone (CS-3150) in Patients with Type 2 Diabetes and Microalbuminuria (ESAX-DN): Phase 3 Randomized Controlled Clinical Trial. Clin. J. Am. Soc. Nephrol. 2020, 15, 1715–1727. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Kashihara, N.; Shikata, K.; Nangaku, M.; Wada, T.; Okuda, Y.; Sawanobori, T. Efficacy and Safety of Esaxerenone (CS-3150) in Japanese Patients with Type 2 Diabetes and Macroalbuminuria: A Multicenter, Single-Arm, Open-Label Phase III Study. Clin. Exp. Nephrol. 2021, 25, 1070–1078. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Ma, S.; Huang, Q.; Fang, Y.; Tan, H.; Chen, Y.; Li, C. Meta-Analysis of the Efficacy and Safety of Finerenone in Diabetic Kidney Disease. Kidney Blood Press. Res. 2022. [CrossRef] [PubMed]
| Study | Study Design | Follow Up | Nr of Patients | Patient Characteristics | Comparator | Back- Ground Treatment | Main Results |
|---|---|---|---|---|---|---|---|
| Sato et al., 2003 [63] | Open-label, single-arm study | 24 weeks | 13 | T2DM, UACR 30–300 mg/g and creatinine clearance > 60 mL/min | - | Trandolapril | ↓ UAE and LVMI for spironolactone (p < 0.05 for both) Non-significant ↑ K (before 4.2 ± 0.3 mEq/L; after 4.3 ± 0.2 mEq/L) |
| Rossing et al., 2005 [65] | Double-blind, cross-over RCT | 8 weeks | 21 | T2DM and UAE > 300 mg/24 h | Placebo | ACEi or ARB | ↓ UAE −33% (95%CI −41%, −25%); 24 h SBP −6 mmHg (95%CI −10, −2); 24 h DBP −4 mmHg (95%CI −6, −2); eGFR −3 mL/min/1.73 m2 (95%CI −0.3, +6.0) for spironolactone |
| Schjoedt et al., 2005 [66] | Double-blind, cross-over RCT | 8 weeks | 20 | T1DM and UAE > 300 mg/24 h | Placebo | ACEi or ARB | ↓ UAE −30% (95%CI −41%, –17%); 24 h SBP −8 mmHg (95%CI −17, +1); 24 h DBP −3 mmHg (95%CI −7, +0.2); eGFR by −3.4 mL/min/ 1.73 m2 (−6.9, 0.1) for spironolactone 1 patient excluded due to hyperK in spironolactone |
| Schjoedt et al., 2006 [67] | Double-blind, cross-over RCT | 8 weeks | 20 | T1DM or T2DM and UAE > 2500 mg/24 h | Placebo | ACEi or ARB | ↓ UAE −32% (95%CI −42%, −21%); 24 h SBP −6 mmHg (95%CI −10, −2); 24 h DBP −4 mmHg (95%CI −6, −2); eGFR −3 mL/min/1.73 m2 (−6.0, +1.0) ↑ K +0.2 mmol/L (95%CI −0.004, +0.5) |
| van den Meiracker, 2006 [68] | Double-blind, parallel-group RCT | 1 year | 59 | T2DM and UAE > 300 mg/24 h or UACR > 20 mg/g | Placebo | ACEi or ARB | ↓ UAE −40.6% (95%CI −57.8%, −23.4%); 24 h SBP −7 mmHg (95%CI −12, −2); 24 h DBP −3 mmHg (95%CI −6, −1) eGFR −12.9 mL/min/1.73 m2 (−16.5, −9.5) for spironolactone; −4.9 mL/min/1.73 m2 (−8.9, −0.8) for placebo 5 patients excluded due to hyperK in spironolactone |
| Saklayen et al., 2008 [69] | Double-blind, cross-over RCT | 7 weeks | 30 | T1DM or T2DM patients with any level of proteinuria | Placebo | ACEi or ARB | ↓ UPCR from 1.80 ± 1.78 to 0.79 ± 0.99 for spironolactone (p = 0.004); from 1.24 ± 1.13 to 1.57 ± 2.13 for placebo (p = 0.35); eGFR from 61.91 ± 23.4 to 53.94 ± 23.58 for spironolactone (p = 0.0001) |
| Mehdi et al., 2009 [70] | Double-blind parallel-group RCT | 48 weeks | 81 | T1DM or T2DM patients and UACR > 300 mg/g | Placebo, Losartan 100 mg | Lisinopril 80 mg | ↓ UACR −34% (95%CI −51%, −11.2%) for spironolactone (p = 0.007 vs. placebo); −16.8% (95%CI −37.3%, +10.5%) for losartan (p = 0.2 vs. placebo); % change in creatinine clearance −13.1% (95%CI −21.3%, −3.9%) for spironolactone; −16.8% (95%CI −23.9%, −9.1%) for losartan; −16.0% (95% CI −23.3%, −7.9%) for placebo HyperK episodes (>6.0 mmol/L): 14 patients in spironolactone (p < 0.001 vs. placebo); 10 in losartan (p = 0.009 vs. placebo); 2 patients in placebo |
| Nielsen et al., 2012 [71] | Double-blind, cross-over RCT | 60 days | 21 | T1D and UAE > 30 mg/day | Placebo | ACEi or ARB | ↓ UACR −60% (range −80% to −21%); eGFR from 78 ± 6 to 72 ± 6 mL/min/1.73 m2 (p = 0.003) HyperK episodes (>5.7 mmol/L): 2 patients in spironolactone group |
| Ziaee et al., 2013 [72] | Parallel-group RCT | 12 weeks | 60 | T2DM and microalbuminuria | Placebo | Enalapril | ↓ UACR from 126 ± 69.3 to 59.3 ± 48.1 for spironolactone (p < 0.001); eGFR from 79.8 ± 18 to 75.6 ± 16.3 mL/min/1.73 m2 for spironolactone (p = 0.6) |
| Esteghamati et al., 2013 [73] | Open-label, parallel-group RCT | 18 months | 136 | T2DM and UAE ≥ 30 mg/day | Enalapril | Losartan | ↓ UAE −60.5 mg (95%CI −148.8, −16.4) for spironolactone; +22.0 mg (95%CI −110.3, +108.9) for placebo (p = 0.017); SBP −8.89 mmHg (95%CI −15.88, −1.89) for spironolactone; −6.08 mmHg (−14.71, +2.57) for placebo (p < 0.001); DBP −4.44 mmHg (95%CI −8.10, −0.79) for spironolactone; −2.86 (−7.06, +1.34) for placebo (p = 0.001); eGFR −10.23 mL/min/1.73 m2 (95%CI −16.69, −3.76) for spironolactone; −9.08 mL/min/1.73 m2 (−16.06, −2.10) for placebo (p = 0.674) |
| Oxlund et al., 2013 [13] | Double-blind, parallel-group RCT | 16 weeks | 119 | T2DM and resistant hypertension | Placebo | ACEi or ARB | ↓ UACR −7.3 mg/g (95%CI −1093, +12.2) for spironolactone; +0 mg/g (95% +7, +146.3) for placebo (p = 0.001); placebo-corrected 24 h SBP −8.9 mmHg (95%CI −13.2, −4.6); placebo-corrected 24 h DBP −3.9 mmHg (95%CI −6.2, −1.7) ↑ K +0.26 mmol/L (95%CI +0.1, +0.4) for spironolactone; +0.02 (95%CI +0.07, +0.10) for placebo (between-group p < 0.001) |
| Kato et al., 2015 [74] | Open-label parallel-group RCT | 8 weeks | 52 | T2DM and UACR 100–2000 mg/g | Placebo | ACEi or ARB | ↓ UACR −33% (95%CI −54%, −22%); eGFR −3.2 ± 9.7 mL/min/1.73 m2 (p = 0.052) |
| Chen et al., 2018 [75] | Open-label, parallel-group RCT | 72 weeks | 244 | T2DM and UAER 20–199 μg/min | Placebo | Irbesartan 150 mg or 300 mg | ↓ UAΕR −30 μg/min (95%CI −54, −15) for spironolactone/irbesartan 300 mg; −30 μg/min (95%CI −51, −12) for spironolactone/irbesartan 150 mg; −23 μg/min (95%CI −35, −12) for irbesartan 300 mg; −15 μg/min (95%CI −24, −11) for irbesartan 150 mg (between-group p < 0.001) |
| Study | Study Design | Follow Up | Nr of Patients | Patient Characteristics | Comparator | Back- Ground Treatment | Main Results |
|---|---|---|---|---|---|---|---|
| Epstein et al., 2006 [77] | Double-blind, parallel-group RCT | 12 weeks | 268 | T2DM and UACR ≥ 50 mg/g | Placebo | Enalapril 20 mg | ↓ UACR −41% for eplerenone 50 mg; −48.4% for eplerenone 100 mg; −7.4% for placebo (p < 0.001 vs. placebo for both) Between-group differences in sustained hyperK (>5.5 mmol/L on two consecutive measurements) p = 0.29; severe hyperK (≥6.0 mmol/L at any timepoint) p = 0.38 |
| Brandt-Jacobsen et al., 2020 (MIRAD trial) [79] | Double-blind, parallel-group RCT | 26 weeks | 140 | T2DM, median UACR 17 mg/g, 12% had eGFR < 60 mL/min/1.73 m2 | Placebo | Antihypertensive treatment | ↓ UACR by −34% for eplerenone vs. placebo (p = 0.005); eGFR −3.5 mL/min/1.73 m2 for eplerenone Between-group differences in episodes of hyperkalemia (≥5.5 mmol/L) p = 0.276 ↑ K by +0.26 mmol/L for eplerenone |
| Mokadem et al., 2020 [78] | Single-blind, parallel-group RCT | 24 weeks | 75 | T2DM and UACR 30–300 mg/g and stage 1 hypertension | Treatment groups: Eplerenone/ramipril combination, ramipril monotherapy, eplerenone monotherapy | ↓ UACR −70% for eplerenone/ramipril; −37% for ramipril; −38% for eplerenone (p < 0.0001 for combination vs. both others) HyperK episodes (>5.5 mmol/L on 2 measurements): 8% for eplerenone/ramipril; 4% for ramipril; 4% for eplerenone (for eplerenone/ramipril vs. others p = 0.5, ramipril vs. eplerenone p = 0.6); for eGFR < 60 mL/min/1.73 m2: ↑ incidence of hyperK for eplerenone/ramipril vs. others (p < 0.05) | |
| Study | Study Design | Follow UP | Nr of Patients | Patient Characteristics | Comparator | Back- Ground Treatment | Main Results |
|---|---|---|---|---|---|---|---|
| Bakris et al., 2015 (ARTS-DN) [80] | Double-blind, parallel-group RCT | 90 days | 821 (4 different finerenone dose groups) | T2DM and UACR 30 to <300 mg/g or >300 mg/g (stratified randomization) | Placebo | ACEi or ARB | ↓ placebo-corrected mean ratio of UACR at day 90 relative to baseline: finerenone 7.5 mg 0.79 (p = 0.004); finerenone 10 mg 0.76 (p = 0.001); finerenone 15 mg 0.67 (p < 0.001); finerenone 20 mg 0.62 (p < 0.001) Significantly ↑ incidence of hyperK episodes leading to study discontinuation: finerenone 7.5 mg 2.1%, finerenone 15 mg 3.2% and finerenone 20 mg 1.7% No significant ↑ in the risk of hyperkalemia for placebo and finerenone 10 mg |
| Katayama et al., 2017 [81] | Double-blind, parallel-group RCT | 90 days | 96 (4 different finerenone dose groups) | T2DM and UACR 30 to <300 mg/g or >300 mg/g (stratified randomization) | Placebo | ACEi or ARB | ↓ LS mean ratio of finerenone to baseline (0.712); LS mean ratio of finerenone to placebo (0.670) for finerenone 20 mg (p = 0.0240) ↑ K for finerenone (+0.025, +0.167 mmol/L) vs. placebo (−0.075 mmol/L) |
| Bakris et al., 2020 (FIDELIO- DKD) [23] | Double-blind, parallel-group RCT | 2.6 years | 5734 | T2DM and: (a) UACR 300–5000 mg/g and eGFR 25–75 mL/min/1.73 m2 or (b) UACR 30–300 mg/g, eGFR 25–60 mL/min/1.73 m2, diabetic retinopathy | Placebo | ACEi or ARB | Primary composite endpoint of kidney failure (ESKD or eGFR < 15 mL/min/1.73 m2), eGFR decrease of ≥40%, renal death: HR 0.82; 95% CI 0.73–0.93 Secondary: kidney failure HR 0.87; 95% CI 0.72–1.05; eGFR decrease of ≥40% HR 0.81; 95% CI 0.72–0.92 Secondary composite endpoint of kidney failure (ESKD or eGFR <15 mL/min/1.73 m2), eGFR decrease of ≥57%, renal death: HR 0.76; 95% CI 0.65–0.90 Secondary composite endpoint of CV death, nonfatal MI/stroke, HHF: HR 0.86; 95% CI 0.75–0.99 Secondary: CV death HR 0.86; 95% 0.68–1.08; nonfatal MI HR 0.80; 95% CI 0.58–1.09; nonfatal stroke: HR 1.03; 95%CI 0.76–1.38; HHF HR 0.86; 95%CI 0.68–1.08HyperK leading to drug discontinuation: 2.3% for finerenone; 0.9% for placebo Pre-specified secondary analysis: new-onset AF HR 0.71, 95%CI 0.53–0.94; fatal/nonfatal stroke after new-onset AF: HR 7.13; 95%CI 4.01–12.70 |
| Pitt et al., 2021 (FIGARO- DKD) [24] | Double-blind, parallel-group RCT | 3.4 years | 7437 | T2DM and: (a) UACR 30–300 mg/g, eGFR ≥ 25–90 mL/min/ 1.73 m2 or (b) UACR 300–5000 mg/g, eGFR ≥ 60 mL/min/1.73 m2 | Placebo | ACEi or ARB | Primary composite endpoint of CV death, nonfatal MI/stroke, HHF: HR 0.87; 95%CI 0.76–0.98 Secondary: HHF HR 0.71; 95%CI 0.56–0.90 Secondary composite endpoint of kidney failure (ESKD or eGFR <15 mL/min/1.73 m2), eGFR decrease of ≥40%, renal death: HR 0.87; 95%CI 0.76–1.01 Secondary: ESKD HR 0.64, 95%CI 0.41–0.995HyperK leading to drug discontinuation: 1.2% finerenone; 0.4% placebo |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alexandrou, M.-E.; Theodorakopoulou, M.P.; Sarafidis, P.A. Role of Mineralocorticoid Receptor Antagonists in Diabetic Kidney Disease. Kidney Dial. 2022, 2, 163-182. https://doi.org/10.3390/kidneydial2020019
Alexandrou M-E, Theodorakopoulou MP, Sarafidis PA. Role of Mineralocorticoid Receptor Antagonists in Diabetic Kidney Disease. Kidney and Dialysis. 2022; 2(2):163-182. https://doi.org/10.3390/kidneydial2020019
Chicago/Turabian StyleAlexandrou, Maria-Eleni, Marieta P. Theodorakopoulou, and Pantelis A. Sarafidis. 2022. "Role of Mineralocorticoid Receptor Antagonists in Diabetic Kidney Disease" Kidney and Dialysis 2, no. 2: 163-182. https://doi.org/10.3390/kidneydial2020019







