Elevated Unfractionated Heparin Requirement in COVID-19 ICU Patients: Exploring Influencing Factors
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Measurements
2.3. Treatment Procedures
2.4. Statistical Analysis
3. Results
3.1. Measurements
3.2. Measurements Within Therapeutic Range of APTT 60–80
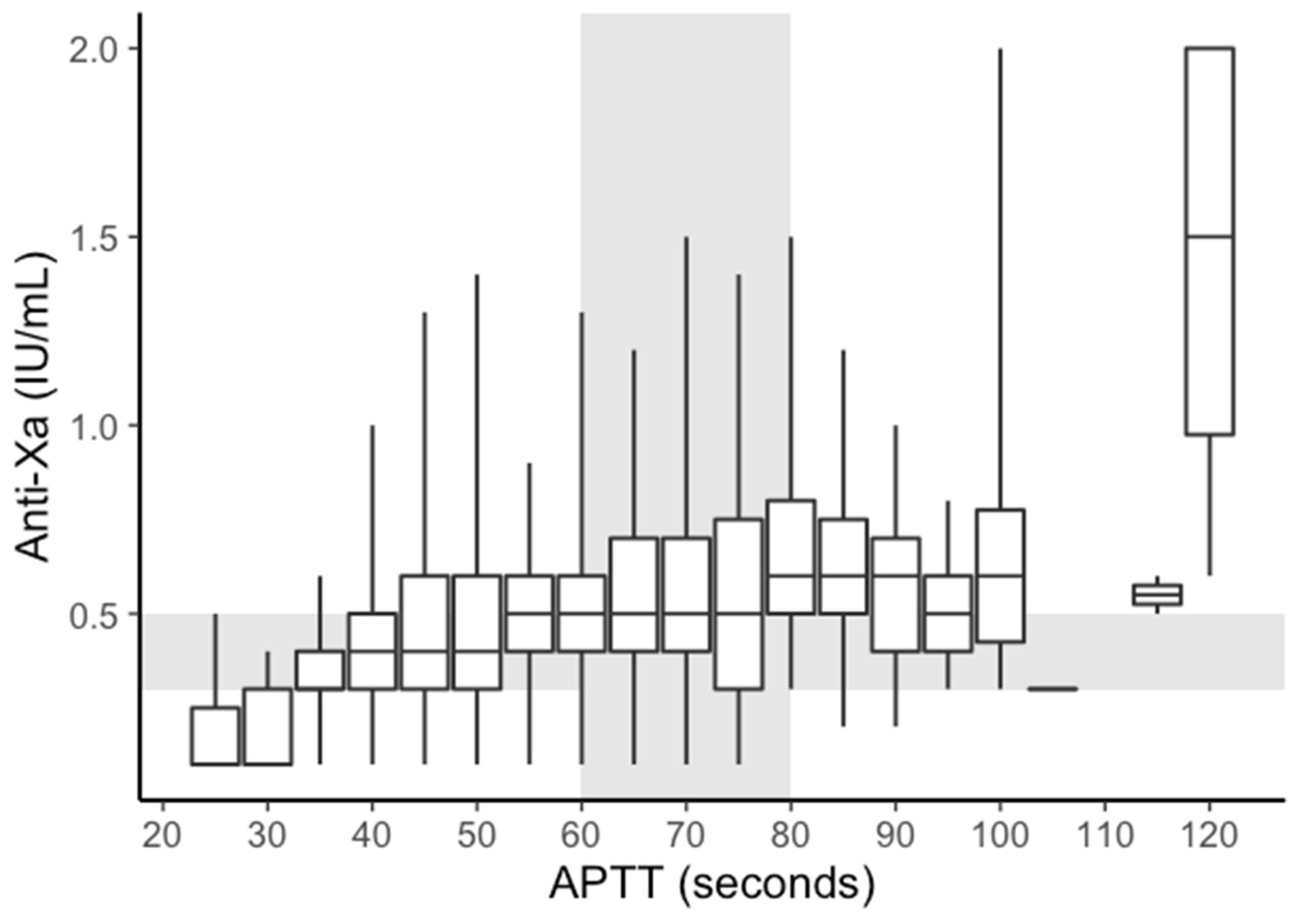
3.3. APTT vs. Anti-Xa
3.4. Association APTT and Various Clinical Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| APTT | Activated Partial Thromboplastin Time |
| AT | Antithrombin |
| BMI | Body Mass Index |
| COPD | Chronic Obstructive Pulmonary Disease |
| CRP | C-Reactive Protein |
| DIC | Disseminated Intravascular Coagulation |
| ICU | Intensive Care Unit |
| IQR | Interquartile Range |
| LUMC | Leiden University Medical Center |
| NICE | National Intensive Care Evaluation |
| NYHA | New York Heart Association |
| PE | Pulmonary Embolism |
| REML | Restricted Maximum Likelihood |
| SAPS | Simplified Acute Physiology Score |
| UFH | Unfractionated Heparin |
Appendix A
| Loading dose 70 IU/Kg (max 5000 IU) Unless: Alteplase (Actilyse) or ECMO/HVAD Starting dose 300 IU/Kg/24 h (max: 30.000 IU/24 h) Target APTT 1,5-3 × prolonged (60–80 s) APTT check every 8 h. (Consult an ICU physician for doses <20.000 of >50.000 IU/24 h) | ||||
| ▼ | ||||
| APTT < 35 s | APTT 35–59 s | APTT 60–81 s | APTT 81–100 s | APTT > 100 s |
| ▼ | ▼ | ▼ | ▼ | |
| Consider bolus consult ICU physician 70 IU/Kg (max 5000 IU) | Consider bolus consult ICU physician 35 IU/Kg (max 2500 IU) | Stop infusion 1 h | ||
| ▼ | ▼ | ▼ | ▼ | |
| Increase Dose 100 IU/Kg/24 h | Increase Dose 75 IU/Kg/24 h | Decrease Dose 75 IU/Kg/24 h | Decrease Dose 100 IU/Kg/24 h | |
| Consider Previous Adjustments When Deciding to Change the Pump Settings Consult ICU Physician for Doses > 50,000 IU/24 h | ||||
|---|---|---|---|---|
| Weight | +100 IU/kg/24 h | +75 IU/kg/24 h | −75 IU/kg/24 h | −100 IU/kg/24 h |
| 40 | +0.4 mL | +0.3 mL | −0.3 mL | −0.4 mL |
| 45 | +0.5 mL | +0.3 mL | −0.3 mL | −0.5 mL |
| 50 | +0.5 mL | +0.4 mL | −0.4 mL | −0.5 mL |
| 55 | +0.6 mL | +0.4 mL | −0.4 mL | −0.6 mL |
| 60 | +0.6 mL | +0.5 mL | −0.5 mL | −0.6 mL |
| 65 | +0.7 mL | +0.5 mL | −0.5 mL | −0.7 mL |
| 70 | +0.7 mL | +0.5 mL | −0.5 mL | −0.7 mL |
| 75 | +0.8 mL | +0.6 mL | −0.6 mL | −0.8 mL |
| 80 | +0.8 mL | +0.6 mL | −0.6 mL | −0.8 mL |
| 85 | +0.9 mL | +0.6 mL | −0.6 mL | −0.9 mL |
| 90 | +0.9 mL | +0.7 mL | −0.7 mL | −0.9 mL |
| 95 | +1.0 mL | +0.7 mL | −0.7 mL | −1.0 mL |
| 100 | +1.0 mL | +0.8 mL | −0.8 mL | −1.0 mL |
| 105 | +1.1 mL | +0.8 mL | −0.8 mL | −1.1 mL |
| 110 | +1.1 mL | +0.8 mL | −0.8 mL | −1.1 mL |
| 115 | +1.2 mL | +0.9 mL | −0.9 mL | −1.2 mL |
| 120 | +1.2 mL | +0.9 mL | −0.9 mL | −1.2 mL |
Appendix B
| APTT *** | Anti-Xa | UFH Dose (Pump Setting) ** |
|---|---|---|
| 60–80 s | 0.3–0.5 IU/mL | Do not change |
| 60–80 s | <0.3 IU/mL | Increase |
| 60–80 s | >0.5 IU/mL * | Decrease |
| <60 s | 0.3–0.5 IU/mL | Increase |
| <60 s | <0.3 IU/mL | Increase |
| <60 s | >0.5 IU/mL | Consultation vascular medicine |
| >80 s | 0.3–0.5 IU/mL | Decrease |
| >80 s | >0.5 IU/mL | Decrease |
| >80 s | <0.3 IU/mL | Consultation vascular medicine |
Appendix C
- aptt = APTT.
- hep = unfractionated heparin.
- crp = C-reactive protein.
- AT = antithrombin.
- Admno = number identifying each individual patient.
| Min | 1Q | Median | 3Q | Max |
| −1.9418 | −0.7953 | −0.1251 | 0.7391 | 2.5093 |
| Groups | Name | Variance | Std. Dev. |
| Admno | (Intercept) | 2.429 | 1.559 |
| Residual | 26.820 | 5.179 | |
| Number of obs: 669, groups: admno, 97 | |||
| Estimate | Std. Error | df | p Value | pr (>|t|) | |
| (Intercept) | 67.260990 | 2.624192 | 97.330919 | 25.631 | <2 × 10−16 *** |
| hep | 0.004203 | 0.001774 | 153.697666 | 2.370 | 0.019 * |
| crp | 0.003248 | 0.002186 | 456.552019 | 1.486 | 0.138 |
| BMI | −0.005614 | 0.075087 | 80.087474 | −0.075 | 0.941 |
| AT | −0.013683 | 0.012709 | 526.896915 | −1.077 | 0.282 |
| Signif. codes: 0 ‘***’; 0.001 ‘**’; 0.01 ‘*’; 0.05 ‘.’; 0.1 ‘ ’ 1. |
| (Intr) | hep | crp | BMI | |
| hep | −0.514 | |||
| crp | −0.154 | −0.213 | ||
| BMI | −0.815 | 0.199 | 0.058 | |
| AT | −0.357 | 0.154 | 0.174 | −0.160 |
References
- WHO. Coronavirus (COVID-19) Dashboard. 2025. Available online: https://covid19.who.int/ (accessed on 24 March 2025).
- Klok, F.A.; Kruip, M.J.H.A.; van der Meer, N.J.M.; Arbous, M.S.; Gommers, D.A.M.P.J.; Kant, K.M.; Kaptein, F.H.J.; van Paassen, J.; Stals, M.A.M.; Huisman, M.V.; et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020, 191, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Connors, J.M.; Levy, J.H. COVID-19 and its implications for thrombosis and anticoagulation. Blood 2020, 135, 2033–2040. [Google Scholar] [PubMed]
- van Dam, L.; Kroft, L.; van der Wal, L.; Cannegieter, S.; Eikenboom, J.; de Jonge, E.; Huisman, M.; Klok, F. Clinical and computed tomography characteristics of COVID-19 associated acute pulmonary embolism: A different phenotype of thrombotic disease? Thromb. Res. 2020, 193, 86–89. [Google Scholar] [CrossRef] [PubMed]
- van der Wal, L.; Kroft, L.; van Dam, L.; Cobbaert, C.; Eikenboom, J.; Huisman, M.; Helmerhorst, H.; Klok, F.; de Jonge, E. Early effects of unfractionated heparin on clinical and radiological signs and D-dimer levels in patients with COVID-19 associated pulmonary embolism: An observational cohort study. Thromb. Res. 2021, 200, 130–132. [Google Scholar] [CrossRef]
- White, D.; MacDonald, S.; Bull, T.; Hayman, M.; de Monteverde-Robb, R.; Sapsford, D.; Lavinio, A.; Varley, J.; Johnston, A.; Besser, M.; et al. Heparin resistance in COVID-19 patients in the intensive care unit. J. Thromb. Thrombolysis 2020, 50, 287–291. [Google Scholar] [CrossRef]
- Vandiver, J.W.; Vondracek, T.G. Antifactor Xa levels versus activated partial thromboplastin time for monitoring unfractionated heparin. Pharmacotherapy 2012, 32, 546–558. [Google Scholar] [CrossRef]
- Adie, S.K.; Farina, N. Impact of COVID-19 on monitoring of therapeutic unfractionated heparin. J. Thromb. Thrombolysis 2021, 51, 827–829. [Google Scholar] [CrossRef]
- Downie, I.; Liederman, Z.; Thiyagarajah, K.; Selby, R.; Lin, Y. Pseudo heparin resistance caused by elevated factor VIII in a critically ill patient. Can. J. Anaesth. 2019, 66, 995–996. [Google Scholar] [CrossRef]
- Artim-Esen, B.; Pericleous, C.; Mackie, I.; Ripoll, V.M.; Latchman, D.; Isenberg, D.; Rahman, A.; Ioannou, Y.; Giles, I. Anti-factor Xa antibodies in patients with antiphospholipid syndrome and their effects upon coagulation assays. Arthritis Res. Ther. 2015, 17, 47. [Google Scholar]
- Devreese, K.M.J.; Verfaillie, C.J.; De Bisschop, F.; Delanghe, J.R. Interference of C-reactive protein with clotting times. Clin. Chem. Lab. Med. 2015, 53, e141–e145. [Google Scholar] [CrossRef]
- Tang, N.; Li, D.; Wang, X.; Sun, Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020, 18, 844–847. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, M.; Zhang, S.; Xia, P.; Cao, W.; Jiang, W.; Chen, H.; Ding, X.; Zhao, H.; Zhang, H.; et al. Coagulopathy and Antiphospholipid Antibodies in Patients with COVID-19. N. Engl. J. Med. 2020, 382, e38. [Google Scholar] [CrossRef]
- Streng, A.S.; Delnoij, T.S.; Mulder, M.M.; Sels, J.W.E.; Wetzels, R.J.; Verhezen, P.W.; Olie, R.H.; Kooman, J.P.; van Kuijk, S.M.; Brandts, L.; et al. Monitoring of Unfractionated Heparin in Severe COVID-19: An Observational Study of Patients on CRRT and ECMO. TH Open 2020, 4, e365–e375. [Google Scholar] [CrossRef]
- Chau, T.; Joseph, M.; Solomon, D.M.; Lee, B.; Igneri, L.A. Heparin Resistance in SARS-CoV-2 Infected Patients with Venous Thromboembolism. Hosp. Pharm. 2022, 57, 737–743. [Google Scholar] [CrossRef]
- Reis, S.; Faske, A.; Monsef, I.; Langer, F.; Müller, O.J.; Kranke, P.; Meybohm, P.; Weibel, S. Anticoagulation in COVID-19 patients-An updated systematic review and meta-analysis. Thromb. Res. 2024, 238, 141–150. [Google Scholar] [CrossRef]
- Arts, D.; de Keizer, N.; Scheffer, G.J.; de Jonge, E. Quality of data collected for severity of illness scores in the Dutch National Intensive Care Evaluation (NICE) registry. Intensive Care Med. 2002, 28, 656–659. [Google Scholar] [CrossRef]
- Bates, D.M.M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Lüdecke, D.; Ben-Shachar, M.S.; Patil, I.; Waggoner, P.; Makowski, D. Performance: An R Package for Assessment, Comparison and Testing of Statistical Models. J. Open Source Softw. 2021, 6, 3139. [Google Scholar] [CrossRef]
- Novelli, C.; Borotto, E.; Beverina, I.; Punzi, V.; Radrizzani, D.; Brando, B. Heparin dosage, level, and resistance in SARS-CoV2 infected patients in intensive care unit. Int. J. Lab. Hematol. 2021, 43, 1284–1290. [Google Scholar] [CrossRef] [PubMed]
- Kostousov, V.; Devaraj, S.; Bruzdoski, K.; Hensch, L.; Hui, S.K.; Teruya, J. C-reactive protein-induced activated partial thromboplastin time prolongation in heparinized samples is attenuated by elevated factor VIII. Int. J. Lab. Hematol. 2021, 43, 139–142. [Google Scholar] [CrossRef]
- Iba, T.; Levy, J.H.; Connors, J.M.; Warkentin, T.E.; Thachil, J.; Levi, M. The unique characteristics of COVID-19 coagulopathy. Critical Care 2020, 24, 360. [Google Scholar] [CrossRef] [PubMed]
- Escher, R.; Breakey, N.; Lammle, B. Severe COVID-19 infection associated with endothelial activation. Thromb. Res. 2020, 190, 62. [Google Scholar] [CrossRef] [PubMed]
- Rostami, M.; Mansouritorghabeh, H.; Parsa-Kondelaji, M. High levels of Von Willebrand factor markers in COVID-19: A systematic review and meta-analysis. Clin. Exp. Med. 2022, 22, 347–357. [Google Scholar] [CrossRef]
- Susen, S.; Tacquard, C.A.; Godon, A.; Mansour, A.; Garrigue, D.; Nguyen, P.; Godier, A.; Testa, S.; Levy, J.H.; Albaladejo, P.; et al. Prevention of thrombotic risk in hospitalized patients with COVID-19 and hemostasis monitoring. Crit. Care 2020, 24, 364. [Google Scholar] [CrossRef]
- Hofmaenner, D.A.; Furfaro, D.; Wild, L.C.; Wendel-Garcia, P.D.; Kassis, E.B.; Pannu, A.; Welte, T.; Erlebach, R.; Stahl, K.; Grandin, E.W.; et al. Reduced anticoagulation strategy is associated with a lower incidence of intracerebral hemorrhage in COVID-19 patients on extracorporeal membrane oxygenation. Intensive Care Med. Exp. 2023, 11, 38. [Google Scholar] [CrossRef]
- Levine, M.N.; Hirsh, J.; Gent, M.; Turpie, A.G.; Cruickshank, M.; Weitz, J.; Anderson, D.; Johnson, M. A randomized trial comparing activated thromboplastin time with heparin assay in patients with acute venous thromboembolism requiring large daily doses of heparin. Arch. Intern. Med. 1994, 154, 49–56. [Google Scholar] [CrossRef]
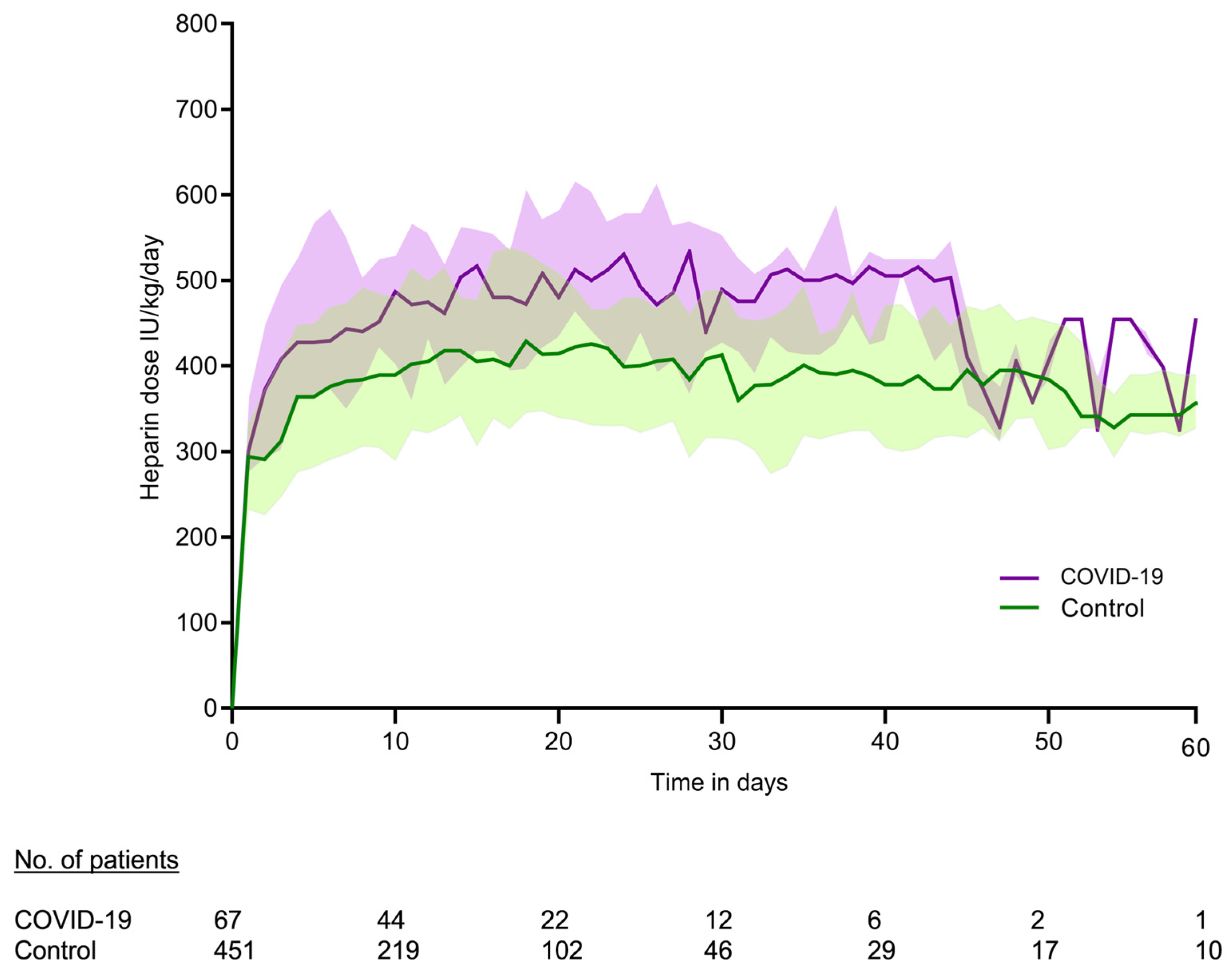

| Variables | COVID-19 Patients (N = 162) | Control Patients (N = 1006) | p-Value |
|---|---|---|---|
| Age (mean (SD)) | 64 (10) | 62 (14) | 0.022 |
| BMI (mean (SD)) | 29 (4) | 27 (6) | <0.001 |
| Sex = Female (%) | 33 (20) | 342 (34) | 0.001 |
| ICU length of stay (days) (median (IQR)) | 15 (10, 29) | 8 (3, 19) | <0.001 |
| Hospital length of stay (days) (median (IQR)) * | 21 (13, 34) | 23 (10, 44) | 0.619 |
| Duration of UFH therapy (days) (median (IQR)) | 9 (5, 18) | 5 (2, 11) | <0.001 |
| ICU mortality (%) * | 58 (36) | 254 (25) | 0.011 |
| Hospital mortality (%) * | 62 (38) | 320 (32) | 0.183 |
| SAPS II score (median (IQR)) * | 45 (35, 59) | 43 (33, 55) | 0.049 |
| Type of admission *, No. (%) | <0.001 | ||
| Medical | 160 (99) | 559 (57) | |
| Emergency surgery | 1 (1) | 141 (14) | |
| Elective surgery | 1 (1) | 282 (29) | |
| Acute diagnosis *‡, No. (%) | <0.001 | ||
| Cardiac (including cardiac surgery) | 3 (2) | 496 (51) | |
| Sepsis | 0 (0) | 53 (5) | |
| Gastrointestinal | 0 (0) | 94 (10) | |
| Pneumonia | 157 (97) | 77 (8) | |
| Respiratory (other) | 2 (2) | 124 (12) | |
| Neurologic | 0 (0) | 24 (2) | |
| Trauma | 0 (0) | 10 (1) | |
| Transplant | 0 (0) | 52 (5) | |
| Other | 0 (0) | 52 (5) | |
| Chronic diagnosis *§, No. (%) | |||
| Chronic kidney failure | 7 (4) | 138 (14) | 0.001 |
| Chronic dialysis | 0 (0) | 32 (3) | 0.038 |
| Metastasized neoplasm | 2 (1) | 25 (3) | 0.460 |
| COPD (drug dependent) | 8 (5) | 68 (7) | 0.441 |
| Chronic respiratory insufficiency | 6 (4) | 27 (3) | 0.675 |
| Cardiovascular insufficiency (NYHA IV) | 2 (1) | 79 (8) | 0.003 |
| Liver cirrhosis | 1 (1) | 43 (4) | 0.037 |
| Diabetes | 32 (20) | 210 (21) | 0.713 |
| Haematological malignancy | 0 (0) | 24 (2) | 0.086 |
| Immunological insufficiency | 1 (1) | 52 (5) | 0.015 |
| Vasoactive drugs at ICU admission | 128 (79) | 779 (79) | 1 |
| Categories | Total | Anti-Xa < 0.3 IU/mL | Anti-Xa 0.3–0.5 IU/mL | Anti-Xa > 0.5 IU/mL |
|---|---|---|---|---|
| APTT < 60 | 2392 (100%) | 556 (23%) | 1066 (45%) | 770 (32%) |
| APTT 60–80 | 1471 (100%) | 190 (13%) | 525 (36%) | 756 (51%) |
| APTT > 80 | 304 (100%) | 23 (8%) | 91 (30%) | 190 (63%) |
| Total | 4167 | 769 | 2785 | 613 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wal, L.I.v.d.; Eikenboom, H.C.J.; Bosma, M.; Klok, F.A.; de Jonge, E. Elevated Unfractionated Heparin Requirement in COVID-19 ICU Patients: Exploring Influencing Factors. COVID 2025, 5, 51. https://doi.org/10.3390/covid5040051
Wal LIvd, Eikenboom HCJ, Bosma M, Klok FA, de Jonge E. Elevated Unfractionated Heparin Requirement in COVID-19 ICU Patients: Exploring Influencing Factors. COVID. 2025; 5(4):51. https://doi.org/10.3390/covid5040051
Chicago/Turabian StyleWal, L. I. van der, H. C. J. Eikenboom, M. Bosma, F. A. Klok, and E. de Jonge. 2025. "Elevated Unfractionated Heparin Requirement in COVID-19 ICU Patients: Exploring Influencing Factors" COVID 5, no. 4: 51. https://doi.org/10.3390/covid5040051
APA StyleWal, L. I. v. d., Eikenboom, H. C. J., Bosma, M., Klok, F. A., & de Jonge, E. (2025). Elevated Unfractionated Heparin Requirement in COVID-19 ICU Patients: Exploring Influencing Factors. COVID, 5(4), 51. https://doi.org/10.3390/covid5040051






