Impact of COVID-19 History on Patients’ Outcome in the Perioperative Period—A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
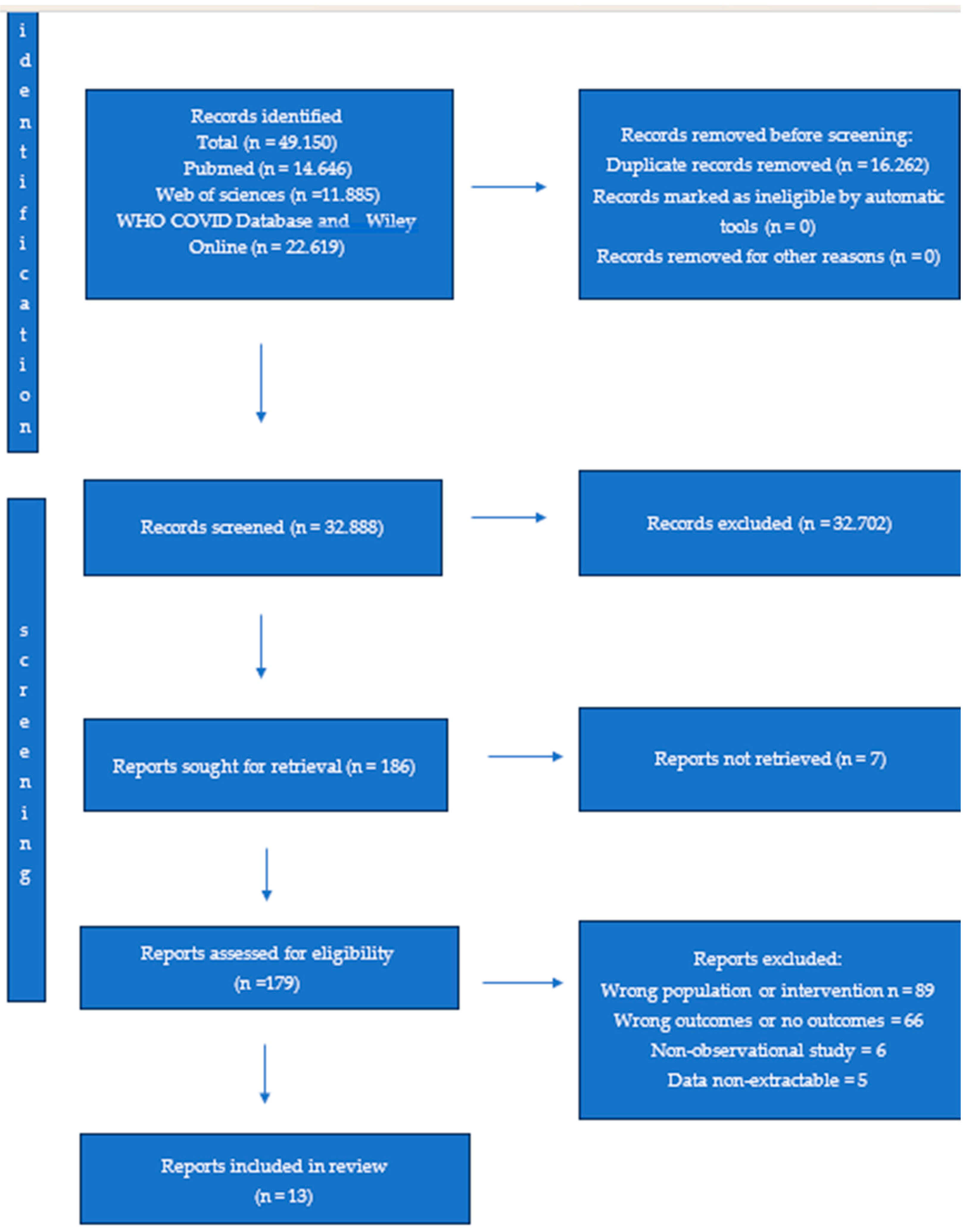
2.1. Search Strategy and Selection Criteria
2.2. Data Extraction
2.3. Data Analysis
3. Results
3.1. Study Characteristics
3.2. Patient Characteristics
3.3. Patient Outcomes
3.4. Risk of Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PRISMA | preferred reporting items for systematic reviews and meta-analyses |
| ICU | intensive care unit |
| MACE | major acute cardiac events |
| AKI | acute kidney injury |
| VTE | venous thromboembolism |
| CABG | coronary artery bypass grafting |
| ARDS | acute respiratory distress syndrome |
| DVT | deep vein thrombosis |
References
- Worldometers. 2025. Available online: https://www.worldometers.info/coronavirus/ (accessed on 15 July 2025).
- Worldometers. 2025. Available online: https://www.worldometers.info/world-population/ (accessed on 15 July 2025).
- Jonker, P.K.C.; van der Plas, W.Y.; Steinkamp, P.J.; Poelstra, R.; Emous, M.; van der Meij, W.; Thunnissen, F.; Bierman, W.F.W.; Struys, M.M.R.F.; de Reuver, P.R.; et al. Dutch Surgical COVID-19 Research Collaborative. Perioperative SARS-CoV-2 infections increase mortality, pulmonary complications, and thromboembolic events: A Dutch, multicenter, matched-cohort clinical study. Surgery 2021, 169, 264–274. [Google Scholar]
- Deng, J.Z.; Chan, J.S.; Potter, A.L.; Chen, Y.W.; Sandhu, H.S.; Panda, N.; Chang, D.C.; Yang, C.J. The Risk of Postoperative Complications After Major Elective Surgery in Active or Resolved COVID-19 in the United States. Ann. Surg. 2022, 275, 242–246. [Google Scholar] [PubMed]
- Argandykov, D.; Dorken-Gallastegi, A.; El Moheb, M.; Gebran, A.; Proaño-Zamudio, J.A.; Bokenkamp, M.; Renne, A.M.; Nepogodiev, D.; Bhangu, A.; Kaafarani, H.M.A.; et al. Is perioperative COVID-19 really associated with worse surgical outcomes? A nationwide COVIDSurg propensity-matched analysis. J. Trauma Acute Care Surg. 2023, 94, 513–524. [Google Scholar] [CrossRef] [PubMed]
- El-Boghdadly, K.; Cook, T.M.; Goodacre, T.; Kua, J.; Blake, L.; Denmark, S.; McNally, S.; Mercer, N.; Moonesinghe, S.R.; Summerton, D.J. SARS-CoV-2 infection, COVID-19 and timing of elective surgery: A multidisciplinary consensus statement on behalf of the Association of Anaesthetists, the Centre for Peri-operative Care, the Federation of Surgical Specialty Associations, the Royal College of Anaesthetists and the Royal College of Surgeons of England. Anaesthesia 2021, 76, 940–946. [Google Scholar]
- Asta, L.; Falco, D.; Benedetto, U.; Porreca, A.; Majri, F.; Angelini, G.D.; Sensi, S.; Di Giammarco, G. Stroke after Cardiac Surgery: A Risk Factor Analysis of 580,117 Patients from UK National Adult Cardiac Surgical Audit Cohort. J. Pers. Med. 2024, 14, 169. [Google Scholar] [CrossRef] [PubMed]
- Classe, J.M.; Dolivet, G.; Evrard, S.; Ferron, G.; Lécuru, F.; Leufflen, L.; Rivoire, M.; Sgarbura, O. Recommandations de la Société française de chirurgie oncologique (SFCO) pour l’organisation de la chirurgie oncologique durant l’épidémie de COVID-19 [French Society for Surgical Oncology (SFCO) guidelines for the management of surgical oncology in the pandemic context of COVID 19]. Bull. Cancer 2020, 107, 524–527. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Br. Med. J. 2021, 372, n71. [Google Scholar]
- Downs, S.H.; Black, N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J. Epidemiol. Community Health 1998, 52, 377–384. [Google Scholar] [CrossRef]
- Gabryel, P.; Zielińska, D.; Skrzypczak, P.; Sielewicz, M.; Campisi, A.; Kasprzyk, M.; Piwkowski, C. Outcomes of lung cancer surgery in patients with COVID-19 history: A single center cohort study. Gen. Thorac. Cardiovasc. Surg. 2023, 71, 175–181. [Google Scholar] [CrossRef]
- Le, S.T.; Kipnis, P.; Cohn, B.; Liu, V.X. COVID-19 Vaccination and the Timing of Surgery Following COVID-19 Infection. Ann. Surg. 2022, 276, e265–e272. [Google Scholar] [CrossRef]
- Kothari, A.N.; DiBrito, S.R.; Lee, J.J.; Caudle, A.S.; Clemens, M.W.; Gottumukkala, V.N.; Katz, M.H.G.; Offodile, A.C.; Uppal, A.; D3CODETeam; et al. Surgical Outcomes in Cancer Patients Undergoing Elective Surgery After Recovering from Mild-to-Moderate SARS-CoV-2 Infection. Ann. Surg. Oncol. 2021, 28, 8046–8053. [Google Scholar] [CrossRef] [PubMed]
- COVIDSurg Collaborative; GlobalSurg Collaborative. Timing of surgery following SARS-CoV-2 infection: An international prospective cohort study. Anaesthesia 2021, 76, 748–758. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, P.; Salunke, B.; Wajekar, A.; Siddique, A.; Daruwalla, K.; Chawathey, S.; Niyogi, D.; Nayak, P.; Divatia, J. Outcomes of elective cancer surgery in COVID-19 survivors: An observational study. J. Surg. Oncol. 2023, 127, 11–17. [Google Scholar] [CrossRef]
- Erçen Diken, Ö.; Hafez, İ.; Tünel, H.A.; Hanedan, M.O.; Alemdaroğlu, U.; Diken, A.İ. The impact of previous COVID-19 pneumonia on postoperative outcomes and complications in coronary artery bypass grafting. Turk. J. Thorac. Cardiovasc. Surg. 2024, 32, 132–140. [Google Scholar] [CrossRef]
- SenthilKumar, G.; Verhagen, N.B.; Sheriff, S.A.; Yang, X.; Figueroa Castro, C.E.; Szabo, A.; Taylor, B.W.; Wainaina, N.; Lauer, K.; Gould, J.C.; et al. Preoperative SARS-CoV-2 infection increases risk of early postoperative cardiovascular complications following noncardiac surgery. Am. J. Physiol. Heart Circ. Physiol. 2023, 324, H721–H731. [Google Scholar] [CrossRef]
- Cheung, M.S.; Ying, L.; Duffy, A.J.; Ghiassi, S.; Nadzam, G.; Roberts, K.E.; Morton, J.M. Performing Bariatric Surgery on Previously COVID+ Patients Is Safe and Effective. Obes. Surg. 2021, 31, 5082–5084. [Google Scholar] [CrossRef] [PubMed]
- Lazzareschi, D.V.; Luo, Y.; Fong, N.; Boscardin, J.; Legrand, M.; Chen, C.L. Postoperative thrombotic events following major surgery in patients with a history of COVID-19: A retrospective cohort analysis of commercially insured beneficiaries in the USA. Can. J. Anesth. 2024, 71, 55–65. [Google Scholar]
- Shen, Z.; Huang, Z.; Zhu, T.; Zhang, J.; Teng, M.; Qing, Y.; Hu, S.; Li, Y.; Xiong, Y.; Shen, J.; et al. Optimal surgical timing for lung cancer following SARS-CoV-2 infection: A prospective multicenter cohort study. BMC Cancer 2024, 24, 1250. [Google Scholar] [CrossRef]
- Fang, D.; Wu, L.; Gan, B.L.; Guo, C.L.; Chen, Z.H.; Zhou, S.A.; Wu, F.; QunXu, L.; Chen, Z.R.; Shi, N.; et al. Impact of prior SARS-CoV-2 infection on postoperative recovery in patients with hepatocellular carcinoma resection. BMC Gastroenterol. 2024, 24, 317. [Google Scholar] [CrossRef]
- Bansal, S.; Giribabu, P.; Sriganesh, K.; Shukla, D. Perioperative outcomes in patients with symptomatic versus asymptomatic previous COVID-19 infection undergoing neurosurgical treatment (post-COVID-19 study). J. Anaesthesiol. Clin. Pharmacol. 2025, 41, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Gabriyelyan, A.V.; Cheveliuk, O.V.; Romanova, S.V.; Kudlai, I.V.; Gergi, M.S.; Moshta, S.S. Evaluation of the Perioperative Period After Off-Pump Coronary Artery Bypass Grafting in Patients with the History of COVID-19. Ukr. J. Cardiovasc. Surg. 2021, 3, 80–85. [Google Scholar] [CrossRef]
- Şahin, İ.; Batur, Ş.; Üstündağ, A.; Arapi, B.; Tel Üstünışık, Ç.; Göksedef, D.; Ömeroğlu, S.N.; İpek, G.; Balkanay, O.O. The Impact of COVID-19 on Graft Vasculopathy and Postoperative Thromboembolism in CABG Patients: A Prospective Controlled Study. Cardiovasc. Toxicol. 2025, 25, 1123–1138. [Google Scholar] [CrossRef]
- Li, H.; Xing, J.; Song, Z.; Fan, Z.; Wen, H.; Liang, S.; Yan, Q.; Feng, H.; Han, S.; Yang, N.; et al. Effect of mild-to-moderate COVID 19 on the incidence and risk factors for deep vein thrombosis in patients with hip fracture: A retrospective study. BMC Surg. 2025, 25, 113. [Google Scholar] [CrossRef] [PubMed]
- Abraham, A.S.; Sasikumar, N.K.; Rajan, S.; Abubaker, R.; Manoharan, K.S.; Kumar, L. Incidence and Severity of Postoperative Complications in Patients Undergoing Surgery Following COVID-19 Infection at a Tertiary Care Center in South India. Anesth. Essays Res. 2022, 16, 268–271. [Google Scholar] [CrossRef]
- Nadarajan, A.R.; George, N.A.; Thomas, S.; Varghese, B.T.; Iype, E.M.; KM, J.K. Impact of COVID-19 on Disease Progression and Postoperative Complications in Patients with Head and Neck Cancer. Indian J. Surg. Oncol. 2023, 16, 491–495. [Google Scholar] [CrossRef]
- Bautista, A.; Madani, M.; Pretorius, V.; Yang, J.; Fernandes, T.; Papamatheakis, D.; Poch, D.; Kerr, K.; Kim, N.H. Pulmonary thromboendarterectomy after COVID-19 infection: USA single center experience. Eur. Respir. J. 2022, 60 (Suppl. S66), 3976. [Google Scholar] [CrossRef]
- Spadaccio, C.; Rose, D.; Candura, D.; Lopez Marco, A.; Cerillo, A.; Stefano, P.; Nasso, G.; Ramoni, E.; Fattouch, K.; Minacapelli, A.; et al. Effect of Hospital-associated SARS-CoV-2 Infections in Cardiac Surgery: A Multicenter Study. Ann. Thorac. Surg. 2024, 117, 213–219. [Google Scholar] [CrossRef]
- Jagdewsing, D.R.; Fahmy, N.S.C.; Chen, X.; Keuzetien, Y.K.; Silva, F.A.; Kang, H.; Xu, Y.; Al-Sharabi, A.; Jagdewsing, S.A.; Jagdewsing, S.A. Postoperative Surgical Site and Secondary Infections in Colorectal Cancer Patients with a History of SARS-CoV-2: A Retrospective Cohort Study. Cureus 2025, 17, e78077. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.T.; Gulati, S.; Joshi, S.; Huang, A.L.; Laskey, D.; Wolf, A.; Taioli, E.; Flores, R.M. The Impact of COVID-19 Infection Prior to Lung Resection on Postoperative Complications. Ann. Thorac. Surg. Short Rep. 2024, 3, 156–160. [Google Scholar] [CrossRef]
- Sharath, S.E.; Kougias, P.; Daviú-Molinari, T.; Faridmoayer, E.; Berger, D.H. Association Between Coronavirus Disease 2019 Vaccination and Mortality After Major Operations. Ann. Surg. 2024, 279, 58–64. [Google Scholar] [CrossRef] [PubMed]
- El-Boghdadly, K.; Cook, T.; Goodacre, T.; Kua, J.; Denmark, S.; Mercer, N.; Summerton, D.J. Timing of elective surgery and risk assessment after sars-cov-2 infection: 2023 update. Anaesthesia 2023, 78, 1147–1152. [Google Scholar] [CrossRef] [PubMed]
| Study | Country | Year of Publishing | Language of the Study | Study Design | Sample Size (Number of Patients) | Type of Surgery | Time (Weeks) from COVID-19 Infection to Surgery | Risk of Bias Assessment (Percentage, %), Down and Black Checklist |
|---|---|---|---|---|---|---|---|---|
| Gabryel (2022) [11] | Poland | 2022 | English | Retrospective, observational, single center cohort study | 72 | Thoracic surgery | 18.65 | 59.37 |
| Le, S.T. (2022) [12] | United States | 2022 | English | Retrospective, observational, single center cohort study | 9170 | Non—cardiac surgery | >8 | 60.93 |
| Kothari (2021) [13] | United States | 2022 | English | Retrospective, observational, single center cohort study, propensity—matched | 114 | Cancer surgery | 7.42 | 60.93 |
| COVIDSurg Collaborative (2021) [14] | United Kingdom | 2021 | English | Prospective observational, cohort study, international, multicentric | 1202 | Cancer surgery, obstetrics | >7 | 62.5 |
| Ranganathan (2023) [15] | India | 2022 | English | Ambi-directional, observational (retrospective and prospective) study | 138 | Cancer surgery | >7 | 64.06 |
| Diken (2024) [16] | Turkiye | 2024 | English | Retrospective, observational, multicentric cohort study | 28 | Cardiac surgery—Coronary artery bypass | 12 | 51.56 |
| SenthilKumar (2023) [17] | United States | 2023 | English | Retrospective, observational, multicentric cohort study | 22,179 | Elective non cardiac surgery | >8 | 60.93 |
| Cheung (2021) [18] | United States | 2021 | English | Retrospective, observational, single center cohort study | 6 | Bariatric surgery | 11.71 | 48.43 |
| Deng (2022) [4] | United States | 2022 | English | Retrospective, observational, multicentric, cohort study | 1633 | Major non-cardiac surgery | >8 | 62.57 |
| Lazzareschi (2024) [19] | United States | 2023 | English | Retrospective, observational, multicentric, cohort study | 2091 | Major non-cardiac surgery | 8.1 | 73.43 |
| Shen (2024) [20] | China | 2024 | English | Prospective observational multicentric, cohort study | 1316 | Lung cancer surgery | >8 | 82.81 |
| Fang (2024) [21] | China | 2024 | English | Retrospective, observational, multicentric, cohort study | 58 | Elective partial hepatectomy | 11.9 | 71.87 |
| Bansal (2024) [22] | India | 2024 | English | Prospective monocentric observational study | 48 | Neurosurgery | 28 | 57.81 |
| Study | RAS (no pts) | Pneumonia (no pts) | BF (no pts) | Reint. (no pts) | PH (no pts) | P.eff. (no pts) | Ptx (no pts) | Atelect. (no pts) | RF (no pts) | PE (no pts) | Prol. AL (no pts) | Empyema (no pts) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gabryel (2022) [11] | 7 | 1 | 1 | 1 | - | - | - | - | - | - | - | - |
| Le, S.T. (2022) [12] | - | 431 | - | - | - | - | - | - | - | - | - | - |
| Kothari (2021) [13] | - | 5 | - | - | 33 | 4 | - | - | - | - | - | - |
| COVIDSurg Collaborative (2021) [14] | - | 42 | - | - | - | - | - | - | - | - | - | - |
| Ranganathan (2023) [15] | - | 1 | - | - | - | 3 | 1 | - | - | - | - | - |
| Diken (2024) [16] | - | - | - | - | - | 4 | - | 5 | - | - | - | - |
| SenthilKumar (2023) [17] | - | - | - | - | - | - | - | - | - | - | - | - |
| Cheung (2021) [18] | - | - | - | - | - | - | - | - | - | - | - | - |
| Deng (2022) [4] | - | 23 | - | - | - | - | - | - | 45 | 16 | - | - |
| Lazzareschi (2024) [19] | - | - | - | - | - | - | - | - | - | - | - | - |
| Shen (2024) [20] | - | 98 | 1 | - - | - | 78 | 31 | - | - | 2 | 46 | 1 |
| Fang (2024) [21] | - | 3 | - | - | - | - | - | 4 | - | - | - | - |
| Bansal (2024) [22] | - | - | - | - | - | - | - | - | - | - | - | - |
| Study | PMI/MACE (no pts) | PCA (no pts) | M.Is (no pts) | Hy.Cr. (no pts) | AFIB (no pts) | AFL (no pts) | VA (no pts) | Carditis (no pts) | Ac.IHD (no pts) | MI (no pts) | AHF (no pts) | CA (no pts) | CS (no pt) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gabryel (2022) [11] | - | - | - | - | - | - | - | - | - | - | - | - | |
| Le, S.T. (2022) [12] | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Kothari (2021) [13] | 4 | 5 | - | - | - | - | - | - | - | - | - | - | - |
| COVIDSurg Collaborative (2021) [14] | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Ranganathan (2023) [15] | - | - | 2 | 1 | - | - | - | - | - | - | - | - | - |
| Diken (2024) [16] | - | - | - | - | 2 | - | - | - | - | - | - | - | - |
| SenthilKumar (2023) [17] | - | - | - | - | 393 | 226 | 44 | 94 | 287 | 217 | 237 | 163 | 95 |
| Cheung (2021) [18] | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Deng (2022) [4] | 37 | - | - | - | - | - | - | - | - | - | - | - | |
| Lazzareschi (2024) [19] | 56 | - | - | - | - | - | - | - | - | - | - | - | - |
| Shen (2024) [20] | - | - | - | - | 2 | - | - | - | - | - | - | - | - |
| Fang (2024) [21] | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Bansal (2024) [22] | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Study | DVT (no. pts) | PE (no. pts) | SVT (no. pts) | AKI (no. of pts) | Stroke (no. pts) | TCI (no. pts) |
|---|---|---|---|---|---|---|
| Gabryel (2022) [11] | - | - | - | - | - | |
| Le, S.T. (2022) [12] | - | - | - | - | - | - |
| Kothari (2021) [13] | 1 | - | - | - | - | |
| COVIDSurg Collaborative (2021) [14] | - | - | - | - | - | - |
| Ranganathan (2023) [15] | - | - | - | 1 | - | - |
| Diken (2024) [16] | - | - | - | 1 | - | - |
| SenthilKumar (2023) [17] | 492 | 517 | 159 | - | 20 | 99 |
| Cheung (2021) [18] | - | - | - | - | - | - |
| Deng (2022) [4] | 28 | - | - | 48 | - | - |
| Lazzareschi (2024) [19] | 44 | - | - | - | - | - |
| Shen (2024) [20] | - | - | - | - | - | - |
| Fang (2024) [21] | - | - | - | - | - | - |
| Bansal (2024) [22] | - | - | - | - | 8 | - |
| Study | Hospitalization Days (No. of Days) | ICU Days (No. of Days) | Mortality (No.) |
|---|---|---|---|
| Gabryel (2022) [11] | 6 (IQR 4–7) | - | 1 |
| Le, S.T. (2022) [12] | - | - | - |
| Kothari (2021) [13] | 4 | - | 1 |
| COVIDSurg Collaborative (2021) [14] | - | - | 18 |
| Ranganathan (2023) [15] | - | - | 1 |
| Diken (2024) [16] | 5.71 ± 3.5 | 1.71 ± 1.74 | 2 |
| SenthilKumar (2023) [17] | - | - | 584 |
| Cheung (2021) [18] | - | - | 0 |
| Deng (2022) [4] | - | - | - |
| Lazzareschi (2024) [19] | - | - | - |
| Shen (2024) [20] | 5 | 3 | 0 |
| Fang (2024) [21] | 8.5 | - | - |
| Bansal (2024) [22] | 5 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Predoi, C.E.; Dascalu, A.; Goicea, R.; Stefan, M.; Filipescu, D.; Iordache, N. Impact of COVID-19 History on Patients’ Outcome in the Perioperative Period—A Systematic Review. COVID 2025, 5, 148. https://doi.org/10.3390/covid5090148
Predoi CE, Dascalu A, Goicea R, Stefan M, Filipescu D, Iordache N. Impact of COVID-19 History on Patients’ Outcome in the Perioperative Period—A Systematic Review. COVID. 2025; 5(9):148. https://doi.org/10.3390/covid5090148
Chicago/Turabian StylePredoi, Cornelia Elena, Alexandru Dascalu, Raluca Goicea, Mihai Stefan, Daniela Filipescu, and Niculae Iordache. 2025. "Impact of COVID-19 History on Patients’ Outcome in the Perioperative Period—A Systematic Review" COVID 5, no. 9: 148. https://doi.org/10.3390/covid5090148
APA StylePredoi, C. E., Dascalu, A., Goicea, R., Stefan, M., Filipescu, D., & Iordache, N. (2025). Impact of COVID-19 History on Patients’ Outcome in the Perioperative Period—A Systematic Review. COVID, 5(9), 148. https://doi.org/10.3390/covid5090148






