Abstract
Rheumatic diseases affect the musculoskeletal system and may also compromise internal organs. The immunosuppressive treatments commonly used in these conditions can weaken the immune system, raising concerns about the efficacy of immunizations, particularly against SARS-CoV-2. This observational and analytical study evaluated the humoral immune response in patients with rheumatic diseases who were vaccinated with a fourth dose of the SARS-CoV-2 vaccine in comparison with a control group and the effect prior COVID-19 infection. The results showed that individuals with a history of COVID-19 infection developed significantly higher levels of neutralizing antibody titers, indicating a stronger immune response. Moreover, the response on the Wuhan strain elicited a more robust humoral response than the Omicron strain in both immunocompromised patients and healthy controls. These findings highlight the impact of previous viral exposure on the effectiveness of immunization in rheumatology patients.
1. Introduction
Since its emergence, the SARS-CoV-2 pandemic has posed an unprecedented global health crisis. By January 2021, more than 778 million people had been infected and over 7 million had died worldwide due to COVID-19 [1] Chile, like many other countries, has endured severe consequences from the pandemic, including the overburdening of its healthcare system and considerable impacts on public and mental health. Vaccination quickly became a cornerstone strategy in mitigating the pandemic’s toll, with extensive evidence demonstrating that COVID-19 vaccines are highly effective in preventing severe disease, hospitalization, and death [2].
However, during the initial phases of global vaccine deployment, uncertainty surrounded the safety and efficacy of COVID-19 vaccines in immunocompromised populations. Among these, patients with autoimmune rheumatic diseases (ARDs), including rheumatoid arthritis (RA), represent a particularly vulnerable group. These patients typically receive long-term immunosuppressive treatments, which not only increase susceptibility to infections but may also attenuate the immune response to vaccinations [3,4,5].
While early research suggested that patients with RA were not at significantly greater risk of severe COVID-19 compared to the general population [6,7,8], more recent and comprehensive studies have revealed that individuals with systemic autoimmune or immune-mediated diseases are indeed at higher risk of adverse outcomes, including hospitalization and mortality [4,5]. The degree of this risk appears closely linked to the specific immunosuppressive therapy in use. Of particular concern is rituximab (RTX), a B-cell-depleting monoclonal antibody widely prescribed for RA and other autoimmune conditions, which has been associated with a significantly impaired humoral response to both SARS-CoV-2 infection and vaccination, as well as increased severity of COVID-19 illness [3,8].
In this context, the present study aims to evaluate the humoral immune response to SARS-CoV-2 vaccines in patients with rheumatic diseases treated at the Hospital Regional de Copiapó. It specifically examines the generation of neutralizing antibody titers in individuals vaccinated with a fourth dose of the SARS-CoV-2 vaccine and assesses the impact of prior SARS-CoV-2 infection. The NAbs titer against SARS-CoV-2 was determined for the original Wuhan strain and Omicron variant. Additionally, the study compares responses between immunosuppressed patients and healthy controls to better understand how immunomodulatory therapies influence vaccine-induced immunity.
2. Materials and Methods
2.1. Study Design and Setting
This study is a quantitative, observational, analytical, cohort, and cross-sectional investigation conducted at the Rheumatology Unit of the Regional Hospital of Copiapó, located in the Atacama Region of northern Chile. The study period spanned from March to July 2022. The primary objective was to evaluate the effect of prior SARS-CoV-2 infection and COVID-19 vaccination on the humoral immune response in patients diagnosed with rheumatic diseases.
2.2. Study Population
The data analyzed in this study were derived from the project “4th Booster-Dose SARS-CoV-2 Heterologous and Homologous Vaccination in Rheumatologic Patients”, [9] which included a total of 341 participants. Of these, 218 were patients with a confirmed diagnosis of immune-mediated rheumatic diseases (IMRDs), and 123 were healthy controls without chronic pathologies.
Non-probabilistic convenience sampling was carried out among patients over 18 years of age attending the rheumatology outpatient clinic at Hospital de Copiapó. Eligible participants were those who had received the fourth dose of the SARS-CoV-2 vaccine. The IMRDs included in the study were rheumatoid arthritis (RA), psoriatic arthritis (PsA), ankylosing spondylitis (AS), systemic lupus erythematosus (SLE), systemic vasculitis (VS), and systemic scleroderma (SS). Patients continued their usual treatments during the vaccination period; however, in specific cases, individuals receiving rituximab were instructed—at the physician’s discretion—to postpone therapy after vaccination.
The control group comprised participants without immunosuppressive conditions who had also received the fourth dose of the SARS-CoV-2 vaccine during the same period. This group consisted predominantly of healthcare professionals, providing a representative comparison sample of the general population.
2.3. Measurement of Neutralizing Antibody Titers
Neutralizing antibody (NAb) titers against SARS-CoV-2 were measured using a pseudovirus-based neutralization assay, as per the guidance of [10] Serum samples were serially diluted and incubated with HIV-1-based pseudoviruses expressing spike proteins from the Wuhan (D614G) or Omicron (BA.1) variants. Following incubation with HEK-ACE2 cells, luciferase activity was quantified to determine neutralization capacity. The 50% inhibitory dilution (ID50) was calculated using nonlinear regression with GraphPad Prism v9.1.2. Samples not reaching 50% neutralization at the lowest dilution were assigned an ID50 of 10.
2.4. Data Collection and Statistical Analysis
Data collected included clinical and demographic information, COVID-19 infection history, vaccination type and schedule (homologous or heterologous), and immunological parameters such as neutralizing antibody presence and levels. Ethical standards were strictly observed, ensuring participant confidentiality and informed consent.
All data were tabulated and analyzed using R software version 4.3.1. Both descriptive and inferential statistics were applied to compare immune responses across different groups.
3. Results
3.1. Demographic Characteristics of the Study Population
The median age of healthy controls was 39 years, while for IMRD patients this was 58 years. Regarding gender distribution, 63.4% of healthy controls were women, while IMRD patients were 84.8%. RA was the most common disease among participants (n = 172), followed by SLE (n = 25). The median duration of the rheumatic disease in patients was 6 years. Samples are measured 1–20 weeks after vaccination, the median of the time window is 35 days.
In terms of therapy, 18.4% of patients were treated with csDMARDs only, and 27% of patients were receiving bDMARDs and no tsDMARDs. Corticosteroids were used by 71.5% of patients. Methotrexate was the most frequent csDMARD, while TNF inhibitors were the most common bDMARD. These details are summarized in Table 1.

Table 1.
Baseline characteristics of patients with rheumatic diseases and controls.
The baseline characteristics of the study participants are summarized in Table 1. Among the 218 patients with immune-mediated rheumatic diseases (IMRDs), rheumatoid arthritis (RA) was the most frequent diagnosis, followed by psoriatic arthritis (PsA), ankylosing spondylitis (AS), systemic lupus erythematosus (SLE), systemic vasculitis (VS), and systemic scleroderma (SS). The median disease duration ranged from 48 to 64 years across the different conditions.
Neutralizing antibody responses stratified by treatment group and prior COVID-19 infection are summarized in Table 2. For the Wuhan strain, antibody titers were generally higher in participants with a history of COVID-19, with the most robust responses observed in healthy controls, followed by patients on csDMARDs. In contrast, patients receiving bDMARDs, tsDMARDs, or corticosteroids exhibited markedly reduced titers. Statistically, no significant differences were observed between COVID-19-positive and COVID-19-negative participants within the csDMARD (p = 0.9), bDMARD (p = 0.4), or corticosteroid groups (p = 0.8). However, among healthy controls, prior infection was associated with significantly higher titers (p = 0.0056).

Table 2.
Neutralizing antibody responses (ID50_{50}50) against SARS-CoV-2 Wuhan and Omicron variants according to treatment and previous COVID-19 infection.
Against the Omicron variant, antibody levels were consistently lower across all groups compared to Wuhan, with the lowest responses detected in patients receiving bDMARDs and corticosteroids. Prior COVID-19 infection conferred a relative advantage, but the effect was less pronounced than for Wuhan. Specifically, no significant differences were found within the csDMARD (p = 0.09) and bDMARD (p = 0.10) groups, whereas corticosteroid-treated patients (p = 0.0003) and healthy controls (p < 0.0001) showed significantly higher titers if previously infected.
These findings suggest that prior SARS-CoV-2 infection enhances neutralizing antibody responses against both Wuhan and Omicron strains, although this effect is blunted in patients undergoing immunosuppressive therapy with csDMARDs and bDMARDs.
3.2. Immunogenicity According to IMRDs SARS-CoV-2
We present neutralizing antibody (ID50) levels against SARS-CoV-2 in patients with rheumatic diseases and in healthy controls, stratified by history of infection (COVID-19 positive or negative). For each subgroup, mean titers and p-values are reported to evaluate statistical significance. The findings are shown in the following Figure 1 and Figure 2.
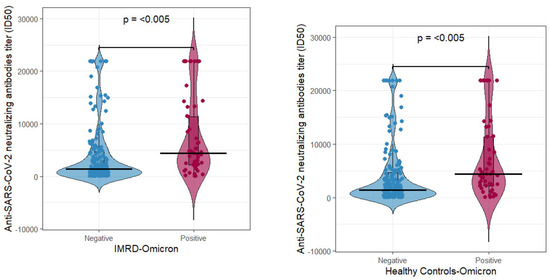
Figure 1.
Neutralizing antibody titers in rheumatologic (left) and control (right) patients determined for the Omicron variant grouped by COVID-19 status (negative or positive).
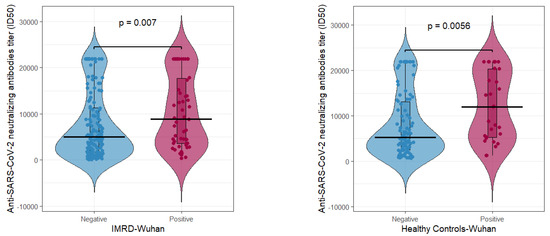
Figure 2.
Neutralizing antibody titers in rheumatologic (left) and control (right) patients determined for the Wuhan variant grouped by COVID-19 status (negative or positive).
For the Omicron strain (Figure 1), rheumatologic patient antibody titers showed significantly higher antibody levels in the COVID-19-positive group (average ≈ 7308) compared to the COVID-19-negative group (average ≈ 3985), with a p-value = 5.091 × 10−5 (left panel). A similar trend was observed in the control group, where the COVID-19-positive subgroup exhibited an average of 10,812 antibody titers compared to 4916 in COVID-19-negative individuals (p = 2.582 × 10−6, right panel).
For the original Wuhan strain (Figure 2), rheumatologic patients with prior COVID-19 infection had significantly higher antibody levels (average ≈ 10,516) than COVID-19-negative individuals (average ≈ 7616), with p = 0.007 (left panel). In the control group, COVID-19-positive individuals exhibited an average of 11,983 antibody titers, while COVID-19-negative controls averaged 7967, a difference that was highly significant (p = 0.0056, right panel).
Across all groups, prior SARS-CoV-2 infection was consistently associated with higher levels of neutralizing antibody titers, a statistically significant pattern observed in both rheumatologic patients and healthy controls (Table 3). When comparing strains effects, individuals without prior infection showed higher antibody levels with the Wuhan strain compared to the Omicron strain—10,812 vs. 4916 in controls and 7308 vs. 3985 in rheumatologic patients. Similarly, among COVID-19-positive individuals, the Wuhan strain elicited stronger responses—11,983 vs. 7967 in controls and 10,516 vs. 7616 in rheumatologic patients. Regarding disease status, rheumatologic patients consistently exhibited lower neutralizing antibody levels than controls, especially following the Omicron strain. Although the difference between groups was not significant for the Wuhan strain, it was statistically significant for the Omicron strain.

Table 3.
Summary of average Anti SARS-CoV-2 neutralizing antibody levels (ID50) by group and COVID status.
4. Discussion
Recent evidence in patients with rheumatoid arthritis indicates that COVID-19 vaccines elicit adequate humoral immunogenicity with an acceptable safety profile [11]. Consistent with this, our results show that prior SARS-CoV-2 infection induces a significantly stronger neutralizing antibody response in both rheumatic patients and healthy controls. These findings were consistent regardless of infection history, supporting the notion that both vaccine strain and prior exposure play critical roles in shaping immune responses [10,12,13]. In healthy controls, higher neutralizing antibody titers were observed following Wuhan vaccination, with prior infection further boosting responses—reflecting the well-documented effect of hybrid immunity [14,15,16,17]. In rheumatic patients, who often experience impaired immune responses due to immunosuppressive therapy, a similar pattern emerged as follows: the Wuhan vaccine elicited a stronger humoral response than the Omicron vaccine. Notably, antibody levels in some rheumatic patients vaccinated with the Wuhan strain approached those of healthy controls who received the Omicron vaccine, underscoring the superior immunogenicity of Wuhan-based formulations [18,19]. These findings are particularly relevant for patients with autoimmune rheumatic diseases (ARDs), where vaccine efficacy is often reduced by treatments such as corticosteroids, methotrexate, and B-cell–depleting agents [3,20]. Prior studies have reported diminished responses in these populations [8]. Our results extend this knowledge by highlighting that vaccine strain also significantly influences immune responses within these vulnerable groups. From a public health perspective, the data suggest that not all vaccine platforms provide equal protection in immunocompromised individuals, and formulations based on the ancestral spike protein may confer superior antibody-mediated protection [21,22]. Furthermore, accounting for prior infection is essential when designing immunization strategies as hybrid immunity enhances both the breadth and durability of protection [23,24,25].
Our results also highlight the impact of methotrexate on vaccine-induced immune responses. In patients with rheumatoid arthritis (RA) receiving methotrexate, we observed a significant difference (p = 0.02) in neutralizing antibody titers against the Omicron variant between COVID-19-positive and -negative individuals, whereas no such difference was seen for the Wuhan strain. Notably, among patients with psoriatic arthritis (PsA), none were on methotrexate, so no comparisons could be made for this subgroup. These findings are in line with previous reports indicating that methotrexate can attenuate vaccine responses, and temporary discontinuation may enhance cellular and humoral immunity without increasing disease activity [11,12].
Several limitations must be acknowledged. Most importantly, the cross-sectional design precludes the evaluation of antibody kinetics and durability over time. This is especially relevant with the emergence of new variants, where longitudinal studies with serial sampling are necessary to assess the persistence and breadth of protection. Additionally, although our sample size allowed meaningful comparisons, larger and more diverse cohorts are needed to fully capture interindividual variability in vaccine responses, particularly across different immunomodulatory therapies. Finally, while this study focused on humoral immunity, cellular responses—especially T-cell–mediated immunity—likely contribute to protection even when neutralizing antibody titers wane or viral variants partially escape recognition [26].
In conclusion, our findings emphasize the critical impact of prior infection on the humoral response in patients with rheumatic diseases. Future prospective studies are warranted to better characterize the durability of protection and to inform optimized vaccination strategies in this vulnerable population.
5. Conclusions
This study demonstrates that prior SARS-CoV-2 infection significantly enhances the immune response across all groups, highlighting the role of hybrid immunity. These findings emphasize the importance of developing tailored immunization strategies that take into account both the vaccine strain and the immunological condition of recipients, particularly in immunocompromised populations such as individuals with autoimmune rheumatic disorders.
Author Contributions
M.J.G.-N.: Conceptualization, Investigation, Writing—original draft, Writing—review and editing, Data curation, Funding acquisition, Methodology, Project administration, Supervision. Y.G.: Formal analysis, Investigation, Software, Supervision, Validation, Writing—original draft, Writing—review and editing, Data curation. J.D.V.: Investigation, Writing—review and editing. F.Z.: Investigation, Writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by FONIS grant number SA21I0078.
Institutional Review Board Statement
The studies involving humans were approved by Proyecto: No 161-2021, Archivo acta: N° 109. UNIVERSIDAD DE CHILE—FACULTAD DE MEDICINA COMITÉ DE ÉTICA DE INVESTIGACIÓN EN SERES HUMANOS. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Informed Consent Statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors without undue reservation.
Acknowledgments
The authors wish to thank all the patients and healthy volunteers who participated in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- World Health Organization (WHO). Coronavirus Disease (COVID-19) Pandemic. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (accessed on 15 July 2025).
- Altarawneh, H.N.; Chemaitelly, H.; Ayoub, H.H.; Hasan, M.R.; Coyle, P.; Yassine, H.M.; Al-Khatib, H.A.; Smatti, M.K.; Coyle, P.; Al-Kanaani, Z.; et al. Effects of previous infection and vaccination on symptomatic Omicron infections. N. Engl. J. Med. 2022, 387, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-J.; Cheng, P.-L.; Huang, W.-N.; Chen, H.-H.; Chen, H.-W.; Chen, J.-P.; Lin, C.-T.; Tang, K.-T.; Hung, W.-T.; Hsieh, T.-Y.; et al. Single-cell RNA sequencing to decipher the immunogenicity of ChAdOx1 nCoV-19/AZD1222 and mRNA-1273 vaccines in patients with autoimmune rheumatic diseases. Front. Immunol. 2022, 13, 920865. [Google Scholar] [CrossRef]
- Bieber, A.; Sagy, I.; Novack, L.; Brikman, S.; Abuhasira, R.; Ayalon, S.; Novofastovski, I.; Abu-Shakra, M.; Mader, R. BNT162b2 mRNA COVID-19 vaccine and booster in patients with autoimmune rheumatic diseases: A national cohort study. Annals of the Rheumatic Diseases. Ann. Rheum. Dis. 2022, 81, 1028–1035. [Google Scholar] [CrossRef]
- Brito-Zerón, P.; Sisó-Almirall, A.; Flores-Chávez, A.; Retamozo, S.; Ramos-Casals, M. SARS-CoV-2 infection in patients with systemic autoimmune diseases. Clin. Exp. Rheumatol. 2021, 39, 676–687. [Google Scholar] [CrossRef]
- Furer, V.; Eviatar, T.; Zisman, D.; Peleg, H.; Paran, D.; Levartovsky, D.; Zisapel, M.; Elalouf, O.; Kaufman, I.; Meidan, R.; et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: A multicentre study. Ann. Rheum. Dis. 2021, 80, 1330–1338. [Google Scholar] [CrossRef]
- Tedeschi, S.K.; Solomon, D.H.; Chen, Y.; Ellrodt, J.; Whelan, M.G.; Stratton, J.; Hayashi, K.; Whiteman, N.B.; Chen, L.; Adejoorin, I.; et al. Humoral and cellular immune responses in individuals with rheumatoid arthritis after a third dose of mRNA COVID-19 vaccine. Semin. Arthritis Rheum. 2023, 59, 152177. [Google Scholar] [CrossRef]
- Gaete-Argel, A.; Saavedra-Alarcón, V.; Sauré, D.; Alonso-Palomares, L.; Acevedo, M.L.; Alarcón, M.; Bueno, S.M.; Kalergis, A.M.; Soto-Rifo, R.; Valiente-Echeverría, F.; et al. Impact of homologous and heterologous boosters in neutralizing antibody response against SARS-CoV-2 D614G and Omicron in solid organ transplant recipients and healthy controls. Front. Immunol. 2023, 14, 1135478. [Google Scholar] [CrossRef]
- Gallardo-Nelson, M.J.; Cruces, M.; Gómez, Y.M.; Fuenzalida, C.; Silva, J.; Aravena-Traipi, L.; Nuñez, E.; Gaete-Angel, A.; Rivas-Yáñez, E.; Kalergis, A.M.; et al. 4th booster-dose SARS-CoV-2 heterologous and homologous vaccination in rheumatological patients. Front. Immunol. 2024, 15, 1427501. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, J.; Shao, R.; Han, X.; Su, C.; Lu, W. Risk and clinical outcomes of COVID-19 in patients with rheumatic diseases: A systematic review and meta-analysis. Rheumatol. Int. 2021, 41, 843–856. [Google Scholar] [CrossRef]
- Martínez-Fleta, P.; Vicente-Rabaneda, E.F.; Triguero-Martínez, A.; Roy-Vallejo, E.; Uriarte-Ecenarro, M.; Gutiérrez-Rodríguez, F.; Quiroga-Colina, P.; Romero-Robles, A.; Montes, N.; García-Castañeda, N.; et al. Beneficial effect of temporary methotrexate interruption on B and T cell responses upon SARS-CoV-2 vaccination in patients with rheumatoid arthritis or psoriatic arthritis. NPJ Vaccines 2024, 9, 21. [Google Scholar] [CrossRef]
- Shirata, M.; Ito, I.; Tanaka, M.; Murata, K.; Murakami, K.; Ikeda, H.; Oi, I.; Hamao, N.; Nishioka, K.; Hayashi, Y.; et al. Impact of methotrexate on humoral and cellular immune responses to SARS-CoV-2 mRNA vaccination in patients with rheumatoid arthritis. Clin. Exp. Med. 2023, 23, 4707–4720. [Google Scholar] [CrossRef] [PubMed]
- Jena, A.; Mishra, S.; Deepak, P.; Kumar-M, P.; Sharma, A.; Patel, Y.; Kennedy, N.A.; Kim, A.H.J.; Sharma, V.; Sebastian, S. Response to SARS-CoV-2 vaccination in immune mediated inflammatory diseases: Systematic review and meta-analysis. Autoimmun. Rev. 2021, 21, 102927. [Google Scholar] [CrossRef] [PubMed]
- Bonelli, M.M.; Mrak, D.; Perkmann, T.; Haslacher, H.; Aletaha, D. SARS-CoV-2 vaccination in rituximab-treated patients: Evidence for impaired humoral but inducible cellular immune response. Ann. Rheum. Dis. 2021, 80, 1355–1356. [Google Scholar] [CrossRef] [PubMed]
- Krasselt, M.; Wagner, U.; Nguyen, P.; Pietsch, C.; Boldt, A.; Baerwald, C.; Pierer, M.; Seifert, O. Humoral and cellular response to COVID-19 vaccination in patients with autoimmune inflammatory rheumatic diseases under real-life conditions. Rheumatology 2022, 61, SI180–SI188. [Google Scholar] [CrossRef]
- Németh, D.; Vágó, H.; Tothfalusi, L.; Ulakcsai, Z.; Becker, D.; Szabó, Z.; Rojkovich, B.; Merkely, B.; Nagy, G. Factors influencing the SARS-CoV-2 infection and vaccination-induced immune response in rheumatoid arthritis. Front. Immunol. 2022, 13, 960001. [Google Scholar] [CrossRef]
- Petty, R.E. Chapter 1—Pediatric Rheumatology: The Study of Rheumatic Diseases in Childhood and Adolescence. In Pediatric Rheumatology; Elsevier: Amsterdam, The Netherlands, 2015; pp. 1–4.e1. [Google Scholar] [CrossRef]
- Strangfeld, A.; Schäfer, M.; Gianfrancesco, M.A.; Lawson-Tovey, S.; Liew, J.; Ljung, L.; Mateus, E.F.; Richez, C.; Santos, M.J.; Schmajuk, G.; et al. Factors associated with COVID-19-related death in people with rheumatic diseases: Results from the COVID-19 Global Rheumatology Alliance. Ann. Rheum. Dis. 2021, 80, 930–942. [Google Scholar] [CrossRef]
- Torres-Rufas, M.; Vicente-Rabaneda, E.F.; Cardeñoso, L.; Gutiérrez, A.; Bong, D.A.; Valero-Martínez, C.; Serra López-Matencio, J.M.; García-Vicuña, R.; González-Gay, M.A.; González-Álvaro, I.; et al. Effectiveness and safety of the COVID-19 vaccine in patients with rheumatoid arthritis in a real-world setting. Vaccines 2024, 12, 672. [Google Scholar] [CrossRef]
- Togt, C.V.D.; Ten Cate, D.F.; den Broeder, N.; Rahamat-Langendoen, J.; van den Bemt, B.V.D.; den Broeder, A.D. Humoral response to COVID-19 vaccines depends on dosage and timing of rituximab in patients with rheumatoid arthritis. Rheumatology 2022, 61, SI175–SI179. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 15 July 2025).
- Kamar, N.; Abravanel, F.; Marion, O.; Couat, C.; Izopet, J.; Del Bello, A. Three doses of an mRNA COVID-19 vaccine in solid-organ transplant recipients. N. Engl. J. Med. 2021, 385, 661–662. [Google Scholar] [CrossRef]
- Premkumar, L.; Segovia-Chumbez, B.; Jadi, R.; Martinez, D.R.; Raut, R.; Markmann, A.J.; Cornaby, C.; Bartelt, L.; Weiss, S.; Park, Y.; et al. The receptor-binding domain of the viral spike protein is an immunodominant and highly specific target of antibodies in SARS-CoV-2 patients. Sci. Immunol. 2022, 5, eabc8413. [Google Scholar] [CrossRef]
- Sattler, A.; Angermair, S.; Stockmann, H.; Heim, K.M.; Khadzhynov, D.; Treskatsch, S.; Halleck, F.; Kreis, M.E.; Kotsch, K. SARS-CoV-2-specific T cell responses and correlations with COVID-19 patient predisposition. J. Clin. Investig. 2021, 131, e145272. [Google Scholar] [CrossRef]
- Wratil, P.R.; Stern, M.; Priller, A.; Willmann, A.; Almanzar, G.; Vogel, E.; Feuerherd, M.; Cheng, C.-C.; Yazici, S.; Christa, C.; et al. Three exposures to the spike protein of SARS-CoV-2 by either infection or vaccination elicit superior neutralizing immunity to all variants of concern. Nat. Med. 2022, 28, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, F.; Weisblum, Y.; Muecksch, F.; Hoffmann, H.-H.; Michailidis, E.; Lorenzi, J.C.C.; Mendoza, P.; Rutkowska, M.; Bednarski, E.; Gaebler, C.; et al. Measuring SARS-CoV-2 neutralizing antibody activity using pseudotyped and chimeric viruses. J. Exp. Med. 2020, 217, e20201181. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).