Impact of COVID-19 on the Risk of Coronary Stent Thrombosis and Restenosis: A Retrospective Angiographic Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Characteristics of the Study Subjects
2.2. Collection of Clinical and Laboratory Parameters
2.3. Methods of Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Batenova, G.; Pivina, L.; Dedov, E.; Dyussupov, A.; Zhumanbayeva, Z.; Smail, Y.; Belikhina, T.; Pak, L.; Ygiyeva, D. Restenosis of Coronary Arteries in Patients with Coronavirus Infection: Case Series. Case Rep. Med. 2023, 2023, 3000420. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A.; et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in COVID-19. N. Engl. J. Med. 2020, 383, 120–128. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pelliccia, F.; Zimarino, M.; Niccoli, G.; Morrone, D.; De Luca, G.; Miraldi, F.; De Caterina, R. In-stent restenosis after percutaneous coronary intervention: Emerging knowledge on biological pathways. Eur. Hear. J. Open 2023, 3, oead083. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zwart, B.; van Werkum, J.; Heestermans, A.; Kelder, J.; Zomer, A.C.; van’t Hof, A.W.J.; Verheugt, F.; Berg, J.T. Triggering mechanisms of stent thrombosis. EuroIntervention 2011, 6, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Antuña, P.; Rivero, F.; del Val, D.; Cuesta, J.; Alfonso, F. Late Coronary Stent Thrombosis in a Patient With Coronavirus Disease 2019. JAMA Cardiol. 2020, 5, 1195–1198. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xiong, T.-Y.; Redwood, S.; Prendergast, B.; Chen, M. Coronaviruses and the cardiovascular system: Acute and long-term implications. Eur. Heart J. 2020, 41, 1798–1800. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lim, J.T.; En, W.L.; Tay, A.T.; Pang, D.; Chiew, C.J.; Ong, B.; Lye, D.C.B.; Tan, K.B. Long-term Cardiovascular, Cerebrovascular, and Other Thrombotic Complications in COVID-19 Survivors: A Retrospective Cohort Study. Clin. Infect. Dis. 2023, 78, 70–79. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Libby, P.; Lüscher, T. COVID-19 is, in the end, an endothelial disease. Eur. Heart J. 2020, 41, 3038–3044. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ayan, M.; Kovelamudi, S.; Al-Hawwas, M. Subacute stent thrombosis in a patient with COVID-19 pneumonia. Bayl. Univ. Med. Cent. Proc. 2020, 34, 175–177. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sheikh, A.B.; Shekhar, R.; Javed, N.; Upadhyay, S. Inferior Wall Myocardial Infarction in Severe COVID-19 Infection: A Case Report. Am. J. Case Rep. 2020, 21, e926101-1–e926101-5. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shi, S.; Qin, M.; Shen, B.; Cai, Y.; Liu, T.; Yang, F.; Gong, W.; Liu, X.; Liang, J.; Zhao, Q.; et al. Association of Cardiac Injury With Mortality in Hospitalized Patients With COVID-19 in Wuhan, China. JAMA Cardiol. 2020, 5, 802–810. [Google Scholar] [CrossRef]
- Shinkai, W.; Hashikata, T.; Kameda, R.; Sato, N.; Minami, Y.; Ako, J. Very late stent thrombosis lacking findings of the typical causes on optical coherence tomography in a patient with SARS-CoV-2. J. Cardiol. Cases 2022, 26, 197–199. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef]
- Pivina, L.; Batenova, G.; Omarov, N.; Ygiyeva, D.; Messova, A.; Alibayeva, G.; Jamedinova, U.; Kurumbayev, R.; Pivin, M. Peculiarities of in-Stent Thrombosis and Restenosis in Coronary Arteries Post-COVID-19: A Systematic Review of Clinical Cases and Case Series. Open Access Emerg. Med. 2025, 17, 15–30. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Skorupski, W.J.; Grygier, M.; Lesiak, M.; Kałużna-Oleksy, M. Coronary Stent Thrombosis in COVID-19 Patients: A Systematic Review of Cases Reported Worldwide. Viruses 2022, 14, 260. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pivina, L.; Orekhov, A.; Batenova, G.; Ygiyeva, D.; Belikhina, T.; Pivin, M.; Dyussupov, A. Assessment of Risk Factors for Coronary Artery Restenosis and Patient Survival During the COVID-19 Pandemic. Healthcare 2025, 13, 1175. [Google Scholar] [CrossRef]
- Shaw, L.J.; Merz, C.N.B.; Pepine, C.J.; Reis, S.E.; Bittner, V.; Kelsey, S.F.; Olson, M.; Johnson, B.D.; Mankad, S.; Sharaf, B.L.; et al. Insights from the NHLBI-Sponsored Women’s Ischemia Syndrome Evaluation (WISE) Study: Part I: Gender differences in traditional and novel risk factors, symptom evaluation, and gender-optimized diagnostic strategies. J. Am. Coll. Cardiol. 2006, 47 (Suppl. S3), S4–S20. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Zeng, C.; Zhang, X.; Yang, C.; Wang, H.; Song, W.; Wang, X.; Fu, C.; Shi, W.; Fang, Y. The characteristics of coronary stenosis in 11,267 patients from Southwest China: A retrospective study. J. Thromb. Thrombolysis 2017, 45, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Hou, J.; Gu, X.; Weng, R.; Zhong, Z.; Liu, S. Incidence and risk factors of in-stent restenosis after percutaneous coronary intervention in patients from southern China. Eur. J. Med. Res. 2022, 27, 12. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, B.; Li, M.; Liu, J.; Xie, L.; Li, J.; Liu, Y.; Niu, C.; Xiao, D.; Li, J.; Zhang, L. Risk Factors and Incidence for In-Stent Restenosis with Drug-Eluting Stent: A Systematic Review and Meta-Analysis. Rev. Cardiovasc. Med. 2024, 25, 458. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Calais, F.; Lagerqvist, B.; Leppert, J.; James, S.K.; Fröbert, O. Proximal coronary artery intervention: Stent thrombosis, restenosis and death. Int. J. Cardiol. 2013, 170, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Xi, H.; Liu, J.; Xu, T.; Li, Z.; Mou, X.; Jin, Y.; Xia, S. Risk investigation of in-stent restenosis after initial implantation of intracoronary drug-eluting stent in patients with coronary heart disease. Front. Cardiovasc. Med. 2023, 10, 1117915. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gupta, P.K.; Balachander, J. Predictor of in-stent restenosis in patients with drug-eluting stent (PRIDE)—A retrospective cohort study. Clin. Investig. Arter. 2021, 33, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Triantafyllis, A.S.; Sfantou, D.; Karapedi, E.; Peteinaki, K.; Kotoulas, S.C.; Saad, R.; Fountoulakis, P.N.; Tsamakis, K.; Tsiptsios, D.; Rallidis, L.; et al. Coronary Implications of COVID-19. Med. Princ. Pract. 2024, 34, 1–12. [Google Scholar] [CrossRef]
- Manolis, T.A.; Manolis, A.A.; Papatheou, D.; Melita, H. COVID-19 Infection: Viral Macro- and Micro-Vascular Coagulopathy and Thromboembolism/Prophylactic and Therapeutic Management. J. Cardiovasc. Pharmacol. Ther. 2020, 26, 12–24. [Google Scholar] [CrossRef]
- Urano, T.; Yasumoto, A.; Yokoyama, K.; Horiuchi, H.; Morishita, E.; Suzuki, Y. COVID-19 and Thrombosis: Clinical Aspects. Curr. Drug Targets 2022, 23, 1567–1572. [Google Scholar] [CrossRef]
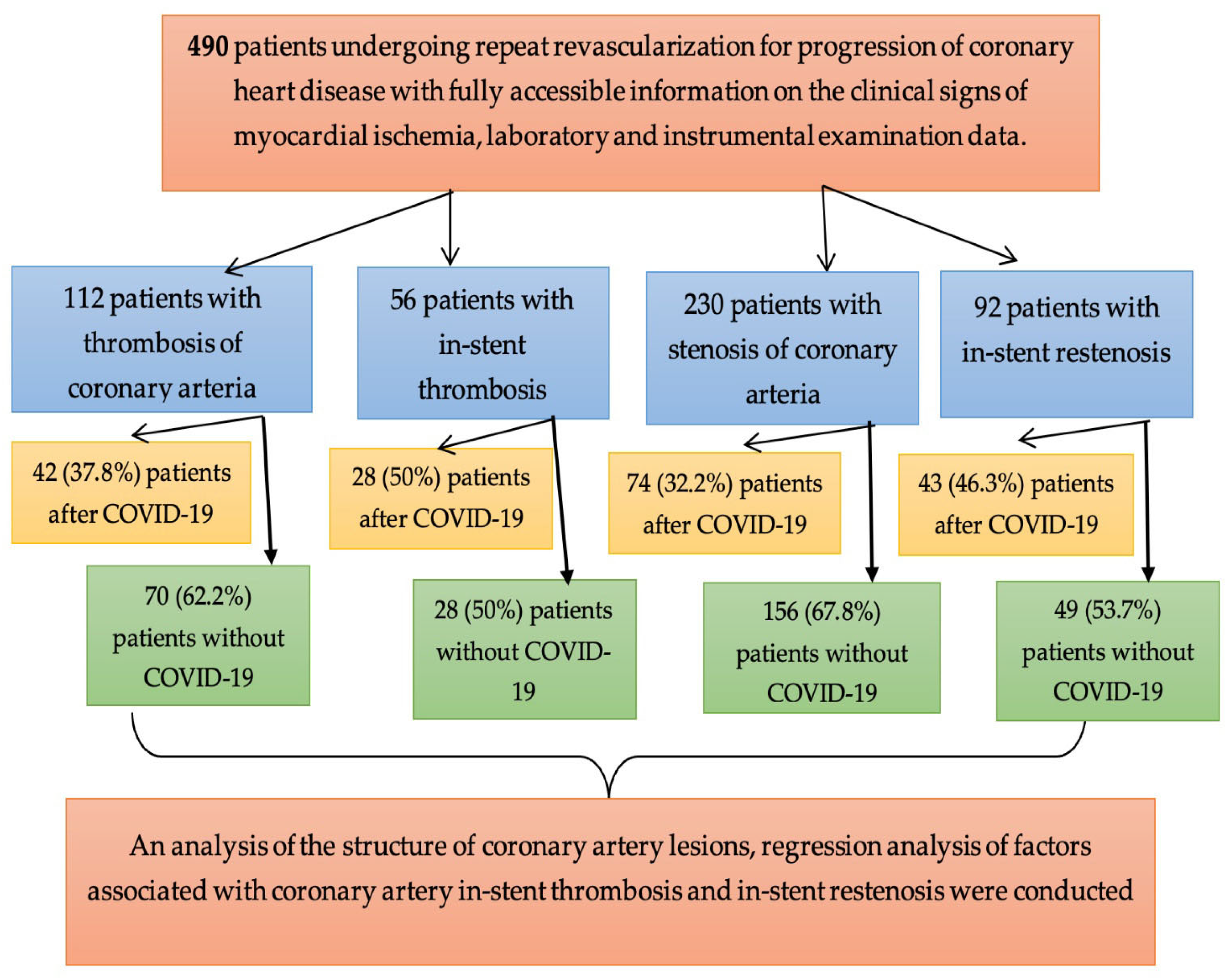
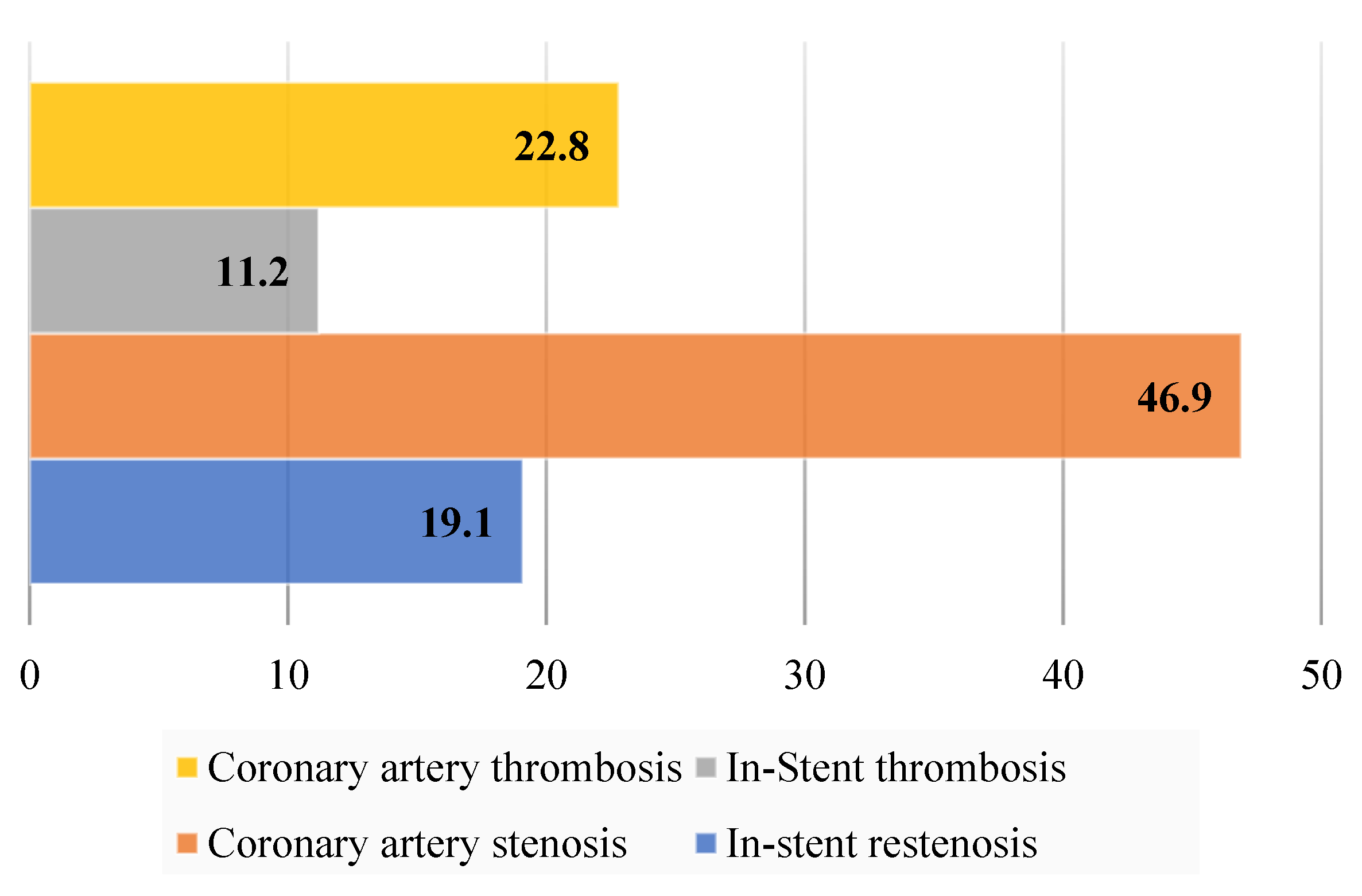
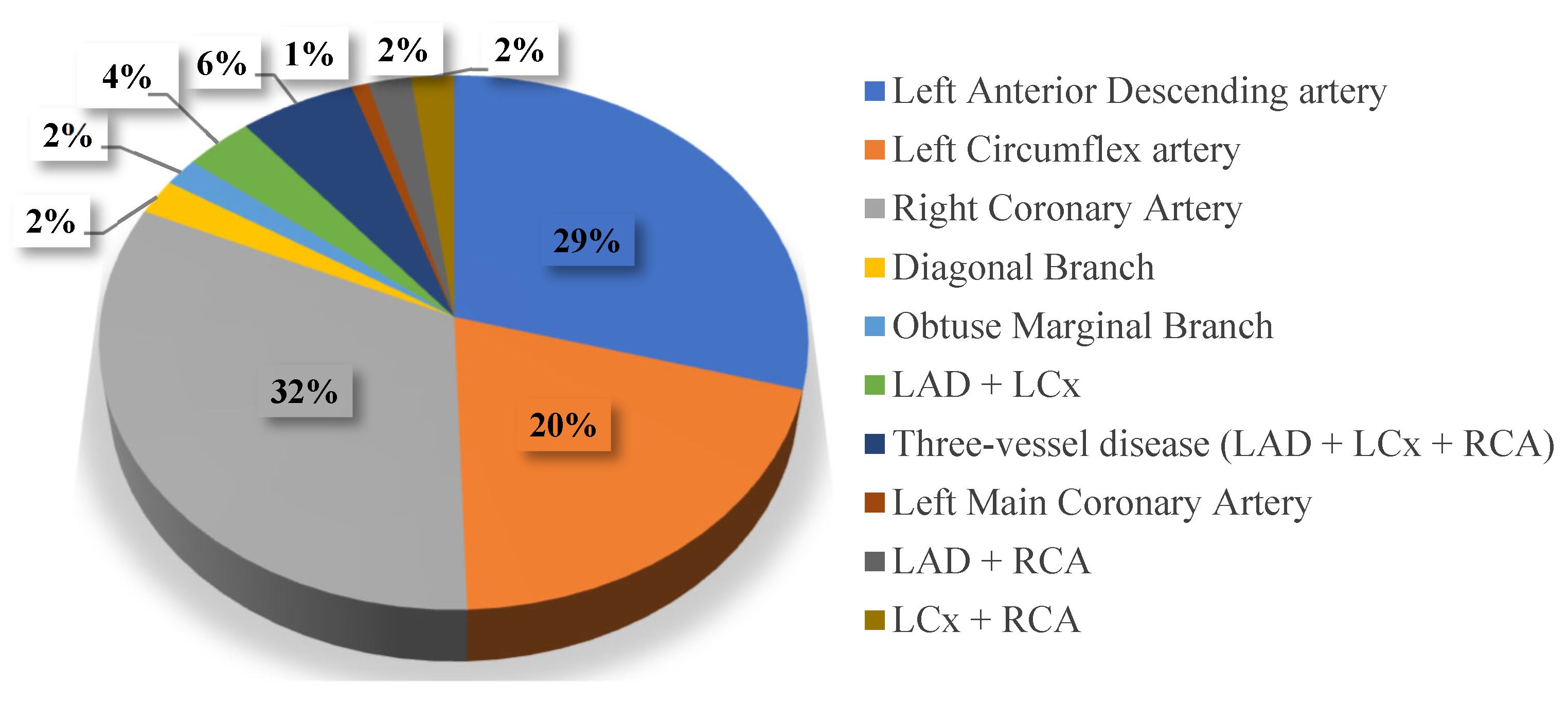
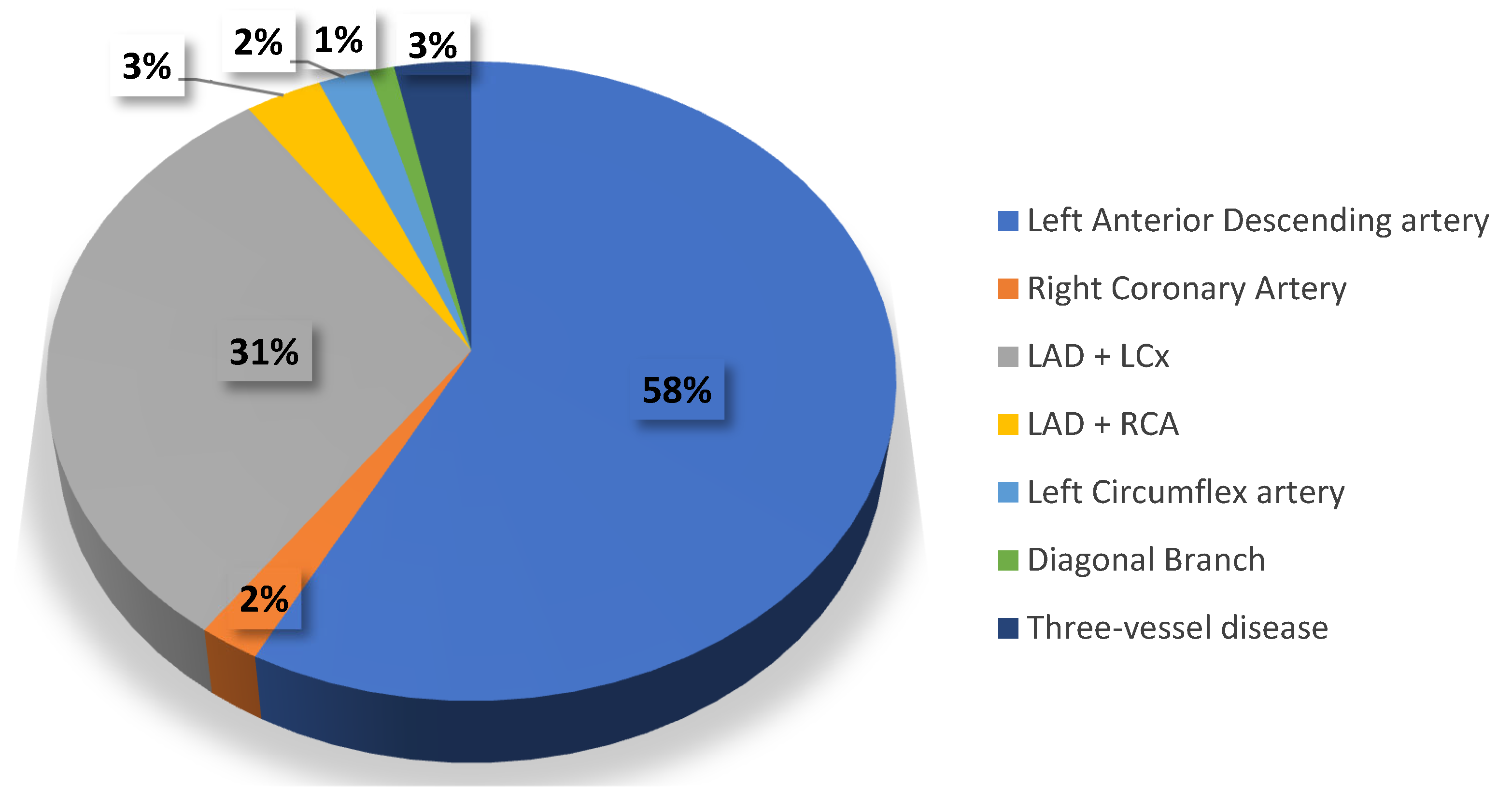
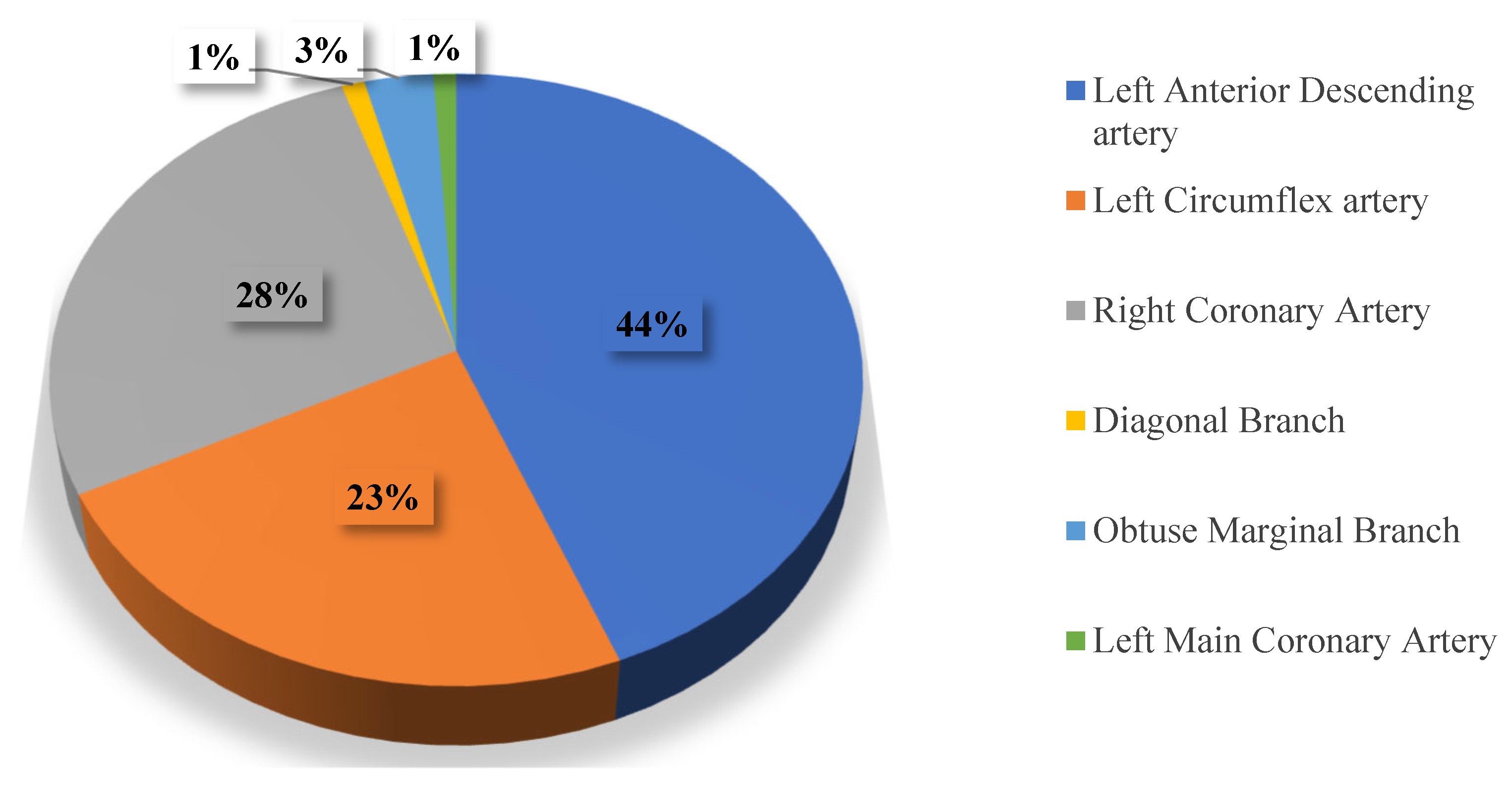
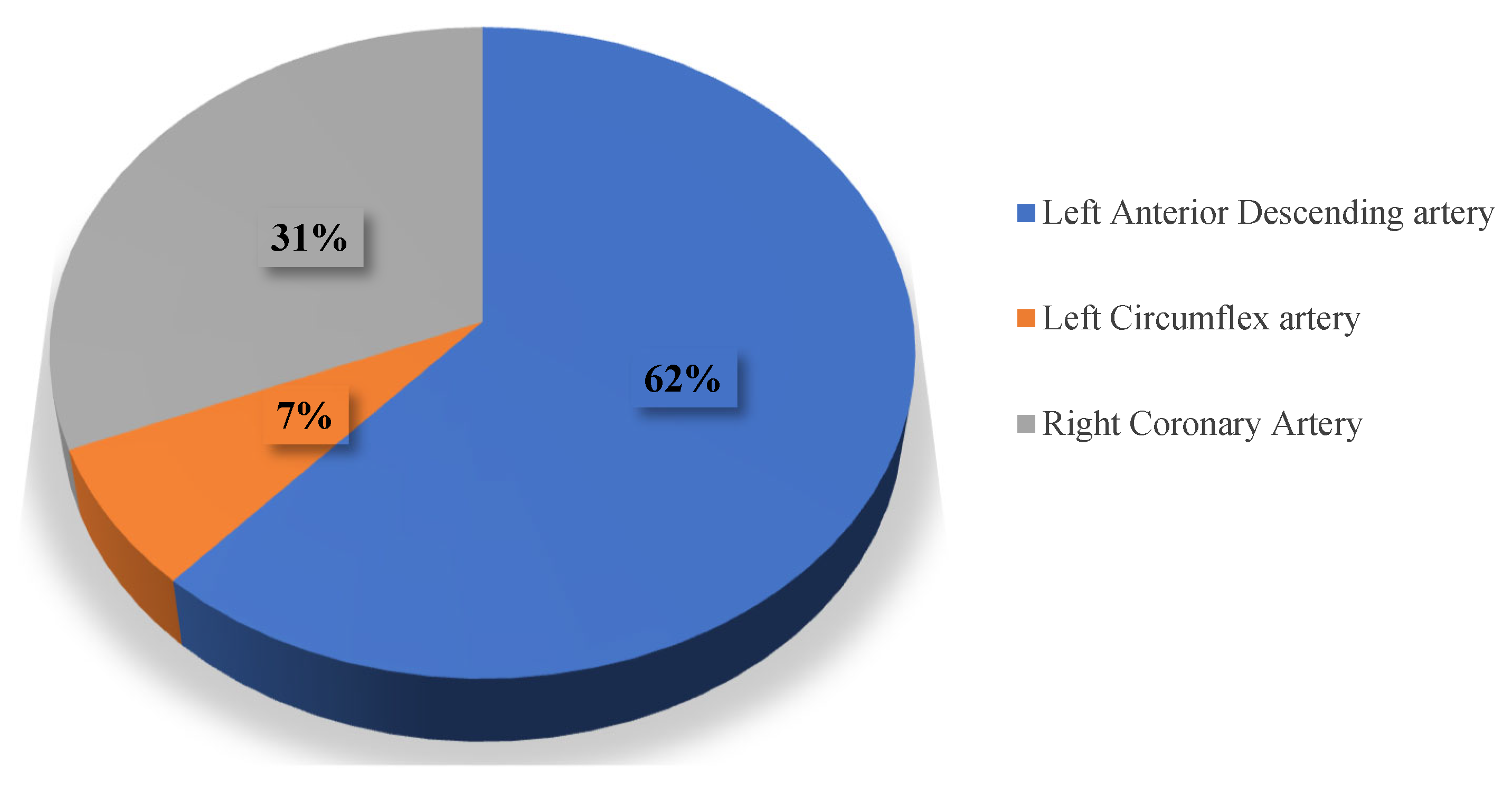
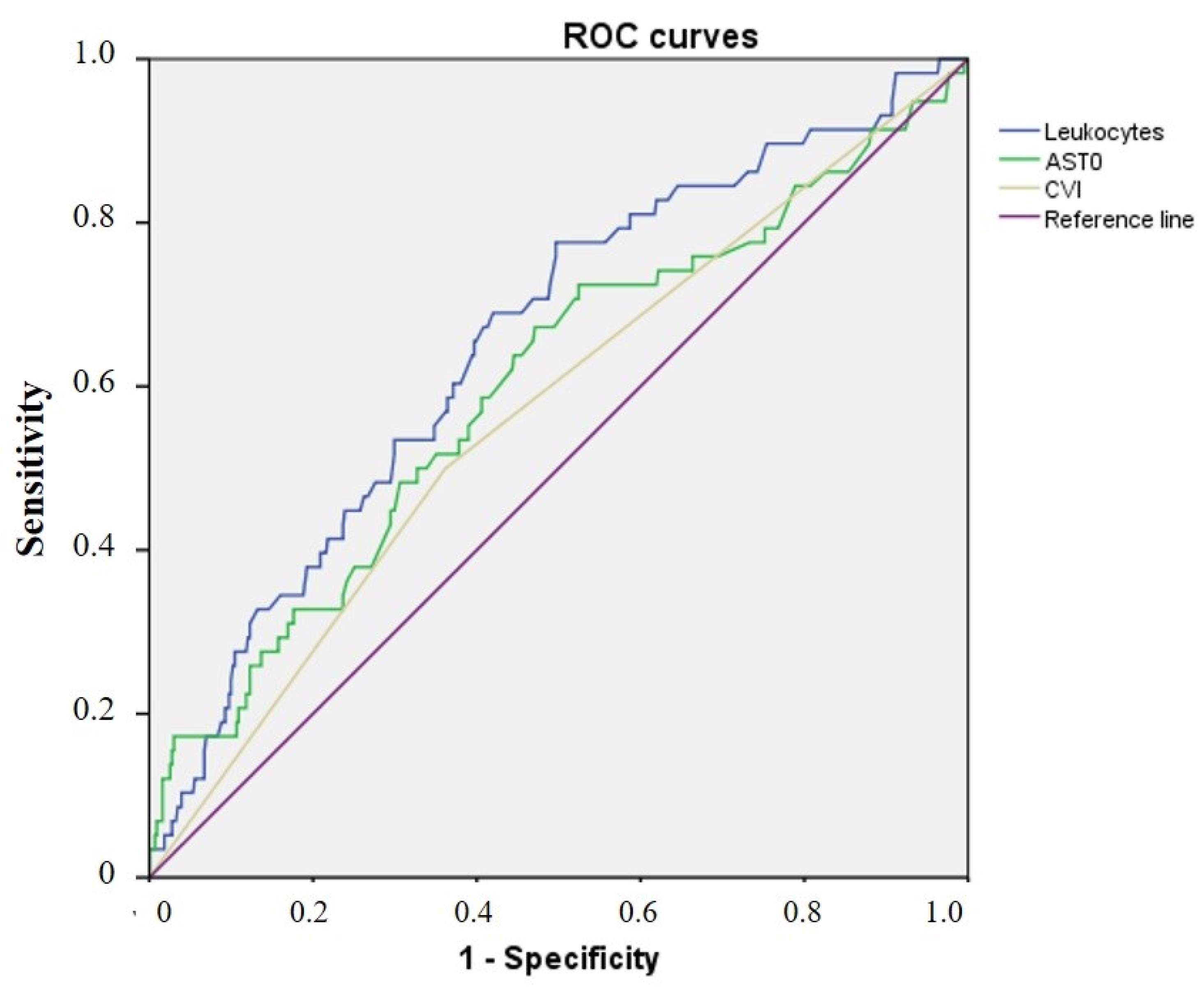
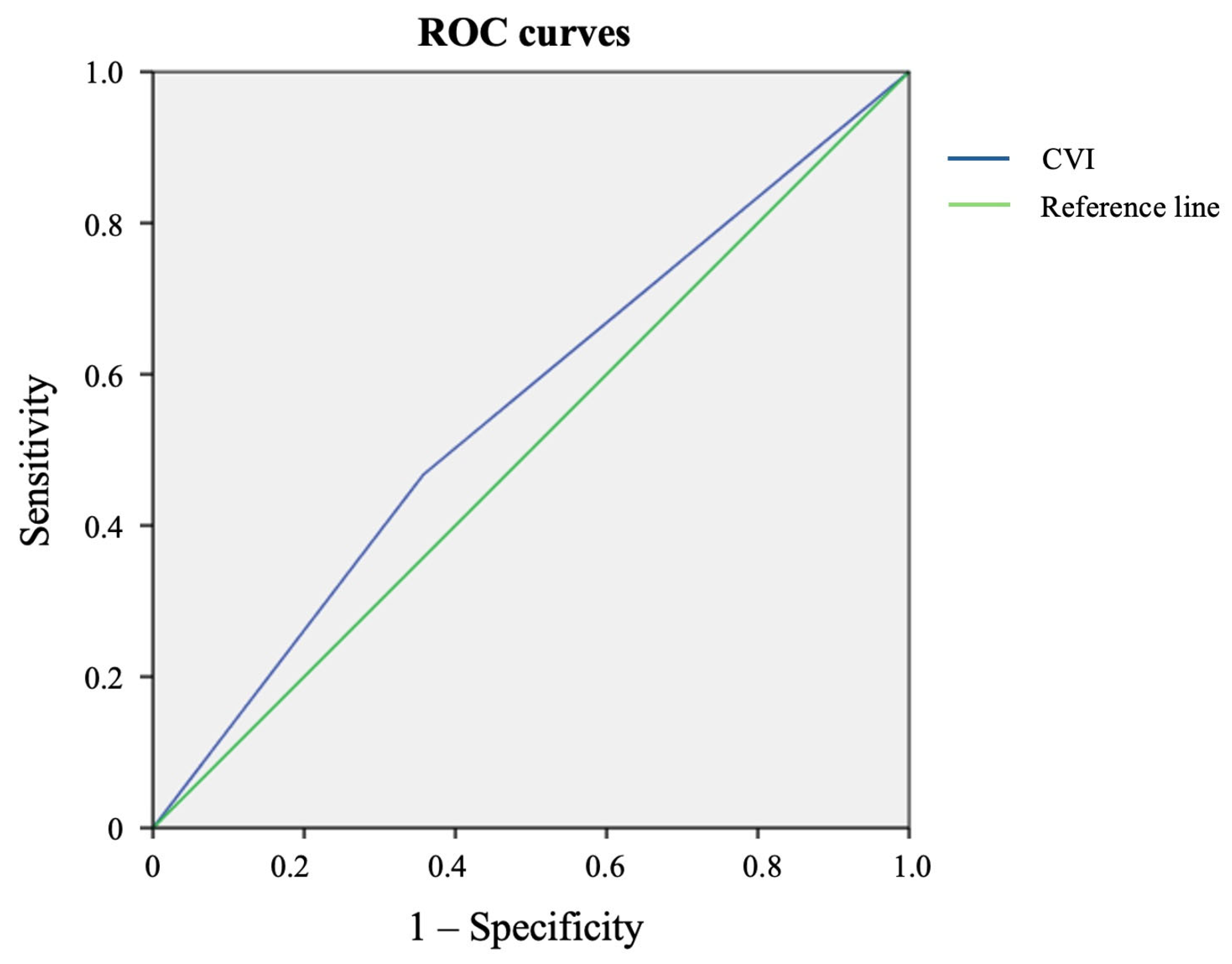
| Parameter | CVI+ (n = 186, 38%) Me, IQR | CVI− (n = 304, 62%) Me, IQR | p |
|---|---|---|---|
| LVEF, % | 50.0 (12.0) | 52.0 (11.0) | 0.098 |
| SBP | 130.0 (22.5) | 130.0 (27.5) | 0.875 |
| DBP | 80.0 (20.0) | 80.0 (10.0) | 0.336 |
| Lymphocytes | 24.9 (13.48) | 24.85 (10.88) | 0.733 |
| neutrophils | 65.1 (16.3) | 65.3 (13.5) | 0.833 |
| Leukocytes | 8.25 (4.13) | 8.02 (3.77) | 0.985 |
| Platelets | 233.5 (70.3) | 231.0 (78.8) | 0.535 |
| Hemoglobin | 141.5 (21.3) | 142.0 (21.8) | 0.555 |
| APTT | 31.0 (7.99) | 28.7 (8.5) | 0.003 |
| Fibrinogen | 3.31 (1.45) | 3.13 (1.297) | 0.023 |
| INR | 0.98 (0.2) | 1.0 (0.17) | 0.122 |
| D-dimer | 484.5 (369.0) | 427.5 (328.5) | 0.005 |
| Troponin | 40.0 (40) | 0 | 0.0001 |
| ALT | 27.6 (19.63) | 23.12 (20.38) | 0.073 |
| AST | 25.0 (21.25) | 22.65 (17.94) | 0.026 |
| Glucose | 6.1 (3.08) | 6.03 (2.0) | 0.181 |
| Urea | 5.64 (2.73) | 5.9 (2.5) | 0.757 |
| Creatinine | 83.9 (30.78) | 87.0 (30.0) | 0.987 |
| CRP | 12.7 (22.4) | 8.21 (10.88) | 0.001 |
| Triglycerides | 1.66 (1.18) | 1.6 (1.29) | 0.576 |
| LDL | 2.86 (1.28) | 2.71 (1.29) | 0.869 |
| HDL | 1.0 (0.342) | 1.02 (0.357) | 0.77 |
| History of AH | 180 (96.8%) | 300 (98.7%) | 0.147 |
| History of DM | 41 (22.0%) | 59 (19.4%) | 0.482 |
| Predictors | Univariate Logistic Regression | Multivariate Logistic Regression | ||
|---|---|---|---|---|
| OR; 95% CI | p | OR; 95% CI | p | |
| LVEF, % | 0.951; 0.925–0.977 | <0.001 * | 0.959; 0.926–0.993 | 0.017 * |
| SBP | 0.988; 0.975–1.000 | 0.059 | 0.984; 0.960–1.009 | 0.209 |
| DBP | 0.982; 0.962–1.002 | 0.082 | 1.013; 0.970–1.058 | 0.555 |
| Lymphocytes | 0.965; 0.936–0.995 | 0.021 * | 0.977; 0.908–1.051 | 0.535 |
| neutrophils | 1.031; 1.004–1.059 | 0.026 * | 0.998; 0.943–1.055 | 0.932 |
| NLR | 1.040; 0.987–1.094 | 0.139 | ||
| Leukocytes | 1.159; 1.074–1.252 | <0.001 * | 1.180; 1.052–1.323 | 0.005 * |
| Platelets | 1.004; 1.001–1.007 | 0.009 * | 1.001; 0.996–1.005 | 0.743 |
| Hemoglobin | 0.985; 0.970–0.999 | 0.037 * | 0.975; 0.956–0.995 | 0.014 * |
| APTT | 1.018; 1.003–1.033 | 0.018 * | 1.021; 1.003–1.039 | 0.025 * |
| Fibrinogen | 0.861; 0.681–1.089 | 0.212 | ||
| INR | 1.474; 1.053–2.061 | 0.023 * | 1.087; 0.699–1.690 | 0.712 |
| D-dimer | 1.000; 1.000–1.001 | 0.103 | ||
| Troponin | 0.958; 0.920–0.996 | 0.033 * | 0.998; 0.972–1.024 | 0.884 |
| ALT | 1.007; 1.000–1.013 | 0.034 * | 1.002; 0.991–1.013 | 0.721 |
| AST | 1.005; 1.002–1.008 | 0.001 * | 1.005; 1.000–1.010 | 0.042 * |
| Glucose | 1.134; 1.054–1.220 | 0.001 * | 1.080; 0.962–1.212 | 0.192 |
| Urea | 1.020; 0.946–1.101 | 0.602 | ||
| Creatinine | 1.001; 0.998–1.004 | 0.420 | ||
| CRP | 1.008; 1.001–1.016 | 0.035 * | 1.009; 0.999–1.020 | 0.085 |
| Triglycerides | 0.893; 0.682–1.169 | 0.409 | ||
| LDL | 1.312; 0.994–1.732 | 0.042 * | 1.421; 1.013–1.992 | 0.056 |
| HDL | 0.485; 0.195–1.208 | 0.120 | ||
| Female gender | 0.383; 0.152–0.965 | 0.042 * | 0.601; 0.294–1.228 | 0.162 |
| History of COVID-19 | 1.763; 1.016–3.059 | 0.044 * | 2.050; 1.041–4.035 | 0.038 * |
| History of AH | 1.216; 0.151–9.777 | 0.854 | ||
| History of DM | 1.420; 0.754–2.675 | 0.278 | ||
| Predictors | Univariate Logistic Regression | Multivariate Logistic Regression | ||
|---|---|---|---|---|
| OR; 95% CI | p | OR; 95% CI | p | |
| SBP | 1.001; 0.990–1.011 | 0.901 | ||
| DBP | 0.994; 0.975–1.012 | 0.500 | ||
| HR | 0.991; 0.975–1.007 | 0.284 | ||
| LVEF | 1.008; 0.983–1.033 | 0.528 | ||
| Lymphocytes | 1.029; 1.004–1.054 | 0.021 * | 1.043; 0.971–1.121 | 0.245 |
| neutrophils | 0.982; 0.962–1.003 | 0.098 | 1.040; 0.978–1.106 | 0.209 |
| NLR | 0.869; 0.766–0.987 | 0.030 * | 0.849; 0.647–1.114 | 0.238 |
| Leukocytes | 0.945; 0.874–1.022 | 0.159 | ||
| Platelets | 1.003; 1.000–1.005 | 0.014 * | 1.004; 1.001–1.007 | 0.072 |
| Hemoglobin | 1.000; 0.990–1.009 | 0.987 | ||
| APTT | 1.000; 0.985–1.016 | 0.982 | ||
| Fibrinogen | 0.992; 0.939–1.047 | 0.761 | ||
| INR | 0.773; 0.432–1.385 | 0.387 | ||
| D-dimer | 1.000; 0.999–1.000 | 0.530 | ||
| Troponin | 0.994; 0.971–1.016 | 0.571 | ||
| ALT | 0.995; 0.987–1.004 | 0.289 | ||
| AST | 0.999; 0.995–1.003 | 0.508 | ||
| Glucose | 0.967; 0.891–1.050 | 0.427 | ||
| Urea | 1.000; 0.934–1.071 | 0.992 | ||
| Creatinine | 0.998; 0.993–1.003 | 0.517 | ||
| CRP | 0.989; 0.976–1.001 | 0.072 | 0.987; 0.973–1.001 | 0.065 |
| Triglycerides | 0.734; 0.558–0.967 | 0.028 * | 0.836; 0.660–1.060 | 0.138 |
| LDL | 1.118; 0.884–1.413 | 0.352 | ||
| HDL | 1.711; 0.989–2.956 | 0.055 | 1.712; 0.931–3.152 | 0.084 |
| Female gender | 0.878; 0.513–1.502 | 0.636 | ||
| History of CVI | 1.576; 0.997–2.492 | 0.052 | 1.720; 1.034–2.863 | 0.037 * |
| History of AH | 2.111; 0.264–16.878 | 0.481 | ||
| History of DM | 1.191; 0.690–2.054 | 0.531 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ygiyeva, D.; Batenova, G.; Belikhina, T.; Orekhov, A.; Pivin, M.; Biakhmetova, Z.; Sadykova, L.; Zhumagaliyev, A.; Pivina, L. Impact of COVID-19 on the Risk of Coronary Stent Thrombosis and Restenosis: A Retrospective Angiographic Study. COVID 2025, 5, 168. https://doi.org/10.3390/covid5100168
Ygiyeva D, Batenova G, Belikhina T, Orekhov A, Pivin M, Biakhmetova Z, Sadykova L, Zhumagaliyev A, Pivina L. Impact of COVID-19 on the Risk of Coronary Stent Thrombosis and Restenosis: A Retrospective Angiographic Study. COVID. 2025; 5(10):168. https://doi.org/10.3390/covid5100168
Chicago/Turabian StyleYgiyeva, Diana, Gulnara Batenova, Tatyana Belikhina, Andrey Orekhov, Maksim Pivin, Zhanerke Biakhmetova, Laila Sadykova, Adilzhan Zhumagaliyev, and Lyudmila Pivina. 2025. "Impact of COVID-19 on the Risk of Coronary Stent Thrombosis and Restenosis: A Retrospective Angiographic Study" COVID 5, no. 10: 168. https://doi.org/10.3390/covid5100168
APA StyleYgiyeva, D., Batenova, G., Belikhina, T., Orekhov, A., Pivin, M., Biakhmetova, Z., Sadykova, L., Zhumagaliyev, A., & Pivina, L. (2025). Impact of COVID-19 on the Risk of Coronary Stent Thrombosis and Restenosis: A Retrospective Angiographic Study. COVID, 5(10), 168. https://doi.org/10.3390/covid5100168






