Point-of-Care Ultrasound (POCUS) for Dialysis Patients: A Step Forward
Abstract
:1. Introduction
- eFAST: extended focused assessment with sonography for trauma [3]
- BLUE: bedside lung ultrasound in emergency settings [4]
- RADIUS: rapid assessment of dyspnea with ultrasound [5]
- RUSH: rapid ultrasound in shock [6]
- FEEL: focused echocardiography in emergency life-support cardiac arrest [7]
- ACES: abdominal and cardiac evaluation with sonography in shock [8]
- Vascular-access assessment and cannulation
- Assessment of volume status
- Hypotensive episodes
- Shortness of breath
- Assessment of respiratory symptoms such as cough and fever
- Assessment of abdominal pain
- Leg edema and pain
- Reduction in time to diagnosis and treatment
- Improvement of patient safety
- Reduction in complication rates
2. First Part: POCUS for Vascular Access
3. Second Part: POCUS for Volume Status and Dry-Weight Assessment
3.1. The Heart
- a pericardial effusion
- diastolic right-ventricular collapse (high specificity)
- systolic right-atrial collapse (earliest sign)
- a plethoric inferior vena cava with minimal respiratory variation (high sensitivity)
- exaggerated respiratory cycle changes in mitral- and tricuspid-valve in-flow velocities as a surrogate for pulsus paradoxus
- Inferior-vena-cava (IVC) diameter and collapsibility
- Hepatic-vein indices of size and flow
- Tricuspid-valve Doppler inflow and tricuspid-valve tissue Doppler
- An A wave above the baseline indicates normal phasicity
- Once the A wave descends below the baseline, there is at least mildly decreased phasicity
- Once the peak of the A wave is at least halfway between the baseline and the peak negative excursion of the waveform, there is at least moderately decreased phasicity.
- When the waveform loses all phasic variation (ie, becomes nonphasic) and no component waves can be distinguished, phasicity is severely decreased.
3.2. The Abdominal Veins (Other Than IVC and Hepatic Veins)
3.3. The Lungs
- Reproducibility
- Precision
- Easy to perform in everyday clinical practice (bedside) by the clinician
- Not ionizing
- Low cost
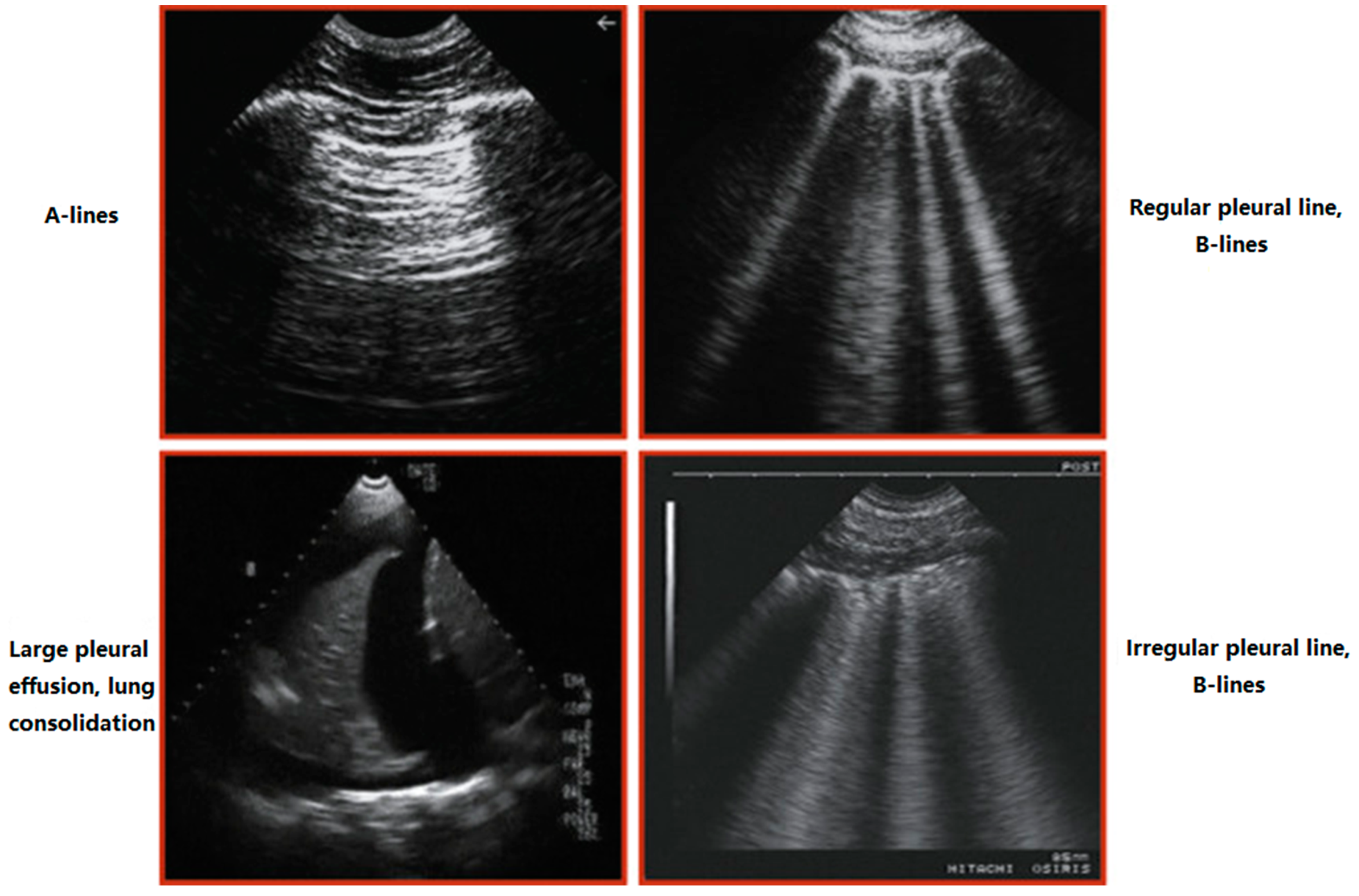
- Hyper-resonant
- Vertical
- Well-defined
- Cover the entire screen
- Comet-tail appearance
- Originate from the pleural line
- Follow pleural sliding
- Erase A-lines
- Diffuse-Bilateral
- ≥ 3 between two ribs
4. Deep Venous Thrombosis (DVT)
4.1. Ultrasound Physics
- Ultrasound wave properties, transducer types
- Introduction to modes
- Image optimization
- Image orientation
- Basics of image interpretation
- Common ultrasound artifacts
4.2. Limited Doppler Echocardiography
4.3. Quantification of Venous Congestion Using Doppler Ultrasound
- Rationale
- Technique
- Components of VExUS:
- Hepatic-vein waveform: genesis, nomenclature of normal waves, transformation with increasing right-atrial pressure, pitfalls, utility of simultaneous electrocardiographic trace
- Portal-vein waveform: normal appearance, transformation with increasing right-atrial pressure, pitfall
4.4. Lung Ultrasound
- Technique
- Sonographic zones of evaluation: rationale
- A and B lines: physics underlying artifact generation
- Pleural effusion: simple effusion, spine sign, recognition of complex/exudative effusions
- Consolidations: differentiating lobar pneumonia and atelectasis, static and dynamic air bronchograms, sub-pleural consolidations
4.5. Focused Cardiac Ultrasound
- Technique: probe and preset selection, acquisition of basic cardiac views and inferior vena cava
- Utility of M-mode and color Doppler
- Cardiac anatomy: gross and sonographic correlation of the basic views
- Evaluation of 5 Es: ejection, effusion, equality, entrance, and exit
- Pitfalls of isolated inferior-vena-cava ultrasound
4.6. Integrative Assessment of Fluid-Volume Status
- Rationale
- Patient studies
- Limitations of basic POCUS and introduction to hemodynamic assessment using Doppler ultrasound
4.7. Sonographic Evaluation of the Dialysis Access
- Principles of spectral Doppler
- Anatomy of vascular access: gross and sonographic correlation in long and short axes
- Technique: probe selection, measurement of depth, diameter, volume flow
- Core pathologies: pseudoaneurysm, hematoma, thrombosis, narrowing and turbulent flow; assessment of maturity of a newly placed access. Detailed assessment of stenosis/vein mapping is beyond the scope of POCUS
4.8. Ultrasound-Guided Procedures
- Temporary hemodialysis catheter placement: probe selection, vessel selection, visualization of the needle tip, technique of catheter insertion, confirmation of correct placement by cardiac ultrasound (rapid atrial-swirl sign)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smallwood, N.; Dachsel, M. Point-of-care ultrasound (POCUS): Unnecessary gadgetery or evidence-based medicine. Clin. Med. 2018, 18, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Takatami, J.; Takeshima, N.; Okuda, K.; Uchimo, T.; Hagiwara, S.; Naguchi, T. Enhanced needle visualization: Advantages and indications of an ultrasound software package. Anaesth. Intensive Care 2012, 40, 856–860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirkpatrick, A.W.; Sirdis, M.; Laupland, L.; Liu, D.; Rowan, K.; Ball, C.G.; Hameed, S.M.; Brown, R.; Simons, R.; Dulchavsky, S.A.; et al. Hand-held thoracic sonography for detecting post-traumatic pneumothoraces: The Extended Focused Assessment with Sonography for Trauma (EFAST). J. Trauma Acute Care Surg. 2004, 57, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, D.; Mezière, G. Relevance of ling ultrasound in the diagnosis of acute respiratory failure: The Blue protocol. Chest 2008, 134, 117–125. [Google Scholar] [CrossRef] [Green Version]
- Manson, W.; Hafez, N.M. The rapid assessment of dyspnea with ultrasound (RADIUS). Ultrasound Clin. 2011, 6, 261–276. [Google Scholar] [CrossRef]
- Perera, P.; Mailhot, M.T.; Riley, D.; Mandaria, M.D. The RUSH exam: Rapid Ultrasound in Shock in the evaluation of critically ill. Emerg. Med. Clin. N. Am. 2010, 628, 29–56. [Google Scholar] [CrossRef]
- Breitkreutz, R.; Walcher, S.; Seeger, F.H. Focus echocardiographic evaluation in resuscitation management: Concept of an advanced life support-conformed algorithm. Crit. Care Med. 2007, 35 (Suppl. S5), S150–S161. [Google Scholar] [CrossRef] [Green Version]
- Atkinson, P.R.T.; McAuley, D.J.; Kendall, R.J.; Abeyakoon, O.; Reid, C.G.; Connolly, J.; Lewis, D. Abdominal and Cardiac Evaluation with sonography in Shock (ACES): An approach by emergency physicians for the use of ultrasound in patients with undifferentiated hypotension. Emerg. Med. J. 2009, 26, 87–91. [Google Scholar] [CrossRef] [Green Version]
- Schoch, M.; Bennett, P.; Currey, J.; Hutchinson, A. POCUS use for vascular access assessment and cannulation in hemodialysis: A scoping review. Semin. Dial. 2020, 33, 355–368. [Google Scholar] [CrossRef]
- ERBP Guideline Development Group on Vascular Access. Clinical practice guideline on peri- and postoperative care of arteriovenous fistulas and grafts for haemodialysis in adults. Nephrol. Dial. Transplant. 2019, 34, ii1–ii42. [Google Scholar] [CrossRef]
- Koratala, A.; Kazory, A. Point of Care Ultrasonography for Objective Assessment of Heart Failure: Integration of Cardiac, Vascular, and Extravascular Determinants of Volume Status. Cardiorenal. Med. 2021, 11, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Gudmundsson, P.; Rydberg, E.; Winter, R.; Willenheimer, R. Visually estimated left ventricular ejection fraction by echocardiography is closely correlated with formal quantitative methods. Int. J. Cardiol. 2005, 101, 209–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matos, J.; Kronzon, I.; Panagopoulos, G.; Perk, G. Mitral annular plane systolic excursion as a surrogate for left ventricular ejection fraction. J. Am. Soc. Echocardiogr. 2012, 25, 969–974. [Google Scholar] [CrossRef] [PubMed]
- Silverstein, J.R.; Laffely, N.H.; Rifkin, R.D. Quantitative estimation of left ventricular ejection fraction from mitral valve E-point to septal separation and comparison to magnetic resonance imaging. Am. J. Cardiol. 2006, 97, 137–140. [Google Scholar] [CrossRef] [PubMed]
- McKaigney, C.J.; Krantz, M.J.; La Rocque, C.L.; Hurst, N.D.; Buchanan, M.S.; Kendall, J.L. E-point septal separation: A bedside tool for emergency physician assessment of left ventricular ejection fraction. Am. J. Emerg. Med. 2014, 32, 493–497. [Google Scholar] [CrossRef]
- Wizemann, V.; Wabel, P.; Chamney, P.; Zaluska, X.; Moissl, U.; Rode, C.; Malecka-Masalska, T.; Marcelli, D. The mortality risk of overhydration in haemodialysis patients. Nephrol. Dial. Transplant. 2009, 24, 1574–1579. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, R. Hypervolemia is associated with increased mortality among hemodialysis patients. Hypertension 2010, 56, 512–517. [Google Scholar] [CrossRef] [Green Version]
- Whalley, G.A.; Doughty, R.N.; Gamble, G.D.; Wright, S.P.; Walsh, H.J.; Muncaster, S.A.; Sharpe, N. Pseudonormal mitral filling pattern predicts hospital re-admission in patients with congestive heart failure. J. Am. Coll. Cardiol. 2002, 39, 1787–1795. [Google Scholar] [CrossRef] [Green Version]
- Hanson, B.G.; Chan, B. The role of point-of-care ultrasound in the diagnosis of pericardial effusion: A single academic center retrospective study. Ultrasound J. 2021, 13, 1–6. [Google Scholar] [CrossRef]
- Reynolds, T.; Appleton, C.P. Doppler flow velocity patterns of the superior vena cava, inferior vena cava, hepatic vein, coronary sinus, and atrial septal defect: A guide for the echocardiographer. J. Am. Soc. Echocardiogr. 1991, 4, 503–512. [Google Scholar] [CrossRef]
- McNaughton, D.A.; Abu-Yousef, M.M. Doppler US of the liver made simple. Radiographics 2011, 31, 161–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sivaciyan, V.; Ranganathan, N. Transcutaneous Doppler jugular venous flow velocity recording. Circulation 1978, 57, 930–939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Appleton, C.P.; Hatle, L.K.; Popp, R.L. Superior vena cava and hepatic vein doppler echocardiography in healthy adults. J. Am. Coll. Cardiol. 1987, 10, 1032–1039. [Google Scholar] [CrossRef] [Green Version]
- Nagueh, M.F.; Kopelen, H.A.; Zoghbi, W.A.; Quinones, M.A.; Nagueh, S.F. Estimation of mean right atrial pressure using tissue Doppler imaging. Am. J. Cardiol. 1999, 84, 1448–1451. [Google Scholar] [CrossRef]
- Sade, L.E.; Gulmez, O.; Eroglu, S.; Sezgin, A.; Muderrisoglu, H. Noninvasive estimation of right ventricular filling pressure by ratio of early tricuspid inflow to annular diastolic velocity in patients with and without recent cardiac surgery. J. Am. Soc. Echocardiogr. 2007, 20, 982–988. [Google Scholar] [CrossRef]
- Patel, A.R.; Alsheikh-Ali, A.A.; Mukherjee, J.; Evangelista, A.; Quraini, D.; Ordway, L.J.; Kuvin, J.T.; DeNofrio, D.; Pandian, N.G. 3D echocardiography to evaluate right atrial pressure in acutely decompensated heart failure correlation with invasive hemodynamics. J. Am. Coll. Cardiol. Imaging 2011, 4, 938–945. [Google Scholar] [CrossRef] [Green Version]
- Abu-Yousef, M.M. Normal and respiratory variations of the hepatic and portal venous duplex Doppler waveforms with simultaneous electrocardiographic correlation. J. Ultrasound Med. 1992, 11, 263–268. [Google Scholar] [CrossRef]
- Baik, S.K. Haemodynamic evaluation by Doppler ultrasonography in patients with portal hypertension: A review. Liver Int. 2010, 30, 1403–1413. [Google Scholar] [CrossRef]
- Lichtenstein, D.; Mezière, G.; Lagoueyte, J.F.; Biderman, P.; Goldstein, I.; Gepner, A. A-Lines and B-Lines. Lung Ultrasound as a Bedside Tool for Predicting Pulmonary Artery Occlusion Pressure in the Critically Ill. Chest 2009, 136, 1014–1020. [Google Scholar] [CrossRef] [Green Version]
- Saad, M.; Kamal, J.; Moussaly, E.; Karam, B.; Mansour, W.; Gobran, E.; Abbasi, S.H.; Mahgoub, A.; Singh, P.; Hardy, R.; et al. Relevance of B-Lines on Lung Ultrasound in Volume Overload and Pulmonary Congestion: Clinical Correlations and Outcomes in Patients on Hemodialysis. Cardiorenal Med. 2018, 8, 83–91. [Google Scholar] [CrossRef]
- Zoccali, C.; Torino, C.; Tripepi, R.; Tripepi, G.; D’Arrigo, G.; Postorino, M.; Gargani, L.; Sicari, R.; Picano, E.; Mallamaci, F. Lung US in CKD Working Group. Pulmonary congestion predicts cardiac events and mortality in ESRD. J. Am. Soc. Nephrol. 2013, 24, 639–646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pourdowlat, G.; Farokhi, F.R. Pleural effusion in hemodialysis patients with chronic kidney disease. Eur. Respir. J. 2012, 39, 889–891. [Google Scholar]
- Eibenberger, K.L.; Dock, W.I.; Ammann, M.E.; Dorffner, R.; Hörmann, M.F.; Grabenwöger, F. Quantification of pleural effusions: Sonography versus radiography. Radiology 1994, 191, 681–684. [Google Scholar] [CrossRef] [PubMed]
- Zoccali, C.; Torino, C.; Mallamaci, F.; Sarafidis, P.; Papagianni, A.; Ekart, R.; Hojs, R.; Klinger, M.; Letachowicz, K.; Fliser, D.; et al. A randomized multicenter trial on a lung ultrasound-guided treatment strategy in patients on chronic hemodialysis with high cardiovascular risk. Kidney Int. 2021, 100, 1325–1333. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.P.; Alonso, J.V.; Garcia, P.C.; Rodriguez, F.R.; Lopez, M.A.A.; Munoz-Villanueva, M.D.C. Comparison of the Accuracy of Emergency Department-Performed Point-of-Care-Ultrasound (POCUS) in the Diagnosis of Lower-Extremity Deep Vein Thrombosis. J. Emerg. Med. 2018, 54, 656–664. [Google Scholar] [CrossRef]
- Vieira, A.L.S.; Pazeli, J.M.; Matos, A.S.; Pereira, A.M.; Pinto, I.R.; Esteves de Oliveira, L.; Guilherme, L.S.; Silva, S.L.A. Ultrasonographic evaluation of deep vein thrombosis related to the central catheter in hemodialytic patients. Ultrasound J. 2022, 14, 1–6. [Google Scholar]
- Koratala, A.; Olaoye, O.A.; Bhavna Bhasin-Chhabra, B.; Kazory, A. A Blueprint for an Integrated Point-of-Care Ultrasound. Kidney 2021, 2, 1669–1676. [Google Scholar] [CrossRef]
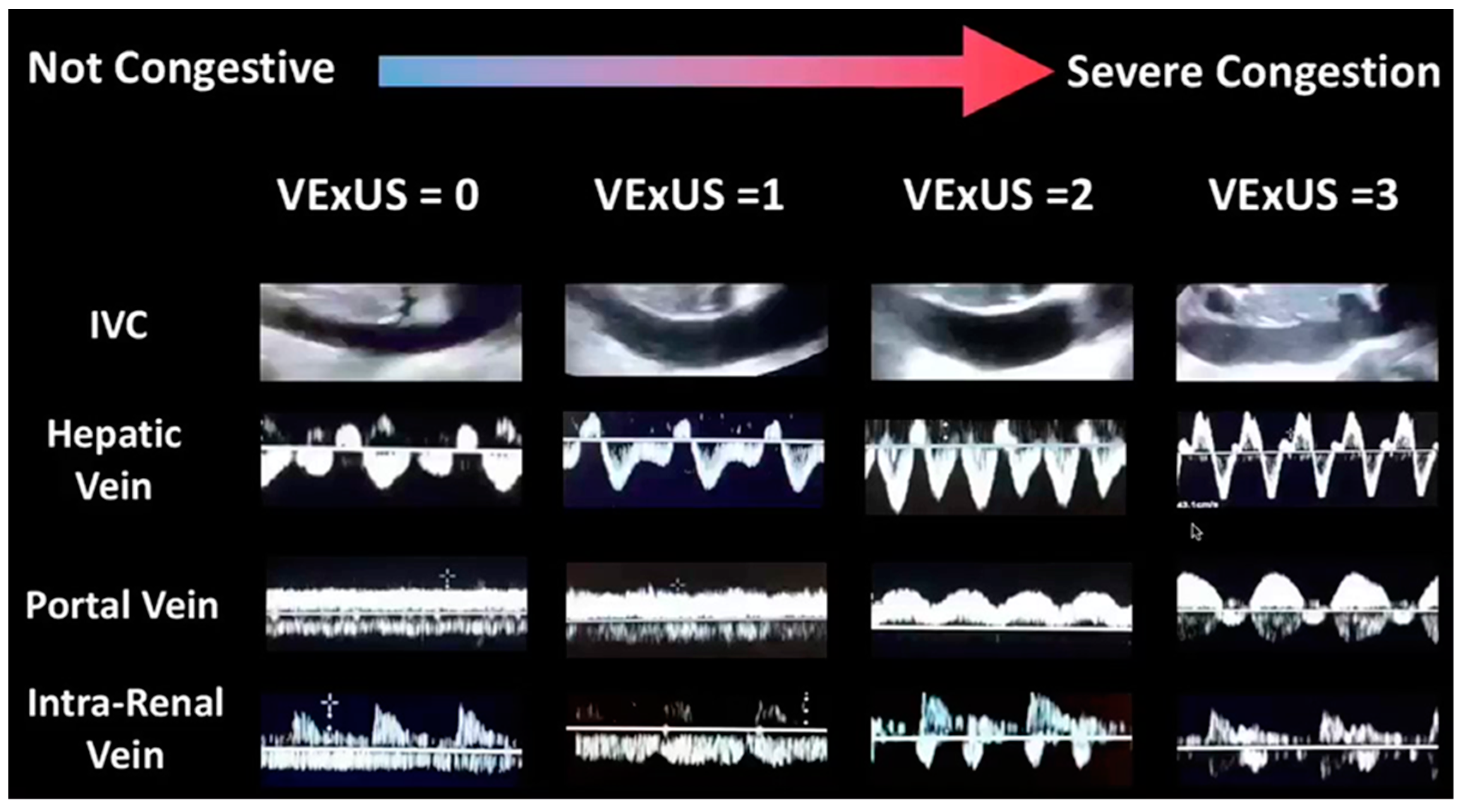
| IVC Diameter | <2 cm | ≥2 cm | ≥2 cm |
|---|---|---|---|
| Hepatic-vein Doppler | S > D (normal) | S < D (mildly abnormal) | S wave reversal (severely abnormal) |
| Portal-vein Doppler | < 30% PI (normal) | 30–49% PI (mildly abnormal) | ≥ 50% PI (severely abnormal |
| data | data | data | |
| Renal-vein Doppler | Continuous monophasic flow (normal) | Discontinuous/biphasic flow with systolic/diastolic phases (mildly abnormal) | Discontinuous monophasic flow with only diastolic phase (severely abnormal) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsangalis, G.; Loizon, V. Point-of-Care Ultrasound (POCUS) for Dialysis Patients: A Step Forward. Kidney Dial. 2022, 2, 140-152. https://doi.org/10.3390/kidneydial2020017
Tsangalis G, Loizon V. Point-of-Care Ultrasound (POCUS) for Dialysis Patients: A Step Forward. Kidney and Dialysis. 2022; 2(2):140-152. https://doi.org/10.3390/kidneydial2020017
Chicago/Turabian StyleTsangalis, Georgios, and Valerie Loizon. 2022. "Point-of-Care Ultrasound (POCUS) for Dialysis Patients: A Step Forward" Kidney and Dialysis 2, no. 2: 140-152. https://doi.org/10.3390/kidneydial2020017
APA StyleTsangalis, G., & Loizon, V. (2022). Point-of-Care Ultrasound (POCUS) for Dialysis Patients: A Step Forward. Kidney and Dialysis, 2(2), 140-152. https://doi.org/10.3390/kidneydial2020017





