Pathophysiology of High Flow Access and Surgical Flow Reduction Procedures
Abstract
1. Introduction
2. Development and Remodeling after AVF Creation
3. Condition of High Flow Access and High-Output Heart Failure
4. Venous Hypertension
5. Dialysis Access Steal Syndrome (DASS)
6. Flow Reduction Procedures
7. Banding
8. Fistula Plication
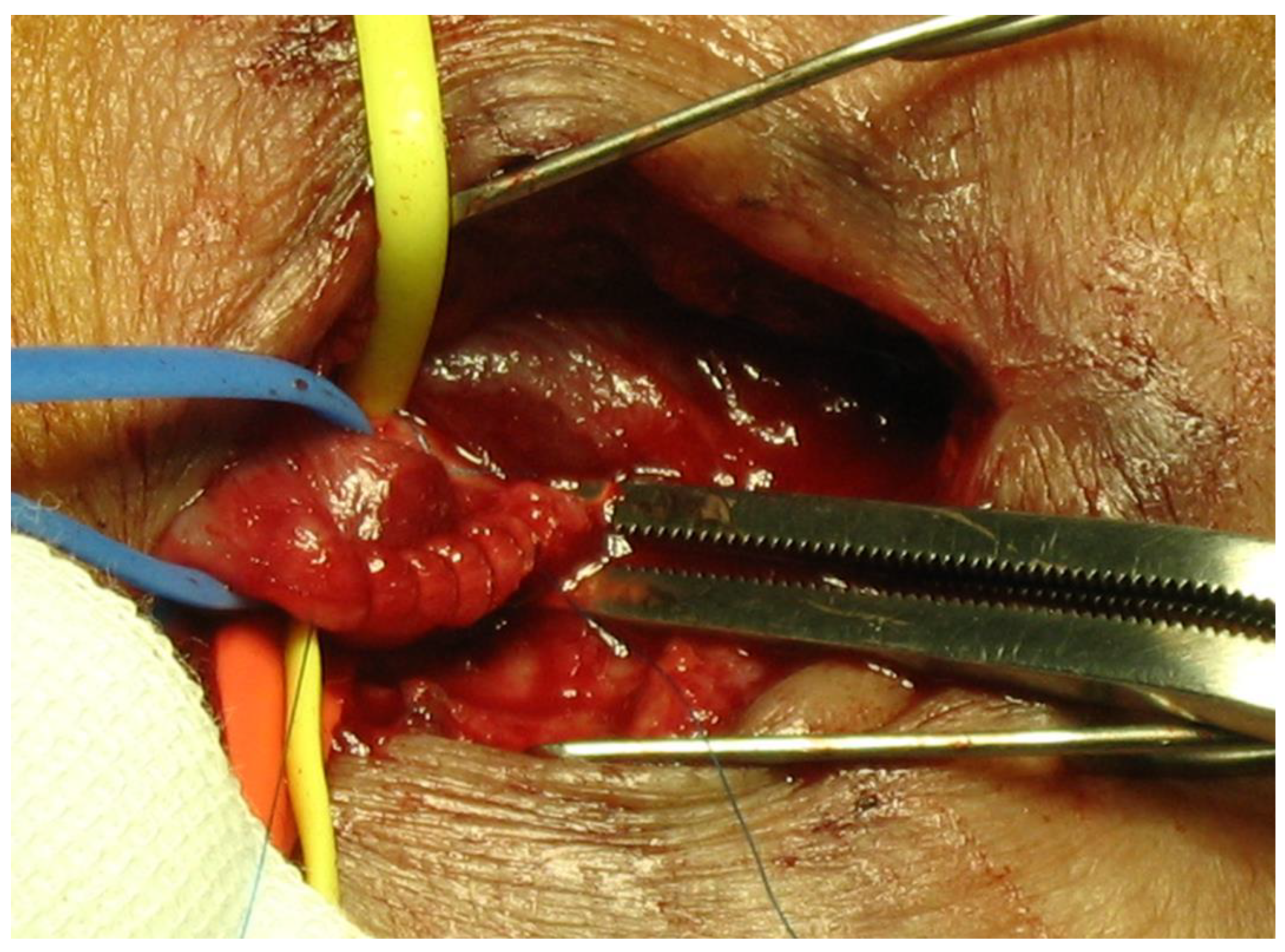
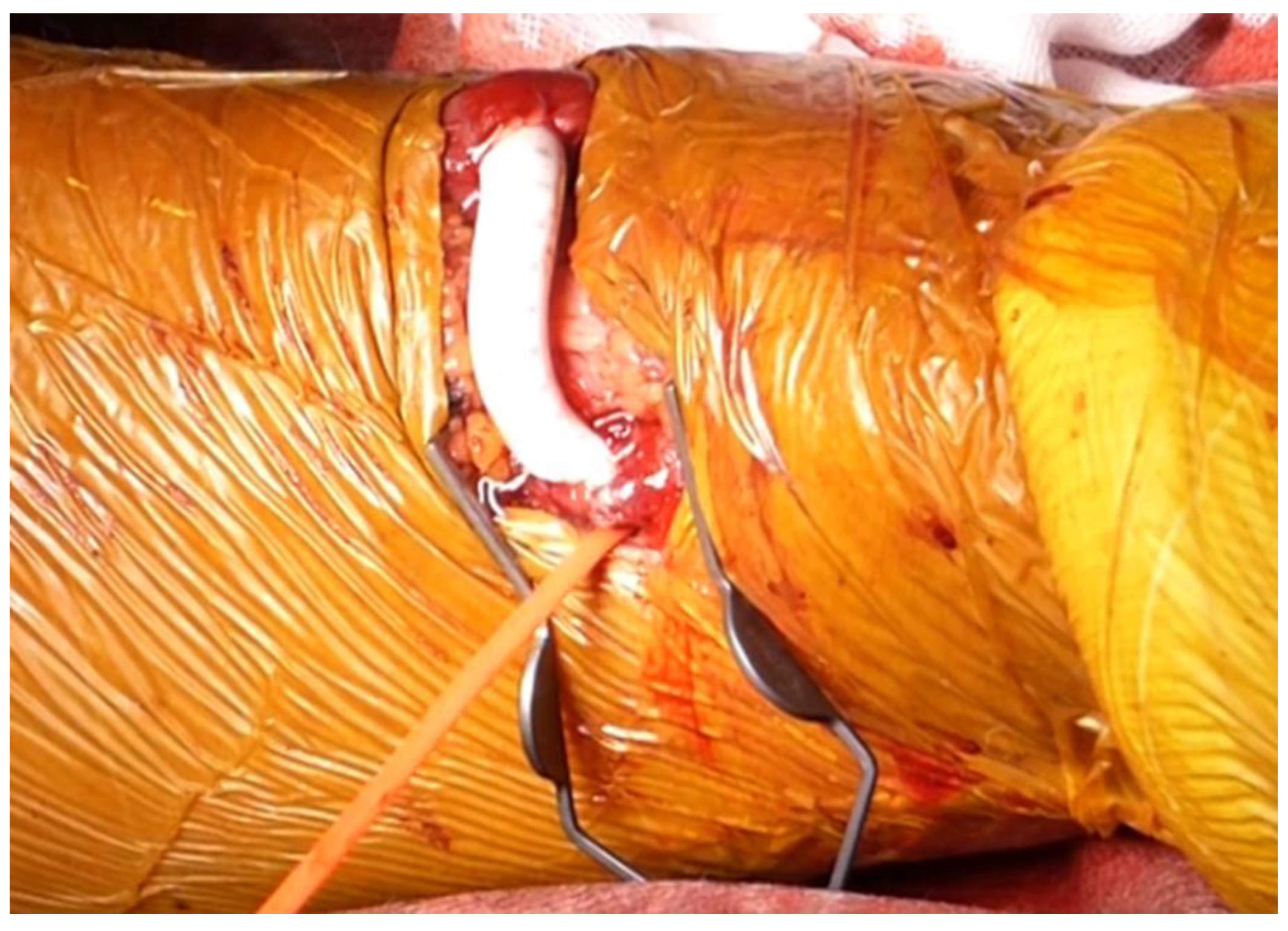
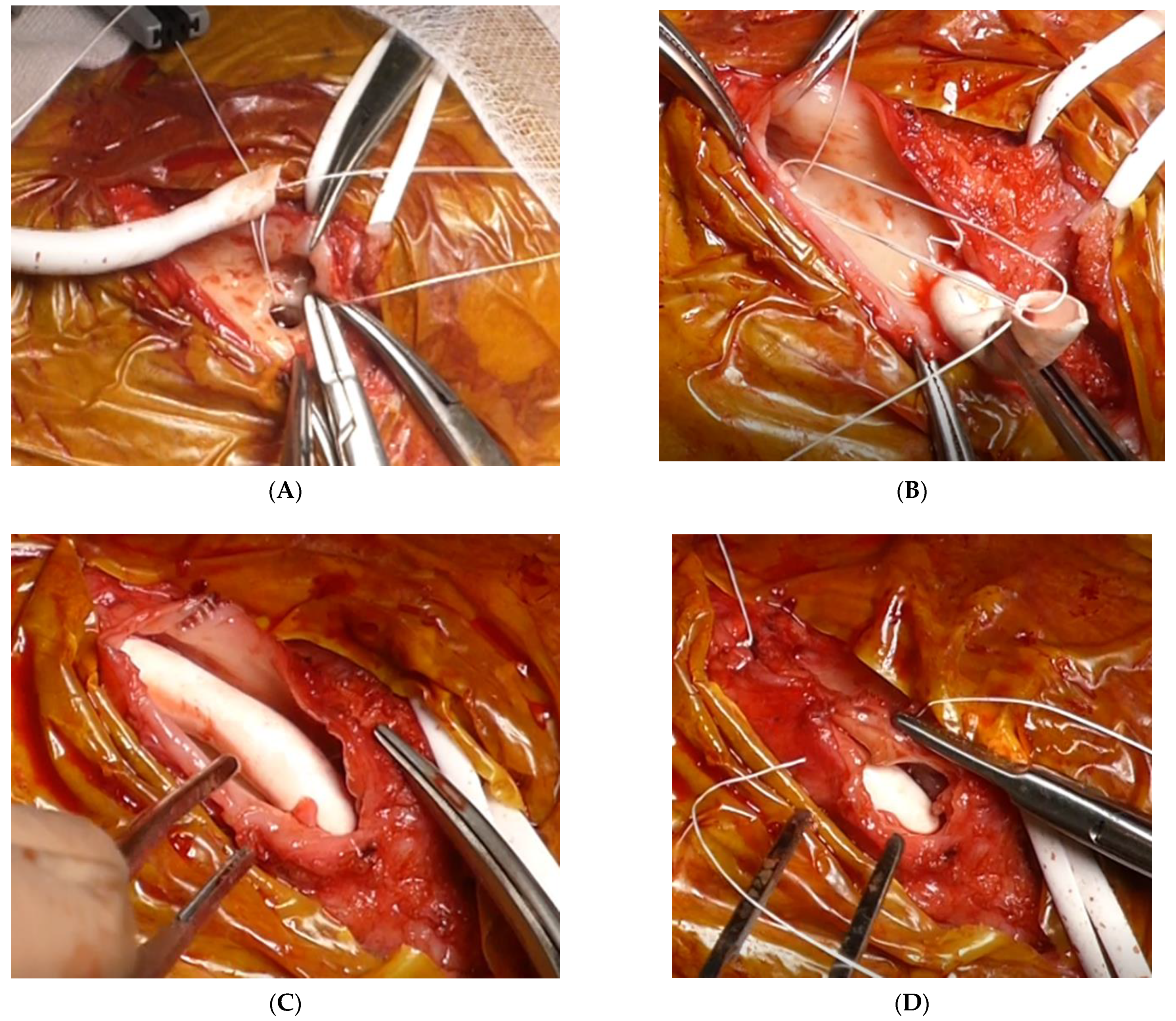
9. Graft Interposition
10. Graft Inclusion Technique
11. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- MacRae, J.M.; Levin, A.; Belenkie, I. The cardiovascular effects of arteriovenous fistulas in chronic kidney disease: A cause for concern? Semin. Dial. 2006, 19, 349–352. [Google Scholar] [CrossRef]
- Basile, C.; Lomonte, C. Pro: The arteriovenous fistula is a blessing of God. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 2012, 27, 3752–3756. [Google Scholar] [CrossRef]
- Roca-Tey, R. Permanent arteriovenous fistula or catheter dialysis for heart failure patients. J. Vasc. Access 2016, 17, S23–S29. [Google Scholar] [CrossRef]
- Kanno, T.; Kamijo, Y.; Hashimoto, K.; Kanno, Y. Outcomes of blood flow suppression methods of treating high flow access in hemodialysis patients with arteriovenous fistula. J. Vasc. Access 2015, 16 (Suppl. S1), S28–S33. [Google Scholar] [CrossRef] [PubMed]
- Torreggiani, M.; Scaramuzzi, M.L.; Manini, A.; Castoldi, F.; Serpieri, N.; Maggi, N.; Sileno, G.; Migotto, C.; Esposito, V.; Montagna, F.; et al. Hemodialysis vascular access: Everything you always wanted to know about it (but were afraid to ask). J. Nephrol. 2013, 26, 836–847. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Suemitsu, K.; Nakamura, J. Superficialization of brachial artery as effective alternative vascular access. J. Vasc. Surg. 2014, 59, 1385–1392. [Google Scholar] [CrossRef] [PubMed]
- Brescia, M.J.; Cimino, J.E.; Appell, K.; Hurwich, B.J. Chronic hemodialysis using venipuncture and a surgically created arteriovenous fistula. N. Engl. J. Med. 1966, 275, 1089–1092. [Google Scholar] [CrossRef] [PubMed]
- Dixon, B.S. Why don’t fistulas mature? Kidney Int. 2006, 70, 1413–1422. [Google Scholar] [CrossRef]
- Dammers, R.; Tordoir, J.H.M.; Welten, R.J.T.H.J.; Kitslaar, P.J.E.H.M.; Hoeks, A.P.G. The effect of chronic flow changes on brachial artery diameter and shear stress in arteriovenous fistulas for hemodialysis. Int. J. Artif. Organs 2002, 25, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Dammers, R.; Tordoir, J.H.M.; Kooman, J.P.; Welten, R.J.T.J.; Hameleers, J.M.M.; Kitslaar, P.J.E.H.M.; Hoeks, A.P.G. The effect of flow changes on the arterial system proximal to an arteriovenous fistula for hemodialysis. Ultrasound Med. Biol. 2005, 31, 1327–1333. [Google Scholar] [CrossRef]
- Roumeliotis, S.; Mallamaci, F.; Zoccali, C. Endothelial Dysfunction in Chronic Kidney Disease, from Biology to Clinical Outcomes: A 2020 Update. J. Clin. Med. 2020, 9, 2359–2386. [Google Scholar] [CrossRef]
- Huynh, N.N.; Chin-Dusting, J. Amino acids, arginase and nitric oxide in vascular health. Clin. Exp. Pharmacol. Physiol. 2006, 33, 1–8. [Google Scholar] [CrossRef]
- Vallance, P.; Hingorani, A. Endothelial nitric oxide in humans in health and disease. Int. J. Exp. Pathol. 1999, 80, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Hou, X.; Wason, C.; Kopel, T.; Cohen, R.A.; Dember, L.M. Smooth Muscle Nitric Oxide Responsiveness and Clinical Maturation of Hemodialysis Arteriovenous Fistulae. Am. J. Pathol. 2017, 187, 2095–2101. [Google Scholar] [CrossRef] [PubMed]
- Berceli, S.A.; Jiang, Z.; Klingman, N.V.; Schultz, G.S.; Ozaki, C.K. Early differential MMP-2 and -9 dynamics during flow-induced arterial and vein graft adaptations. J. Surg. Res. 2006, 134, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.-Y.; Chen, Y.-S.; Ma, M.-C.; Chen, C.-F. Remodeling of experimental arteriovenous fistula with increased matrix metalloproteinase expression in rats. J. Vasc. Surg. 2007, 45, 804–811. [Google Scholar] [CrossRef]
- Achneck, H.E.; Sileshi, B.; Li, M.; Partington, E.J.; Peterson, D.A.; Lawson, J.H. Surgical aspects and biological considerations of arteriovenous fistula placement. Semin. Dial. 2010, 23, 25–33. [Google Scholar] [CrossRef]
- Ben Driss, A.; Benessiano, J.; Poitevin, P.; Levy, B.I.; Michel, J.B. Arterial expansive remodeling induced by high flow rates. Am. J. Physiol. 1997, 272, H851–H858. [Google Scholar] [CrossRef]
- Jie, K.; Feng, W.; Boxiang, Z.; Maofeng, G.; Jianbin, Z.; Zhaoxuan, L.; Yangyi, Z.; Liang, C.; Haobo, S.; Wensheng, L.; et al. Identification of Pathways and Key Genes in Venous Remodeling After Arteriovenous Fistula by Bioinformatics Analysis. Front. Physiol. 2020, 11, 565240. [Google Scholar] [CrossRef]
- Panagrosso, M.; Bracale, U.M.; Del Guercio, L.; Viscardi, A.; Peluso, A.; Dinoto, E. Case report of a large cephalic vein aneurysm inducing heart failure in a renal transplant patient with radio-cephalic fistula for haemodialysis. Int. J. Surg. Case Rep. 2020, 77S, S162–S165. [Google Scholar] [CrossRef]
- Dember, L.M.; Imrey, P.B.; Beck, G.J.; Cheung, A.K.; Himmelfarb, J.; Huber, T.S.; Kusek, J.W.; Roy-Chaudhury, P.; Vazquez, M.A.; Alpers, C.E.; et al. Objectives and design of the hemodialysis fistula maturation study. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2014, 63, 104–112. [Google Scholar] [CrossRef]
- Allon, M.; Lok, C.E. Dialysis fistula or graft: The role for randomized clinical trials. Clin. J. Am. Soc. Nephrol. 2010, 5, 2348–2354. [Google Scholar] [CrossRef]
- Dember, L.M.; Beck, G.J.; Allon, M.; Delmez, J.A.; Dixon, B.S.; Greenberg, A.; Himmelfarb, J.; Vazquez, M.A.; Gassman, J.J.; Greene, T.; et al. Effect of clopidogrel on early failure of arteriovenous fistulas for hemodialysis: A randomized controlled trial. JAMA 2008, 299, 2164–2171. [Google Scholar] [CrossRef] [PubMed]
- Huijbregts, H.J.T.; Bots, M.L.; Wittens, C.H.A.; Schrama, Y.C.; Moll, F.L.; Blankestijn, P.J. Hemodialysis arteriovenous fistula patency revisited: Results of a prospective, multicenter initiative. Clin. J. Am. Soc. Nephrol. 2008, 3, 714–719. [Google Scholar] [CrossRef]
- Roy-Chaudhury, P.; Arend, L.; Zhang, J.; Krishnamoorthy, M.; Wang, Y.; Banerjee, R.; Samaha, A.; Munda, R. Neointimal hyperplasia in early arteriovenous fistula failure. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2007, 50, 782–790. [Google Scholar] [CrossRef] [PubMed]
- Corpataux, J.-M.; Haesler, E.; Silacci, P.; Ris, H.B.; Hayoz, D. Low-pressure environment and remodelling of the forearm vein in Brescia-Cimino haemodialysis access. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 2002, 17, 1057–1062. [Google Scholar] [CrossRef] [PubMed]
- Roy-Chaudhury, P.; Sukhatme, V.P.; Cheung, A.K. Hemodialysis vascular access dysfunction: A cellular and molecular viewpoint. J. Am. Soc. Nephrol. 2006, 17, 1112–1127. [Google Scholar] [CrossRef]
- Vaes, R.H.D.; Wouda, R.; van Loon, M.; van Hoek, F.; Tordoir, J.H.; Scheltinga, M.R. Effectiveness of surgical banding for high flow in brachial artery-based hemodialysis vascular access. J. Vasc. Surg. 2015, 61, 762–766. [Google Scholar] [CrossRef] [PubMed]
- Basile, C.; Lomonte, C.; Vernaglione, L.; Casucci, F.; Antonelli, M.; Losurdo, N. The relationship between the flow of arteriovenous fistula and cardiac output in haemodialysis patients. Nephrol. Dial. Transpl. 2008, 23, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Sequeira, A.; Tan, T.-W. Complications of a High-flow Access and Its Management. Semin. Dial. 2015, 28, 533–543. [Google Scholar] [CrossRef]
- Korsheed, S.; Eldehni, M.T.; John, S.G.; Fluck, R.J.; McIntyre, C.W. Effects of arteriovenous fistula formation on arterial stiffness and cardiovascular performance and function. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 2011, 26, 3296–3302. [Google Scholar] [CrossRef]
- Ori, Y.; Korzets, A.; Katz, M.; Erman, A.; Weinstein, T.; Malachi, T.; Gafter, U. The contribution of an arteriovenous access for hemodialysis to left ventricular hypertrophy. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2002, 40, 745–752. [Google Scholar] [CrossRef]
- Dundon, B.K.; Torpey, K.; Nelson, A.J.; Wong, D.T.; Duncan, R.F.; Meredith, I.T.; Faull, R.J.; Worthley, S.G.; Worthley, M.I. The deleterious effects of arteriovenous fistula-creation on the cardiovascular system: A longitudinal magnetic resonance imaging study. Int. J. Nephrol. Renovasc. Dis. 2014, 7, 337–345. [Google Scholar] [PubMed]
- McIntyre, C.W. Effects of hemodialysis on cardiac function. Kidney Int. 2009, 76, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Derthoo, D.; Belmans, A.; Claes, K.; Bammens, B.; Ciarka, A.; Droogné, W.; Vanhaecke, J.; Van Cleemput, J.; Janssens, S. Survival and heart failure therapy in chronic dialysis patients with heart failure and reduced left ventricular ejection fraction: An observational retrospective study. Acta Cardiol. 2013, 68, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Duque, J.C.; Gomez, C.; Tabbara, M.; Alfonso, C.E.; Li, X.; Vazquez-Padron, R.I.; Asif, A.; Lenz, O.; Briones, P.L.; Salman, L.H. The impact of arteriovenous fistulae on the myocardium: The impact of creation and ligation in the transplant era. Semin. Dial. 2015, 28, 305–310. [Google Scholar] [CrossRef]
- Yamada, S.; Ishii, H.; Takahashi, H.; Aoyama, T.; Morita, Y.; Kasuga, H.; Kimura, K.; Ito, Y.; Takahashi, R.; Toriyama, T.; et al. Prognostic value of reduced left ventricular ejection fraction at start of hemodialysis therapy on cardiovascular and all-cause mortality in end-stage renal disease patients. Clin. J. Am. Soc. Nephrol. 2010, 5, 1793–1798. [Google Scholar] [CrossRef]
- Voorzaat, B.M.; van Schaik, J.; Siebelink, H.-M.J.; Tordoir, J.H.; Rotmans, J.I. The pros and cons of preserving a functioning arteriovenous fistula after kidney transplantation. J. Vasc. Access 2016, 17 (Suppl. S1), S16–S22. [Google Scholar] [CrossRef]
- Balamuthusamy, S.; Jalandhara, N.; Subramanian, A.; Mohanaselvan, A. Flow reduction in high-flow arteriovenous fistulas improve cardiovascular parameters and decreases need for hospitalization. Hemodial. Int. 2016, 20, 362–368. [Google Scholar] [CrossRef]
- Van Duijnhoven, E.C.; Cheriex, E.C.; Tordoir, J.H.; Kooman, J.P.; van Hooff, J.P. Effect of closure of the arteriovenous fistula on left ventricular dimensions in renal transplant patients. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 2001, 16, 368–372. [Google Scholar] [CrossRef]
- Movilli, E.; Viola, B.F.; Brunori, G.; Gaggia, P.; Camerini, C.; Zubani, R.; Berlinghieri, N.; Cancarini, G. Long-term effects of arteriovenous fistula closure on echocardiographic functional and structural findings in hemodialysis patients: A prospective study. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2010, 55, 682–689. [Google Scholar] [CrossRef]
- Aitken, E.; Kerr, D.; Geddes, C.; Berry, C.; Kingsmore, D. Cardiovascular changes occurring with occlusion of a mature arteriovenous fistula. J. Vasc. Access 2015, 16, 459–466. [Google Scholar] [CrossRef]
- Nojima, T.; Motomiya, Y. Graft Inclusion Technique: A New Flow Reduction Procedure for High Flow Arteriovenous Fistulae. Ann. Vasc. Dis. 2018, 11, 202–209. [Google Scholar] [CrossRef]
- Neville, R.F.; Abularrage, C.J.; White, P.W.; Sidawy, A.N. Venous hypertension associated with arteriovenous hemodialysis access. Semin. Vasc. Surg. 2004, 17, 50–56. [Google Scholar] [CrossRef]
- Georgakarakos, E.I.; Kapoulas, K.C.; Georgiadis, G.S.; Tsangaris, A.S.; Nikolopoulos, E.S.; Lazarides, M.K. An overview of the hemodynamic aspects of the blood flow in the venous outflow tract of the arteriovenous fistula. J. Vasc. Access 2012, 13, 271–278. [Google Scholar] [CrossRef]
- Kim, M.J.; Yun, S.; Song, D.; Cho, S.W.; Goo, D.E.; Kim, Y.J.; Choi, D. Alternative venous outflow by brachial to jugular vein vascular access for hemodialysis in the exhausted upper extremities. J. Vasc. Access 2015, 16, 269–274. [Google Scholar] [CrossRef]
- Henry, J.C.; Sachdev, U.; Hager, E.; Dillavou, E.; Yuo, T.; Makaroun, M.; Leers, S.A. Cephalic vein transposition is a durable approach to managing cephalic arch stenosis. J. Vasc. Access 2017. [Google Scholar] [CrossRef]
- Sivananthan, G.; Menashe, L.; Halin, N.J. Cephalic arch stenosis in dialysis patients: Review of clinical relevance, anatomy, current theories on etiology and management. J. Vasc. Access 2014, 15, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.S.; Peden, E.K. Dialysis-associated steal syndrome (DASS). J. Vasc. Access 2017, 18, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Beathard, G.A.; Spergel, L.M. Hand ischemia associated with dialysis vascular access: An individualized access flow-based approach to therapy. Semin. Dial. 2013, 26, 287–314. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Yuo, T.H.; Konig, G., 4th; Dillavou, E.; Leers, S.A.; Chaer, R.A.; Cho, J.S.; Makaroun, M.S. Treatment strategies of arterial steal after arteriovenous access. J. Vasc. Surg. 2011, 54, 162–167. [Google Scholar] [CrossRef]
- DeCaprio, J.D.; Valentine, R.J.; Kakish, H.B.; Awad, R.; Hagino, R.T.; Clagett, G.P. Steal syndrome complicating hemodialysis access. Cardiovasc. Surg. 1997, 5, 648–653. [Google Scholar] [CrossRef]
- Gkotsis, G.; Jennings, W.C.; Malik, J.; Mallios, A.; Taubman, K. Treatment of High Flow Arteriovenous Fistulas after Successful Renal Transplant Using a Simple Precision Banding Technique. Ann. Vasc. Surg. 2016, 31, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.G.; Gawad, K.A.; Strate, T.; Pfalzer, B.; Izbicki, J.R. T-banding: A technique for flow reduction of a hyperfunctioning arteriovenous fistula. J. Vasc. Surg. 2006, 43, 402–405. [Google Scholar] [CrossRef] [PubMed][Green Version]
- De Palma, J.R.; Vannix, R.; Bahuth, J.; Abukurah, A. “Steal” syndrome, ischemia, congestive failure and periperhal neuropathy. Proc. Clin. Dial. Transplant Forum 1973, 3, 9–11. [Google Scholar]
- Anderson, C.B.; Groce, M.A. Banding of arteriovenous dialysis fistulas to correct high-output cardiac failure. Surgery 1975, 78, 552–554. [Google Scholar]
- Van Hoek, F.; Scheltinga, M.; Luirink, M.; Pasmans, H.; Beerenhout, C. Banding of hemodialysis access to treat hand ischemia or cardiac overload. Semin. Dial. 2009, 22, 204–208. [Google Scholar] [CrossRef]
- Odland, M.D.; Kelly, P.H.; Ney, A.L.; Andersen, R.C.; Bubrick, M.P. Management of dialysis-associated steal syndrome complicating upper extremity arteriovenous fistulas: Use of intraoperative digital photoplethysmography. Surgery 1991, 110, 664–670. [Google Scholar]
- Bourquelot, P.; Gaudric, J.; Turmel-Rodrigues, L.; Franco, G.; Van Laere, O.; Raynaud, A. Proximal radial artery ligation (PRAL) for reduction of flow in autogenous radial cephalic accesses for haemodialysis. Eur. J. Vasc. Endovasc. Surg. 2010, 40, 94–99. [Google Scholar] [CrossRef][Green Version]
- Chemla, E.S.; Morsy, M.; Anderson, L.; Whitemore, A. Inflow reduction by distalization of anastomosis treats efficiently high-inflow high-cardiac output vascular access for hemodialysis. Semin. Dial. 2007, 20, 68–72. [Google Scholar] [CrossRef]
- Loh, T.M.; Bennett, M.E.; Peden, E.K. Revision using distal inflow is a safe and effective treatment for ischemic steal syndrome and pathologic high flow after access creation. J. Vasc. Surg. 2016, 63, 441–444. [Google Scholar] [CrossRef]
- Leake, A.E.; Winger, D.G.; Leers, S.A.; Gupta, N.; Dillavou, E.D. Management and outcomes of dialysis access-associated steal syndrome. J. Vasc. Surg. 2015, 61, 754–760. [Google Scholar] [CrossRef] [PubMed]
- Goel, N.; Miller, G.A.; Jotwani, M.C.; Licht, J.; Schur, I.; Arnold, W.P. Minimally Invasive Limited Ligation Endoluminal-assisted Revision (MILLER) for treatment of dialysis access-associated steal syndrome. Kidney Int. 2006, 70, 765–770. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.S.; Davies, M.G.; Nassar, G.M.; Naoum, J.J. Open Repair and Venous Inflow Plication of the Arteriovenous Fistula Is Effective in Treating Vascular Steal Syndrome. Ann. Vasc. Surg. 2015, 25, 927–933. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.A.; Goel, N.; Friedman, A.; Khariton, A.; Jotwani, M.C.; Savransky, Y.; Khariton, K.; Arnold, W.P.; Preddie, D.C. The MILLER banding procedure is an effective method for treating dialysis-associated steal syndrome. Kidney Int. 2010, 77, 359–366. [Google Scholar] [CrossRef]
- Sheaffer, W.W.; Hangge, P.T.; Chau, A.H.; Alzubaidi, S.J.; Knuttinen, M.-G.; Naidu, S.G.; Ganguli, S.; Oklu, R.; Davila, V.J. Minimally Invasive Limited Ligation Endoluminal-Assisted Revision (MILLER): A Review of the Available Literature and Brief Overview of Alternate Therapies in Dialysis Associated Steal Syndrome. J. Clin. Med. 2018, 7, 128–137. [Google Scholar] [CrossRef]
- Shintaku, S.; Kawanishi, H.; Moriishi, M.; Banshodani, M.; Ago, R.; Tsuchiya, S. Modified MILLER banding procedure for managing high-flow access and dialysis-associated steal syndrome. J. Vasc. Access 2015, 16, 227–232. [Google Scholar] [CrossRef]
- Yaghoubian, A.; de Virgilio, C. Plication as primary treatment of steal syndrome in arteriovenous fistulas. Ann. Vasc. Surg. 2009, 23, 103–107. [Google Scholar] [CrossRef]
- Murray, B.M.; Rajczak, S.; Herman, A.; Leary, D. Effect of surgical banding of a high-flow fistula on access flow and cardiac output: Intraoperative and long-term measurements. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2004, 44, 1090–1096. [Google Scholar] [CrossRef]
- Hashimoto, T.; Akagi, D.; Yamamoto, S.; Suhara, M.; Deguchi, J.-O.; Sato, O. Short Interposition with a Small-Diameter Prosthetic Graft for Flow Reduction of a High-Flow Arteriovenous Fistula. J. Vasc. Surg. 2021, 73, 285–290. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nojima, T.; Motomiya, Y. Pathophysiology of High Flow Access and Surgical Flow Reduction Procedures. Kidney Dial. 2021, 1, 36-46. https://doi.org/10.3390/kidneydial1010007
Nojima T, Motomiya Y. Pathophysiology of High Flow Access and Surgical Flow Reduction Procedures. Kidney and Dialysis. 2021; 1(1):36-46. https://doi.org/10.3390/kidneydial1010007
Chicago/Turabian StyleNojima, Takehisa, and Yasuki Motomiya. 2021. "Pathophysiology of High Flow Access and Surgical Flow Reduction Procedures" Kidney and Dialysis 1, no. 1: 36-46. https://doi.org/10.3390/kidneydial1010007
APA StyleNojima, T., & Motomiya, Y. (2021). Pathophysiology of High Flow Access and Surgical Flow Reduction Procedures. Kidney and Dialysis, 1(1), 36-46. https://doi.org/10.3390/kidneydial1010007





