Vascular Access, Complications and Survival in Incident Hemodialysis Patients
Abstract
:1. Introduction
2. Methods
- -
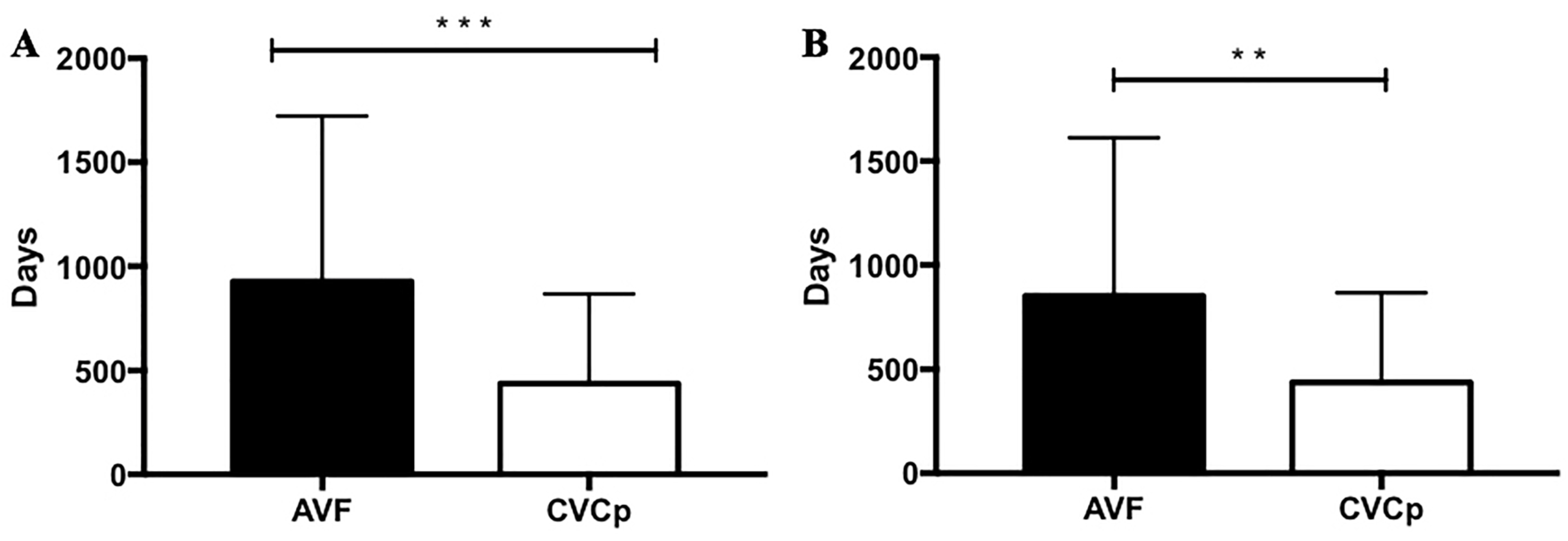
- Its survival, which was considered as the time from placement or from the beginning of renal replacement therapy (“utilization time”) to its definitive failure (i.e., the need to replace it with a new vascular access), independently of the procedures performed to maintain access patency in case of malfunctioning or thrombosis;
- -
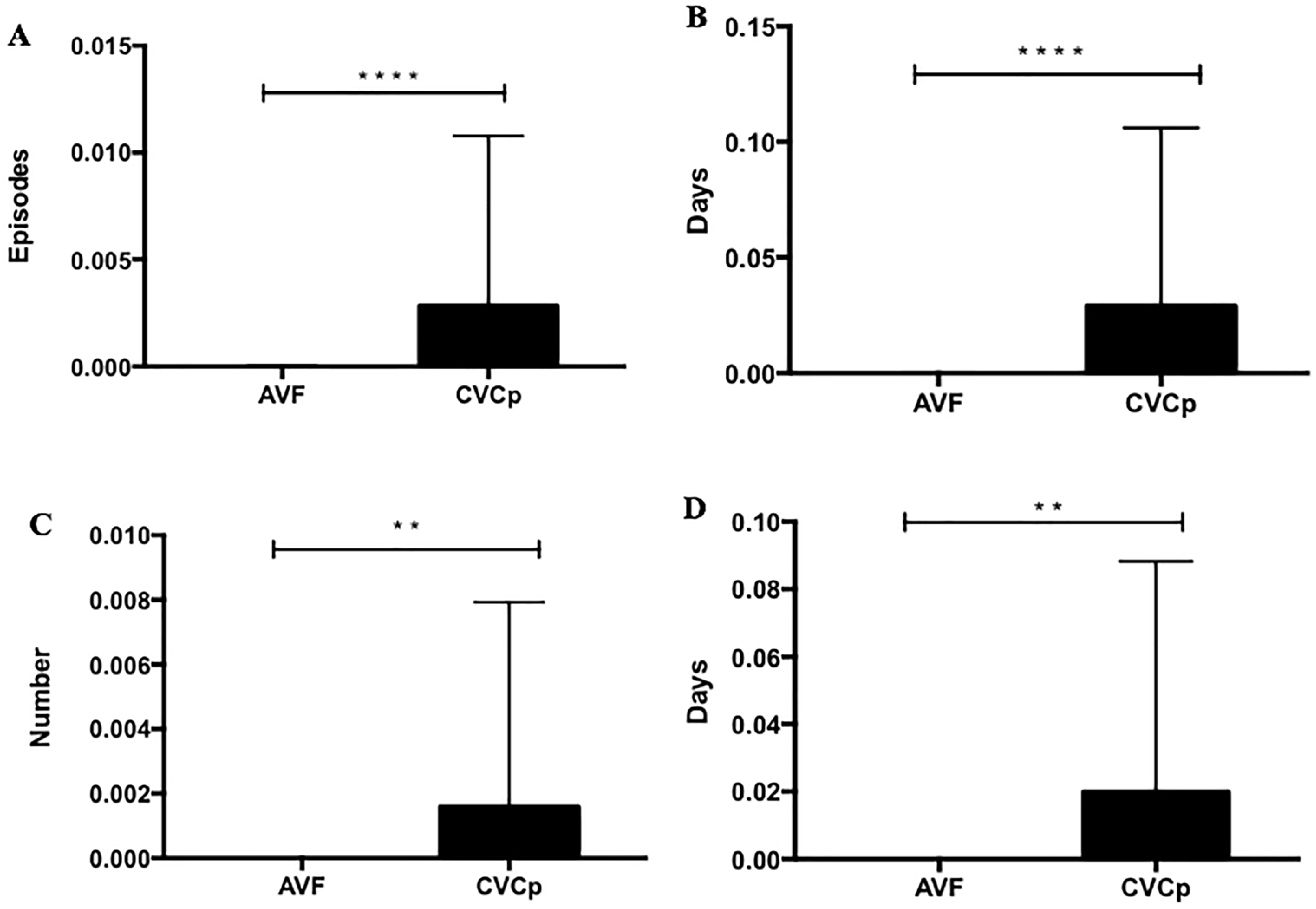
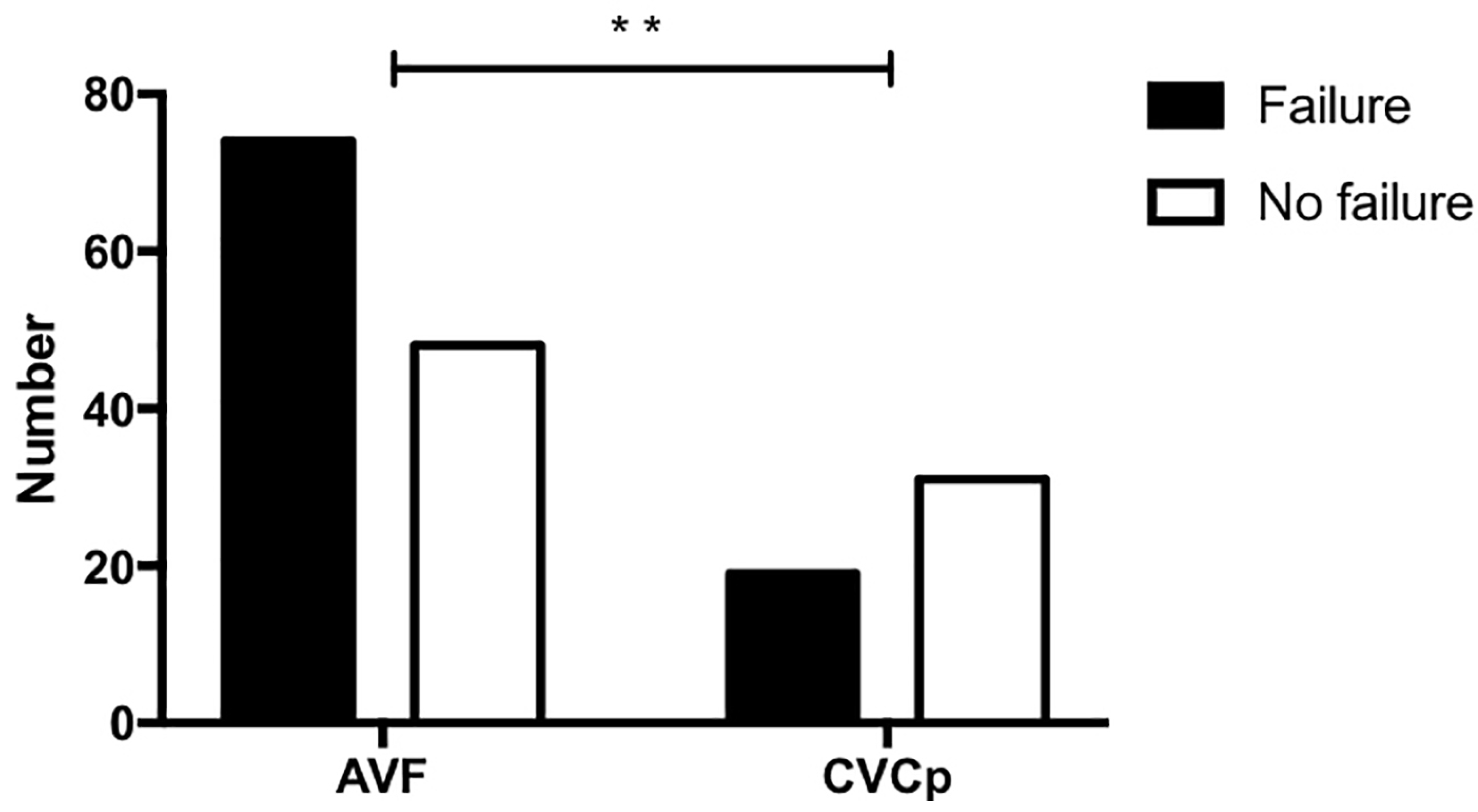
- The number of failures (defined as the inability to use the access to dialyze the patient and the need to intervene to restore patency or to replace it);
- -
- The number of infections (comprising CVC-related bacteremia, exit-site infection, and tunnel infection);
- -
- The number of infection days (defined as days of antibiotic therapy);
- -
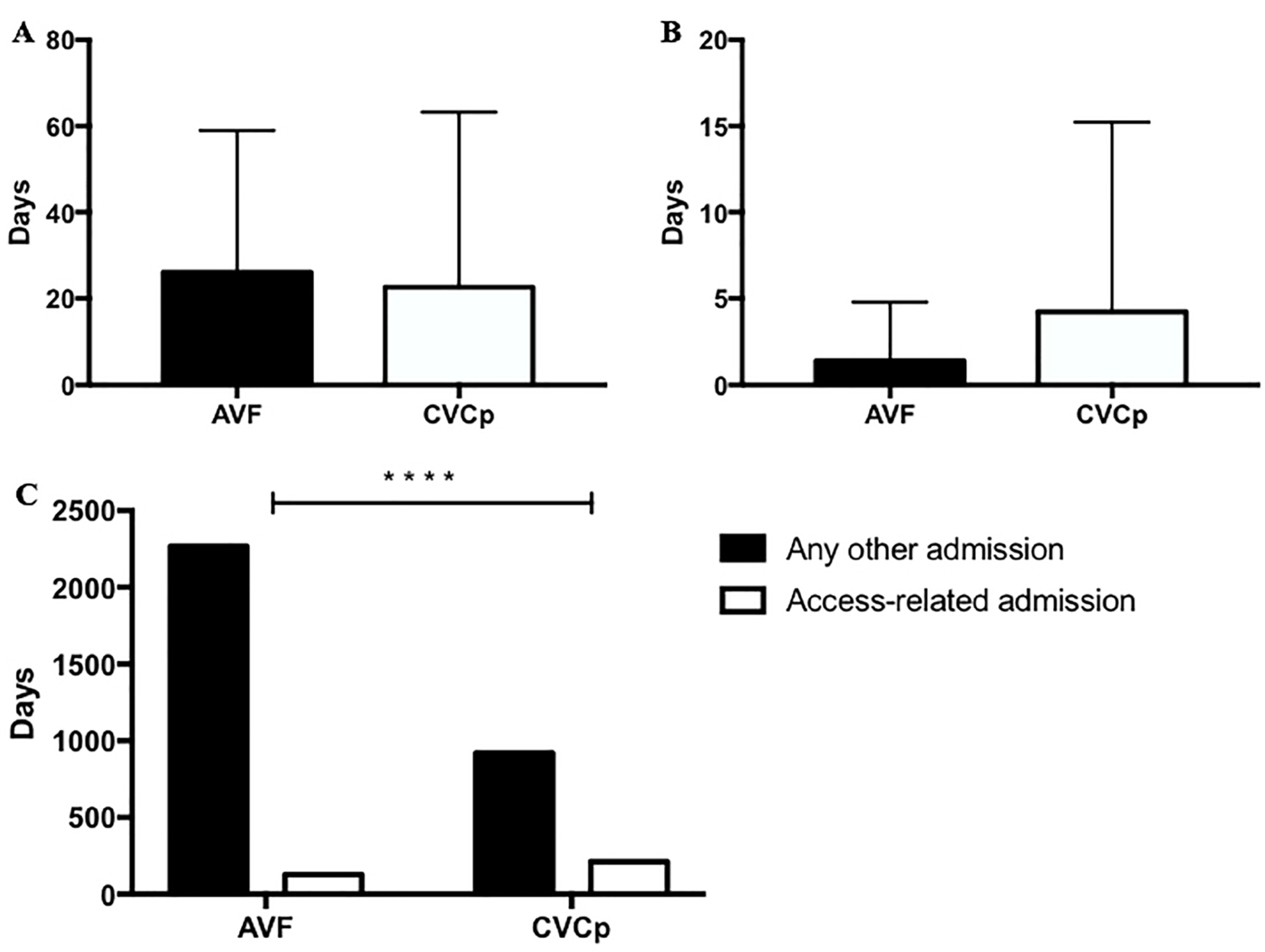
- The number of hospital admissions related to the access or for any other cause;
- -
- The length of stay for every admission period.
2.1. Statistics
2.2. Informed Consent
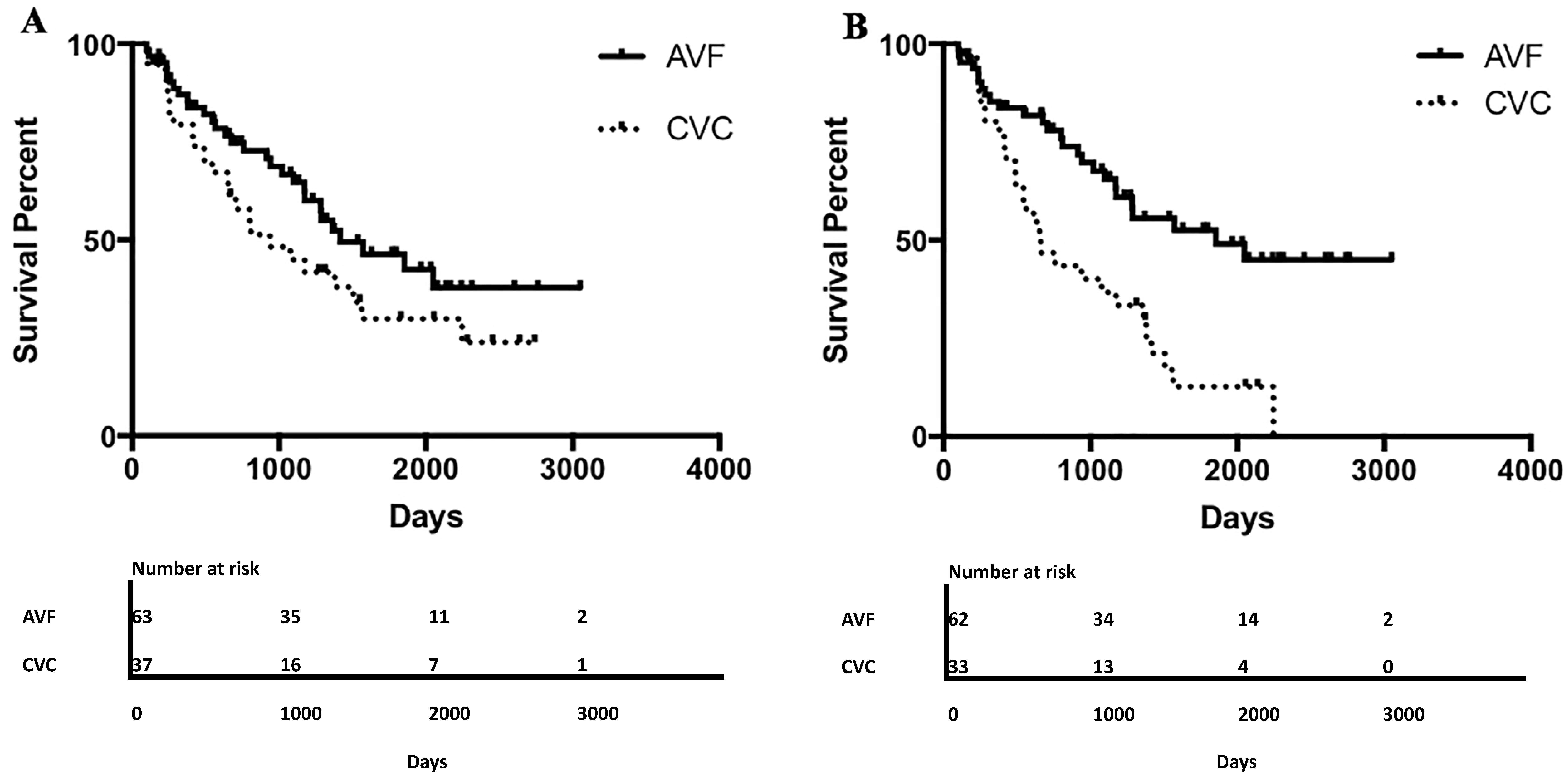
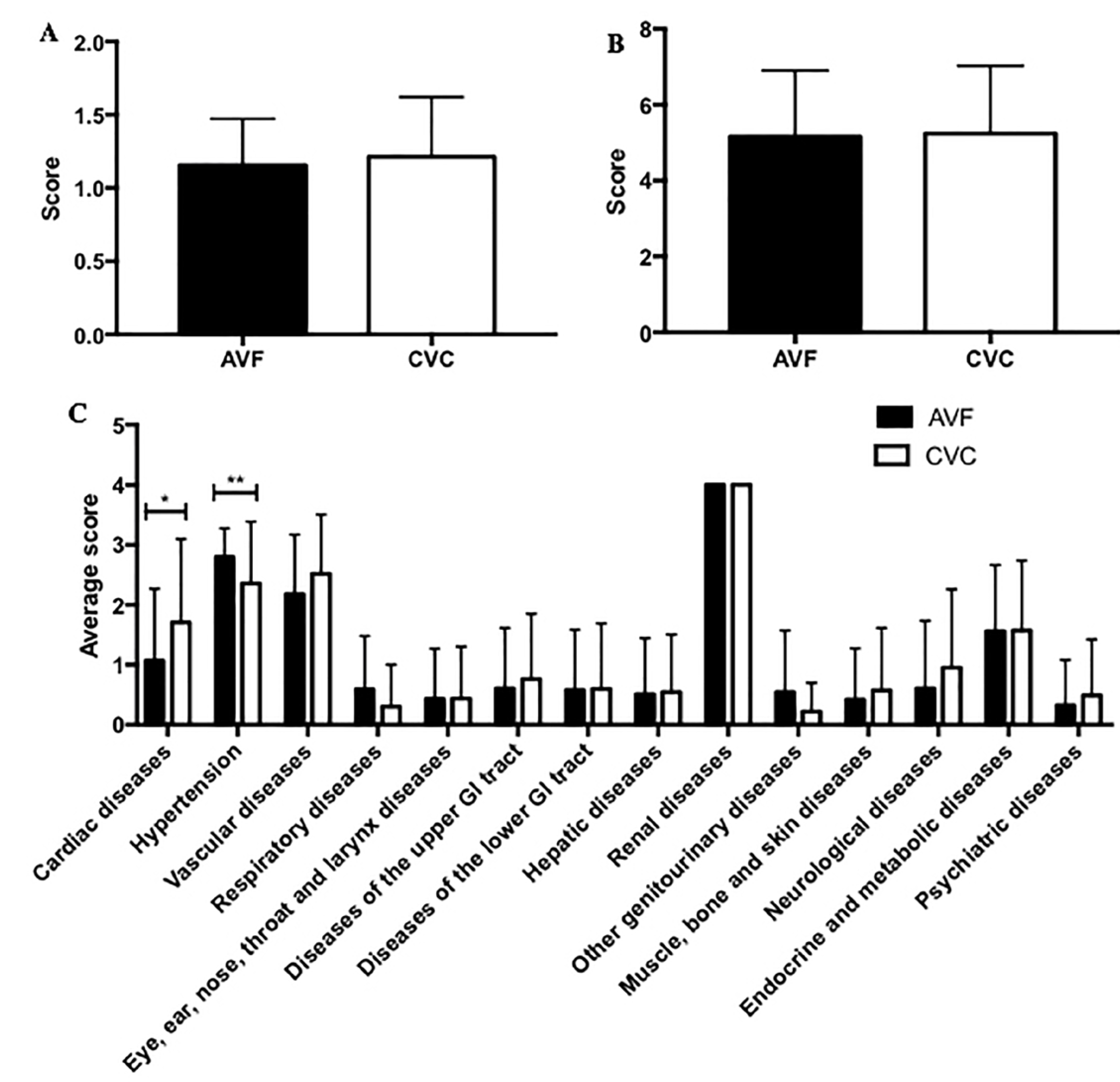
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Clark, T.W.; Hirsch, D.A.; Jindal, K.J.; Veugelers, P.J.; LeBlanc, J. Outcome and prognostic factors of restenosis after percutaneous treatment of native hemodialysis fistulas. J. Vasc. Interv. Radiol. JVIR 2002, 13, 51–59. [Google Scholar] [CrossRef]
- Nordio, M.; Limido, A.; Conte, F.; Di Napoli, A.; Quintaliani, G.; Reboldi, G.; Sparacino, V.; Postorino, M. Italian Registry Dialysis and Transplant 2011–2013. G. Ital. Di Nefrol. Organo Uff. Della Soc. Ital. Di Nefrol. 2016, 33. Available online: https://pubmed.ncbi.nlm.nih.gov/27374391/ (accessed on 2 August 2021).
- Santoro, D.; Benedetto, F.; Mondello, P.; Pipito, N.; Barilla, D.; Spinelli, F.; Ricciardi, C.A.; Cernaro, V.; Buemi, M. Vascular access for hemodialysis: Current perspectives. Int. J. Nephrol. Renov. Dis. 2014, 7, 281–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torreggiani, M.; Scaramuzzi, M.L.; Manini, A.; Castoldi, F.; Serpieri, N.; Maggi, N.; Sileno, G.; Migotto, C.; Esposito, V.; Montagna, F.; et al. Hemodialysis vascular access: Everything you always wanted to know about it (but were afraid to ask). J. Nephrol. 2013, 26, 836–847. [Google Scholar] [CrossRef] [PubMed]
- Schwab, S.; Besarab, A.; Beathard, G.N.K.F.; Brouwer, D.; Etheredge, E.; Hartigan, M.; Levine, M.; McCann, R.; Sherman, R.; Trerotola, S. NKF-DOQI clinical practice guidelines for vascular access. National Kidney Foundation-Dialysis Outcomes Quality Initiative. Am. J. Kidney Dis. 1997, 30, S150–S191. [Google Scholar]
- Ethier, J.H.; Lindsay, R.M.; Barre, P.E.; Kappel, J.E.; Carlisle, E.J.; Common, A. Clinical practice guidelines for vascular access. Canadian Society pf Nephrology. J. Am. Soc. Nephrol. JASN 1999, 10 (Suppl. 13), S297–S305. [Google Scholar] [PubMed]
- National Kidney Foundation Anemia Working Group. KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Anemia in Chronic Kidney Disease. Am. J. Kidney Dis. 2006, 47, S11–S145. [Google Scholar] [CrossRef] [Green Version]
- Pisoni, R.L.; Young, E.W.; Dykstra, D.M.; Greenwood, R.N.; Hecking, E.; Gillespie, B.; Wolfe, R.A.; Goodkin, D.A.; Held, P.J. Vascular access use in Europe and the United States: Results from the DOPPS. Kidney Int. 2002, 61, 305–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vassalotti, J.A.; Jennings, W.C.; Beathard, G.A.; Neumann, M.; Caponi, S.; Fox, C.H.; Spergel, L.M. Fistula first breakthrough initiative: Targeting catheter last in fistula first. Semin. Dial. 2012, 25, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Pisoni, R.L.; Zepel, L.; Port, F.K.; Robinson, B.M. Trends in US Vascular Access Use, Patient Preferences, and Related Practices: An Update from the US DOPPS Practice Monitor with International Comparisons. Am. J. Kidney Dis. 2015, 65, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Golestaneh, L. Decreasing hospitalizations in patients on hemodialysis: Time for a paradigm shift. Semin. Dial. 2018, 31, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Roy-Chaudhury, P.; Sukhatme, V.P.; Cheung, A.K. Hemodialysis vascular access dysfunction: A cellular and molecular viewpoint. J. Am. Soc. Nephrol. JASN 2006, 17, 1112–1127. [Google Scholar] [CrossRef] [PubMed]
- Pastan, S.; Soucie, J.M.; McClellan, W.M. Vascular access and increased risk of death among hemodialysis patients. Kidney Int. 2002, 62, 620–626. [Google Scholar] [CrossRef] [Green Version]
- Daugirdas, J.T. Second generation logarithmic estimates of single-pool variable volume Kt/V: An analysis of error. J. Am. Soc. Nephrol. JASN 1993, 4, 1205–1213. [Google Scholar] [CrossRef] [PubMed]
- Linn, B.S.; Linn, M.W.; Gurel, L. Cumulative illness rating scale. J. Am. Geriatr. Soc. 1968, 16, 622–626. [Google Scholar] [CrossRef]
- Blunt, I.; Bardsley, M.; Strippoli, G.F. Pre-dialysis hospital use and late referrals in incident dialysis patients in England: A retrospective cohort study. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 2015, 30, 124–129. [Google Scholar] [CrossRef] [Green Version]
- Kjellstrand, C.M. The Achilles’ heel of the hemodialysis patient. Arch. Intern. Med. 1978, 138, 1063–1064. [Google Scholar] [CrossRef] [PubMed]
- Polkinghorne, K.R.; McDonald, S.P.; Atkins, R.C.; Kerr, P.G. Vascular access and all-cause mortality: A propensity score analysis. J. Am. Soc. Nephrol. JASN 2004, 15, 477–486. [Google Scholar] [CrossRef] [Green Version]
- DeSilva, R.N.; Sandhu, G.S.; Garg, J.; Goldfarb-Rumyantzev, A.S. Association between initial type of hemodialysis access used in the elderly and mortality. Hemodial. Int. 2012, 16, 233–241. [Google Scholar] [CrossRef]
- Orimo, H. Reviewing the definition of elderly. Nihon Ronen Igakkai Zasshi 2006, 43, 27–34. [Google Scholar] [CrossRef] [Green Version]
- Beard, J.R.; Officer, A.M.; Cassels, A.K. The World Report on Ageing and Health. Gerontologist 2016, 56 (Suppl. 2), S163–S166. [Google Scholar] [CrossRef] [Green Version]
- Esposito, C.; Torreggiani, M.; Arazzi, M.; Serpieri, N.; Scaramuzzi, M.L.; Manini, A.; Grosjean, F.; Esposito, V.; Catucci, D.; La Porta, E.; et al. Loss of renal function in the elderly Italians: A physiologic or pathologic process? J. Gerontol. A Biol. Sci. Med. Sci. 2012, 67, 1387–1393. [Google Scholar] [CrossRef] [Green Version]
- Kramer, A.; Boenink, R.; Stel, V.S.; Santiuste de Pablos, C.; Tomović, F.; Golan, E.; Kerschbaum, J.; Seyahi, N.; Ioanou, K.; Beltrán, P.; et al. The ERA-EDTA Registry Annual Report 2018: A summary. Clin. Kidney J. 2020, 14, 107–123. [Google Scholar] [CrossRef]
- Sands, J.J. Vascular access: The past, present and future. Blood Purif. 2009, 27, 22–27. [Google Scholar] [CrossRef]
- Kim, D.H.; Park, J.I.; Lee, J.P.; Kim, Y.L.; Kang, S.W.; Yang, C.W.; Kim, N.H.; Kim, Y.S.; Lim, C.S. The effects of vascular access types on the survival and quality of life and depression in the incident hemodialysis patients. Ren. Fail. 2020, 42, 30–39. [Google Scholar] [CrossRef] [Green Version]

| AVF | CVC | p-Value | |
|---|---|---|---|
| Number of patients | 63 | 37 | |
| Sex (M/F) | 47/16 | 22/15 | 0.1239 |
| Age (Years) | 68.43 ± 13.07 | 69.14 ± 16.48 | 0.5779 |
| Follow-up (days) | 1111.00 ± 731.30 | 980.70 ± 797.70 | 0.2722 |
| Weight (kg) | 74.06 ± 15.57 | 66.70 ± 11.65 | 0.0144 |
| BMI (kg/m2) | 27.31 ± 4.43 | 24.77 ± 3.02 | 0.0483 |
| Hb (g/dl) | 10.19 ± 1.33 | 10.08 ± 2.35 | 0.7599 |
| Total serum proteins (g/dl) | 6.39 ± 0.69 | 6.31 ± 0.80 | 0.5972 |
| Albumin (g/dl) | 3.45 ± 0.51 | 3.29 ± 0.68 | 0.1933 |
| Serum calcium (mg/dl) | 8.92 ± 0.55 | 8.79 ± 0.45 | 0.2825 |
| Serum phosphorus (mg/dl) | 4.86 ± 0.93 | 4.66 ± 0.86 | 0.2554 |
| PTH (pg/mL) | 241.80 ± 116.00 | 244.60 ± 144.20 | 0.9095 |
| Kt/V | 1.35 ± 0.31 | 1.30 ± 0.25 | 0.3969 |
| Diabetes (yes/no) | 23/40 | 14/23 | >0.9999 |
| Hypertension (yes/no) | 63/0 | 31/6 | 0.0020 |
| Peripheral artery disease (yes/no) | 15/48 | 7/30 | 0.6255 |
| Coronary artery disease or chronic ischemic heart disease (yes/no) | 25/38 | 28/9 | 0.0008 |
| Late referrals (yes/no) | 0/63 | 6/31 | 0.0020 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torreggiani, M.; Bernasconi, L.; Colucci, M.; Accarino, S.; Pasquinucci, E.; Esposito, V.; Sileno, G.; Esposito, C. Vascular Access, Complications and Survival in Incident Hemodialysis Patients. Kidney Dial. 2021, 1, 88-99. https://doi.org/10.3390/kidneydial1020013
Torreggiani M, Bernasconi L, Colucci M, Accarino S, Pasquinucci E, Esposito V, Sileno G, Esposito C. Vascular Access, Complications and Survival in Incident Hemodialysis Patients. Kidney and Dialysis. 2021; 1(2):88-99. https://doi.org/10.3390/kidneydial1020013
Chicago/Turabian StyleTorreggiani, Massimo, Lucia Bernasconi, Marco Colucci, Simone Accarino, Ettore Pasquinucci, Vittoria Esposito, Giuseppe Sileno, and Ciro Esposito. 2021. "Vascular Access, Complications and Survival in Incident Hemodialysis Patients" Kidney and Dialysis 1, no. 2: 88-99. https://doi.org/10.3390/kidneydial1020013
APA StyleTorreggiani, M., Bernasconi, L., Colucci, M., Accarino, S., Pasquinucci, E., Esposito, V., Sileno, G., & Esposito, C. (2021). Vascular Access, Complications and Survival in Incident Hemodialysis Patients. Kidney and Dialysis, 1(2), 88-99. https://doi.org/10.3390/kidneydial1020013








