sCD40L-Mediated Platelet Activation and Thromboinflammation During SARS-CoV-2 Infection: Clinical and Experimental Evidence
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection and Clinical Characterization
2.2. In Vitro Platelet Activation and Measurement of sCD40L
2.3. Human Platelet Preparation and Aggregation Assay
2.4. Mice Handling
2.5. Thrombosis Model
2.6. Statistical Analysis
3. Results
3.1. Clinical and Hematological Characteristics
3.2. Platelets Release sCD40L in COVID-19
3.3. Platelet Aggregation in Response to Thrombotic Stimulus
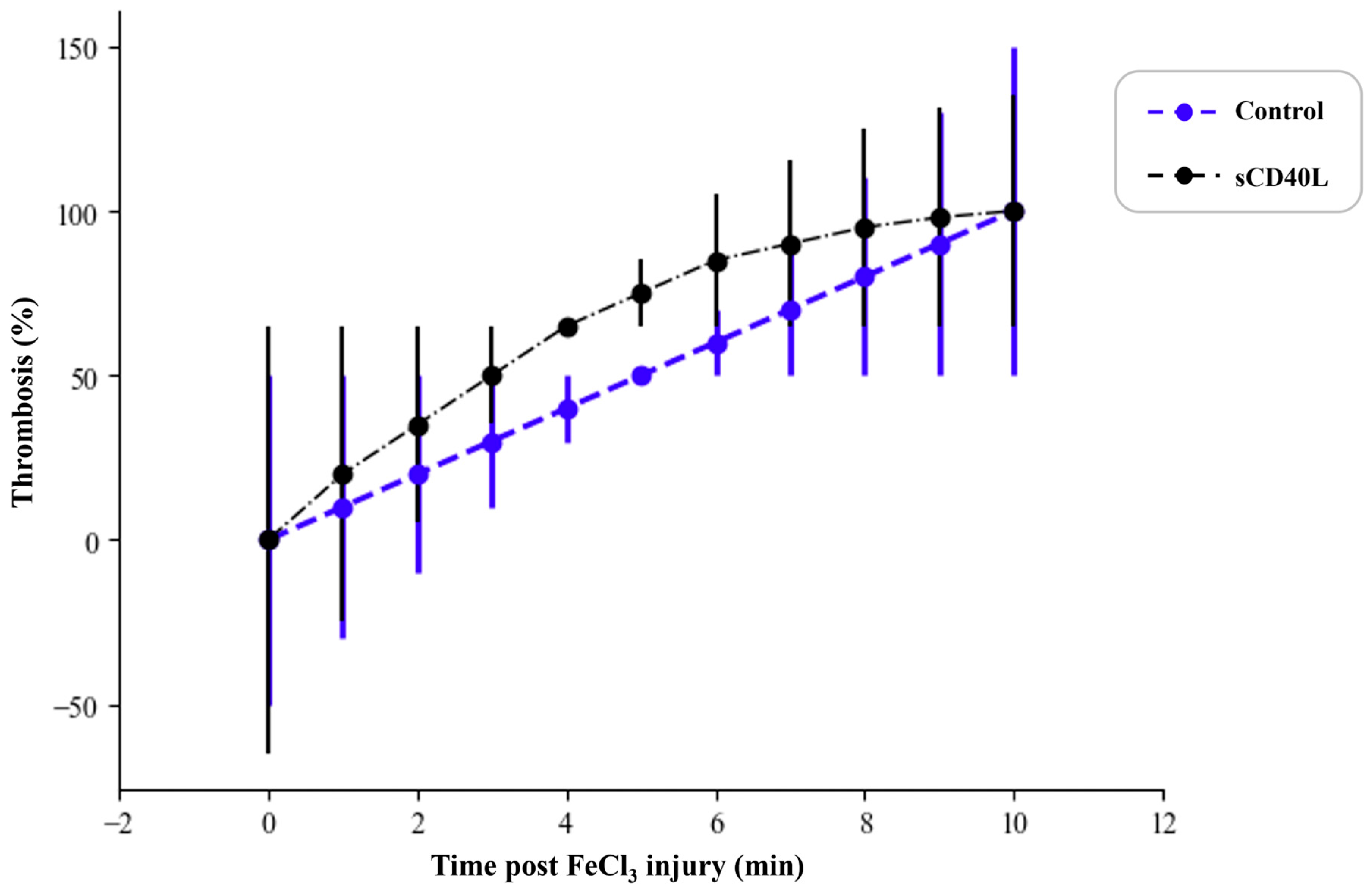
3.4. Effect of sCD40L on Thrombus Formation in a Mouse Thrombosis Model
4. Discussion
4.1. The Dual Function of sCD40L in Inflammation and Thrombosis
4.2. Comparative Analysis with Existing Studies
4.3. Potential Mechanisms Underlying sCD40L-Mediated Thrombosis
4.4. Therapeutic Implications and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J. COVID-19: Consider Cytokine Storm Syndromes and Immunosuppression. Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.-W.; Schmitz, J.E.; Persing, D.H.; Stratton, C.W. Laboratory Diagnosis of COVID-19: Current Issues and Challenges. J. Clin. Microbiol. 2020, 58, e00512-20. [Google Scholar] [CrossRef] [PubMed]
- Mellema, R.A.; Crandell, J.; Petrey, A.C. Platelet Dysregulation in the Pathobiology of COVID-19. Hämostaseologie 2021, 42, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Manne, B.K.; Denorme, F.; Middleton, E.A.; Portier, I.; Rowley, J.W.; Stubben, C.; Petrey, A.C.; Tolley, N.D.; Guo, L.; Cody, M.; et al. Platelet Gene Expression and Function in Patients with COVID-19. Blood 2020, 136, 1317–1329. [Google Scholar] [CrossRef] [PubMed]
- Zaid, Y.; Puhm, F.; Allaeys, I.; Naya, A.; Oudghiri, M.; Khalki, L.; Limami, Y.; Zaid, N.; Sadki, K.; Ben El Haj, R.; et al. Platelets Can Associate with SARS-Cov-2 RNA and Are Hyperactivated in COVID-19. Circ. Res. 2020, 127, 1404–1418. [Google Scholar] [CrossRef] [PubMed]
- Zuily, S.; Dufrost, V.; Wahl, D. Thrombose Artérielle et Veineuse Au Cours Du COVID-19. Arch. Mal. Coeur Vaiss. Prat. 2020, 2020, 16–19. [Google Scholar] [CrossRef] [PubMed]
- Mach, F.; Schönbeck, U.; Libby, P. CD40 Signaling in Vascular Cells: A Key Role in Atherosclerosis? Atherosclerosis 1998, 137, S89–S95. [Google Scholar] [CrossRef] [PubMed]
- Cognasse, F.; Duchez, A.C.; Audoux, E.; Ebermeyer, T.; Arthaud, C.A.; Prier, A.; Eyraud, M.A.; Mismetti, P.; Garraud, O.; Bertoletti, L.; et al. Platelets as Key Factors in Inflammation: Focus on CD40L/CD40. Front. Immunol. 2022, 13, 825892. [Google Scholar] [CrossRef] [PubMed]
- Fard, M.B.; Fard, S.B.; Ramazi, S.; Atashi, A.; Eslamifar, Z. Thrombosis in COVID-19 Infection: Role of Platelet Activation-Mediated Immunity. Thromb. J. 2021, 19, 59. [Google Scholar] [CrossRef] [PubMed]
- Bendas, G.; Gobec, M.; Schlesinger, M. Modulating Immune Responses: The Double-Edged Sword of Platelet CD40L. Semin. Thromb. Hemost. 2024, s-0044-1791512. [Google Scholar] [CrossRef]
- Lippi, G.; Mattiuzzi, C.; Bovo, C.; Plebani, M. Current Laboratory Diagnostics of Coronavirus Disease 2019 (COVID-19). Acta Bio Med. 2020, 91, 137. [Google Scholar]
- American Thoracic Society; Infectious Diseases Society of America. Guidelines for the Management of Adults with Hospital-Acquired, Ventilator-Associated, and Healthcare-Associated Pneumonia. Am. J. Respir. Crit. Care Med. 2005, 171, 388–416. [Google Scholar] [CrossRef] [PubMed]
- McFadyen, J.D.; Stevens, H.; Peter, K. The Emerging Threat of (Micro)Thrombosis in COVID-19 and Its Therapeutic Implications. Circ. Res. 2020, 127, 571–587. [Google Scholar] [CrossRef] [PubMed]
- Publications Office of the European Union, Luxembourg. European Parliament & Council of the European Union. Directive 2010/63/EU of 22 September 2010 on the Protection of Animals Used for Scientific Purposes. Off. J. Eur. Union 2010, 276, 33–79. [Google Scholar]
- Yacoub, D.; Hachem, A.; Théorêt, J.-F.; Gillis, M.-A.; Mourad, W.; Merhi, Y. Enhanced Levels of Soluble CD40 Ligand Exacerbate Platelet Aggregation and Thrombus Formation through a CD40-Dependent Tumor Necrosis Factor Receptor-Associated Factor-2/Rac1/P38 Mitogen-Activated Protein Kinase Signaling Pathway. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2424–2433. [Google Scholar] [CrossRef] [PubMed]
- Cipollone, F.; Mezzetti, A.; Porreca, E.; Di Febbo, C.; Nutini, M.; Fazia, M.; Falco, A.; Cuccurullo, F.; Davì, G. Association Between Enhanced Soluble CD40L and Prothrombotic State in Hypercholesterolemia: Effects of Statin Therapy. Circulation 2002, 106, 399–402. [Google Scholar] [CrossRef] [PubMed]
- Mach, F.; Schönbeck, U.; Sukhova, G.K.; Atkinson, E.; Libby, P. Reduction of Atherosclerosis in Mice by Inhibition of CD40 Signalling. Nature 1998, 394, 200–203. [Google Scholar] [CrossRef] [PubMed]
- Schönbeck, U.; Libby, P. The CD40/CD154 Receptor/Ligand Dyad. Cell. Mol. Life Sci. 2001, 58, 4–43. [Google Scholar] [CrossRef] [PubMed]
- Philippe, A.; Chocron, R.; Bonnet, G.; Yatim, N.; Sutter, W.; Hadjadj, J.; Weizman, O.; Guerin, C.L.; Mirault, T.; Fauvel, C.; et al. Platelet Activation and Coronavirus Disease 2019 Mortality: Insights from Coagulopathy, Antiplatelet Therapy and Inflammation. Arch. Cardiovasc. Dis. 2023, 116, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Zaid, Y.; Guessous, F.; Puhm, F.; Elhamdani, W.; Chentoufi, L.; Morris, A.C.; Cheikh, A.; Jalali, F.; Boilard, E.; Flamand, L. Platelet Reactivity to Thrombin Differs between Patients with COVID-19 and Those with ARDS Unrelated to COVID-19. Blood Adv. 2021, 5, 635–639. [Google Scholar] [CrossRef] [PubMed]
- Allaoui, A.; Khawaja, A.A.; Badad, O.; Naciri, M.; Lordkipanidzé, M.; Guessous, F.; Zaid, Y. Platelet Function in Viral Immunity and SARS-CoV-2 Infection. Semin. Thromb. Hemost. 2021, 47, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Sciaudone, A.; Corkrey, H.; Humphries, F.; Koupenova, M. Platelets and SARS-CoV-2 During COVID-19: Immunity, Thrombosis, and Beyond. Circ. Res. 2023, 132, 1272–1289. [Google Scholar] [CrossRef] [PubMed]
- Hachem, A.; Yacoub, D.; Zaid, Y.; Mourad, W.; Merhi, Y. Involvement of Nuclear Factor κB in Platelet CD40 Signaling. Biochem. Biophys. Res. Commun. 2012, 425, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. TLR Signaling. Semin. Immunol. 2007, 19, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Banchereau, J.; Bazan, F.; Blanchard, D.; Brière, F.; Galizzi, J.P.; van Kooten, C.; Liu, Y.J.; Rousset, F.; Saeland, S. The CD40 Antigen and Its Ligand. Annu. Rev. Immunol. 1994, 12, 881–922. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Goerlich, C.E.; Zhang, T.; Lewis, B.G.T.; Hershfeld, A.; Mohiuddin, M.M. CD40-CD40L Blockade: Update on Novel Investigational Therapeutics for Transplantation. Transplantation 2023, 107, 1472–1481. [Google Scholar] [CrossRef] [PubMed]
- Inwald, D.P.; McDowall, A.; Peters, M.J.; Callard, R.E.; Klein, N.J. CD40 Is Constitutively Expressed on Platelets and Provides a Novel Mechanism for Platelet Activation. Circ. Res. 2003, 92, 1041–1048. [Google Scholar] [CrossRef] [PubMed]
- Lievens, D.; Zernecke, A.; Seijkens, T.; Soehnlein, O.; Beckers, L.; Munnix, I.C.A.; Wijnands, E.; Goossens, P.; van Kruchten, R.; Thevissen, L.; et al. Platelet CD40L Mediates Thrombotic and Inflammatory Processes in Atherosclerosis. Blood 2010, 116, 4317. [Google Scholar] [CrossRef] [PubMed]
- Garlichs, C.D.; John, S.; Schmeisser, A.; Eskafi, S.; Stumpf, C.; Karl, M.; Goppelt-Struebe, M.; Schmieder, R.; Daniel, W.G. Upregulation of CD40 and CD40 Ligand (CD154) in Patients with Moderate Hypercholesterolemia. Circulation 2001, 104, 2395–2400. [Google Scholar] [CrossRef] [PubMed]
- Henn, V.; Slupsky, J.R.; Gräfe, M.; Anagnostopoulos, I.; Förster, R.; Müller-Berghaus, G.; Kroczek, R.A. CD40 Ligand on Activated Platelets Triggers an Inflammatory Reaction of Endothelial Cells. Nature 1998, 391, 591–594. [Google Scholar] [CrossRef] [PubMed]
- Schönbeck, U.; Sukhova, G.K.; Shimizu, K.; Mach, F.; Libby, P. Inhibition of CD40 Signaling Limits Evolution of Established Atherosclerosis in Mice. Proc. Natl. Acad. Sci. USA 2000, 97, 7458–7463. [Google Scholar] [CrossRef] [PubMed]
- Pamukcu, B.; Lip, G.Y.H.; Snezhitskiy, V.; Shantsila, E. The CD40-CD40L System in Cardiovascular Disease. Ann. Med. 2011, 43, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Zaid, Y.; Lahlimi, Q.; Khalki, L.; Zaid, N.; Oudghiri, M.; Cheikh, A.; Naya, A.; Merhi, Y.; Guessous, F. Aspirin Use Reduces Platelet Hyperreactivity and Degranulation in COVID-19 Patients. Semin. Thromb. Hemost. 2023, 49, 92–96. [Google Scholar] [CrossRef] [PubMed]



| Index No. (Median [IQR]) | Healthy Controls n = 100 | Patients with COVID-19 | p Value | |
|---|---|---|---|---|
| Severe n = 58 | Non-Severe n = 102 | |||
| Female/male | 50/50 | 28/30 | 53/49 | -- |
| Age (n), y | 52.3 ± 25.9 | 59 ± 24.68 | 56 ± 18.91 | 0.1095 |
| Weight (n), kg | 80.7 ± 20.0 | 76.85 ± 20.34 | 82.23 ± 16.73 | 0.1924 |
| Platelet value at admission (n), ×106/mL | 253.7 ± 121.5 | 187.37 ± 85.7 | 239.16 ± 44.22 | <0.0001 |
| ALT value at admission (n), U/L | 25.5 ± 16.3 | 35.26 ± 13.59 | 34.05 ± 10.22 | 0.0223 |
| AST value at admission (n), U/L | 28.7 ± 19.6 | 38.18 ± 12.61 | 36.86 ± 14.53 | 0.0003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allaoui, A.; Atifi, F.; Mabrouk, M.; Ourradi, Z.; Chami, A.; Labied, S.; Ammara, M.; Naya, A.; Zaid, Y. sCD40L-Mediated Platelet Activation and Thromboinflammation During SARS-CoV-2 Infection: Clinical and Experimental Evidence. COVID 2025, 5, 112. https://doi.org/10.3390/covid5080112
Allaoui A, Atifi F, Mabrouk M, Ourradi Z, Chami A, Labied S, Ammara M, Naya A, Zaid Y. sCD40L-Mediated Platelet Activation and Thromboinflammation During SARS-CoV-2 Infection: Clinical and Experimental Evidence. COVID. 2025; 5(8):112. https://doi.org/10.3390/covid5080112
Chicago/Turabian StyleAllaoui, Afaf, Farah Atifi, Meryem Mabrouk, Zineb Ourradi, Abir Chami, Salma Labied, Mounia Ammara, Abdallah Naya, and Younes Zaid. 2025. "sCD40L-Mediated Platelet Activation and Thromboinflammation During SARS-CoV-2 Infection: Clinical and Experimental Evidence" COVID 5, no. 8: 112. https://doi.org/10.3390/covid5080112
APA StyleAllaoui, A., Atifi, F., Mabrouk, M., Ourradi, Z., Chami, A., Labied, S., Ammara, M., Naya, A., & Zaid, Y. (2025). sCD40L-Mediated Platelet Activation and Thromboinflammation During SARS-CoV-2 Infection: Clinical and Experimental Evidence. COVID, 5(8), 112. https://doi.org/10.3390/covid5080112






