Abstract
This review provides a comprehensive summary of evidence to explore the role and value of differential diagnosis in the management of Acute Respiratory Infections (ARIs) through point-of-care (POC) rapid testing in a post-pandemic scenario, paying particular attention to coronavirus disease 2019 (COVID-19), influenza, and respiratory syncytial virus (RSV). The document builds on a review of literature and policies and a process of validation and feedback by a group of seven experts from Latin America (LATAM). Evidence was collected to understand scientific and policy perspectives on the differential diagnosis of ARIs and POC rapid testing, with a focus on seven countries: Argentina, Brazil, Chile, Colombia, Costa Rica, Mexico, and Peru. The evidence indicates that POC rapid testing can serve to improve ARI case management, epidemiological surveillance, research and innovation, and evidence-based decision-making. With multiple types of rapid tests available for POC, decisions regarding which tests to use require the consideration of the testing purpose, available resources, and test characteristics regarding accuracy, accessibility, affordability, and results turnaround time. Based on the understanding of the current situation, this document provides a set of recommendations for the implementation of POC rapid testing in LATAM, supporting decision-making and guiding efforts by a broad range of stakeholders.
1. Scope and Methodology
This document seeks to explore and position the role and value of differential diagnosis in the management of Acute Respiratory Infections (ARIs) through point-of-care (POC) rapid testing in a coronavirus disease 2019 (COVID-19) post-pandemic scenario, paying particular attention to COVID-19, influenza, and respiratory syncytial virus (RSV). The document provides an overview of the types of tests available for POC testing, the value of differential diagnosis for the management of ARIs, the policies and current recommendations by international organizations for ARI testing, and the challenges and barriers to implementing POC rapid testing. Based on the available evidence, the document provides a set of actionable solutions (recommendations) to the challenges and barriers identified. By illustrating a path forward, the recommendations can support decision-making and guide efforts by a broad range of stakeholders, including governments, the academic community, and international organizations.
The methodology employed to build this document derives from a review of literature and policies, followed by a process of validation and feedback with a group of seven experts on relevant fields. Experts were selected based on their academic merit and experience. Their disciplinary backgrounds include infectious diseases, public health, diagnostics, and microbiology. An in-depth understanding of diagnostics, the ARI policy landscape, and the COVID-19 pandemic were deemed essential.
Global, regional, and country-level evidence from seven focus countries—Argentina, Brazil, Chile, Colombia, Costa Rica, Mexico, and Peru—was collated and analyzed between 13 March and 5 July 2023. Data and evidence came from diverse sources, prioritizing peer-reviewed pieces, and official governmental and international organizations’ sources. Sources were selected and prioritized to capture the following dimensions:
- Multidimensional impact of ARIs in the region and focus countries.
- Opportunities and challenges for the management of ARIs in a COVID-19 post-pandemic scenario.
- Scientific perspectives and positions on the role and value of testing, POC rapid testing, and differential diagnosis for the management of ARIs.
- The current international guidelines and recommendations regarding testing for ARIs, including POC rapid testing regionally and in focus countries.
- Key policies, frameworks, and recommendations for the management of ARIs regionally and in focus countries.
The information was synthesized in a working document which was then discussed, reviewed, and validated by all experts during three online panel sessions (2, 6, and 21 June 2023) and rounds of offline review. All participating experts approved the final document.
2. Background and Introduction
On 31 December 2019, Chinese authorities reported a novel coronavirus causing a cluster of pneumonia-like cases. The virus was later identified as the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), and the disease caused by this new virus is COVID-19. By March 2020, only three months later, the World Health Organization (WHO) characterized the COVID-19 outbreak as a pandemic [1]. In July and October 2020, the European Medicines Agency (EMA) and the U.S. Food and Drug Administration (FDA) approved the first COVID-19 treatment (remdesivir) [2,3]. With the objective of putting a halt to the pandemic, LATAM countries began vaccination during December 2020 and January 2021 [1]. Finally, in May 2023, the WHO declared an end to COVID-19 as a global health emergency [4].
The timeline of the measures taken and the key events that characterized the influenza pandemic ten years earlier are similar, especially regarding the accelerated development and introduction of treatment and immunization technologies (Figure 1). The first human infection of the latest registered pandemic of influenza A (H1N1) virus was recorded in California on 15 April 2009. Only a few days later, on 27 April, the FDA issued the emergency authorization of two neuraminidase inhibitors (zanamivir and oseltamivir phosphate) for the treatment and prophylaxis of influenza [5]. By June 2009, the WHO characterized the influenza outbreak as a pandemic [6,7]. In July of the same year, clinical trials to develop a vaccine began, and by September, both the FDA and EMA announced the approval of four vaccines [6,7,8]. Since then, and as of today, annual vaccination is recommended for high-risk groups [6,7]. As the number of cases decreased, the WHO declared an end to the pandemic on 10 July 2010 [6,7].
Likewise, the development and introduction of tests for COVID-19 and influenza happened within the first month of the first recorded case [6,9]. As cases increased and demand rose, health systems had to quickly adapt to ensure the availability of tests and laboratory capacity to process samples [10,11]. The influenza and COVID-19 pandemics led to changes in the diagnostic algorithms, prioritizing the novel virus. After the influenza pandemic, the diagnostic algorithm remained without significant changes from 2009 to 2020, when testing priorities rapidly shifted due to the arrival of COVID-19 [12,13,14]. While SARS-CoV-2 continues to be predominant, testing for this pathogen will continue to be prioritized [15].
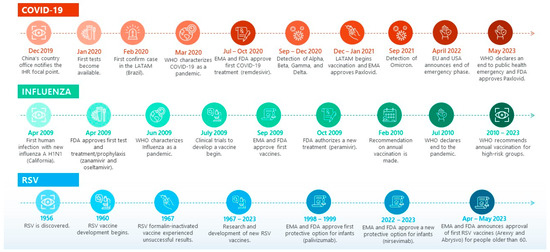

Figure 1.
ARI key events timeline. Source: elaborated based on reviewed reports and literature [1,2,3,4,5,6,7,8,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33].
In contrast, the timeline of key events for RSV shows a pattern of slower progress, mainly attributed to knowledge gaps regarding immunological protection and the unsuccessful results of RSV formalin-inactivated vaccine development experienced in 1967 [20]. In a turn of events, over the past decade there has been significant progress in the knowledge of RSV molecular and structural biology and the human immune response, leading to several promising monoclonal antibodies (mAbs) [34] and RSV vaccines undergoing clinical trials [35]. These efforts have culminated in the approval by the EMA and FDA of two protective options for infants: palivizumab (approved by the FDA in 1998 and by EMA in 1999) [32] and nirsevimab (approved by the EMA in September 2022 and by the FDA in July 2023) [22,33], as well as the first RSV vaccines (Arexvy and Abrysvo) for people older than 60, between April and May 2023 [23,24,27].
While the WHO declared the end of COVID-19 as a public health emergency, this does not mean that COVID-19 is no longer a global threat; only the acute phase of the pandemic is over [4]. COVID-19 will not disappear, but countries will be able to transition towards integrating COVID-19-related measures into routine health services and programs, eventually reaching an endemic stage [36]. Endemicity does not necessarily mean a disease is rare or mild, posing no public health risks. Rather, it means that infection rates remain static, even if high SARS-CoV-2 variants will likely continue to emerge, some of which might be more transmissible and immune-evasive [37]. While it is true that population immunity against SARS-CoV-2 continues to accumulate and may help compensate for the impact of such a scenario, SARS-CoV-2 evolution is inherently unpredictable. It is possible that a recombinant could emerge with high transmissibility linked to intrinsic biology and novel antigenic properties [38]. Thus, while countries transition to a post-pandemic scenario, COVID-19 efforts, including testing and surveillance, should continue as a priority.
Endemicity is an epidemiological term used to describe a state of constant presence and/or usual prevalence of a disease or infectious agent in a population within a geographic area [39]. When an infection becomes endemic, there are different ways in which immunity provides protection without eliminating the virus. In the case of SARS-CoV-2, where neither vaccination nor infection warrants life-long immunity, understanding how different aspects of protection (reduction in susceptibility to infection and reduction in pathology) wane with time and how they are boosted by natural infection and vaccination is critical [40,41]. As the world moves into an endemic stage, countries will also face the challenge of increasing lagging vaccination rates [42,43], while at the same time having the opportunity to improve the technology and reach of testing [44]. In this context, the value of innovative testing technologies, such as rapid tests (including multiplex tests), might become evident [44].
2.1. Vaccines and Treatments Available and in the Pipeline for ARIs
An overview of the vaccines and treatments available and in the pipeline for ARIs demonstrates a considerable investment in innovation. The development of new technologies might open an opportunity to question the role and value of the differential diagnosis of ARIs, as diagnosis might be a gatekeeper to access adequate treatment.
There are currently three types of vaccines available against COVID-19: mRNA vaccines, protein subunits vaccines, and viral vector vaccines [45]. As of May 2023, there are currently four vaccines undergoing the review/market authorization process in the EMA [46]. The same number have received emergency use authorization by the FDA [47]. Regarding treatments, there are currently three main types available: antiviral medication, immune modulators, and mAbs. Antiviral medicines are used in mild to moderate COVID-19 cases for people who are more likely to get very sick and can be administrated through oral and IV infusion forms [48]. Immune modulators are prescribed to help suppress hyperinflammation when COVID-19 triggers a hyperactive reaction of the immune system. These drugs are used to treat adults and children who are hospitalized and require supplemental oxygen or mechanical ventilation [49]. mAbs are proteins made in laboratories that act like antibodies. Laboratory-made mAbs help stimulate the immune system and are a prime example of personalized therapeutics. While both the FDA and EMA have authorized antiviral medications and immune modulators [3,49,50,51], the FDA has not yet authorized mAbs for COVID-19 [49]. As of October 2022, there are 111 COVID-19 treatments in the research and development phase according to the EMA, of which 62 are antiviral medicines [51]. Similarly, according to data from the FDA (as of January 2023), there are 720 drug-development programs in the planning stages, and 440 trials have been reviewed [47].
For influenza, there are multiple types of vaccines available in the market, including standard-dose flu shots, cell-based flu shots, recombinant flu shots, high-dose flu shots, adjuvanted flu shots, and live attenuated flu nasal spray vaccines. Influenza vaccine composition is updated regularly according to the incidence of circulating variants from the previous year (these may or may not be new) [52]. Regarding treatment, antiviral medicines for influenza can be used to prevent or treat infection. There are two classes of antiviral agents that are globally approved and available: neuraminidase inhibitors (NAIs) and M2 inhibitors (adamantanes) [53,54]. Since adamantanes are not active against influenza B strains and there is widespread resistance among H1N1 and H3N2 influenza A strains [53,55], NAIs are the only influenza antivirals currently recommended by the WHO [54]. There is only a small number of alternative agents with potential effectiveness against NAI-resistant strains [56]; thus, the development of novel drugs with a broad spectrum, better bioavailability, easier administrative pathways, and fewer adverse effects is crucial [54,57]. So far, the FDA has authorized three NAIs and one adamantane [57,58], while the EMA has authorized two NAIs and no adamantanes [53]. At the time of this study, there are six influenza antiviral medicines that are in the pipeline and have received EMA advice [59].
Regarding RSV, there are currently two vaccines (Arexvy and Abrysvo) recommended for older adults recently approved by the EMA and FDA [23,24,27,60], one for pregnant women and newborns from birth through 6 months through passive protection (Abrysvo) [60,61] and one preventive option for infants up to 24 months (nirsevimab) [22,33]. In light of the global RSV vaccine development pipeline, the WHO developed a guideline in 2020 to facilitate the international development and assessment of candidate RSV vaccines [21,62]. As of January 2023, there are 12 vaccines and mAbs in phases 2 and 3 of clinical trials [63].
2.2. Misdiagnosis of ARIs and Its Impact on Drug Resistance
While resistance to antiviral drugs is often a consequence of virus evolution, a natural phenomenon, evidence indicates a growing drug resistance due to drug-induced selective pressure [55,56,64]. Antimicrobial resistance seems to be accelerated due to the inappropriate use of antimicrobials, as well as their excessive prescription [65]. In fact, antibiotics are frequently unnecessarily administered for ARIs [66,67], often due to incorrect diagnoses, apprehension regarding bacterial co-infections, or dismissal of the detrimental effects of unnecessary antibiotic use [67]. Evidence indicates that up to half of patients’ use of antibiotics is unnecessary or inappropriate [65]. Thus, there is a need to improve patient treatment stewardship, prescription guidelines, and monitoring resistance [64,66,68]. Better and adequate use of treatments requires timely and accurate diagnosis, for which access to sensitive and timely diagnostic tests is particularly important [66,69]. This benefit has been documented in influenza cases, where rapid diagnostic testing has helped reduce the unnecessary use of antibiotics in positive cases and led to adequate treatment of bacterial infections in negative cases [70].
3. Impact of ARIs and COVID-19 in LATAM
As of 22 November 2023, the WHO reported 772,166,517 confirmed cases of COVID-19, leading to 6,981,263 deaths worldwide [71]. According to the reported number of cumulated deaths, the Americas profile as the worst-hit region of the world, even though it only accounts for 8.4% of the world’s population [72]. Deaths reported in the region sum to 2,983,561, which equates to approximately 42.7% of confirmed associated deaths worldwide [71]. Within the Americas, LATAM countries have been hit the hardest by the COVID-19 pandemic. Whereas reported deaths per million people are 875.38 globally, in the Americas this figure increases to 2689.55 for North America and Central America, and 3105.43 for South America (as of 18 November 2023) [73]. Excess mortality estimates also confirm the disproportionate effect of COVID-19 in the region. Excess deaths in LATAM (combining 2020 and 2021) are estimated at 2,273,620, which represents 15% of the total excess deaths in the world [74].
Based on cumulative deaths per million inhabitants, as of 18 November 2023, Peru (6511.89) and Brazil (3272.71) are disproportionately affected compared to countries like Costa Rica (1819.78) and Mexico (2625.69) [73]. According to excess mortality, Peru and Mexico suffer more than other countries in the region, recording estimates of 45.50% and 34.35%, respectively [74]. Current data on the burden of diseases of COVID-19 must be considered carefully, as some LATAM countries stopped reporting and/or updating COVID-19 cases and deaths on 31 May 2023. This can lead to an artificial perception of a drop in both measures [75].
Regarding testing, according to COVID-19 tests applied per 1000 inhabitants, it is possible to see that Chile had a stronger testing strategy, making tests available to more of the population when compared to other countries in the region (see column 6 of Table 1) [76,77]. Similarly, regarding vaccination, Chile has the highest vaccination rate among the focus countries, with a rate of 92.68%, 20 percentage points above the LATAM estimate. Mexico, on the other hand, is the only focus country that performed below LATAM estimates, recording a vaccination rate of 63.09% (see column 8 of Table 1) [71].

Table 1.
Impact of COVID-19 in focus countries: Argentina, Brazil, Chile, Colombia, Costa Rica, Mexico, and Peru.
Nevertheless, a cautious and critical perspective is essential when looking at official COVID-19 statistics, as circumstances might be much worse. Countries in the region faced challenges regarding the detection and reporting of cases and deaths, which may have led to an under-registry of COVID-19 cases. This reality might considerably limit efforts to compare impact across countries. LATAM countries use two systems of collection and reporting of COVID-19-associated deaths, one from civil registries (e.g., Brazil) and the other from health system reports derived from surveillance systems and the synthesis of hospital reports and clinical histories (e.g., Mexico) [80,81]. Even though countries followed Pan American Health Organization (PAHO) recommendations to certify and code deaths [82], figures are not fully comparable between countries given the influence of data collection and publication systems [80]. Furthermore, data are also subject to changes and updates. For example, most countries consider within their protocols the recodification of deaths due to new test results and change accordingly in the registry or update the death certificates [83,84,85,86,87].
In terms of the burden of the disease of other ARIs, during the COVID-19 pandemic, influenza and RSV activity declined globally and most notably at the onset [12,88]. Nonetheless, this decline was also heterogeneous across countries and trimesters between March 2020 and September 2021, according to demographic, socio-economic, weather, and COVID-19 characteristics [88]. The observed reduction in the community prevalence of non-SARS-CoV-2 ARIs during the COVID-19 pandemic is undeniably multifactorial. Many authors attribute this reduction to changes in the circumstances derived from the implementation of SARS-CoV-2 control measures, such as the use of non-pharmaceutical interventions, changes in health behaviors, reductions in people’s mobility (travel), virus-specific transmission factors, changes in testing priorities and surveillance systems, and repurposing of hospitals and test centers [12,13,14,89,90,91,92]. However, changes in the prevalence of non-SARS-CoV-2 ARIs could also be associated with the displacement of other ARI viruses as a novel agent is incorporated (in this case SARS-CoV-2). This is better known as the theory of the ecological niche of viruses, referring to the place each virus occupies in the ecosystem as it dynamically varies according to weather conditions and the presence of other pathogens. According to this theory, the incorporation of a new seasonal virus usually causes a displacement of other viruses (positive interactions between viruses might also be observed) [93,94].
Looking beyond the pandemic, the literature demonstrates a gap in the reporting of respiratory infections in the region. The quality of the data and the possible under-reporting of influenza morbidity and mortality limits the possibility of accurately calculating the burden of disease [95]. Similarly, for RSV, there is a considerable scarcity of data in LATAM [96,97]. National and subnational surveillance is weak in most parts of the world due to the limited capacities of the National Influenza Centers (NICs), insufficient funds, lack of intersectoral coordination, and varying commitment to surveillance by local governments [98].
To investigate the viruses in circulation, the WHO established the Worldwide Influenza Centre in 1948 and the Global Influenza Surveillance and Response System (GISRS) in 1952 [99]. The GISRS is a global mechanism of surveillance, preparedness, and response, undertaking epidemiology and disease monitoring and global alerts for novel influenza viruses and other respiratory pathogens, and collects evidence from the NICs [100]. Surveillance systems enabled the WHO to issue recommendations regarding influenza vaccine composition, which has been performed annually since 1973 and biannually since 1999. Today, the WHO issues two different sets of recommendations every year: one for the northern hemisphere in February and one for the southern hemisphere in September [101].
The WHO piloted a surveillance strategy for RSV based on the GISRS [102]. The pilot took place in two phases (the first covering 2016–2018, and the second between 2018 and 2021) [103]. Today, most LATAM countries use their Severe Acute Respiratory Infection (SARI) and influenza-like illness (ILI) systems to identify possible RSV cases [104]. RSV surveillance data efforts aim to collect evidence on the more severe cases, virus types, seasonality, risk groups, and disease burden, using this evidence to support public health measures and inform RSV vaccination policy [102,104]. Nonetheless, evidence suggests the under-reporting of RSV as algorithms for respiratory diseases only consider RSV testing in limited scenarios [105,106].
According to FluNet’s (a global web-based tool for influenza virological surveillance that records data from the NICs) latest report (2023) [107], influenza and SARS-CoV-2 are the two highest respiratory virologic pathogens in the Americas based on the percentage of recorded cases, accounting for approximately 7% and 13%, respectively. Within the Americas, Central America is the subregion with the highest percent of positivity for SARS-CoV-2 (17.1%) and influenza (14.9%). Notably, approximately 12% of respiratory cases in the Americas correspond to other unidentified respiratory viruses, accounting for 32% of recorded cases in Central America. Even though ARIs are surveilled through the GISRS and are included in epidemiological surveillance weekly reports, the data reported by NICs are heterogeneous. This often creates an inability to recognize many different respiratory viruses, challenging the possibility of capturing the real burden of ARIs and the accurate comparison between countries.
Regarding focus countries, Brazil records the highest burden in terms of prevalence and incidence of both upper and lower respiratory infections, followed by Colombia in the case of upper respiratory infections and Peru for lower respiratory infections (Table 2) [108]. According to evidence on the cumulative percent positivity rate of influenza and RSV (2022), Mexico profiles as the country most affected by influenza (34%), and Argentina and Chile for RSV (7%) [109]. It is important to note that a high positivity rate can be observed when there is a high number of positive tests, but the same holds if the number of total tests is too low [110]. When comparing the number of samples for influenza, Mexico has 10,314, while Argentina has more than 20,000 samples [110].

Table 2.
Impact of respiratory infections, influenza, and RSV in focus countries: Argentina, Brazil, Chile, Colombia, Costa Rica, Mexico, and Peru.
It is also important to consider the implications directly associated with long COVID and other COVID-19 sequels (e.g., multisystem inflammatory syndrome [111]). Long-COVID’s prevalence in LATAM might reach 29 million cases [112]. Characterized by multiple symptoms such as chronic fatigue, lung damage, anxiety, and depression [113,114], long COVID is a multidimensional disability that negatively impacts physical, mental, and cognitive health, affecting daily activities and social, family, and employment relationships [113]. Often, patients suffering from long COVID lack an understanding of their condition, leading them to not seek help or prioritize recovery [115]. In LATAM, where health systems often fail to adequately prevent and control chronic diseases, the impact is expected to be higher [116]. Thus, there is a pressing need to design and implement measures to address this new pandemic [112]. The unveiling of long COVID has come alongside an increased understanding of long-term sequels of other respiratory diseases as well. For example, sequels associated with RSV on children’s developmental trajectory of reduced lung function have also been evidenced in the literature [117,118].
The COVID-19 pandemic’s long-lasting impacts go beyond health, transcending social and economic dimensions. In LATAM, the pandemic led to an economic contraction and a regression in social indicators. In 2020, LATAM experienced a 6.8% drop in Gross Domestic Product (GDP) and a 7.7% drop in GDP per capita, the largest annual decline observed in the 120-year statistical history of the region [119,120,121]. Due to an increase in the unemployment rate (3% between 2019 and 2020) [119,122] and declining income, people’s capacity to access basic services worsened, leading to a rise in poverty and extreme poverty in the region. According to estimates from 2021, approximately 86 million people are living in extreme poverty, and 201 million people are living in poverty, which represents 13.8% and 32.1% of the total LATAM population, respectively [119,120,121]. The pandemic also increased social inequalities [122]. COVID-19 did not affect all population groups equally; women, children, and essential workers were most impacted [122]. As countries struggled to cope with the pandemic, the region also witnessed an eruption of social protests and a shift in political trends characterized by an increase in authoritarian practices and corruption, weakened democratic institutions, politicized judicial systems, and overall high levels of crime and violence [120].
Other ARIs also have negative socio-economic implications in LATAM. A literature review on influenza, for example, concluded that there is a significant economic burden related to hospitalization, treatment, and other resource expenses [95]. In South America, ARIs have been found to negatively impact health and productivity, representing a cost of USD 834 million, 0.024% of the combined GDP of countries [123]. Similar to the socio-economic spillover of the COVID-19 pandemic, a study on the impact of the influenza pandemic of 2009 in Mexico found that the costs associated with medical care during the pandemic were a smaller fraction than the costs associated with the impact on other sectors due to the measures taken to prevent the transmission [124].
Many valuable lessons can be learned from the COVID-19 pandemic regarding the management of public health emergencies and the pivotal role health plays in sustainable development and well-being. The pandemic exacerbated long-standing challenges faced by health systems across the LATAM region, including the fragmentation of services, inequalities in access, and limited funding and capacity for responding to public health emergencies [122]. The pandemic highlighted that comprehensive health policies are necessary to keep health at the center of sustainable development [125,126], and reinforced the urgent need to restructure health systems, prioritizing a people-centered model based on Universal Health Coverage, guaranteed through more public health expenditure and financial sustainability [126,127,128].
The disruption of ongoing health services, such as screenings and immunization programs, caused by the pandemic [122], as well as behavioral changes (e.g., vaccine hesitancy), calls for measures directed at restoring and reinforcing health programs [127,128,129,130]. Such measures must pay particular attention to mental health and populations that have been disproportionally affected [126,131,132]. Pandemic recovery efforts must be accompanied by global and regional cooperation and coordination mechanisms and frameworks to respond to and prevent public health emergencies. Based on lessons learned from the COVID-19 pandemic, governments across the globe should develop a new pandemic agreement, update the current International Health Regulations (IHR), create a Global Health Fund, and implement national strategies for the prevention and preparedness of respiratory virus-based epidemics and pandemics using an integrated approach [125].
4. Overview of Testing Options Available for ARIs
There are currently four primary types of tests available for respiratory infections: (1) nucleic acid amplification tests (NAATs), which detect the genetic material (nucleic acids) of the virus using upper respiratory specimens; (2) antigen tests, which detect the nucleocapsid protein antigen; (3) serology tests, which detect the presence of antibodies; and (4) viral culture tests, which use culture-based systems for virus isolation.
Testing options can also be organized according to their design to measure or detect a single pathogen or multiple pathogens. While most ARI tests are designed to detect only a single pathogen, such as COVID-19 or influenza, multiplex tests can simultaneously detect or identify multiple pathogens in a single sample [133]. There are two types of rapid multiplex tests currently available that are relevant for POC: antigen and molecular. Multiplex diagnostic tests, sometimes called combo tests, are a particularly valuable tool to reduce misdiagnosis or incomplete diagnosis of infectious diseases that have shared symptoms and clinical features, such as SARS-CoV-2, influenza A or B, and RSV [133,134,135]. Ideally, the diagnosis of infections should be approached by testing for all the potential pathogens rather than testing for just the most likely pathogen and then conducting other tests if the results are negative [133].
4.1. Types of Tests Available for Detecting COVID-19
There are currently three types of tests available for the detection of SARS-CoV-2: NAATs, antigen tests, and serology tests. NAATs detect the genetic material of the virus, in this case the ribonucleic acid sequences, using upper respiratory specimens [136,137]. The reverse transcription-quantitative chain reaction (RT-qPCR) is the reference method for the detection (diagnosis) of current SARS-CoV-2 infection [136]. RT-qPCR tests are commonly applied in laboratory facilities by trained professionals, as sensitivity is higher under these conditions [137]. Moreover, RT-qPCR requires ideal storage conditions for the samples to guarantee sensitivity [138]. Given these conditions, while RT-qPCR testing takes 30 min to 4 h (depending on the test), transportation of specimens might be required. Thus, RT-qPCR test results are usually available within 24 h [136].
Although the RT-qPCR is the most common type of amplification technique used to diagnose COVID-19, its use for POC remains limited due to the potential of error amplification and sequence mismatch [139,140,141] and mandatory requirements for thermal cycling conditions [142]. An effective rapid alternative for POC is the nucleic acid amplification method called isothermal amplification [143]. The loop-mediated isothermal amplification (LAMP) and the nicking enzyme-assisted reaction (NEAR) are two rapid nucleic acid amplification techniques that have gained recent traction.
LAMP is a DNA amplification method that, in combination with reverse transcription (RT-LAMP), has been successfully used for the detection of SARS-CoV-2 [144]. RT-LAMP is a viable alternative to RT-qPCR given its high specificity and sensitivity, cost-effectiveness, minimal instrumentation requirements, and fast turnaround time (typically within 30 min) [145,146]. Nonetheless, this technology has its limitations, namely the difficulty of designing new assays and the risk of false positives (which will require more strict control measures than RT-qPCR) [146]. The risk of false positives has been associated with unintentional primer cross-reactivity at concentrations that result in a quantification cycle (Cq) of 38 and above by RT-qPCR (with matching samples) [147,148] and the premature color change of pH-based dyes for colorimetry [146,149,150]. Recent studies, however, suggest that RT-LAMP can reliably detect viruses in samples that amplify by RT-qPCR at Cq < 30, reaching similar or better sensitivity than RT-qPCR [145,147]. Evidence also indicates promising efforts to reduce the risk of false positives associated with pH-based dyes by using custom saliva stabilization solutions or an alternative extraction method (nucleic acid extraction) [149,151,152,153,154].
NEAR is a novel technique that uses nicking enzymes to improve ordinary isothermal amplification, creating a very promising automated rapid option for POC [146,155]. NEAR has at least three main advantages: the potential for high sensitivity, easy application, and clinically relevant turnaround time. NEAR uses two enzymes, nicking endonuclease and DNA polymerase, for DNA amplification [156,157], with the second having shown improved sensitivity in the past [158]. Since NEAR tests can take place inside the manufacturer’s instrument, they are easy for non-laboratory staff to use, requiring only the instrument and a cartridge to be applied [146]. Given the small size of the amplicon compared to other molecular tests, NEAR has also reduced the results turnaround time significantly (to approximately 5 min for positive results and 15 min for negative results) [159]. Finally, NEAR also seems to adapt better to different temperatures, likely due to the use of different primers, polymerases, and nicking enzymes [146]. Among the disadvantages of NEAR is the risk of false negatives at higher Cq values (usually above 35) and under some conditions, such as the dilution associated with the use of viral transport media prior to amplification [146,160,161,162].
Antigen tests detect viral proteins using upper respiratory specimens. Most often presented in a lateral flow immunoassay (LFIA) format, antigen-detection rapid diagnostic tests (Ag-RDTs) are used to diagnose current SARS-CoV-2 infection. Test sensitivity is higher when performed within five to seven days after symptoms onset [163,164]. Given the brief window of opportunity to provide life-saving treatments, such as Paxlovid, antigen tests might profile as a suitable alternative for timely diagnosis. Paxlovid, which is currently approved for use on mild-to-moderate COVID-19 cases in adults who are at high risk of severe disease (including hospitalization or death), should be initiated within five days after symptoms start [26,28]. Antigen tests are available for professional use, and self-testing is applicable both in hospital and POC settings (home facility, primary care, physician office, pharmacy, etc.), with results available within 15–30 min [136]. Thus, although not as sensitive as RT-qPCR, rapid antigen tests provide a fast, inexpensive, portable, and effective method of testing in laboratory and non-laboratory settings [165]. Due to their conditions and costs, they have been the preferred tests used for diagnosis of SARS-CoV-2 acute infection in LATAM (for more details please see Section 7).
Nevertheless, antigen tests also have certain limitations. Evidence indicates a variable sensitivity among LFIA rapid antigen tests and a generally lower sensitivity when compared to NAAT [166,167,168,169,170,171], resulting in an ongoing debate over the utility of these tests (especially considering that the WHO recommends an 80% sensitivity and ≥97% specificity for these tests) [172]. One of the main factors leading to decreased sensitivity of these tests is the emergence of new virus variants [170,171], inspiring the development of innovative methods to enhance sensitivity. A study comparing the performance of rapid antigen tests for the detection of different SARS-CoV-2 variants and sub-variants found that a test using a flow immunoassay meter was able to detect more virus variants than other tests, which might be due to the meter facilitating a lower limit of detection compared to other options [173,174].
Sensitivity in relation to viral concentration values shows that sensitivity of rapid antigen tests decreases dramatically with increasing Cq value (decreasing viral load), leading to more false-negative results [173,175]. Although there is no definitive Cq value threshold beyond which antigen tests consistently result in false negatives, evidence indicates that rapid antigen tests are frequently negative in RT-qPCR-positive samples with Cq values above 24–28 [176] and have a 100% correlation to RT-qPCR at Cq values ≤ 22 [177]. This is particularly relevant since the Centers for Disease Control and Prevention (CDC) indicates that a Cq value > 33 might reflect a non-contagious stage [178]. Thus, early detection using rapid antigen tests is recommended. Nevertheless, efforts have also been made to create an LFIA test that could detect SARS-CoV-2 in low concentrations through the implementation of sensitivity-boosting strategies such as an increase in antibody concentration in the test line, and the insertion of an intermembrane between the conjugate pad and the nitrocellulose membrane to increase antibody–antigen interaction time, showing promising results [179].
Serology tests detect antibodies generated against the virus from prior infection or vaccination using serum/plasma or whole blood specimens. SARS-CoV-2 antibodies are usually detectable one or two weeks after infection or vaccination. Serology testing is not recommended as a standalone test to identify an active SARS-CoV-2 infection but can be used for retrospective diagnosis, surveillance, and research purposes. Results are usually available within 24 h when performed in hospital settings and within 10–30 min in POC settings [180,181,182].
4.2. Types of Tests Available for Detecting Influenza
There are currently four main types of tests available for the detection of influenza: NAATs, antigen tests, serology tests, and viral cultures. The most common NAATs for the detection of influenza are the rapid molecular assays, since they present sensitivity and specificity values close to those of RT-qPCR, with certain advantages for application at POC [70,183]. These rapid tests, eligible for POC, are usually able to detect influenza type A and type B (wide range of targets) in approximately 30–60 min (instead of the 24 h turnaround time of RT-qPCR). Rapid molecular assays are applied using a nasal swab by medical staff, not necessarily a lab technician, to run a molecular test [70]. Other molecular assays can detect and discriminate between infections with influenza A and B viruses and identify specific seasonal influenza A virus subtypes. The results may take from 45 min to several hours depending on the assay. Among the influenza molecular tests are multiplex assays, particularly useful for the management of critical patients, such as severely immunosuppressed individuals [184,185].
Influenza antigen tests can be divided into two categories: rapid antigen influenza diagnostic tests (RIDTs) and immunofluorescence antigen detection assays. While some RIDTs are approved for use in outpatient settings, others must be used only in a moderately complex clinical setting. RIDTs can differentiate between influenza types (A and B) but do not provide information on influenza type A subtypes. RIDT results are often available in 10 to 15 min, and negative results are recommended to be confirmed with molecular assays. The immunofluorescence antigen detection test delivers results in approximately two to four hours. Like RIDTs, these tests can distinguish between influenza A and B, but not subtypes [184,185].
Serology tests for influenza are not recommended for clinical decision-making but can be used for research, monitoring, and surveillance purposes. A single serum specimen is not reliable for differentiating antibodies for influenza A or B. These tests are not recommended for clinical diagnosis as this would require paring acute and convalescent sera collected two to three weeks apart [184,185].
Viral culture tests for influenza are not recommended to inform clinical management due to their lengthy turnaround time. Shell-vial tissue culture results may take one to three days and traditional tissue-cell viral culture three to ten days. Viral culture methods, however, have an important public health role. Viral culture tests allow for extensive antigenic and genetic characterization of influenza viruses. They are essential for the surveillance and characterization of new seasonal influenza A and B virus strains, facilitating critical information for the biannual review of influenza vaccine composition [184,186].
4.3. Types of Tests Available for Detecting RSV
The main types of tests used for the detection of RSV are the same as for influenza, including NAATs, antigen tests, serology tests, and viral cultures. NAATs for RSV detection are more sensitive than viral culture and antigen testing. NAATs are the recommended method for diagnosing RSV in infants, young children, and the elderly. They are mostly used for critical patients in hospital settings, according to the diagnostics algorithm in their respective countries [19]. Antigen tests are considered an effective method for diagnosing RSV infection in infants and young children. The sensitivity of antigen detection tests generally ranges from 80% to 90% in this age group. Antigen tests are not sensitive for older children and adults as they may have lower viral loads in their respiratory specimens [19]. Serology tests and viral culture for RSV are not used routinely to diagnose infection but may be used by public health officials to track RSV infections [187]. Viral culture for RSV is particularly costly, difficult to perform, and has a lengthy turnaround time, limiting its clinical role. However, viral culture methods may be useful for public health surveillance purposes [19].
While RSV is one of the most common causes of significant respiratory illness in young children and older adults [188], RSV testing is not routinely recommended and often not performed [189,190]. Evidence indicates that this is, at least in part, due to the limited availability of treatment and prophylaxis options [69] and the common under-appreciation of the severity of RSV for some populations [189,190,191]. Since antibiotics are often unnecessarily administered for ARIs due to incorrect diagnoses [66,67], RSV diagnostic testing could help improve antibiotic prescription. Furthermore, diagnosing will also help reduce the current limited availability of real-world RSV epidemiology data [192,193]. In the absence of this information, decision-makers often rely on estimates gathered through prospective studies which are often limited to a small sample size and short study periods [193,194].
4.4. Types of Tests Eligible for POC Rapid Testing
Table 3 summarizes the types of tests eligible for POC rapid testing according to ARI. The table also provides a high-level overview of the advantages and disadvantages associated with the use of antigen rapid tests, the alternative considered one of the most suitable for POC in LATAM. It is important to highlight that while some of these disadvantages are associated with the intrinsic characteristics of such tests, measures can be undertaken to improve performance. These have been presented in Section 4.1.

Table 3.
Types and characteristics of ARI POC rapid tests.
Rapid antigen testing might be one of the most suitable alternatives for POC testing in many LATAM countries. While molecular testing continues to be the recommended method for the diagnosis of COVID-19, the broad use of this method is constrained in low-resource settings due to limited testing capacity, shortages of reagents/supplies, lack of skilled personnel, long turnaround times, and high costs [200,201]. A study on the optimal use of rapid testing in low-resource countries found that the inclusion of Ag-RDTs in testing strategies was cost-effective and critical in increasing timely testing access. The study found that, regardless of the epidemic phase, all countries sampled had insufficient molecular testing capacity to meet the calculated required testing demand within a relevant clinical time (48 h turnaround time) [201]. Furthermore, two studies in Brazil evaluating the replacement of RT-qPCR with Ag-RDTs found that they profile as a cost-effective alternative for the expansion of testing, combating COVID-19, and reducing the impact on the local economy [202,203]. One of these studies found a reduction in the total cost per patient of between USD 130.43 to USD 166.97 and unwanted clinical outcomes (avoiding 2406 to 3208 new cases of COVID-19, 457 to 609 hospitalizations, and 172 to 230 deaths per 38,000 antigen tests performed) [203]. Moreover, maintaining the use of RT-qPCR as the first choice for diagnosing COVID-19 in working-age patients was found to potentially lead to an additional USD 207,515.14 in management costs in the municipality of Itaberá [202]. Evidence from high-resource countries, such as Germany and Italy, have also found economic benefits in using rapid antigen tests in both emergency rooms [204] and COVID-19 testing services [205].
However, additional cost-effectiveness studies are needed to validate the accuracy of the tests as part of such economic evaluations [202] and the role treatment pathways have on potential benefits based on actual practice [206]. Ultimately cost-effectiveness information on POC rapid testing should be used alongside other considerations, such as budget impact and feasibility, as part of a transparent decision-making process [207]. Decisions on the use of rapid molecular and antigen options, and/or the combination of both (e.g., antigen testing for initial screening and molecular testing in case of negative results) should consider cost-effectiveness, saturating testing demand, molecular testing capacity, test accuracy, and testing turnaround times. This is particularly important as new rapid molecular testing technologies become available. While rapid molecular testing is in general more expensive than RT-qPCR, studies from high-resource countries have shown promising results on the cost-effectiveness of such methods in emergency rooms and hospital settings [208,209].
Aside from rapid antigen and rapid molecular tests, POC testing can be supported by the use of rapid multiplex tests [210,211]. Multiplex testing allows for simultaneous on-site detection of different analytes using a single specimen, one of the main reasons why multiplex platforms have recently gained attention, especially for resource-limited settings [212]. There are two main types of rapid multiplex tests currently available for ARIs. The first is the NAAT, a rapid multiplex PCR [213,214]. These tests include various combinations such as influenza A, influenza B, and SARS-CoV-2; influenza A, influenza B, RSV, and SARS-CoV-2; and 20 of the most common respiratory viruses and bacteria causing upper respiratory illness. Information provided through these tests may be used for diagnosis, clinical management, and epidemiological surveillance (including the burden of disease virus surveillance) [215]. The second type is the rapid multiplex antigen test. The most common combinations of these tests include SARS-CoV-2, influenza A, and influenza B [213,216]. These tests can easily be implemented at POC with minimal training [172,173,174]. Rapid multiplex antigen tests can be used for diagnosis, in clinical correlation with patient history and other diagnostic information. As for epidemiological surveillance, these tests can support monitoring of the burden of disease.
5. What Is POC Rapid Testing?
In this section, we present a brief description of POC rapid testing, where (in what settings) this strategy can be implemented, and the benefits this strategy might bring. Using James H. Nichols’ (2020) definition, POC testing involves performing a test outside of laboratory conditions, closer to the site of patient care [217], seeking to better identify or manage chronic diseases and acute infections [218]. POC testing can be performed and interpreted by health personnel or by the individual, a family member, or a caregiver of the individual being tested [195]. In the context of ARIs, POC testing can be used to diagnose current or detect past SARS-CoV-2, influenza, and RSV infections [19,184,195]. Based on the experience of COVID-19, POC testing can be implemented in various settings, including but not limited to physician offices, urgent care facilities, pharmacies, school health clinics, long-term care facilities and nursing homes, temporary locations including drive-through sites managed by local organizations, home self-testing, and other locations such as cruise ships and national and subnational borders [195].
The use of POC testing has several advantages, enabling decentralized, rapid, sensitive, and low-cost diagnosis [219]. Studies demonstrate that effective treatments for confirmed COVID-19 have the potential to offer value for money to healthcare systems, especially if they confer a survival benefit and reduce the need for hospitalization. In this context, diagnostic tests are more likely to be cost-effective if they can provide accurate results quickly [220]. While clinical evidence on the cost-effectiveness of POC testing for COVID-19 is limited and immature [220], even studies using high-cost methods, such as rapid molecular tests, show considerable cost-saving benefits down the line [208,209]. For example, a study found that the use of rapid COVID-19 molecular testing in an emergency department and shock room led to a reduction of USD 285.23 in direct costs in admissions with subsequent surgery, and USD 79.02 without surgery [208]. As evidence grows, a common model for assessing the value for money of COVID-19 diagnostics and treatments, able to capture decision points applicable to different settings and use all available evidence (including real-world evidence), would be beneficial [220].
According to the literature, POC testing can help achieve four main goals [198,217,221]:
- Disease identification: facilitates identifying the disease in a quick manner, allowing decisions to be made regarding adequate treatment and care, which in turn can reduce hospital follow-up visits.
- Disease monitoring: allows monitoring of the disease, including aspects such as the response to medicines.
- Behavior modification: contributes to patients’ capacity to modify behaviors to avoid further transmission swiftly and to improve the patient’s outcome.
- Reduced barriers to care: can also help reduce disparities in access to diagnosis in remote settings.
6. The Role and Value of POC Rapid Testing in the Diagnosis and Management of ARIs in a Post-Pandemic Scenario
This section provides an overview of the role and value of POC rapid testing for the diagnosis and management of ARIs, particularly in a COVID-19 post-pandemic scenario. As the world moves into a post-pandemic scenario, all types of tests will continue to have a critical function from a public health perspective. This is in part determined by certain COVID-19 characteristics. The fact that asymptomatic and presymptomatic populations drive transmission, the duration of infectiousness, the persistent emergence of variants of concern, and the potential for reinfection, makes diagnostic testing a key tool to prevent further infection. In a post-pandemic scenario, COVID-19 testing can be used to (1) improve case management, (2) inform public health policy decision-making, (3) control outbreaks and prevent infections, and (4) support surveillance efforts [197]. With such great value, there is an undeniable pressing need to continue investing in the development of diagnostic technologies and advocacy for broader access to differential diagnosis and testing.
Differential diagnosis of ARIs through POC testing can improve the clinical management of cases, as it supplies health practitioners with critical information to provide adequate and timely treatment and care. In particular, evidence indicates that timely and decentralized diagnosis can reduce the unnecessary or prolonged use of antibiotics (antimicrobial stewardship); improve antiviral prescribing; reduce recurrent infections and persistent secondary infections, hospital admissions, and burden on secondary and tertiary health facilities; and shorten hospital or emergency department lengths of stay [70,133,222,223,224,225,226,227,228]. A systematic review that examines the effects of influenza POC testing found that diagnosis resulted in significantly higher rates of antiviral prescription [70]. Since antivirals are most clinically beneficial if taken within 48 h of symptom onset [229,230,231], faster result turnaround times (facilitated through POC testing) [232,233,234] are especially critical for their effective use [70].
By removing diagnostic uncertainty, POC testing was also found to reduce unnecessary antibiotic prescriptions (in positive influenza cases) and allow bacterial infection to be treated promptly (in negative influenza cases) [70]. This is particularly important as patient-treatment stewardship can help reduce the risks of antibiotic resistance, both at patient and large-scale levels [64,66,68,69,70]. The value of differential diagnosis for case management of influenza might be more easily acknowledged by decision-makers given the availability of treatments and antiviral prophylactics for the general population, unlike RSV [22,69,189,190,191,222,223].
POC testing can also help reduce emergency room length of stay, which can in turn lead to improving clinical outcomes by preventing nosocomial spread (intrahospital room assignment) and easing the burden on the healthcare system. The latter is particularly important as emergency room length of stay can be influenced by hospital capacity conditions, including the availability of beds, overcrowding, and efficiency of healthcare providers, among others. To enhance this positive impact, decision-makers will need to update management protocol and emergency room collaboration to improve clinical decision-making and patient workflow [70].
Furthermore, rapid testing at POC can be an effective way of addressing inequalities in access to diagnosis (a common challenge in LATAM) by reducing the barriers that negatively impact vulnerable communities and rural areas [219,226,235]. By improving access and turnaround times, decentralized testing can help curb the spread of the infection through early diagnosis and optimize infection-control practices [223,226,235,236]. Thus, POC rapid testing can play a role in policy-making, tailoring the response to pandemics, epidemics, and outbreaks according to the needs of each context and epidemiological moment. Diagnostic tests will serve as the eyes and ears of the health care system, sounding alarms about unusual disease patterns or outbreaks that enable an early response [197].
From a policy perspective, information gathered through POC rapid testing can allow for the evaluation of measures used and guide the planning and implementation of programs (including resource allocation) to help prevent and control disease [237]. In this way, POC testing can empower states to adopt newer, faster, and tailored technologies and tracing methods by building rapidly reactive health systems [226,235,236], and the scientific community can continue learning about the viruses (including routes of transmission and immunity) by supplying and analyzing much-needed data [219].
As countries move from pandemic response to living with the virus, one of the main roles of testing will shift toward surveillance efforts. The pandemic unveiled the need for countries to invest in diagnostic and surveillance systems, as well as data connectivity, so that clinicians and policymakers have tools at their disposal to practice precision medicine and rapidly investigate early alerts of possible outbreaks [197]. In LATAM, the PAHO recognized the need to adjust the current ARI surveillance systems to, among other goals, guarantee correct monitoring of the transmission, severity, and impact of COVID-19, as well as the immune response to sequels or episodes after infection [238]. The PAHO also recognized the role of both sentinel and non-sentinel surveillance systems, and the WHO called for continuing the triangulation of sentinel-generated data with other sources (e.g., event-based surveillance, non-sentinel surveillance, and mortality surveillance) [238,239]. Data generated through POC testing, if recorded and reported correctly, can support surveillance efforts. ARI surveillance through POC testing would improve the understanding of the real burden of these diseases, motivating further investment in research and development of new technologies, including vaccines and treatments [240,241].
LATAM has made outstanding progress in strengthening the information and surveillance systems of ARIs during the COVID-19 pandemic. It is essential to continue investing in diagnostic testing and the integration of information systems. This is relevant for all technologies, including ultra-sophisticated detection methods and sequencing technologies at higher levels, but also POC diagnostics at the community level [197]. Evidence points to rapid antigen and serological tests as the most cost-effective alternative to scale COVID-19 POC testing [242,243]. Rapid molecular tests that do not require sophisticated instruments could be an alternative if the higher risk of cross-contamination can be mitigated [242].
Finally, there are many elements that need to be considered for the adequate implementation of POC rapid testing strategies. A study identified 18 key enablers for the successful and rapid implementation of decentralized POC testing [226]:
- National policy, guidelines, and implementation plans.
- Strong governance and consultation.
- Champions from government, community, and health services.
- Shared responsibilities between the POC program and jurisdictional stakeholders.
- Staggered roll-out to learn lessons from the first tier of sites.
- Transparent but strict inclusion criteria due to limited test supply.
- Funding for diagnostics and personal protective equipment.
- Local supply of quality control and external quality assurance materials.
- Robust quality-control development, overcoming cold-chain barriers.
- Use of platforms already in place by a subset of health services.
- Reactive supply chain systems.
- Program website for rapid dissemination of program resources.
- Flexible connectivity systems.
- Referral pathways with accredited pathology providers.
- Capacity-building for health-care workers through a comprehensive set of procedures, posters, and other resources.
- Training and competency assessments delivered virtually, meaning no face-to-face contact is required.
- Monitoring and evaluation systems, including a real-time dashboard to enable management of stock and monitoring of the implementation progress.
- Flexibility in the implementation model to meet different jurisdictional and health service needs.
7. Current Recommendations on the Use of POC Rapid Testing for the Diagnosis and Management of ARIs
Having explored the role and value of POC testing, in this section we provide an overview of the current global, regional, and national policies and recommendations relevant to POC rapid testing. Regarding COVID-19, as of 5 July 2023, the WHO continues to recommend the use of both NAATs and Ag-RDTs for the diagnosis of COVID-19, with the first being defined as the gold standard. However, Ag-RDTs are recommended in settings where NAAT testing capacity is limited [164], a common reality among LATAM countries. In fact, evidence indicates that Ag-RDTs are currently the tests of choice for diagnostic purposes in the focus countries (Table 4) [85,244,245,246,247,248,249,250]. Furthermore, the WHO recognizes the value of Ag-RDTs for POC testing. According to the organization, Ag-RDTs are recommended for community settings, as they do not require sophisticated clinical and laboratory conditions. The organization recommends that in such cases, Ag-RDTs should be performed and interpreted by trained operators, ensuring the accuracy of the results [164]. During the pandemic, antigen tests were largely distributed in many countries, sometimes without proper quality verification or even formal market approval (public health authorities enforced exceptions by fast-tracking approvals through Emergency Use Authorizations) [251]. This may have caused the circulation of low-sensitivity tests in certain countries [252,253].
Following WHO recommendations, the CDC put forward a guide for POC SARS-CoV-2 rapid testing [195]. The guide provides information on the regulatory requirements for POC settings, the collection of samples, and the conditions required to perform rapid tests safely and adequately. In terms of regulatory requirements, the CDC regulates POC testing through four different types of Clinical Laboratory Improvement Amendments (CLIA) certificates. A CLIA-certified laboratory or testing site is obliged to report all positive diagnostic and screening results to the person who was tested or its healthcare provider but is not required to report negative test results. The testing site or laboratory also must report the positive test results to their state, tribal, local, and territorial health department systems [195].
To support the country’s transition from pandemic response to living with the virus, the WHO issued a Strategic Preparedness and Response Plan in May 2023 (2023–2025). According to this plan, testing is transitioning to priority risk groups and individuals with moderate or severe symptoms [254]. In this context, widespread screening of asymptomatic individuals is not currently recommended [255], unless for specific groups at a higher risk due to exposure, such as contacts of confirmed cases [255].
Regarding influenza, the WHO recommends applying laboratory diagnostic testing to differentiate an infection with influenza from other ARIs, outside of epidemic situations and in periods of low activity [18]. Similarly, the CDC conveys the importance of diagnostic testing to differentiate between influenza and COVID-19, particularly as both cannot be differentiated based solely on symptoms [256]. However, during periods of increased influenza activity, the CDC does not recommend diagnostic testing in outpatients. Under such circumstances, testing is only recommended when it can help inform clinical management and decision-making, such as when patients are being admitted to hospital and arrangement of rooms to avoid further intrahospital spreading [184]. WHO and CDC recommendations are similar for RSV. Laboratory testing is only advised to differentiate from other viral respiratory and bacterial infections when the disease is severe or when a patient is admitted to the hospital. Mild and asymptomatic presentations of the disease, or during seasonal outbreaks, are not tested [19,21].
As for surveillance, the WHO calls for countries to maintain core surveillance activities by applying multiple approaches, including sentinel, environmental, participatory, seroepidemiology, and event-based surveillance, among others [254]. The WHO recommends that SARS-CoV-2 testing be integrated into existing respiratory disease surveillance activities, including the GISRS and the Global Coronavirus Laboratory Network (CoViNet) [254]. Furthermore, countries are encouraged to continue strengthening their capacities for genomic surveillance and real-time data collection [254]. Aligned with the WHO, the PAHO has already integrated COVID-19 into the surveillance report of influenza and other ARIs [104].
In this context, the use of multiplex assays is considered a potential asset by the WHO, PAHO, and CDC to support surveillance efforts [15,100,257]. In 2021, the PAHO published a guiding document for the implementation of influenza + SARS-CoV-2 multiplex RT-PCR assay into influenza and COVID-19 integrated surveillance activities [15]. While multiplex assays are currently not recommended for universal COVID-19 surveillance as SARS-CoV-2 is still predominant, it is considered that under a scenario of high or very high influenza community transmission, SARS-CoV-2 confirmation should be prioritized [15]. The CDC recognized the value of multiplex assays for differential diagnosis and surveillance efforts (particularly the influenza + SARS-CoV-2 multiplex) as it helps differentiate SARS-CoV-2, influenza A, and/or influenza B viruses in one test [258]. Undeniably, multiplex assays may assist the process of integrating COVID-19 testing services with testing for other respiratory illnesses such as influenza and RSV [100,257].
Table 4 summarizes the current landscape of relevant policies for the management of ARIs in focus countries. Notably, all countries currently include respiratory illnesses in their National Health Plans and have specific national policies for both respiratory illnesses and influenza. On the contrary, none of the countries have a national policy for RSV. Given the call by international organizations [238] to integrate COVID-19 into ongoing services, we found that by July 2023, most of the countries of interest had incorporated COVID-19 into the National Policy for Respiratory Illnesses (at least by the protocols for surveillance), with Costa Rica and Peru lagging behind [85,103,244,257,259,260,261,262]. While policy progress for respiratory infections is evident, the challenges of implementing said policies remain a concern, particularly regarding the allocation of sufficient resources.


Table 4.
National guidelines and recommendations for the management and testing of ARIs in focus countries: Argentina, Brazil, Chile, Colombia, Costa Rica, Mexico, and Peru.
Table 4.
National guidelines and recommendations for the management and testing of ARIs in focus countries: Argentina, Brazil, Chile, Colombia, Costa Rica, Mexico, and Peru.
| Country | National Health Plan Includes Respiratory Illnesses | National Policy Program for RSV | National Policy Program for Influenza | National Policy for Respiratory Illnesses (NPRI) | NPRI Integrates COVID-19, Influenza, and RSV a | Current Preferred Diagnostic Method for COVID-19 | Purpose of COVID-19 Rapid Testing | Current Recommendations Relevant to ARI Multiplex Testing | Are Multiplex Tests Available in the Market? b |
|---|---|---|---|---|---|---|---|---|---|
| Argentina | Yes [244] | No | Yes [244] | Yes [244] | Yes [244] | Antigen test [244] | Diagnosis, clinical management, surveillance, and control [244] | Recommended for pediatrics (less than 5 years old) and hospitalized patients [244] | Yes [263,264] |
| Brazil | Yes [265] | No | Yes [266,267] | Yes [268] | Partial c [259] | Antigen test [245,246] | Diagnosis, surveillance, and control [245,246] | No mention of multiplex tests in the national guidelines [245,246] | Yes [269] |
| Chile | Yes [270] | No | Yes [271] | Yes [272] | Yes [260] | Antigen test [247] | Diagnosis, surveillance, and control [247,273] | No mention of multiplex tests in the national guidelines | Yes [274] |
| Colombia | Yes [275] | No | Yes [276,277] | Yes [278,279] | Yes d [261] | Antigen and PCR tests [248] | Diagnosis and surveillance [248] | Recommended for hospitalized patients with a negative PCR for COVID-19 [261] | Yes [280] |
| Costa Rica | Yes e [281] | No | Yes [282] | Yes [282] | No | Antigen test [249] | Diagnosis and surveillance [249] | No mention of multiplex tests in the national guidelines [249] | Yes f [283] |
| Mexico | Yes [284] | No | Yes [285] | Yes [85] | Yes [85,262] | Antigen and PCR test [85,286] | Diagnosis and surveillance [85,262] | Recommended in serious cases and deaths covering only 10% of cases [85] | Yes [287] |
| Peru | Yes [288] | No | Yes [289] | Yes [290] | No [250,290] | Antigen test [250] | Diagnosis Surveillance [250] | No mention of multiplex tests in the national guidelines [250] | Yes [291] |
a Assess integration of COVID-19, influenza, and RSV in NPRI. Yes, if all three integrate; partial, if only two integrated; no, if none integrated. b Assess approval of multiplex tests (for at least two pathogens) by the regulatory agencies. c Focused on COVID-19 but includes information for surveillance of influenza and other respiratory viruses. d Plan 2016–2020 outdated, no new plan available. e Specific protocol for surveillance. f Costa Rica has bioequivalence for the approval of products from the agencies of other focus countries included in this research that have approved multiplex tests [292]. Source: Elaborated based on available data from official governmental sources (Ministry of Health, National Health Institutes, Departments of Surveillance, National Regulatory Agencies).
According to current recommendations, the purpose of testing in focus countries centers around diagnosis and epidemiological surveillance [85,244,245,246,247,248,249,250,262,273]. Regarding the use of multiplex tests, only Argentina, Colombia, and Mexico currently have clear recommendations for the use of these assays. Even within these countries, the role of multiplex tests is circumscribed to specific high-risk population groups [85,244,261]. While conditions on when to use multiplex tests for respiratory diseases are not explicit in relevant guidelines, evidence indicates that multiplex tests (SARS-CoV-2, influenza A and B, and other respiratory pathogens), including rapid methods, are currently approved by the regulatory agencies in all focus countries [263,264,269,274,280,283,287,291].
8. Challenges and Barriers to POC Rapid Testing of ARIs in a Post-Pandemic Scenario
A reflection on the value and role of POC rapid testing for ARIs would not be complete without acknowledging the challenges and barriers to the implementation of this strategy identified by international organizations, the scientific and academic community, and governments. Based on the evidence, concerns can be categorized into four groups: challenges and barriers related to (1) intrinsic test limitations and characteristics, (2) the availability of tests and capacity to implement POC rapid testing strategies, (3) the capacity to make adequate use of POC rapid testing results for surveillance purposes, and (4) policies and regulations for POC rapid testing.
8.1. Challenges and Barriers Related to Intrinsic Tests Limitations and Characteristics
Each testing methodology has both benefits and limitations; therefore, decisions on which type of tests to use for POC are based on a tradeoff between test sensitivity, costs, turnaround times, and application requirements. While molecular tests are the gold standard for ARI diagnosis due to their high sensitivity, test results may not be available in a relevant time frame to inform clinical management at POC [293]. Some of the conditions for application, such as the type of equipment needed and sample storage requirements, may limit their application for outpatient or emergency care [293]. Furthermore, while there are some rapid options available, such as for influenza able to detect the virus type A and type B in a reasonable timeframe for POC (15–30 min) [184], molecular tests are in general more expensive.
Although antigen tests are more affordable and easier to implement and use, they are less sensitive than molecular tests [196]. The sensitivity of antigen tests varies according to different factors, including the assay applied [294], the timeframe of sampling after exposure, age groups due to the viral load (as is the case with RSV antigen tests for example), and community prevalence of the virus (populations with low expected prevalence) [198,295]. Less sensitivity may lead to a higher risk of false negative results in people with low viral loads [294]. Thus, in some cases, diagnostic confirmation by a molecular test is required [198]. Use of these tests is not recommended in settings or populations with low expected prevalence of disease and where confirmatory testing by molecular is not readily available [296].
While serology tests have also been considered as an alternative for POC [195], they are not recommended for the diagnosis of an active infection and have limited value for case management [297,298]. The presence of antibodies should not be equated to the individual’s immunity or an active infection [219]. Furthermore, serology tests have not been evaluated to assess the level of protection, which means that if interpreted incorrectly, there is a potential risk of increased transmission due to the false sense of security [219]. Evidence also indicates the possibility of cross-reactivity with other coronaviruses (in the case of COVID-19), posing a challenge to the use of these tests for surveillance purposes [299].
Finally, all test types need constant performance checks. This is particularly challenging for the use of multiplex at POC. Performance assessment of multiplex assays is needed across all known variants at the time of validation, considering simultaneously the potential impact of future variants [219]. The use of rapid multiplex tests at POC is further limited by the conditions needed for their use and current availability and access in the region. Although multiplex tests allow for the detection of different analytes simultaneously on-site [212], not all types of multiplex tests are eligible for POC, as many require laboratories that are certified to perform high-complexity tests (especially for molecular multiplex assays) [210,211]. Eligible rapid multiplex PCR tests for POC can detect a broader range of analyte combinates than multiplex rapid antigen tests; however, only a few options are currently available [213,214]. On the other hand, multiplex rapid antigen tests are more affordable and easier to use at POC [213,216]. Multiplex rapid antigen testing can be conducted outside of a laboratory setting with minimal training [166,196,197]. Finally, the design of better and context-appropriate multiplex techniques is also bounded by the limited availability of epidemiological information on the community circulation of ARIs.
8.2. Challenges and Barriers Regarding the Availability of Tests and Capacity to Implement POC Rapid Testing Strategies
Evidence indicates that POC testing devices have in general limited availability relative to their need in developing countries [300]. Furthermore, major gaps in access to diagnostics have been observed, especially when looking at primary care settings [301]. Access is affected by the high costs of tests, uncertainty regarding who covers their cost, heterogeneous pre-existing infrastructure, and limited availability of financial resources [300,301]. This is particularly concerning as the current health expenditure (% of GDP) for LATAM and the Caribbean is considerably lower than the global value (8.6 and 10.9, respectively, according to data from 2020), and is polarized, ranging from 3.2 in Haiti to 12.4 in Cuba [302]. This means that many countries in the region might struggle to cover the costs associated with POC testing, including adequate capacity building. Furthermore, these circumstances might also position LATAM rural populations at a higher risk. Although transmission may be lower in small cities, access to diagnostics remains essential given the limited capacity these contexts have to manage severe cases and control transmission [122,303].
Other challenges regarding the implementation of POC testing include ensuring adequate use of the tests, from the collection of the sample to the interpretation of the results [219]. Storage conditions of devices may also impact the quality of the results [304]. Furthermore, depending on the type of test used, the limited availability of qualified personnel might be a concern, as the healthcare expert ratio to the general population is relatively low in LATAM [300]. Efforts to build healthcare professional capacity are impeded by significant time constraints and high turnover rates among health service staff [300]. Regarding the interpretation of the test results, the accuracy of RIDTs depends largely on the conditions under which they are used. Minimizing false positive or false negative results must be a consideration [198].
8.3. Challenges and Barriers Regarding the Capacity to Make Adequate Use of POC Rapid Testing Results for Surveillance Purposes
Existing surveillance systems of respiratory viruses face the challenge of integrating COVID-19 into their schemes. According to current recommendations, sentinel surveillance should be one of many sources of information used to triangulate data, including event-based surveillance, non-sentinel surveillance, and mortality surveillance [237,238,239]. Genomic surveillance continues to be essential in a post-pandemic scenario, providing critical information for monitoring the evolution and distribution of circulating variants, and unveiling their association with severity, comorbidities, and age groups, among other risk factors [238]. Genomic surveillance must actively look for emerging agents and new variations in viruses already reported in circulation, and collect samples from different sources, such as humans, animals, and the environment [197,254].
A good surveillance system will need to be able to couple all these conditions in an environment of constrained resources. Failure to address these challenges may lead to under-ascertainment, under-reporting, lack of timeliness of the reports, and incompleteness of the surveillance data [237,305]. There is a need to establish homogeneous regional guidelines and ensure technical support to guarantee that lessons learned, and capacities acquired during the COVID-19 pandemic, translate into better surveillance practices [238].
Reporting and completeness of surveillance data are also restricted by challenges in the registry and reporting of results, especially due to limited capacity and connectivity in remote areas [226]. Reported results sometimes cannot be confirmed due to the inadequate execution of testing and voluntary anonymous reporting. This in turn limits the type and quality of information available to take action during periods of high disease prevalence, such as for case investigation or contact tracing [294].
8.4. Challenges and Barriers Related to Policies and Regulations for POC Rapid Testing
There is a general absence of clear regulatory standards for introducing POC tests. Currently, POC testing is only addressed by laboratory guidelines [300]. There is a need for a regulatory framework that supports access and reimbursement of these technologies. The lack of inclusion of tests in Essential Diagnostic Lists generates uncertainty as to who should cover the tests, negatively impacting access [301]. Moreover, policies for POC testing will need to cover aspects from procurement and approval of tests to the registry of results for surveillance purposes [226]. Moving into a post-pandemic scenario, funding for POC testing might be limited. In this context, testing strategies will need to be strategically deployed to guarantee access to those who need them the most [219]. As to the question of whether differential diagnosis should be a public health priority, there is an imperative need to demonstrate the value and cost-effectiveness of testing strategies, understanding the opportunity for saving costs and reducing suffering down the line, as further burden of the disease on the health system and society is prevented [219].
9. Policy Recommendations
Based on the reviewed evidence, this document puts forward a set of 24 recommendations for the adequate inclusion and implementation of POC testing strategies in LATAM countries in the context of a post-pandemic scenario. The first group of recommendations identifies actions needed to develop evidence and address knowledge gaps. The second and third groups seek to strengthen the capacity to implement POC rapid testing and guarantee adequate means of implementation. Finally, the fourth group addresses the inclusion of POC rapid testing in the local and regional respiratory policies. The recommendations seek to support decision-making in a variety of contexts and guide efforts by a broad range of stakeholders. Considering LATAM’s diverse realities, the following recommendations serve as an ‘umbrella’ that countries can choose from and use according to their needs, priorities, and resources.
9.1. Actions to Develop Evidence and Resolve Knowledge Gaps
- (a)
- There is a need to continue developing evidence on the cost-effectiveness of ARI POC rapid testing. Research institutes and the academic community, coordinated and motivated by governments, should undertake further studies that can provide insights into the value of differential diagnostics for respiratory infections. These studies could focus on generating evidence on the different POC rapid testing methods and their value for clinical management, prognosis, and surveillance.
- (b)
- Governments should commit to and implement measures and policies to actively identify the causing agents of ARI cases in the region, providing a more complete picture of the challenges and priorities that need to be addressed through POC testing, including the use of rapid tests and multiplex tests.
- (c)
- Governments should promote and conduct longitudinal and multicenter studies to overcome the knowledge gaps for the cost-effective use of multiplex tests at POC. Regional collaboration, under the leadership of flagship research centers, might help overcome logistical, resource, and capacity challenges to run such studies individually. As a result, recommendations should be made to enhance the adequate use of these tests for case management, surveillance activities, and public health policy decision-making. Studies should explore the potential benefits of using multiplex tests at POC in terms of costs saved by the health system, including costs associated with the course of the diseases (e.g., hospitalization, multiple interactions with healthcare providers, etc.).
- (d)
- Efforts to resolve knowledge gaps to understand the value of differential diagnostics at POC should pay particular attention to the multidimensional socio-economic impact of ARIs. Studies should also ensure that measures are taken to enhance the comparability of data across countries, allowing evidence to be shared across the region. Countries that have the capacity, ability, and resources to implement studies to develop knowledge and resolve gaps should collaborate with countries that require support, to share knowledge and evidence that can be extrapolated to inform policy decision-making.
- (e)
- Funding the research and development of new tests should be prioritized as new ARI virus variants will continue to emerge that might impact the accuracy of existing tests. Research and development strategies should consider performance verification and validation against potential future variants.
- (f)
- Test innovation efforts should consider the multiple uses of these technologies, including those beyond diagnostic (e.g., tests that are able to provide a prognosis). Tests should be accompanied by detailed guidelines to ensure their adequate use and interpretation.
9.2. Actions to Strengthen Capacity to Implement POC Rapid Testing
- (a)
- The use of antigen or molecular rapid tests for POC differential diagnosis should be considered according to the health systems’ capacity (including laboratory and technical capacity), resources, and costs. Given persistent financial constraints in the health sector in many LATAM countries and the advantages outlined by antigen rapid tests, they profile as the more suitable alternative for POC testing in the region.
- (b)
- Decisions regarding the use of antigen or molecular rapid tests for differential diagnosis need to balance and consider the use of the information provided by such tests, their cost-effectiveness, and other considerations, such as budget impact, feasibility of its implementation in actual practice, testing demand, laboratory capacity, tests accuracy, and testing turnaround times. Using a combination of both for different purposes and contexts might be considered (e.g., using molecular tests for sentinel surveillance purposes and antigen tests for POC diagnostics and case management, or using antigen tests for initial screening and molecular tests in case of negative results).
- (c)
- Governments should allocate dedicated resources to implement an ARI POC diagnostic strategy, addressing aspects of health workforce capacity-building, regulation and procurement of quality diagnostic tests, accessibility, and research and development. In context with limited financial resources, governments might benefit from building public-private partnerships to support addressing capacity-building concerns.
- (d)
- Governments should install and promote a training strategy on POC testing to guarantee that healthcare providers have the necessary skills and knowledge to guarantee the proper use, implementation, and interpretation of POC rapid tests. Training opportunities should be provided at different levels of care and with a particular focus on primary health care.
- (e)
- Governments should prioritize strengthening local capacities and mechanisms for genomic and metagenomic surveillance to be able to timely identify new pathogens associated with respiratory disease outbreaks. This may require investment in infrastructure, laboratory capacity, and technology.
- (f)
- Governments and international organizations should ensure the integration of ARI surveillance systems, both at national and international levels, promoting the interconnectivity between the different surveillance agencies in the LATAM region. Surveillance systems should capture and associate variants with severity, comorbidities, and age groups, among other risk factors.
- (g)
- Governments should ensure that the information collected through POC rapid testing is integrated with a broader health information platform, enhancing the opportunity to continue learning about the risk factors and health impact of COVID-19 and other ARIs.
9.3. Actions to Ensure Adequate Means of Implementation
- (a)
- International organizations (e.g., the PAHO and Southern Common Market) and professional societies should provide guidance and support to national decision-makers on the use of POC rapid tests across different settings and conditions.
- (b)
- International organizations and Ministries of Foreign Relations should align and provide guidelines to regulatory agencies in the region to ensure that approval procedures guarantee high-quality tests are available in the territories. Approval processes should be standardized across the region and ensure tests include information about the conditions and limitations of each methodology.
- (c)
- Policymakers, payers, medical societies, and healthcare providers should form a cross-functional partnership to collaborate on the ongoing development of knowledge related to the diagnosis of respiratory infections.
- (d)
- There should be a multistakeholder strategy for healthcare system strengthening, improved market sustainability, and integration of differential diagnostics into existing epidemic and pandemic response and preparedness plans. This strategy should be informed and supported by governments, medical societies, academic communities, and universities, among others.
9.4. Actions for the Inclusion of POC Rapid Testing in Respiratory Policies
- (a)
- Governments should consider using POC rapid testing to support case management. The differential diagnosis of ARIs at POC might positively impact the clinical management of high-risk patients and the management of disease in the general population when treatments are available, as well as reducing unnecessary or prolonged antibiotic courses (improved antimicrobial stewardship) and hospital admissions.
- (b)
- Given the risks of long COVID and COVID-19-related sequels, as well as sequels from other ARIs, the use of POC rapid testing should be prioritized to promote the early diagnosis of cases and prevent the further spread of infections.
- (c)
- Governments should consider using POC rapid testing to support the monitoring of infections and diseases as well as surveillance efforts. Evidence generated through POC rapid testing can be used for policy decision-making purposes. Evidence collected through POC testing can help monitor the burden of disease over time, control transmission, and prevent and control future outbreaks.
- (d)
- Governments should provide regulatory standards for POC rapid testing considering the conditions for approval, implementation, and the information registry. Regulatory standards will contribute to guaranteeing the quality of the tests (including sensitivity) and proper implementation, contributing to the accuracy of the results. Regulatory standards should be the norm in both the public and private sectors.
- (e)
- Creating a consistent regulatory framework for the standardized approval of COVID-19 rapid tests across LATAM countries might be beneficial. This could include collaborating to establish a regional body similar to EMA for harmonizing the approval process; developing standardized technical requirements for the validation and registration of tests (including sensitivity, specificity, sample type, and testing conditions); creating a common technical dossier format for test manufacturers to submit; establishing and strengthening mutual recognition agreements between countries; and developing comprehensive regulatory guidelines that detail the approval process, including pre-market evaluation, quality control, post-market surveillance, and transparent decision-making, among others.
- (f)
- Governments should consider including POC rapid tests in their national Essential Diagnostic Lists, based on the recognition of the value of diagnosis and disease monitoring and surveillance. Civil societies and patient advocacy groups could advocate for this inclusion.
- (g)
- Governments should include clear guidelines regarding POC rapid testing in relevant respiratory infections policies. Guidelines should specify which test to use, and in what setting, considering test characteristics of sensitivity, accuracy, accessibility, affordability, and the test result turnaround time. The guidelines should also address strategies to reduce access inequalities in the territories.
10. Conclusions
In this document, we provide a comprehensive overview of POC testing, including its benefits, available options, limitations, and challenges. POC testing is an essential tool for the adequate management of ARIs in a COVID-19 post-pandemic scenario, guaranteeing better ARI clinical management and health outcomes. However, evidence also highlights several challenges to the implementation of this strategy, mainly associated with the uncertainty on how to operationalize testing policies in a post-pandemic scenario. Based on the challenges identified, this document puts forward a set of actionable solutions for the implementation of POC rapid testing in LATAM countries. Recommendations illustrate a path forward and can support critical decision-making, guiding efforts by a broad range of stakeholders, including governments, researchers, and academic institutions, among other relevant stakeholders.
There is indisputable evidence of the role and value of POC testing strategies, especially for LATAM countries. Information gathered through POC rapid testing can serve to improve case management, epidemiological surveillance, research and innovation, and evidence-based decision-making. With multiple types of rapid tests available for POC, decisions regarding which tests to use will require careful consideration of the testing purpose and resources available, while also balancing test characteristics regarding accuracy, accessibility, affordability, and results turnaround time. The transition from a COVID-19 pandemic to a post-pandemic scenario risks the prioritization and funding for ARI testing. International organizations have voiced clear concerns and recognized the pivotal role testing will continue to play in the management of COVID-19 and other ARIs moving forward. The benefits of continuing to invest in testing policies may outweigh the costs associated with the economic burden imposed on health systems down the line.
Author Contributions
All authors contributed equally to this work. C.A.A.-M., E.S.A.d.A., E.B., A.M.K., J.E.M.M., P.T. and C.U.-G. served as experts during the online panel sessions and the rounds of offline review. K.A.N.C. facilitated and coordinated the discussion session, rounds of review, and review of the literature to draft the manuscript with experts. All authors have read and agreed to the published version of the manuscript.
Funding
The authors disclose the receipt of financial support from Abbott Laboratories for the research and the discussion process that was part of developing this paper. The authors independently drafted the manuscript’s contents and recommendations, and this manuscript is their product.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors acknowledge the contributions of Hilary Felton (HF), Patricia Salazar (PS), Nadia Vranjac (NV), and Luisa Morales Cabral (LMC) from Policy Wisdom LLC for their support during the process of developing this document and the online discussion sessions. The assistance provided by HF, PS, NV, and LMC was covered by their regular functions at Policy Wisdom LLC.
Conflicts of Interest
Abbott funded the online panel sessions held to develop this final document. The funder sponsored a third-party consulting agency, Policy Wisdom LLC, to facilitate the sessions and coordinate the development of this document but had no role in the agenda of the meetings, nor the design and drafting of the document. The views expressed in this document are exclusively those of the authors and are not influenced by any external parties or sponsors. The authors contributed to their personal capacities, and the recommendations included herein do not necessarily reflect the official positions of their employers or institutions of affiliation.
References
- World Health Organization (WHO). Archived: WHO Timeline—COVID-19. 27 April 2020. Available online: https://www.who.int/news/item/27-04-2020-who-timeline---covid-19 (accessed on 13 August 2023).
- U.S. Food & Drug Administration. FDA Approves First Treatment for COVID-19. 2020. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-covid-19 (accessed on 13 August 2023).
- European Medicines Agency. COVID-19 Treatments: Authorized. 2023. Available online: https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines/treatments-covid-19/covid-19-treatments-authorised (accessed on 3 May 2023).
- United Nations. WHO Chief Declares End to COVID-19 as a Global Health Emergency. UN News, 5 May 2023. Available online: https://news.un.org/en/story/2023/05/1136367 (accessed on 13 August 2023).
- U.S. Food and Drug Administration (FDA). Authorizations of Emergency Use of Certain Antiviral Drugs-Zanamivir and Oseltamivir Phosphate; Availability. 2009. Available online: https://www.federalregister.gov/documents/2009/08/04/E9-18568/authorizations-of-emergency-use-of-certain-antiviral-drugs-zanamivir-and-oseltamivir-phosphate (accessed on 12 August 2023).
- Centers for Disease Control and Prevention (CDC). Influenza Historic Timeline. 2019. Available online: https://www.cdc.gov/flu/pandemic-resources/pandemic-timeline-1930-and-beyond.htm (accessed on 10 August 2023).
- European Centre for Disease Prevention and Control (ECDC). Timeline on the Pandemic (H1N1) 2009. August 2010. Available online: https://www.ecdc.europa.eu/en/seasonal-influenza/2009-influenza-h1n1-timeiline (accessed on 10 August 2023).
- Yu, Y.; Garg, S.; Yu, P.A.; Kim, H.-J.; Patel, A.; Merlin, T.; Redd, S.; Uyeki, T.M. Peramivir Use for Treatment of Hospitalized Patients With Influenza A(H1N1)Pdm09 Under Emergency Use Authorization, October 2009–June 2010. Clin. Infect. Dis. 2012, 55, 8–15. [Google Scholar] [CrossRef] [PubMed][Green Version]
- European Centre for Disease Prevention and Control (ECDC). Coronavirus Disease 2019 (COVID-19) Pandemic: Increased Transmission in the EU/EEA and the UK—Seventh Update; ECDC: Stockholm, Sweden, 2020. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/RRA-seventh-update-Outbreak-of-coronavirus-disease-COVID-19.pdf (accessed on 10 August 2023).
- Pabbaraju, K.; Wong, A.A.; Douesnard, M.; Ma, R.; Gill, K.; Dieu, P.; Fonseca, K.; Zelyas, N.; Tipples, G.A. A Public Health Laboratory Response to the Pandemic. J. Clin. Microbiol. 2020, 58, e01110-20. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Ten Years of Gains: A Look Back at Progress Since the 2009 H1N1 Pandemic. 2019. Available online: https://www.cdc.gov/flu/spotlights/2018-2019/decade-since-h1n1-pandemic.html (accessed on 12 August 2023).
- Chow, E.J.; Uyeki, T.M.; Chu, H.Y. The Effects of the COVID-19 Pandemic on Community Respiratory Virus Activity. Nat. Rev. Microbiol. 2022, 21, 195–210. [Google Scholar] [CrossRef]
- Bish, D.R.; Bish, E.K.; El-Hajj, H.; Aprahamian, H. A Robust Pooled Testing Approach to Expand COVID-19 Screening Capacity. PLoS ONE 2021, 16, e0246285. [Google Scholar] [CrossRef] [PubMed]
- Hills, T.; Kearns, N.; Kearns, C.; Beasley, R. Influenza Control during the COVID-19 Pandemic. Lancet 2020, 396, 1633–1634. [Google Scholar] [CrossRef] [PubMed]
- Pan American Health Organization (PAHO). Guidance for the Implementation of the Influenza and SARS-CoV-2 Multiplex RT-PCR Assay into the Influenza and COVID-19 Integrated Surveillance. 2021 May. Available online: https://www.paho.org/en/documents/guidance-implementation-influenza-and-sars-cov-2-multiplex-rt-pcr-assay-influenza-and (accessed on 10 August 2023).
- European Centre for Disease Prevention and Control (ECDC). SARS-CoV-2 Variants of Concern as of 10 August 2023. 2023. Available online: https://www.ecdc.europa.eu/en/covid-19/variants-concern (accessed on 10 August 2023).
- European Commission. COVID-19: Commission Calls on Member States to Step Up Preparedness for the Next Pandemic Phase. 2022. Available online: https://ec.europa.eu/commission/presscorner/detail/en/ip_22_2646 (accessed on 10 August 2023).
- World Health Organization (WHO). Influenza (Seasonal). 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal) (accessed on 13 August 2023).
- Centers for Disease Control and Prevention (CDC). Respiratory Syncytial Virus Infection (RSV). For Healthcare Providers. 2023. Available online: https://www.cdc.gov/rsv/clinical/index.html#clinical%20 (accessed on 10 August 2023).
- Acosta, P.L.; Caballero, M.T.; Polack, F.P. Brief History and Characterization of Enhanced Respiratory Syncytial Virus Disease. Clin. Vaccine Immunol. 2016, 23, 189–195. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Respiratory Syncytial Virus (RSV) Disease. Available online: https://www.who.int/teams/health-product-policy-and-standards/standards-and-specifications/vaccine-standardization/respiratory-syncytial-virus-disease (accessed on 13 August 2023).
- European Medicines Agency (EMA). New Medicine to Protect Babies and Infants from Respiratory Syncytial Virus (RSV) Infection. 2022. Available online: https://www.ema.europa.eu/en/news/new-medicine-protect-babies-infants-respiratory-syncytial-virus-rsv-infection (accessed on 10 August 2023).
- European Medicines Agency (EMA). First Vaccine to Protect Older Adults from Respiratory Syncytial Virus (RSV) Infection. 2023. Available online: https://www.ema.europa.eu/en/news/first-vaccine-protect-older-adults-respiratory-syncytial-virus-rsv-infection#:~:text=EMA%20has%20recommended%20a%20marketing,respiratory%20syncytial%20virus%20(RSV) (accessed on 10 August 2023).
- U.S. Food and Drug Administration (FDA). FDA Approves First Respiratory Syncytial Virus (RSV) Vaccine. 2023. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-respiratory-syncytial-virus-rsv-vaccine (accessed on 12 August 2023).
- U.S. Food and Drug Administration (FDA). FDA Approves First Oral Antiviral for Treatment of COVID-19 in Adults. 2023. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-oral-antiviral-treatment-covid-19-adults#:~:text=Today%2C%20the%20U.S.%20Food%20and,19%2C%20including%20hospitalization%20or%20death (accessed on 12 August 2023).
- European Medicine Agency (EMA). Paxlovid Received Conditional Marketing Authorization. 2023. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/paxlovid#:~:text=Paxlovid%20received%20a%20conditional%20marketing,authorisation%20on%2024%20February%202023 (accessed on 10 August 2023).
- U.S. Food and Drug Administration (FDA). BLA Approval-ABRYSVO. Collegeville. May 2023. Available online: https://www.fda.gov/media/168890/download (accessed on 12 August 2023).
- U.S. Food and Drug Administration (FDA). Frequently Asked Questions on the Emergency Use Authorization for Paxlovid for Treatment of COVID-19. July 2023. Available online: https://www.fda.gov/media/155052/download (accessed on 12 August 2023).
- LaRotta, J.; Escobar, O.; Ávila-Aguero, M.L.; Torres, J.P.; Sini de Almeida, R.; del Carmen Morales, G.; Srivastava, A. COVID-19 in Latin America: A Snapshot in Time and the Road Ahead. Infect. Dis. Ther. 2023, 12, 389–410. [Google Scholar] [CrossRef]
- Public Health England. PHE Novel Coronavirus Diagnostic Test Rolled Out across UK. Government UK. 2020. Available online: https://www.gov.uk/government/news/phe-novel-coronavirus-diagnostic-test-rolled-out-across-uk (accessed on 13 August 2023).
- European Medicines Agency (EMA). Pandemic Influenza A(H1N1)v Vaccines Authorised via the Core Dossier Procedure Explanatory Note on Scientific Considerations Regarding the Licensing of Pandemic A(H1N1)v Vaccines; EMA: London, UK, 2009. Available online: https://www.ema.europa.eu/en/documents/medicine-qa/explanatory-note-scientific-considerations-regarding-licensing-pandemic-ah1n1v-vaccines_en.pdf (accessed on 10 August 2023).
- Simoes, E.A.F.; Groothius, J.R. Respiratory Syncytial Virus Prophylaxis—The Story so Far. Respir. Med. 2002, 96, S15–S24. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food & Drug Administration. FDA Approves New Drug to Prevent RSV in Babies and Toddlers. 2023. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-new-drug-prevent-rsv-babies-and-toddlers (accessed on 10 August 2023).
- Malik, B.; Ghatol, A. Understanding How Monoclonal Antibodies Work. 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK572118/ (accessed on 13 August 2023).
- Mejias, A.; Rodríguez-Fernández, R.; Oliva, S.; Peeples, M.E.; Ramilo, O. The Journey to a Respiratory Syncytial Virus Vaccine. Ann. Allergy Asthma Immunol. 2020, 125, 36–46. [Google Scholar] [CrossRef]
- United Nations. COVID-19: With Booster Doses, Tests and Preparation, We Could End the Pandemic This Year (Translated from Spanish: COVID-19: Con Dosis de Refuerzo, Pruebas y Preparación, Podemos Poner Fin a La Pandemia Este Año). UN News. 2022. Available online: https://news.un.org/es/story/2022/09/1515191 (accessed on 13 August 2023).
- Klobucista, C.; Ferragamo, M. When Will COVID-19 Become Endemic? Council on Foreign Relations. 2023. Available online: https://www.cfr.org/in-brief/when-will-covid-19-become-endemic (accessed on 10 August 2023).
- Carabelli, A.M.; Peacock, T.P.; Thorne, L.G.; Harvey, W.T.; Hughes, J.; de Silva, T.I.; Peacock, S.J.; Barclay, W.S.; de Silva, T.I.; Towers, G.J.; et al. SARS-CoV-2 Variant Biology: Immune Escape, Transmission and Fitness. Nat. Rev. Microbiol. 2023, 21, 162–177. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Introduction to Epidemiology. 2018. Available online: https://www.cdc.gov/training/publichealth101/epidemiology.html (accessed on 10 August 2023).
- Antia, R.; Halloran, M.E. Transition to Endemicity: Understanding COVID-19. Immunity 2021, 54, 2172–2176. [Google Scholar] [CrossRef]
- Siggins, M.K.; Thwaites, R.S.; Openshaw, P.J.M. Durability of Immunity to SARS-CoV-2 and Other Respiratory Viruses. Trends Microbiol. 2021, 29, 648–662. [Google Scholar] [CrossRef]
- Mendoza, R.U.; Hartigan-Go, K.Y.; Brillantes, A.B.; Ruiz, K.E.V.; Baysic, I.S.; Valenzuela, S.A. Public Policy (Not the Coronavirus) Should Shape What Endemic Means. J. Glob. Health 2022, 12, 03050. [Google Scholar] [CrossRef] [PubMed]
- Biancolella, M.; Colona, V.L.; Mehrian-Shai, R.; Watt, J.L.; Luzzatto, L.; Novelli, G.; Reichardt, J.K.V. COVID-19 2022 Update: Transition of the Pandemic to the Endemic Phase. Hum. Genom. 2022, 16, 19. [Google Scholar] [CrossRef] [PubMed]
- Potter, S. When COVID-19 Is Endemic, What’s the Role of Testing? PBS Wisconsin, 2022. Available online: https://pbswisconsin.org/news-item/when-covid-19-is-endemic-whats-the-role-of-testing/ (accessed on 13 August 2023).
- Centers for Disease Control and Prevention (CDC). Overview of COVID-19 Vaccines. 2023. Available online: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines/overview-COVID-19-vaccines.html (accessed on 10 August 2023).
- European Medicines Agency (EMA). COVID-19 Medicines. Available online: https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/covid-19-medicines (accessed on 10 August 2023).
- U.S. Food and Drug Administration (FDA). Coronavirus Treatment Acceleration Program (CTAP). 2023. Available online: https://www.fda.gov/drugs/coronavirus-covid-19-drugs/coronavirus-treatment-acceleration-program-ctap (accessed on 12 August 2023).
- Administration for Strategic Preparedness & Response (ASPR). What Are the Possible Treatment Options for COVID 19? US Department of Health and Human Services. Available online: https://aspr.hhs.gov/COVID-19/Treatments/Pages/Possible-Treatment-Options-for-COVID19.aspx#oral-antivirals (accessed on 13 August 2023).
- U.S. Food & Drug Administration (FDA). Coronavirus (COVID-19)|Drugs. 2023. Available online: https://www.fda.gov/drugs/emergency-preparedness-drugs/coronavirus-covid-19-drugs (accessed on 13 August 2023).
- Centers for Disease Control and Prevention (CDC). COVID-19 Treatments and Medications. 2023. Available online: https://www.cdc.gov/coronavirus/2019-ncov/your-health/treatments-for-severe-illness.html (accessed on 13 August 2023).
- European Medicines Agency (EMA). COVID-19 Medicines under Evaluation. Available online: https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/covid-19-medicines/covid-19-medicines-under-evaluation (accessed on 13 August 2023).
- Centers for Disease Control and Prevention (CDC). Seasonal Flu Vaccines. 2022. Available online: https://www.cdc.gov/flu/prevent/flushot.htm#:~:text=Several%20different%20brands%20of%20standard,(muscle)%20with%20a%20needle (accessed on 13 August 2023).
- European Medicines Agency (EMA). Pandemic Influenza. Available online: https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/pandemic-influenza (accessed on 10 August 2023).
- European Medicines Agency (EMA). Antiviral Medicines for Pandemic Influenza. Available online: https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/pandemic-influenza/antiviral-medicines-pandemic-influenza (accessed on 10 August 2023).
- Pizzorno, A.; Padey, B.; Terrier, O.; Rosa-Calatrava, M. Drug Repurposing Approaches for the Treatment of Influenza Viral Infection: Reviving Old Drugs to Fight Against a Long-Lived Enemy. Front. Immunol. 2019, 10, 531. [Google Scholar] [CrossRef] [PubMed]
- Sarker, A.; Gu, Z.; Mao, L.; Ge, Y.; Hou, D.; Fang, J.; Wei, Z.; Wang, Z. Influenza-Existing Drugs and Treatment Prospects. Eur. J. Med. Chem. 2022, 232, 114189. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration (FDA). Influenza (Flu) Antiviral Drugs and Related Information. 2022. Available online: https://www.fda.gov/drugs/information-drug-class/influenza-flu-antiviral-drugs-and-related-information#:~:text=There%20are%20four%20FDA%2Dapproved,against%20recently%20circulating%20influenza%20viruses.&text=Two%20older%20drugs%2C%20amantadine%20(generic,of%20influenza%20A%20virus%20infection (accessed on 12 August 2023).
- Centers for Disease Control and Prevention (CDC). Influenza Antiviral Drug Resistance. Available online: https://www.cdc.gov/flu/treatment/antiviralresistance.htm (accessed on 10 August 2023).
- Lampejo, T. Influenza and Antiviral Resistance: An Overview. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 1201–1208. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency (EMA). Abrysvo. 2023. Available online: https://www.ema.europa.eu/en/medicines/human/summaries-opinion/abrysvo (accessed on 12 August 2023).
- Tin, A. First RSV Vaccine to Protect Infants Wins Backing of FDA Panel. CBS News, 2023. Available online: https://www.cbsnews.com/news/fda-rsv-vaccine-to-protect-infants-pfizer/ (accessed on 12 August 2023).
- World Health Organization (WHO). Annex 2 Guidelines on the Quality, Safety and Efficacy of Respiratory Syncytial virus Vaccines. 2020. Available online: https://cdn.who.int/media/docs/default-source/biologicals/vaccine-standardization/respiratory-syncytial-virus-(rsv)-vaccines/annex_2_rsv_vaccines_trs_1024.pdf?sfvrsn=5d7aefa7_3&download=true (accessed on 13 August 2023).
- PATH. RSV Vaccine and mAb Snapshot. 2023. Available online: https://www.path.org/resources/rsv-vaccine-and-mab-snapshot/ (accessed on 13 August 2023).
- Smyk, J.M.; Szydłowska, N.; Szulc, W.; Majewska, A. Evolution of Influenza Viruses—Drug Resistance, Treatment Options, and Prospects. Int. J. Mol. Sci. 2022, 23, 12244. [Google Scholar] [CrossRef] [PubMed]
- Hernández-González, D.G.; Rodríguez-Muñoz, L.; Solórzano-Santos, F. Impact of the Use of Multiplex PCR on Etiological Diagnosis and Treatment of Acute Respiratory Infections in a Private Hospital of the North of the Country. Gac. Med. Mex. 2021, 157, 160–165. [Google Scholar] [CrossRef]
- Havers, F.P.; Hicks, L.A.; Chung, J.R.; Gaglani, M.; Murthy, K.; Zimmerman, R.K.; Jackson, L.A.; Petrie, J.G.; McLean, H.Q.; Nowalk, M.P.; et al. Outpatient Antibiotic Prescribing for Acute Respiratory Infections During Influenza Seasons. JAMA Netw. Open 2018, 1, e180243. [Google Scholar] [CrossRef]
- Tonkin-Crine, S.; Yardley, L.; Little, P. Antibiotic Prescribing for Acute Respiratory Tract Infections in Primary Care: A Systematic Review and Meta-Ethnography. J. Antimicrob. Chemother. 2011, 66, 2215–2223. [Google Scholar] [CrossRef]
- Obolski, U.; Kassem, E.; Na’amnih, W.; Tannous, S.; Kagan, V.; Muhsen, K. Unnecessary Antibiotic Treatment of Children Hospitalised with Respiratory Syncytial Virus (RSV) Bronchiolitis: Risk Factors and Prescription Patterns. J. Glob. Antimicrob. Resist. 2021, 27, 303–308. [Google Scholar] [CrossRef]
- Allen, K.E.; Beekmann, S.E.; Polgreen, P.; Poser, S.; St. Pierre, J.; Santibañez, S.; Gerber, S.I.; Kim, L. Survey of Diagnostic Testing for Respiratory Syncytial Virus (RSV) in Adults: Infectious Disease Physician Practices and Implications for Burden Estimates. Diagn. Microbiol. Infect. Dis. 2018, 92, 206–209. [Google Scholar] [CrossRef] [PubMed]
- Egilmezer, E.; Walker, G.J.; Bakthavathsalam, P.; Peterson, J.R.; Gooding, J.J.; Rawlinson, W.; Stelzer-Braid, S. Systematic Review of the Impact of Point-of-Care Testing for Influenza on the Outcomes of Patients with Acute Respiratory Tract Infection. Rev. Med. Virol. 2018, 28, e1995. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard. 2023. Available online: https://data.who.int/dashboards/covid19/deaths?n=c (accessed on 22 November 2023).
- Gamba, M.R.; LeBlanc, T.T.; Vázquez, D.; dos Santos, E.P.; Franco, O.H. Health Emergency Preparedness and Response Capacity in Latin America and the Caribbean. Am. J. Public Health 2022, 112, S572. [Google Scholar] [CrossRef] [PubMed]
- Our World in Data. Coronavirus (COVID-19) Deaths-Cumulative Confirmed COVID-19 Cases per Million People. 2023. Available online: https://ourworldindata.org/covid-deaths#explore-the-global-data-on-confirmed-covid-19-deaths (accessed on 24 November 2023).
- Organisation for Economic Cooperation and Development; The World Bank. Health at a Glance: Latin America and the Caribbean; OECD Publishing: Paris, France, 2023. [Google Scholar] [CrossRef]
- Pan American Health Organization (PAHO). PAHO Biweekly COVID-19 Epidemiological Update—31st May 2023; PAHO: Washington, DC, USA, 2023; Available online: https://www.paho.org/en/documents/paho-biweekly-covid-19-epidemiological-update-31st-may-2023 (accessed on 13 August 2023).
- Our World in Data. Cumulative COVID-19 Tests per 1000 People. 2023. Available online: https://ourworldindata.org/covid-deaths#explore-the-global-data-on-confirmed-covid-19-deaths (accessed on 24 November 2023).
- Our World in Data. Tests Conducted per Confirmed Case of COVID-19, 7-Day Rolling Average. 2023. Available online: https://ourworldindata.org/coronavirus-testing (accessed on 24 November 2023).
- Our World in Data. Excess Mortality: Cumulative Deaths from All Causes Compared to Projection Based on Previous Years. 2023. Available online: https://ourworldindata.org/covid-deaths#explore-the-global-data-on-confirmed-covid-19-deaths (accessed on 24 November 2023).
- Our World in Data. The Our World in Data COVID-19 Testing Dataset: Source Information Country by Country. 2023. Available online: https://ourworldindata.org/coronavirus-testing (accessed on 24 November 2023).
- Garcia, J.; Torres, C.; Castro, A.; Rousset Yepez, B. The Registration of COVID-19 Associated Deaths: Who Is Included in the Statistics? ALAP (Asociación Latinoamericana de Población): Rio de Janeiro, Brazil, 2021; Available online: https://archined.ined.fr/view/AX3H6p8-Qw0312HDrH9d (accessed on 13 August 2023).
- Binstock, G.; Nathan, M.; Pardo, I.; Peláez, E. Challegens for the Advance of the 2030 Agenda in the Latin America and the Caribbean in the Gramework of COVID-19 (Translated from Spanish: Desafíos Para El Avance de La Agenda 2030 En América Latina y El Caribe En El Marco de La COVID-19); ALAP (Asociación Latinoamericana de Población): Rio de Janeiro, Brazil, 2021; Available online: https://lac.unfpa.org/es/publications/desaf%C3%ADos-para-el-avance-de-la-agenda-2030-en-am%C3%A9rica-latina-y-el-caribe-en-el-marco-de (accessed on 13 August 2023).
- Pan American Health Organization (PAHO). International Guideline for the Certification and Classification (Coding) of COVID-19 as a Cause of Death, April 20th, 2020. (Translated from Spanish: Orientación Internacional Para La Certificación y Clasificación (Codificación) del COVID-19 Como Causa de Muerte, 20 de Abril de 2020); PAHO: Washington, DC, USA, 2020; Available online: https://iris.paho.org/handle/10665.2/52848 (accessed on 13 August 2023).
- National Health Institute (INS) Colombia. ABC of the Mortality Registry by COVID in Colombia (Translated from Spanish: ABECÉ del Registro de Mortalidad Por COVID En Colombia); National Health Institute (INS) Colombia: Bogota, Colombia, 2023. Available online: https://www.ins.gov.co/BibliotecaDigital/abece-Registro-mortalidad-Covid.pdf (accessed on 12 August 2023).
- Ministry of Health Argentina. COVID-19 Disease. Guide for the Codification of Death Causes. (Translated from Spanish: Enfermedad Por COVID-19 Guía para la Codificación de Las Causas de Muerte); Ministry of Health Argentina: Buenos Aires, Argentina, 2020. Available online: https://www.argentina.gob.ar/sites/default/files/certifdef_covid19_cace_2.pdf (accessed on 13 August 2023).
- Health Secretariat Mexico. Standarized Guideline for the Epidemiological and Laboratory Surveillance of Viral Respiratory Disease (Translated from Spanish: Lineamiento Estandarizado Para La Vigilancia Epidemiológica y Por Laboratorio de La Enfermedad Respiratoria Viral); Health Secretariat Mexico: Mexico City, Mexico, 2022. Available online: https://www.gob.mx/cms/uploads/attachment/file/715444/Lineamiento_VE_y_Lab_Enf_Viral_05042022.pdf (accessed on 13 August 2023).
- Advisory Council COVID-19 Ministry of Health Chile. Surveillance, Registry and Death Certificates during the COVID-19 Pandemic (Translated from Spanish: Vigilancia, Registro y Certificación de Defunciones Durante La Pandemia de COVID-19); Advisory Council COVID-19 Ministry of Health Chile: Santiago, Chile, 2020; Available online: https://ciperchile.cl/wp-content/uploads/Minuta-Vigilancia-y-registro-de-defunciones-durante-la-pandemia-de-COVID-19_12-Junio.pdf (accessed on 13 August 2023).
- Brazil Civil Registry. Deaths with Suspicion or Confirmation of COVID-19 by Sex and Age (Translated from Portuguese: Óbitos com Suspeita ou Confirmação de COVID-19 por Sexo e Faixa etária). Transparency Portal Brazil. 2020. Available online: https://transparencia.registrocivil.org.br/especial-covid (accessed on 13 August 2023).
- Bonacina, F.; Boëlle, P.-Y.; Colizza, V.; Lopez, O.; Thomas, M.; Poletto, C. Global Patterns and Drivers of Influenza Decline during the COVID-19 Pandemic. Int. J. Infect. Dis. 2023, 128, 132–139. [Google Scholar] [CrossRef]
- Achangwa, C.; Park, H.; Ryu, S.; Lee, M.-S. Collateral Impact of Public Health and Social Measures on Respiratory Virus Activity during the COVID-19 Pandemic 2020–2021. Viruses 2022, 14, 1071. [Google Scholar] [CrossRef] [PubMed]
- Olsen, S.J.; Azziz-Baumgartner, E.; Budd, A.P.; Brammer, L.; Sullivan, S.; Pineda, R.F.; Cohen, C.; Fry, A.M. Decreased Influenza Activity During the COVID-19 Pandemic—United States, Australia, Chile, and South Africa, 2020. MMWR Morb. Mortal. Wkly Rep. 2020, 69, 1305–1309. [Google Scholar] [CrossRef]
- Chuang, Y.-C.; Lin, K.-P.; Wang, L.-A.; Yeh, T.-K.; Liu, P.-Y. The Impact of the COVID-19 Pandemic on Respiratory Syncytial Virus Infection: A Narrative Review. Infect. Drug Resist. 2023, 16, 661–675. [Google Scholar] [CrossRef]
- Sabeena, S.; Ravishankar, N.; Robin, S. The Impact of COVID-19 Pandemic on Influenza Surveillance: A Systematic Review and Meta-Analysis. Indian J. Public Health 2022, 66, 458. [Google Scholar] [CrossRef]
- Bermúdez Barrezueta, L.; Gutiérrez Zamorano, M.; López-Casillas, P.; Brezmes-Raposo, M.; Sanz Fernández, I.; Pino Vázquez, M.d.l.A. Influence of the COVID-19 Pandemic on the Epidemiology of Acute Bronchiolitis. Enferm. Infecc. Microbiol. Clin. 2023, 41, 348–351. [Google Scholar] [CrossRef]
- Nickbakhsh, S.; Mair, C.; Matthews, L.; Reeve, R.; Johnson, P.C.D.; Thorburn, F.; von Wissmann, B.; Reynolds, A.; McMenamin, J.; Gunson, R.N.; et al. Virus–Virus Interactions Impact the Population Dynamics of Influenza and the Common Cold. Proc. Natl. Acad. Sci. USA 2019, 116, 27142–27150. [Google Scholar] [CrossRef] [PubMed]
- Savy, V.; Ciapponi, A.; Bardach, A.; Glujovsky, D.; Aruj, P.; Mazzoni, A.; Gibbons, L.; Ortega-Barría, E.; Colindres, R.E. Burden of Influenza in Latin America and the Caribbean: A Systematic Review and Meta-Analysis. Influenza Other Respir. Viruses 2013, 7, 1017–1032. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Lopardo, G.; Scarpellini, B.; Stein, R.T.; Ribeiro, D. Systematic Review on Respiratory Syncytial Virus Epidemiology in Adults and the Elderly in Latin America. Int. J. Infect. Dis. 2020, 90, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Bardach, A.; Rey-Ares, L.; Cafferata, M.L.; Cormick, G.; Romano, M.; Ruvinsky, S.; Savy, V. Systematic Review and Meta-Analysis of Respiratory Syncytial Virus Infection Epidemiology in Latin America. Rev. Med. Virol. 2014, 24, 76–89. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Gupta, T.; Gupta, N. Global Respiratory Virus Surveillance: Strengths, Gaps, and Way Forward. Int. J. Infect. Dis. 2022, 121, 184–189. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). History of the Influenza Vaccine. Available online: https://www.who.int/news-room/spotlight/history-of-vaccination/history-of-influenza-vaccination (accessed on 12 August 2023).
- World Health Organization (WHO). Global Influenza Surveillance and Response System (GISRS). 2023. Available online: https://www.who.int/initiatives/global-influenza-surveillance-and-response-system (accessed on 13 August 2023).
- Barberis, I.; Martini, M.; Iavarone, F.; Orsi, A. Available Influenza Vaccines: Immunization Strategies, History and New Tools for Fighting the Disease. J. Prev. Med. Hyg. 2016, 57, E41–E46. [Google Scholar] [PubMed]
- Pebody, R.; Moyes, J.; Hirve, S.; Campbell, H.; Jackson, S.; Moen, A.; Nair, H.; Simões, E.A.F.; Smith, P.G.; Wairagkar, N.; et al. Approaches to Use the WHO Respiratory Syncytial Virus Surveillance Platform to Estimate Disease Burden. Influenza Other Respir. Viruses 2020, 14, 615–621. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Global Influenza Programme-WHO Launches Phase II of the Global Respiratory Syncytial Virus Surveillance; WHO: Geneva, Switzerland, 2022; Available online: https://www.who.int/teams/global-influenza-programme/global-respiratory-syncytial-virus-surveillance#:~:text=WHO%20launches%20phase%20II%20of%20the%20Global%20Respiratory%20Syncytial%20Virus%20Surveillance&text=(d)%20generate%20a%20robust%20understanding,representation%20in%20all%20WHO%20Regions (accessed on 13 August 2023).
- Pan American Health Organization (PAHO). Influenza and Other Respiratory Viruses: Surveillance in the Americas 2021; PAHO: Washington, DC, USA, 2022; Available online: https://iris.paho.org/bitstream/handle/10665.2/56544/9789275124994_eng.pdf?sequence=3&isAllowed=y (accessed on 13 August 2023).
- Broor, S.; Campbell, H.; Hirve, S.; Hague, S.; Jackson, S.; Moen, A.; Nair, H.; Palekar, R.; Rajatonirina, S.; Smith, P.G.; et al. Leveraging the Global Influenza Surveillance and Response System for Global Respiratory Syncytial Virus Surveillance—Opportunities and Challenges. Influenza Other Respir. Viruses 2020, 14, 622–629. [Google Scholar] [CrossRef]
- Sah, R.; Zaman, K.; Mohanty, A.; Al-Ahdal, T.; Awad, H.; Padhi, B.K.; Bhargava, A. Respiratory Syncytial Virus with Ongoing COVID-19: Is It an Emerging Threat? Ann. Med. Surg. 2023, 85, 67–70. [Google Scholar] [CrossRef]
- Pan American Health Organization (PAHO). FluNet Home Page 2010–2023. 2023. Available online: https://ais.paho.org/phip/viz/ed_flu.asp (accessed on 13 August 2023).
- Institute for health and metrics and evaluation (IHME). Global Disease Burden-Respiratory Infections. 2019. Available online: https://vizhub.healthdata.org/gbd-results/ (accessed on 12 August 2023).
- Pan American Health Organization (PAHO) FluNet. Cumulative Percent Positivity for Flu, RSV, and SARS-CoV-2, for 2022 by Country. 2023. Available online: https://ais.paho.org/phip/viz/ed_flu.asp (accessed on 13 August 2023).
- Dowdy, D.; D’Souza, G. COVID-19 Testing: Understanding the “Percent Positive”. Available online: https://publichealth.jhu.edu/2020/covid-19-testing-understanding-the-percent-positive#:~:text=The%20percent%20positive%20will%20be,haven’t%20been%20tested%20yet (accessed on 12 August 2023).
- Centers for Disease Control and Prevention (CDC). About Multisystem Inflammatory Syndrome (MIS). 2023. Available online: https://www.cdc.gov/mis/about.html (accessed on 10 August 2023).
- Sakhamuri, S.M.; Jankie, S.; Pinto Pereira, L.M. Calling on Latin America and the Caribbean Countries to Recognise the Disability from Long COVID. Lancet Reg. Health-Am. 2022, 15, 100362. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Moreno, C.A.; Pineda, J.; Bareño, A.; Espitia, R.; Rengifo, P. Long COVID-19 in Latin America: Low Prevalence, High Resilience or Low Surveillance and Difficulties Accessing Health Care? Travel Med. Infect. Dis. 2023, 51, 102492. [Google Scholar] [CrossRef]
- Rodriguez-Morales, A.J.; Lopez-Echeverri, M.C.; Perez-Raga, M.F.; Quintero-Romero, V.; Valencia-Gallego, V.; Galindo-Herrera, N.; López-Alzate, S.; Sánchez-Vinasco, J.D.; Gutiérrez-Vargas, J.J.; Mayta-Tristan, P.; et al. The Global Challenges of the Long COVID-19 in Adults and Children. Travel Med. Infect. Dis. 2023, 54, 102606. [Google Scholar] [CrossRef] [PubMed]
- The World Bank Group, United Nations Development Programme (UNDP). Long COVID: The Extended Effects of the Pandemic on Labor Markets in Latin America and the Caribbean. 2022. Available online: https://documents1.worldbank.org/curated/en/099900007072289098/pdf/P1758390cd83e707b0845f0450936b8882b.pdf (accessed on 13 August 2023).
- Angarita-Fonseca, A.; Torres-Castro, R.; Benavides-Cordoba, V.; Chero, S.; Morales-Satán, M.; Hernández-López, B.; Salazar-Pérez, R.; Larrateguy, S.; Sanchez-Ramirez, D.C. Exploring Long COVID Condition in Latin America: Its Impact on Patients’ Activities and Associated Healthcare Use. Front. Med. 2023, 10, 1168628. [Google Scholar] [CrossRef] [PubMed]
- Baraldi, E.; Bonadies, L.; Manzoni, P. Evidence on the Link between Respiratory Syncytial Virus Infection in Early Life and Chronic Obstructive Lung Diseases. Am. J. Perinatol. 2020, 37, S26–S30. [Google Scholar] [CrossRef]
- Verwey, C.; Nunes, M.C.; Dangor, Z.; Madhi, S.A. Pulmonary Function Sequelae after Respiratory Syncytial Virus Lower Respiratory Tract Infection in Children: A Systematic Review. Pediatr. Pulmonol. 2020, 55, 1567–1583. [Google Scholar] [CrossRef]
- The World Bank COVID-19. (Coronavirus) Response-The World Bank in Latin America and the Caribbean. Available online: https://www.worldbank.org/en/region/lac/coronavirus (accessed on 13 August 2023).
- Congressional Research Service. Latin America and the Caribbean: Impact of COVID-19; Congressional Research Service: Washington, DC, USA, 2022. Available online: https://sgp.fas.org/crs/row/IF11581.pdf (accessed on 10 August 2023).
- Organisation for Economic Co-Operation and Development (OECD). OECD Policy Responses to Coronavirus (COVID-19) COVID-19 in Latin America and the Caribbean: Regional Socio-Economic Implications and Policy Priorities; OECD: Paris, France, 2020; Available online: https://www.oecd.org/coronavirus/policy-responses/covid-19-in-latin-america-and-the-caribbean-regional-socio-economic-implications-and-policy-priorities-93a64fde/ (accessed on 13 August 2023).
- Economic Commission for Latin America and the Caribbean (ECLAC). The Sociodemographic Impacts of the COVID-19 Pandemic in Latin America and the Caribbean; ECLAC: Santiago, Chile, 2022; Available online: https://www.cepal.org/en/publications/47923-sociodemographic-impacts-covid-19-pandemic-latin-america-and-caribbean (accessed on 13 August 2023).
- Mosegui, G.B.G.; Antoñanzas, F.; de Mello Vianna, C.M. Cost of Lost Productivity from Acute Respiratory Infections in South America. Rev. Panam. De Salud Pública 2023, 47, 1. [Google Scholar] [CrossRef]
- Economic Commission for Latin America and the Caribbean (ECLAC). Preliminary Evaluation of the Impact of the Influenza H1N1-Document Developed by the Team of ECLAC-PAHO-WHO, as a Request and with the Support of the Government of Mexico (Translated from Spanish: Evaluación Preliminar Del Impacto En México de La Influenza AH1N1, Documento Elaborado Por El Equipo Conjunto CEPAL/OPS-OMS a Solicitud y Con El Apoyo Del Gobierno de México; ECLAC: Santiago, Chile, 2010; Available online: https://www.cepal.org/pt-br/node/19750 (accessed on 13 August 2023).
- Sachs, J.D.; Karim, S.S.A.; Aknin, L.; Allen, J.; Brosbøl, K.; Colombo, F.; Barron, G.C.; Espinosa, M.F.; Gaspar, V.; Gaviria, A.; et al. The Lancet Commission on Lessons for the Future from the COVID-19 Pandemic. Lancet 2022, 400, 1224–1280. [Google Scholar] [CrossRef] [PubMed]
- Cid, C.; Marinho, M.L. Two Years of the COVID-19 Pandemic in Latin America and the Caribbean (Translated from Spanish: Dos Años de Pandemia de COVID-19 En América Latina y El Caribe); Comisión Económica para América Latina y el Caribe: Santiago, Chile, 2022; Available online: https://www.cepal.org/es/publicaciones/47914-anos-pandemia-covid-19-america-latina-caribe-reflexiones-avanzar-sistemas-salud (accessed on 13 August 2023).
- Fisher, D.; Suri, S.; Carson, G. What Comes next in the COVID-19 Pandemic? Lancet 2022, 399, 1691–1692. [Google Scholar] [CrossRef] [PubMed]
- Pan American Health Organization (PAHO). Advancing towards Universal Health in Latin America and the Caribbean: Lessons from the COVID-19 Pandemic. 2022. Available online: https://www.paho.org/en/stories/advancing-towards-universal-health-latin-america-and-caribbean-lessons-covid-19-pandemic (accessed on 13 August 2023).
- Kruse, M.H.; Durstine, A.; Evans, D.P. Effect of COVID-19 on Patient Access to Health Services for Noncommunicable Diseases in Latin America: A Perspective from Patient Advocacy Organizations. Int. J. Equity Health 2022, 21, 45. [Google Scholar] [CrossRef]
- United Nations. Development Programme (UNDP); World Bank an Inequal Recovery: Taking Pulse of Latin America and the Caribbean after the Pandemic (Translated from Spanish: Una Recuperación Desigual: Tomando El Pulso de América Latina y El Caribe Después de La Pandemia). Available online: https://www.undp.org/sites/g/files/zskgke326/files/migration/latinamerica/55c219b7a3249badb633859cc85fd94f4b2c7be80af62137d5be4d22a9a3378f.pdf (accessed on 13 August 2023).
- Gallegos, M.; Consoli, A.; Ferrari, I.F.; Cervigni, M.; de Castro, V.; Martino, P.; Caycho, T.; Razumovskiy, A. COVID-19: Psychosocial Impact and Mental Health in Latin America. Fractal (Niterói) 2022, 33, 226–232. [Google Scholar] [CrossRef]
- Zhang, S.X.; Batra, K.; Xu, W.; Liu, T.; Dong, R.K.; Yin, A.; Delios, A.Y.; Chen, B.Z.; Chen, R.Z.; Miller, S.; et al. Mental Disorder Symptoms during the COVID-19 Pandemic in Latin America—A Systematic Review and Meta-Analysis. Epidemiol. Psychiatr. Sci. 2022, 31, e23. [Google Scholar] [CrossRef] [PubMed]
- Otoo, J.A.; Schlappi, T.S. REASSURED Multiplex Diagnostics: A Critical Review and Forecast. Biosensors 2022, 12, 124. [Google Scholar] [CrossRef] [PubMed]
- Domnich, A.; Bruzzone, B.; Trombetta, C.-S.; De Pace, V.; Ricucci, V.; Varesano, S.; Garzillo, G.; Ogliastro, M.; Orsi, A.; Icardi, G. Rapid Differential Diagnosis of SARS-CoV-2, Influenza A/B and Respiratory Syncytial Viruses: Validation of a Novel RT-PCR Assay. J. Clin. Virol. 2023, 161, 105402. [Google Scholar] [CrossRef] [PubMed]
- Khorramdelazad, H.; Kazemi, M.H.; Najafi, A.; Keykhaee, M.; Zolfaghari Emameh, R.; Falak, R. Immunopathological Similarities between COVID-19 and Influenza: Investigating the Consequences of Co-Infection. Microb. Pathog. 2021, 152, 104554. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Testing for SARS-CoV-2 Infection and Immunity. 2021. Available online: https://www.who.int/docs/default-source/coronaviruse/1_diagnostic-testing_a40858ba4cdeb844218acf06d5cffffa8b.pdf?sfvrsn=8b8894bf_1 (accessed on 13 August 2023).
- Centers for Disease Control and Prevention (CDC). Nucleic Acid Amplification Tests (NAATs). 2023. Available online: https://www.cdc.gov/coronavirus/2019-ncov/lab/naats.html (accessed on 10 August 2023).
- Yilmaz Gulec, E.; Cesur, N.P.; Yesilyurt Fazlioğlu, G.; Kazezoğlu, C. Effect of Different Storage Conditions on COVID-19 RT-PCR Results. J. Med. Virol. 2021, 93, 6575–6581. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, M.W.; Walsh, T.J. PCR Methodology and Applications for the Detection of Human Fungal Pathogens. Expert. Rev. Mol. Diagn. 2016, 16, 1025–1036. [Google Scholar] [CrossRef] [PubMed]
- Tabatabaei, M.S.; Islam, R.; Ahmed, M. Applications of Gold Nanoparticles in ELISA, PCR, and Immuno-PCR Assays: A Review. Anal. Chim. Acta 2021, 1143, 250–266. [Google Scholar] [CrossRef]
- Fakruddin, M.; Mannan, K.B.; Chowdhury, A.; Mazumdar, R.; Hossain, M.; Islam, S.; Chowdhury, M. Nucleic Acid Amplification: Alternative Methods of Polymerase Chain Reaction. J. Pharm. Bioallied Sci. 2013, 5, 245. [Google Scholar] [CrossRef]
- Obande, G.A.; Banga Singh, K.K. Current and Future Perspectives on Isothermal Nucleic Acid Amplification Technologies for Diagnosing Infections. Infect. Drug Resist. 2020, 13, 455–483. [Google Scholar] [CrossRef]
- Cao, S.; Tang, X.; Chen, T.; Chen, G. Types and Applications of Nicking Enzyme-Combined Isothermal Amplification. Int. J. Mol. Sci. 2022, 23, 4620. [Google Scholar] [CrossRef]
- Thompson, D.; Lei, Y. Mini Review: Recent Progress in RT-LAMP Enabled COVID-19 Detection. Sens. Actuators Rep. 2020, 2, 100017. [Google Scholar] [CrossRef]
- Amaral, C.; Antunes, W.; Moe, E.; Duarte, A.G.; Lima, L.M.P.; Santos, C.; Gomes, I.L.; Afonso, G.S.; Vieira, R.; Teles, H.S.S.; et al. A Molecular Test Based on RT-LAMP for Rapid, Sensitive and Inexpensive Colorimetric Detection of SARS-CoV-2 in Clinical Samples. Sci. Rep. 2021, 11, 16430. [Google Scholar] [CrossRef] [PubMed]
- Khan, P.; Aufdembrink, L.M.; Engelhart, A.E. Isothermal SARS-CoV-2 Diagnostics: Tools for Enabling Distributed Pandemic Testing as a Means of Supporting Safe Reopenings. ACS Synth. Biol. 2020, 9, 2861–2880. [Google Scholar] [CrossRef] [PubMed]
- Choi, G.; Moehling, T.J.; Meagher, R.J. Advances in RT-LAMP for COVID-19 Testing and Diagnosis. Expert. Rev. Mol. Diagn. 2023, 23, 9–28. [Google Scholar] [CrossRef]
- Huang, X.; Tang, G.; Ismail, N.; Wang, X. Developing RT-LAMP Assays for Rapid Diagnosis of SARS-CoV-2 in Saliva. EBioMedicine 2022, 75, 103736. [Google Scholar] [CrossRef]
- Yang, Q.; Meyerson, N.R.; Clark, S.K.; Paige, C.L.; Fattor, W.T.; Gilchrist, A.R.; Barbachano-Guerrero, A.; Healy, B.G.; Worden-Sapper, E.R.; Wu, S.S.; et al. Saliva TwoStep for Rapid Detection of Asymptomatic SARS-CoV-2 Carriers. medRxiv 2021. [Google Scholar] [CrossRef]
- Uribe-Alvarez, C.; Lam, Q.; Baldwin, D.A.; Chernoff, J. Low Saliva PH Can Yield False Positives Results in Simple RT-LAMP-Based SARS-CoV-2 Diagnostic Tests. PLoS ONE 2021, 16, e0250202. [Google Scholar] [CrossRef]
- Dao Thi, V.L.; Herbst, K.; Boerner, K.; Meurer, M.; Kremer, L.P.; Kirrmaier, D.; Freistaedter, A.; Papagiannidis, D.; Galmozzi, C.; Stanifer, M.L.; et al. A Colorimetric RT-LAMP Assay and LAMP-Sequencing for Detecting SARS-CoV-2 RNA in Clinical Samples. Sci. Transl. Med. 2020, 12, eabc7075. [Google Scholar] [CrossRef] [PubMed]
- Kellner, M.J.; Ross, J.J.; Schnabl, J.; Dekens, M.P.S.; Matl, M.; Heinen, R.; Grishkovskaya, I.; Bauer, B.; Stadlmann, J.; Menéndez-Arias, L.; et al. A Rapid, Highly Sensitive and Open-Access SARS-CoV-2 Detection Assay for Laboratory and Home Testing. Front. Mol. Biosci. 2022, 9, 801309. [Google Scholar] [CrossRef]
- Haque, M.F.U.; Bukhari, S.S.; Ejaz, R.; Zaman, F.U.; Sreejith, K.R.; Rashid, N.; Umer, M.; Shahzad, N. A Novel RdRp-Based Colorimetric RT-LAMP Assay for Rapid and Sensitive Detection of SARS-CoV-2 in Clinical and Sewage Samples from Pakistan. Virus Res. 2021, 302, 198484. [Google Scholar] [CrossRef]
- He, Y.; Xie, T.; Tong, Y. Rapid and Highly Sensitive One-Tube Colorimetric RT-LAMP Assay for Visual Detection of SARS-CoV-2 RNA. Biosens. Bioelectron. 2021, 187, 113330. [Google Scholar] [CrossRef] [PubMed]
- Nie, S.; Roth, R.B.; Stiles, J.; Mikhlina, A.; Lu, X.; Tang, Y.-W.; Babady, N.E. Evaluation of Alere i Influenza A and B for Rapid Detection of Influenza Viruses A and B. J. Clin. Microbiol. 2014, 52, 3339–3344. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.-N.; Jiang, D.; Wang, X.; Liu, Y.; Wei, D. Recent Progress on Rapid Diagnosis of COVID-19 by Point-of-Care Testing Platforms. Chin. Chem. Lett. 2023, 108688. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, Z.; Zhu, J.; Li, C.; Fang, Z.; Chen, K.; Zhang, Y. An Updated Review of SARS-CoV-2 Detection Methods in the Context of a Novel Coronavirus Pandemic. Bioeng. Transl. Med. 2023, 8, e10356. [Google Scholar] [CrossRef] [PubMed]
- James, A.; Alawneh, J. COVID-19 Infection Diagnosis: Potential Impact of Isothermal Amplification Technology to Reduce Community Transmission of SARS-CoV-2. Diagnostics 2020, 10, 399. [Google Scholar] [CrossRef] [PubMed]
- Rhoads, D.D.; Cherian, S.S.; Roman, K.; Stempak, L.M.; Schmotzer, C.L.; Sadri, N. Comparison of Abbott ID Now, DiaSorin Simplexa, and CDC FDA Emergency Use Authorization Methods for the Detection of SARS-CoV-2 from Nasopharyngeal and Nasal Swabs from Individuals Diagnosed with COVID-19. J. Clin. Microbiol. 2020, 58, e00760-20. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Zinger, T.; Inglima, K.; Woo, K.; Atie, O.; Yurasits, L.; See, B.; Aguero-Rosenfeld, M.E. Performance of Abbott ID Now COVID-19 Rapid Nucleic Acid Amplification Test Using Nasopharyngeal Swabs Transported in Viral Transport Media and Dry Nasal Swabs in a New York City Academic Institution. J. Clin. Microbiol. 2020, 58, e01136-20. [Google Scholar] [CrossRef]
- Roumani, F.; Azinheiro, S.; Sousa, H.; Sousa, A.; Timóteo, M.; Varandas, T.; Fonseca-Silva, D.; Baldaque, I.; Carvalho, J.; Prado, M.; et al. Optimization and Clinical Evaluation of a Multi-Target Loop-Mediated Isothermal Amplification Assay for the Detection of SARS-CoV-2 in Nasopharyngeal Samples. Viruses 2021, 13, 940. [Google Scholar] [CrossRef]
- Smithgall, M.C.; Scherberkova, I.; Whittier, S.; Green, D.A. Comparison of Cepheid Xpert Xpress and Abbott ID Now to Roche Cobas for the Rapid Detection of SARS-CoV-2. J. Clin. Virol. 2020, 128, 104428. [Google Scholar] [CrossRef]
- Chen, C.-C.; Chen, S.-Y.; Fang, S.-B.; Lu, S.-C.; Bai, C.-H.; Wang, Y.-H. Diagnostic Accuracy of SARS-CoV-2 Antigen Test in the Pediatric Population: A Systematic Review and Meta-Analysis. Pediatr. Neonatol. 2023, 64, 247–255. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Antigen-Detection in the Diagnosis of SARS-CoV-2 Infection. Interim Guidance. 6 October 2021. Available online: https://apps.who.int/iris/rest/bitstreams/1376869/retrieve (accessed on 13 August 2023).
- Korenkov, M.; Poopalasingam, N.; Madler, M.; Vanshylla, K.; Eggeling, R.; Wirtz, M.; Fish, I.; Dewald, F.; Gieselmann, L.; Lehmann, C.; et al. Evaluation of a Rapid Antigen Test To Detect SARS-CoV-2 Infection and Identify Potentially Infectious Individuals. J. Clin. Microbiol. 2021, 59. [Google Scholar] [CrossRef]
- Dinnes, J.; Deeks, J.J.; Adriano, A.; Berhane, S.; Davenport, C.; Dittrich, S.; Emperador, D.; Takwoingi, Y.; Cunningham, J.; Beese, S.; et al. Rapid, Point-of-Care Antigen and Molecular-Based Tests for Diagnosis of SARS-CoV-2 Infection. Cochrane Database Syst. Rev. 2020, 8, CD013705. [Google Scholar] [CrossRef] [PubMed]
- Bekliz, M.; Adea, K.; Essaidi-Laziosi, M.; Sacks, J.A.; Escadafal, C.; Kaiser, L.; Eckerle, I. SARS-CoV-2 Rapid Diagnostic Tests for Emerging Variants. Lancet Microbe 2021, 2, e351. [Google Scholar] [CrossRef]
- Bekliz, M.; Adea, K.; Puhach, O.; Perez-Rodriguez, F.; Marques Melancia, S.; Baggio, S.; Corvaglia, A.-R.; Jacquerioz, F.; Alvarez, C.; Essaidi-Laziosi, M.; et al. Analytical Sensitivity of Eight Different SARS-CoV-2 Antigen-Detecting Rapid Tests for Omicron-BA.1 Variant. Microbiol. Spectr. 2022, 10, e00853-22. [Google Scholar] [CrossRef] [PubMed]
- Corman, V.M.; Haage, V.C.; Bleicker, T.; Schmidt, M.L.; Mühlemann, B.; Zuchowski, M.; Jo, W.K.; Tscheak, P.; Möncke-Buchner, E.; Müller, M.A.; et al. Comparison of Seven Commercial SARS-CoV-2 Rapid Point-of-Care Antigen Tests: A Single-Centre Laboratory Evaluation Study. Lancet Microbe 2021, 2, e311–e319. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food & Drug Administration. SARS-CoV-2 Viral Mutations: Impact on COVID-19. Tests. Available online: https://www.fda.gov/medical-devices/coronavirus-covid-19-and-medical-devices/sars-cov-2-viral-mutations-impact-covid-19-tests (accessed on 28 January 2024).
- Stanley, S.; Hamel, D.J.; Wolf, I.D.; Riedel, S.; Dutta, S.; Contreras, E.; Callahan, C.J.; Cheng, A.; Arnaout, R.; Kirby, J.E.; et al. Limit of Detection for Rapid Antigen Testing of the SARS-CoV-2 Omicron and Delta Variants of Concern Using Live-Virus Culture. J. Clin. Microbiol. 2022, 60, e00140-22. [Google Scholar] [CrossRef]
- World Health Organization. Antigen-Detection in the Diagnosis of SARS-CoV-2 Infection. Interim Guidance. Available online: https://www.who.int/publications/i/item/antigen-detection-in-the-diagnosis-of-sars-cov-2infection-using-rapid-immunoassays (accessed on 28 January 2024).
- Nasrallah, G.K.; Ali, F.; Younes, S.; Al Khatib, H.A.; Al-Thani, A.A.; Yassine, H.M. Enhancing the Sensitivity of Rapid Antigen Detection Test (RADT) of Different SARS-CoV-2 Variants and Lineages Using Fluorescence-Labeled Antibodies and a Fluorescent Meter. Heliyon 2023, 9, e17179. [Google Scholar] [CrossRef]
- Urusov, A.E.; Zherdev, A.V.; Dzantiev, B.B. Towards Lateral Flow Quantitative Assays: Detection Approaches. Biosensors 2019, 9, 89. [Google Scholar] [CrossRef]
- Lee, J.; Song, J.-U.; Shim, S.R. Comparing the Diagnostic Accuracy of Rapid Antigen Detection Tests to Real Time Polymerase Chain Reaction in the Diagnosis of SARS-CoV-2 Infection: A Systematic Review and Meta-Analysis. J. Clin. Virol. 2021, 144, 104985. [Google Scholar] [CrossRef]
- Platten, M.; Hoffmann, D.; Grosser, R.; Wisplinghoff, F.; Wisplinghoff, H.; Wiesmüller, G.; Schildgen, O.; Schildgen, V. SARS-CoV-2, CT-Values, and Infectivity—Conclusions to Be Drawn from Side Observations. Viruses 2021, 13, 1459. [Google Scholar] [CrossRef]
- Paul, G.; Plecko, T.; Sethi, S.; Schilling, T.; Wienand, O.; Jürgensen, J.S.; Menzel, C.U. Klinische Performance Eines Neuen SARS-CoV-2-Antigen-Tests in Der Notaufnahme Eines Maximalversorgers. Epidemiol. Bull. 2021, 3, 10–15. [Google Scholar]
- Centers for Disease Control and Prevention (CDC). Ending Isolation and Precautions for People with COVID-19: Interim Guidance. Available online: https://www.cdc.gov/coronavirus/2019-ncov/hcp/duration-isolation.html (accessed on 28 January 2024).
- Nicollete, D.R.P.; Benedetti, R.; Valença, B.A.; Kuniyoshi, K.K.; de Jesus, T.C.S.; Gevaerd, A.; Santiago, E.B.; de Almeida, B.M.M.; Júnior, S.R.R.; Figueredo, M.V.M. Enhancing a SARS-CoV-2 Nucleocapsid Antigen Test Sensitivity with Cost Efficient Strategy through a Cotton Intermembrane Insertion. Sci. Rep. 2023, 13, 4690. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Coronavirus Disease (COVID-19): Serology, Antibodies and Immunity. 2020. Available online: https://www.who.int/news-room/questions-and-answers/item/coronavirus-disease-covid-19-serology (accessed on 13 August 2023).
- World Health Organization (WHO). “Solidarity II” Global Serologic Study for COVID-19. 2021. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/global-research-on-novel-coronavirus-2019-ncov/solidarity-2-global-serologic-study-for-covid-19 (accessed on 13 August 2023).
- World Health Organization (WHO). Population-Based Age-Stratified Seroepidemiological Investigation Protocol for COVID-19 Virus Infection; WHO: Geneva, Switzerland, 2020; Available online: https://apps.who.int/iris/bitstream/handle/10665/331656/WHO-2019-nCoV-Seroepidemiology-2020.1-eng.pdf?sequence=1&isAllowed=y (accessed on 13 August 2023).
- World Health Organization (WHO). Guidelines for the Clinical Management of Severe Illness from Influenza Virus Infections; WHO: Geneva, Switzerland, 2022; Available online: https://apps.who.int/iris/handle/10665/352453 (accessed on 13 August 2023).
- Centers for Disease Control and Prevention (CDC). Overview of Influenza Testing Methods. 2020. Available online: https://www.cdc.gov/flu/professionals/diagnosis/overview-testing-methods.htm (accessed on 10 August 2023).
- Vemula, S.; Zhao, J.; Liu, J.; Wang, X.; Biswas, S.; Hewlett, I. Current Approaches for Diagnosis of Influenza Virus Infections in Humans. Viruses 2016, 8, 96. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Recommendations Announced for Influenza Vaccine Composition for the 2023–2024 Northern Hemisphere Influenza Season. 2023. Available online: https://www.who.int/news/item/24-02-2023-recommendations-announced-for-influenza-vaccine-composition-for-the-2023-2024-northern-hemisphere-influenza-season (accessed on 13 August 2023).
- Testing. Respiratory Syncytial Virus (RSV) Testing. Testing. 2020. Available online: https://www.testing.com/tests/respiratory-syncytial-virus-rsv-testing/ (accessed on 13 August 2023).
- Shi, T.; McAllister, D.A.; O’Brien, K.L.; Simoes, E.A.F.; Madhi, S.A.; Gessner, B.D.; Polack, F.P.; Balsells, E.; Acacio, S.; Aguayo, C.; et al. Global, Regional, and National Disease Burden Estimates of Acute Lower Respiratory Infections Due to Respiratory Syncytial Virus in Young Children in 2015: A Systematic Review and Modelling Study. Lancet 2017, 390, 946–958. [Google Scholar] [CrossRef]
- Ackerson, B.; Tseng, H.F.; Sy, L.S.; Solano, Z.; Slezak, J.; Luo, Y.; Fischetti, C.A.; Shinde, V. Severe Morbidity and Mortality Associated With Respiratory Syncytial Virus Versus Influenza Infection in Hospitalized Older Adults. Clin. Infect. Dis. 2019, 69, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Binder, W.; Thorsen, J.; Borczuk, P. RSV in Adult ED Patients: Do Emergency Providers Consider RSV as an Admission Diagnosis? Am. J. Emerg. Med. 2017, 35, 1162–1165. [Google Scholar] [CrossRef] [PubMed]
- Branche, A.R.; Falsey, A.R. Respiratory Syncytial Virus Infection in Older Adults: An Under-Recognized Problem. Drugs Aging 2015, 32, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Kim, L.; Rha, B.; Abramson, J.S.; Anderson, L.J.; Byington, C.L.; Chen, G.L.; DeVincenzo, J.; Edwards, K.M.; Englund, J.A.; Falsey, A.R.; et al. Identifying Gaps in Respiratory Syncytial Virus Disease Epidemiology in the United States Prior to the Introduction of Vaccines. Clin. Infect. Dis. 2017, 65, 1020–1025. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.T.; Nduaguba, S.O.; Diaby, V.; Choi, Y.; Winterstein, A.G. RSV Testing Practice and Positivity by Patient Demographics in the United States: Integrated Analyses of MarketScan and NREVSS Databases. BMC Infect. Dis. 2022, 22, 681. [Google Scholar] [CrossRef] [PubMed]
- Rha, B.; Curns, A.T.; Lively, J.Y.; Campbell, A.P.; Englund, J.A.; Boom, J.A.; Azimi, P.H.; Weinberg, G.A.; Staat, M.A.; Selvarangan, R.; et al. Respiratory Syncytial Virus–Associated Hospitalizations Among Young Children: 2015–2016. Pediatrics 2020, 146, e20193611. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Guidance for SARS-CoV-2 Rapid Testing Performed in Point-of-Care Settings. 2022. Available online: https://www.cdc.gov/coronavirus/2019-ncov/lab/point-of-care-testing.html (accessed on 10 August 2023).
- Infectious Diseases Society of America (IDSA); Centers for Disease Control and Prevention (CDC). Diagnostics- Rapid Testing. 2023. Available online: https://www.idsociety.org/covid-19-real-time-learning-network/diagnostics/#/+/0/publishedDate_na_dt/desc/ (accessed on 12 August 2023).
- Peeling, R.W.; Heymann, D.L.; Teo, Y.-Y.; Garcia, P.J. Diagnostics for COVID-19: Moving from Pandemic Response to Control. Lancet 2022, 399, 757–768. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Rapid Influenza Diagnostic Tests. 2016. Available online: https://www.cdc.gov/flu/professionals/diagnosis/clinician_guidance_ridt.htm (accessed on 10 August 2023).
- Yin, N.; Van Nuffelen, M.; Bartiaux, M.; Préseau, T.; Roggen, I.; Delaunoy, S.; Mahadeb, B.; Dahma, H.; Busson, L.; Vandenberg, O.; et al. Clinical Impact of the Rapid Molecular Detection of RSV and Influenza A and B Viruses in the Emergency Department. PLoS ONE 2022, 17, e0274222. [Google Scholar] [CrossRef] [PubMed]
- Favresse, J.; Douxfils, J.; Henry, B.; Lippi, G.; Plebani, M. Clinical Chemistry and Laboratory Medicine Celebrates 60 Years—Narrative Review Devoted to the Contribution of the Journal to the Diagnosis of SARS-CoV-2. Clin. Chem. Lab. Med. (CCLM) 2023, 61, 811–821. [Google Scholar] [CrossRef] [PubMed]
- Girdwood, S.J.; Carmona, S.; Hannay, E.; Nichols, B. Cost-Effectiveness of SARS-CoV-2 Rapid Antigen Testing in Lowresource Settings. Top. Antivir. Med. 2021, 29, 269. [Google Scholar]
- Cedro, V.Q.M.; de Lima Gomes, S.; Simões, A.C.C.D.; do Valle Lovato Sverzut, T.; Bertti, K.C.X.; Tristão, M.T.; Cavalcanti, Y.W.; Câmara, J.V.F.; Pereira, A.C. Cost-Effectiveness Analysis of COVID-19 Tests in the Unified Health System. Cost. Eff. Resour. Alloc. 2023, 21, 64. [Google Scholar] [CrossRef] [PubMed]
- de Araújo, E.S.A.; Condursi, J.R.; Garmatter, L.P.L. Análise Econômica Da Incorporação Do Teste Rápido de Antígeno Para Covid-19 versus RT-PCR Como Estratégia de Diagnóstico de Pacientes Sintomáticos No Pronto Atendimento de Uma Operadora de Saúde Do Brasil. Braz. J. Infect. Dis. 2022, 26, 101781. [Google Scholar] [CrossRef]
- Diel, R.; Nienhaus, A. Point-of-Care COVID-19 Antigen Testing in German Emergency Rooms—A Cost-Benefit Analysis. Pulmonology 2022, 28, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Pighi, L.; Henry, B.M.; Mattiuzzi, C.; De Nitto, S.; Salvagno, G.L.; Lippi, G. Cost-Effectiveness Analysis of Different COVID-19 Screening Strategies Based on Rapid or Laboratory-Based SARS-CoV-2 Antigen Testing. Clin. Chem. Lab. Med. (CCLM) 2023, 61, e168–e171. [Google Scholar] [CrossRef]
- Bonnet, G.; Bimba, J.; Chavula, C.; Chifamba, H.N.; Divala, T.; Lescano, A.G.; Majam, M.; Mbo, D.; Suwantika, A.A.; Tovar, M.A.; et al. “We Usually See a Lot of Delay in Terms of Coming for or Seeking Care”: An Expert Consultation on COVID Testing and Care Pathways in Seven Low- and Middle-Income Countries. BMC Health Serv. Res. 2023, 23, 1288. [Google Scholar] [CrossRef]
- Bertram, M.Y.; Lauer, J.A.; De Joncheere, K.; Edejer, T.; Hutubessy, R.; Kieny, M.-P.; Hill, S.R. Cost–Effectiveness Thresholds: Pros and Cons. Bull. World Health Organ. 2016, 94, 925–930. [Google Scholar] [CrossRef]
- Stolberg-Stolberg, J.; Jacob, E.; Kuehn, J.; Hennies, M.; Hafezi, W.; Freistuehler, M.; Koeppe, J.; Friedrich, A.W.; Katthagen, J.C.; Raschke, M.J. COVID-19 Rapid Molecular Point-of-Care Testing Is Effective and Cost-Beneficial for the Acute Care of Trauma Patients. Eur. J. Trauma Emerg. Surg. 2023, 49, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Diel, R.; Nienhaus, A. Cost–Benefit of Real-Time Multiplex PCR Testing of SARS-CoV-2 in German Hospitals. Int. J. Env. Res. Public Health 2023, 20, 3447. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Li, H.; Tang, Y.; Yu, F.; Ma, C.; Zhang, H.; Pang, L.; Zhao, H.; Wang, L. Multiplex Tests for Respiratory Tract Infections: The Direct Utility of the FilmArray Respiratory Panel in Emergency Department. Can. Respir. J. 2020, 2020, 6014563. [Google Scholar] [CrossRef] [PubMed]
- El-Nawawy, A.A.; Antonios, M.A.; Tawfik, M.E.; Meheissen, M.A. Comparison of a Point-of-Care FilmArray Test to Standard-of-Care Microbiology Test in Diagnosis of Healthcare Associated Infections in a Tertiary Care Pediatric Intensive Care Unit. Antibiotics 2022, 11, 453. [Google Scholar] [CrossRef] [PubMed]
- Dincer, C.; Bruch, R.; Kling, A.; Dittrich, P.S.; Urban, G.A. Multiplexed Point-of-Care Testing—XPOCT. Trends Biotechnol. 2017, 35, 728–742. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Table 4. Multiplex Assays Authorized for Simultaneous Detection of Influenza Viruses and SARS-CoV-2 by FDA. 2020. Available online: https://www.cdc.gov/flu/professionals/diagnosis/table-flu-covid19-detection.html (accessed on 10 August 2023).
- Clark, T.W.; Lindsley, K.; Wigmosta, T.B.; Bhagat, A.; Hemmert, R.B.; Uyei, J.; Timbrook, T.T. Rapid Multiplex PCR for Respiratory Viruses Reduces Time to Result and Improves Clinical Care: Results of a Systematic Review and Meta-Analysis. J. Infect. 2023, 86, 462–475. [Google Scholar] [CrossRef] [PubMed]
- Boukli, N.; Flamand, C.; Chea, K.L.; Heng, L.; Keo, S.; Sour, K.; In, S.; Chhim, P.; Chhor, B.; Kruy, L.; et al. One Assay to Test Them All: Comparing Multiplex Assays for Expansion of Respiratory Virus Surveillance. Medrxiv 2023. [Google Scholar] [CrossRef]
- Kang, T.; Hyun Cha, J.; Kim, J.; Kim, K.J.; Nam, M.; Nam, M.H.; Kim, D.W.; Cho, Y.; Kyu Lee, C.; Gyu Yun, S. Evaluation of Multiplex Rapid Antigen Test for the Detection of SARS-CoV-2 and Influenza A/B in Respiratory Samples. 2023. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=4351273 (accessed on 12 August 2023). [CrossRef]
- Nichols, J.H. Point-of-Care Testing. In Contemporary Practice in Clinical Chemistry; Elsevier: Amsterdam, The Netherlands, 2020; pp. 323–336. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Biological Risk Management for Point-of-Care Testing Sites. Available online: https://www.cdc.gov/csels/dls/point-of-care-testing.html (accessed on 13 August 2023).
- Valera, E.; Jankelow, A.; Lim, J.; Kindratenko, V.; Ganguli, A.; White, K.; Kumar, J.; Bashir, R. COVID-19 Point-of-Care Diagnostics: Present and Future. ACS Nano 2021, 15, 7899–7906. [Google Scholar] [CrossRef]
- Izadi, R.; Hatam, N.; Baberi, F.; Yousefzadeh, S.; Jafari, A. Economic Evaluation of Strategies against Coronavirus: A Systematic Review. Health Econ. Rev. 2023, 13, 18. [Google Scholar] [CrossRef]
- National Community Pharmacist Association (NCPA). Point-of-Care Testing (POCT). 2023. Available online: https://ncpa.org/point-care-testing-poct (accessed on 13 August 2023).
- American Society for Microbiology. Making Sense of Respiratory Viral Panel Results. 2020. Available online: https://asm.org/Articles/2020/March/Making-Sense-of-Respiratory-Viral-Panel-Results (accessed on 10 August 2023).
- Hanson, K.E.; Azar, M.M.; Banerjee, R.; Chou, A.; Colgrove, R.C.; Ginocchio, C.C.; Hayden, M.K.; Holodiny, M.; Jain, S.; Koo, S.; et al. Molecular Testing for Acute Respiratory Tract Infections: Clinical and Diagnostic Recommendations From the IDSA’s Diagnostics Committee. Clin. Infect. Dis. 2020, 71, 2744–2751. [Google Scholar] [CrossRef]
- Gentilotti, E.; De Nardo, P.; Cremonini, E.; Górska, A.; Mazzaferri, F.; Canziani, L.M.; Hellou, M.M.; Olchowski, Y.; Poran, I.; Leeflang, M.; et al. Diagnostic Accuracy of Point-of-Care Tests in Acute Community-Acquired Lower Respiratory Tract Infections. A Systematic Review and Meta-Analysis. Clin. Microbiol. Infect. 2022, 28, 13–22. [Google Scholar] [CrossRef]
- Antoñanzas, F.; Juárez-Castelló, C.A.; Rodríguez-Ibeas, R. Using Point-of-Care Diagnostic Testing for Improved Antibiotic Prescription: An Economic Model. Health Econ. Rev. 2021, 11, 29. [Google Scholar] [CrossRef]
- Hengel, B.; Causer, L.; Matthews, S.; Smith, K.; Andrewartha, K.; Badman, S.; Spaeth, B.; Tangey, A.; Cunningham, P.; Saha, A.; et al. A Decentralised Point-of-Care Testing Model to Address Inequities in the COVID-19 Response. Lancet Infect. Dis. 2021, 21, e183–e190. [Google Scholar] [CrossRef]
- Bouzid, D.; Casalino, E.; Mullaert, J.; Laurent, O.; Duval, X.; Lescure, F.X.; Peiffer Smadja, N.; Tubiana, S.; Armand Lefèvre, L.; Descamps, D.; et al. Added Value of Rapid Respiratory Syndromic Testing at Point of Care versus Central Laboratory Testing: A Controlled Clinical Trial. J. Antimicrob. Chemother. 2021, 76, iii20–iii27. [Google Scholar] [CrossRef] [PubMed]
- Levin-Reisman, I.; Brauner, A.; Ronin, I.; Balaban, N.Q. Epistasis between Antibiotic Tolerance, Persistence, and Resistance Mutations. Proc. Natl. Acad. Sci. USA 2019, 116, 14734–14739. [Google Scholar] [CrossRef] [PubMed]
- Fiore, A.; Fry, A.; Shay, D.; Gubareva, L.; Bresee, J.; Uyeki, T.; Centers for Disease Control and Prevention (CDC). Antiviral Agents for the Treatment and Chemoprophylaxis of Influenza—Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm. Rep. 2011, 60, 1–24. [Google Scholar] [PubMed]
- Randomised Trial of Efficacy and Safety of Inhaled Zanamivir in Treatment of Influenza A and B Virus Infections. The MIST (Management of Influenza in the Southern Hemisphere Trialists) Study Group. Lancet 1998, 352, 1877–1881. [Google Scholar]
- Treanor, J.J.; Hayden, F.G.; Vrooman, P.S.; Barbarash, R.; Bettis, R.; Riff, D.; Singh, S.; Kinnersley, N.; Ward, P.; Mills, R.G.; et al. Efficacy and Safety of the Oral Neuraminidase Inhibitor Oseltamivir in Treating Acute Influenza. JAMA 2000, 283, 1016. [Google Scholar] [CrossRef]
- Brendish, N.J.; Malachira, A.K.; Armstrong, L.; Houghton, R.; Aitken, S.; Nyimbili, E.; Ewings, S.; Lillie, P.J.; Clark, T.W. Routine Molecular Point-of-Care Testing for Respiratory Viruses in Adults Presenting to Hospital with Acute Respiratory Illness (ResPOC): A Pragmatic, Open-Label, Randomised Controlled Trial. Lancet Respir. Med. 2017, 5, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Li-Kim-Moy, J.; Dastouri, F.; Rashid, H.; Khandaker, G.; Kesson, A.; McCaskill, M.; Wood, N.; Jones, C.; Zurynski, Y.; Macartney, K.; et al. Utility of Early Influenza Diagnosis through Point-of-Care Testing in Children Presenting to an Emergency Department. J. Paediatr. Child. Health 2016, 52, 422–429. [Google Scholar] [CrossRef]
- Andrews, D.; Chetty, Y.; Cooper, B.S.; Virk, M.; Glass, S.K.; Letters, A.; Kelly, P.A.; Sudhanva, M.; Jeyaratnam, D. Multiplex PCR Point of Care Testing versus Routine, Laboratory-Based Testing in the Treatment of Adults with Respiratory Tract Infections: A Quasi-Randomised Study Assessing Impact on Length of Stay and Antimicrobial Use. BMC Infect. Dis. 2017, 17, 671. [Google Scholar] [CrossRef]
- Drain, P.K. Rapid Diagnostic Testing for SARS-CoV-2. N. Engl. J. Med. 2022, 386, 264–272. [Google Scholar] [CrossRef]
- PATH. Decentralizing Testing for COVID-19 and Beyond. 2021. Available online: https://www.path.org/articles/decentralizing-testing-covid-19-and-beyond/ (accessed on 13 August 2023).
- Groseclose, S.L.; Buckeridge, D.L. Public Health Surveillance Systems: Recent Advances in Their Use and Evaluation. Annu. Rev. Public Health 2017, 38, 57–79. [Google Scholar] [CrossRef] [PubMed]
- Pan American Health Organization (PAHO). Final Report Ad Hoc Expert Consultation in the Region of the Americas: Challenges, Gaps and next Steps in COVID 19 Surveillance and Its Integration in to Influenza and Other Respiratory Viruses Surveillance; PAHO: Washington, DC, USA, 2022; Available online: https://www.paho.org/en/documents/final-report-ad-hoc-expert-consultation-region-americas-challenges-gaps-and-next-steps (accessed on 13 August 2023).
- World Health Organization (WHO). End-to-End Integration of SARS-CoV-2 and Influenza Sentinel Surveillance: Revised Interim Guidance. January 2022. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-Integrated_sentinel_surveillance-2022.1 (accessed on 13 August 2023).
- Fernandes, R.S.; de Oliveira Silva, J.; Gomes, K.B.; Azevedo, R.B.; Townsend, D.M.; de Paula Sabino, A.; Branco de Barros, A.L. Recent Advances in Point of Care Testing for COVID-19 Detection. Biomed. Pharmacother. 2022, 153, 113538. [Google Scholar] [CrossRef] [PubMed]
- de Lusignan, S.; Hoang, U.; Liyanage, H.; Tripathy, M.; Sherlock, J.; Joy, M.; Ferreira, F.; Diez-Domingo, J.; Clark, T. Using Point of Care Testing to Estimate Influenza Vaccine Effectiveness in the English Primary Care Sentinel Surveillance Network. PLoS ONE 2021, 16, e0248123. [Google Scholar] [CrossRef] [PubMed]
- Sakthivel, D.; Delgado-Diaz, D.; McArthur, L.; Hopper, W.; Richards, J.S.; Narh, C.A. Point-of-Care Diagnostic Tools for Surveillance of SARS-CoV-2 Infections. Front. Public Health 2021, 9, 766871. [Google Scholar] [CrossRef] [PubMed]
- Iliescu, F.S.; Ionescu, A.M.; Gogianu, L.; Simion, M.; Dediu, V.; Chifiriuc, M.C.; Pircalabioru, G.G.; Iliescu, C. Point-of-Care Testing—The Key in the Battle against SARS-CoV-2 Pandemic. Micromachines 2021, 12, 1464. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health Argentina. Guide for the Epidemiological Surveillance and Recommendations for the Prevention and Control of Acute Respiratory Infections 2023 (Translated from Spanish: Guía Para La Vigilancia Epidemiológica y Recomendaciones Para La Prevención y Control de Las Infecciones Respiratorias Agudas 2023); Ministry of Health Argentina: Buenos Aires, Argentina, 2023. Available online: https://bancos.salud.gob.ar/sites/default/files/2023-05/guia-vigilancia-ira_2023.pdf (accessed on 13 August 2023).
- Ministry of Health Brazil. National Plan for the Testing Expansion for COVID-19 (Translated from Portuguese: Plano Nacional Da Expansão Da Testagem Para COVID-19); Ministry of Health Brazil: Brasilia, Brazil, 2022. Available online: https://www.gov.br/saude/pt-br/centrais-de-conteudo/publicacoes/cartilhas/2021/plano-nacional-de-expansao-da-testagem-para-covid-19.pdf/@@download/file (accessed on 13 August 2023).
- Ministry of Health Brazil. 14th Technical Report National Plan for the Testing Expansion for COVID-19 (Translated from Portuguese: 14° Informe Técnico Plano Nacional Da Expansão Da Testagem Para COVID-19); Ministry of Health Brazil: Brasilia, Brazil, 2022. Available online: https://www.gov.br/saude/pt-br/coronavirus/distribuicao-de-testes/pautas-de-distribuicao/14o-informe-tecnico-do-plano-nacional-de-expansao-da-testagem-para-covid-19-14a-pauta-de-distribuicao (accessed on 13 August 2023).
- Ministry of Health Chile. Testing, Tracing and Isolation Strategy (Translated from Spanish: Estrategio Testeo, Trazabilidad y Aislamiento); Ministry of Health Chile: Santiago, Chile, 2021. Available online: https://www.minsal.cl/wp-content/uploads/2021/03/GUIA_ESTRATEGIA_TTA.pdf (accessed on 13 August 2023).
- Ministry of Health and Social Protection Colombia. Guidelines for the Use of SARS-CoV-2 Diagnostic Tests in Colombia (Translated from Spanish: Lineamientos Para El Uso de Pruebas Diagnósticas Para SARS-CoV-2-En Colombia); Ministry of Health and Social Protection Colombia: Bogota, Colombia, 2022. Available online: https://www.minsalud.gov.co/sites/rid/Lists/BibliotecaDigital/RIDE/VS/PP/ET/gips21-lineamientos-uso-pruebas-diagnosticas-sars-cov-2-covid19-2021.pdf (accessed on 13 August 2023).
- Ministry of Health Costa Rica. LS-SS-012. Guidelines for the Use of the Alternative Tests (Antigen, and Isothermic Molecular Tests) from the Gold Standard (RTPCR) for the COVID-19 Diagnosis (Translated from Spanish: LS-SS-012. Lineamientos Generales Para El Uso de Pruebas Alternativas (Antígeno, Pruebas Moleculares Isotérmicas) al Estándar de Oro (RTPCR) Para El Diagnóstico de COVID-19); Ministry of Health Costa Rica: San José, Costa Rica, 2023. Available online: https://www.ministeriodesalud.go.cr/index.php/biblioteca-de-archivos-left/documentos-ministerio-de-salud/vigilancia-de-la-salud/normas-protocolos-guias-y-lineamientos/situacion-nacional-covid-19/lineamientos-especificos-covid-19/lineamientos-de-servicios-de-salud/5399-version-7-03-marzo-2022-lineamientos-generales-para-el-uso-de-pruebas-alternativas-antigeno-pruebas-moleculares-isotermicas-al-estandar-de-oro-rtpcr-para-el-diagnostico-de-covid-19/file (accessed on 12 August 2023).
- Ministry of Health Peru. Technical Document: Prevention, Diagnosis, and Treatment of People Affected by COVID-19 in Peru. (Translated from Spanish: Documento Técnico Prevención, Diagnóstico, y Tratamiento de Personas Afectadas Por COVID-19 En El Perú); Ministry of Health Peru: Lima, Peru, 2020. Available online: https://cdn.www.gob.pe/uploads/document/file/582567/Prevencio%CC%81n__Diagno%CC%81stico_y_Tratamiento_de_personas_afectadas_por_COVID-19_en_el_Peru%CC%81_.PDF?v=1588182165 (accessed on 13 August 2023).
- Freire-Paspuel, B.; Garcia-Bereguiain, M.A. Low Clinical Performance of the Isopollo COVID-19 Detection Kit (M Monitor, South Korea) for RT-LAMP SARS-CoV-2 Diagnosis: A Call for Action against Low Quality Products for Developing Countries. Int. J. Infect. Dis. 2021, 104, 303–305. [Google Scholar] [CrossRef] [PubMed]
- Morales-Jadan, D.; Castro-Rodriguez, B.; Viteri-Dávila, C.; Orlando, S.A.; Bruno, A.; Perez, F.; Garcia-Bereguiain, M.A. The Quality of Commercial SARS-CoV-2 Nucleic Acid Tests in Ecuador: Lessons from COVID-19 Pandemic for Advancing Social Equity through Microbiology. Front. Cell. Infect. Microbiol. 2023, 13, 1179786. [Google Scholar] [CrossRef]
- Hernández, C.; Florez, C.; Castañeda, S.; Ballesteros, N.; Martínez, D.; Castillo, A.; Muñoz, M.; Gomez, S.; Rico, A.; Pardo, L.; et al. Evaluation of the Diagnostic Performance of Nine Commercial RT-PCR Kits for the Detection of SARS-CoV-2 in Colombia. J. Med. Virol. 2021, 93, 5618–5622. [Google Scholar] [CrossRef]
- World Health Organization (WHO). From Emergency Response to Long-Term COVID-19 Disease Management: Sustaining Gains Made during the COVID-19 Pandemic. 2023. Available online: https://www.who.int/publications/i/item/WHO-WHE-SPP-2023.1 (accessed on 13 August 2023).
- World Health Organization (WHO). Recommendations for National SARS-CoV-2 Testing Strategies and Diagnostic Capacities Interim Guidance. 25 June 2021. Available online: https://apps.who.int/iris/bitstream/handle/10665/342002/WHO-2019-nCoV-lab-testing-2021.1-eng.pdf?sequence=1&isAllowed=y (accessed on 13 August 2023).
- Centers for Disease Control and Prevention (CDC). Similarities and Differences between Flu and COVID-19. Available online: https://www.cdc.gov/flu/symptoms/flu-vs-covid19.htm (accessed on 13 August 2023).
- World Health Organization (WHO). WHO Policy Brief: COVID-19 Testing 14 September 2022; WHO: Geneva, Switzerland, 2022; Available online: https://apps.who.int/iris/rest/bitstreams/1465979/retrieve (accessed on 13 August 2023).
- Centers for Disease Control and Prevention (CDC). CDC’s Influenza SARS-CoV-2 Multiplex Assay. 2022. Available online: https://www.cdc.gov/coronavirus/2019-ncov/lab/multiplex.html (accessed on 13 August 2023).
- Ministry of Health Brazil. Guide for Epidemiological Surveillance- Public Health National Emergency by Coronavirus 2019-Disease: Surveillance of Acute Respiratory Syndromes (Translated from Portuguese: Guia de Vigilância Epidemiologica—Emergênciacia de Saúde Pública de Importância Nacional Pela Doença Pelo Coronavírus 2019: Vigilância de Síndromes Respiratorias Agudas); Ministry of Health Brazil: Brasilia, Brazil, 2022. Available online: https://www.gov.br/saude/pt-br/coronavirus/publicacoes-tecnicas/guias-e-planos/guia-de-vigilancia-epidemiologica-covid-19/@@download/file (accessed on 13 August 2023).
- Public Health Institute Ministry of Health Chile. Respiratory virus Surveillance (Translated from Spanish: Vigilancia de Virus Respiratorios). 2023. Available online: https://www.ispch.gob.cl/virusrespiratorios/ (accessed on 13 August 2023).
- National Health Institute (INS) Colombia. Protocol for Acute Respiratory Infection Surveillance (Translated from Spanish: Protocolo de Vigilancia de Infección Respiratoria Aguda (IRA)); National Health Institute (INS) Colombia: Bogota, Colombia, 2022. Available online: https://www.ins.gov.co/buscador-eventos/Lineamientos/PRO_IRA.pdf (accessed on 13 August 2023).
- Health Secretariat Mexico. Guideline for the Surveillance by Laboratory of Respiratory Viruses (Translated from Spanish: Lineamientos para la Vigilancia por Laboratorios de Virus Respiratorios); Health Secretariat Mexico: Mexico City, Mexico, 2022. Available online: https://www.gob.mx/cms/uploads/attachment/file/727495/LVL_Virus_respiratorios__Mayo_2022_.pdf (accessed on 13 August 2023).
- National Administration of Drugs F and MD (ANMAT) A. PanbioTM COVID-19/Flu A&B Rapid Panel 62FK10 (Nasopharyngeal)-Abbott. Orlaweg. November 2023. Available online: https://helena.anmat.gob.ar/uploads/pdfs/IF-2023-06015124-APN-INPM%20ANMAT.pdf?rnd=61674e08-dd23-4ed7-970e-381ba820af8e (accessed on 13 August 2023).
- National Administration of Drugs F and MD (ANMAT) A. Accordance Declaration 1252-177 (Translated from Spanish: Declaración de conformidad-1252-177). Argentina. 2020. Available online: https://helena.anmat.gob.ar/uploads/pdfs/dc_24138_30638975994_2516.pdf?rnd=a71b0beb-2e4c-4868-b085-c0ea280087f4 (accessed on 13 August 2023).
- Ministry of Health Brazil. National Health Plan 2020-2023 (Translated from Portuguese: Plano Nacional de Saúde 2020–2023); Ministry of Health Brazil: Brasilia, Brazil, 2020. Available online: https://bvsms.saude.gov.br/bvs/publicacoes/plano_nacional_saude_2020_2023.pdf (accessed on 13 August 2023).
- Ministry of Health Brazil. Guide for the Laboratory Network of Influenza Surveillance in Brazil (Translated from Portuguese: Guia para a Rede Laboratorial de Vigilância de Influenza no Brasil); Ministry of Health Brazil: Brasilia, Brazil, 2016. Available online: https://bvsms.saude.gov.br/bvs/publicacoes/guia_laboratorial_influenza_vigilancia_influenza_brasil.pdf (accessed on 13 August 2023).
- Ministry of Health Brazil. Influenza Treatment Protocol: 2017 (Translated from Portuguese: Protocolo de Tratamento de Influenza: 2017); Ministry of Health Brazil: Brasilia, Brazil, 2018. Available online: https://bvsms.saude.gov.br/bvs/publicacoes/protocolo_tratamento_influenza_2017.pdf (accessed on 13 August 2023).
- Federal District Government Brazil. Plan for the Confrontation of Childhood Respiratory Diseases in the Federal District (Translated from Portuguese: Plano de Enfrentamento para as Doencas Respiratorias da Infancia no Distrito Federal); Federal District Government Brazil: Brasilia, Brazil, 2023. Available online: https://www.saude.df.gov.br/documents/37101/0/SEI_GDF+-+107165154+-+Plano+de+A%C3%A7%C3%A3o.pdf/26544adc-aec5-af5c-2080-d9015b894b62?t=1679598372243 (accessed on 13 August 2023).
- Brazilian Health Regulatory Agency (Anvisa). Consultation of Health Products- Coronavirus Brazilian Health Regulatory Agency- ANVISA (Translated from Portuguese: Consultas Produtos para Saúde-Coronavirus ANVISA—Agencia Nacional de Vigilancia Sanitaria). 2023. Available online: https://consultas.anvisa.gov.br/#/saude/q/?nomeTecnico=coronav%C3%ADru (accessed on 13 August 2023).
- Ministry of Health Chile. National Health Strategy for the Health Obectives 2030 (Translated from Spanish: Estrategia Nacional de Salud para los Objetivos Sanitarios al 2030). 2022. Available online: https://www.minsal.cl/wp-content/uploads/2022/03/Estrategia-Nacional-de-Salud-2022-MINSAL-V8.pdf (accessed on 13 August 2023).
- Ministry of Health Chile. Clinical Practice Guideline for the Prevention, Diagnosis, and Clinical Management of Influenza Cases (Translated from Spanish: Guía de Práctica Clinica Prevención, Diagnóstico, y Manejo Clínico de Casos de Influenza). 2014. Available online: https://diprece.minsal.cl/wrdprss_minsal/wp-content/uploads/2015/02/GUIA-CLINICA-INFLUENZA-2014.pdf (accessed on 13 August 2023).
- Ministry of Health Chile. Acute Respiratory Infections (Translated from Spanish: Enfermedades Respiratorias Agudas). 2023. Available online: https://diprece.minsal.cl/programas-de-salud/programas-enfermedades-transmisibles/enfermedades-respiratorias/ (accessed on 13 August 2023).
- Ministry of Health Chile. Update of the Operational Definitions for COVID-19 Surveillance in the Framework of the Plan “We Keep Taking Care of Ourselves” (Translated from Spanish: Actualización de las definiciones operativas para la vigilancia de COVID-19 en el marco del Plan “Seguimos Cuidandónos”; Ministry of Health Chile: Santiago, Chile, 2022. Available online: https://www.minsal.cl/wp-content/uploads/2022/11/ORD-4915-19-10-2022.pdf (accessed on 13 August 2023).
- Public Health Institute, C. List of tests for detection of antigens SARS-CoV-2 of the National Regulatory Authorities from the International Forum of Medical Devices Regulators (Translated from Spanish: Listado de tests para detección de antígenos SARS-CoV-2 de la Autoridades Reguladoras Nacionales pertenecientes al Foro Internacional de Reguladores de Dispositivos Médicos). 2021. Available online: https://www.ispch.cl/wp-content/uploads/2021/12/Lista-Test-Antigenos-Covid-al-28.12.21.pdf (accessed on 13 August 2023).
- Ministry of Health and Social Protection. Public Health Decennial Plan 2022-2031 (Translated from Spanish: Plan Decenal Salud Pública 2022–2031). 2023. Available online: https://www.minsalud.gov.co/plandecenal/Paginas/PDSP-2022-2031.aspx (accessed on 13 August 2023).
- Ministry of Health and Social Protection Colombia. Guidelines for the Prevention, Diagnosis, Management and Control of Influenza Cases. (Translated from Spanish: Lineamientos para la Prevención, Diagnóstico, Manejo y Control de casos de Influenza). 2018. Available online: https://www.minsalud.gov.co/sites/rid/Lists/BibliotecaDigital/RIDE/VS/PP/lineamientos-prevencion-diagnostico-manejo-control-casos-influenza.pdf (accessed on 13 August 2023).
- Ministry of Health and Social Protection Colombia. Influenza Antipandemic Plan (Plan Antipandemia Influenza). 2010. Available online: https://www.minsalud.gov.co/salud/Paginas/PlanAntipandemiadeInfluenza.aspx (accessed on 13 August 2023).
- National Health Institute (INS) Colombia. Guidelines for Laboratory Surveillance of Respiratory Viruses (Translated from Spanish: Lineamientos para la Vigilancia por Laboratorio de Virus Respiratorios); National Health Institute (INS) Colombia: Bogota, Colombia, 2021. Available online: https://www.ins.gov.co/buscador-eventos/Informacin%20de%20laboratorio/Lineamientos%20para%20la%20vigilancia%20por%20Laboratorio%20de%20virus%20respiratorios.pdf (accessed on 13 August 2023).
- National Health Institute (INS) Colombia. Guide for the laboratory surveillance of influenza and other respiratory viruses (Translated from Spanish: Guía para la vigilancia por laboratorio del virus de la influenza y otros virus respiratorios) [Internet]; National Health Institute (INS) Colombia: Bogota, Colombia, 2017. Available online: https://www.ins.gov.co/buscador/Informacin%20de%20laboratorio/Guia%20para%20la%20Vigilancia%20por%20Laboratorio%20de%20Virus%20Respiratorios.pdf (accessed on 13 August 2023).
- National Food and Drug Surveillance Institute (INVIMA) Colombia. Nucleic Acid Tests- Diagnosis Reactives and RUO (Translated from Spanish: Pruebas de Ácidos Núcleicos- Reactivos de Diagnóstico y RUO). Available online: https://www.invima.gov.co/documents/20143/4061863/Reactivos+de+Diagno%CC%81stico+y+RUO+%28Pruebas+PCR%2C+Kit+de+Extraccio%CC%81n%2C+Kit+de+Purificacio%CC%81n%2C+etc%29_mayo-2021.pdf/ (accessed on 13 August 2023).
- Ministry of Health Costa Rica, Government of Costa Rica. National Health Plan 2016–2020 (Translated from Spanish: Plan Nacional de Salud Costa Rica). 2016. Available online: https://www.ministeriodesalud.go.cr/index.php/biblioteca-de-archivos-left/documentos-ministerio-de-salud/ministerio-de-salud/planes-y-politicas-institucionales/planes-institucionales/planes-planes-institucionales/709-plan-nacional-de-salud-2016-2020/file (accessed on 13 August 2023).
- Ministry of Health Costa Rica; Costa Rica’s Institute for Research and Teaching in Health and Nutrition (INCIENSA); Costa Rica Social Security. National Protocol for the Surveillance of People with Influenza and other Respiratory Viruses (Translated from Spanish: Protocolo Nacional para la Vigilancia de Personas con Influenza y Otras Viruses Respiratorias). 2018. Available online: https://www.ministeriodesalud.go.cr/index.php/biblioteca-de-archivos-left/documentos-ministerio-de-salud/vigilancia-de-la-salud/normas-protocolos-guias-y-lineamientos/inmunoprevenibles/1828-protocolo-nacional-para-la-vigilancia-de-personas-con-influenza-y-otras-virosis-respiratorias/file (accessed on 13 August 2023).
- Ministry of Health Costa Rica. Laboratories with Authorization for the Use of Antigen SARS-CoV-2 Tests (Translated from Spanish: Laboratorios con Aval de Salud para Realizar Pruebas de Antígeno de SARS-CoV-2). 2021. Available online: https://www.ministeriodesalud.go.cr/index.php/prensa/43-noticias-2021/839-laboratorios-con-aval-de-salud-para-realizar-pruebas-de-antigeno-de-sars-cov-2 (accessed on 13 August 2023).
- Health Secretariat Mexico. Health Secretariat Sector Program Derived from the National Development Plan of Development 2019–2024 (Translated from Spanish: Programa Sectorial derivado del Plan Nacional de Desarrollo 2019–2024). 2020. Available online: https://dof.gob.mx/nota_detalle.php?codigo=5598474&fecha=17/08/2020#gsc.tab=0 (accessed on 13 August 2023).
- Health Secretariat Mexico. National Plan for the Preparation and Response towards the Stational Influenza Increase or towards an Influenza Pandemic (Translated from Spanish: Plan Nacional para la Preparación y Respuesta Ante la Intensificación de la Influenza Estacional o Ante una Pandemia de Influenza). 2013. Available online: http://www.cenaprece.salud.gob.mx/programas/interior/emergencias/descargas/pdf/Plan_Nacional_Influenza.pdf (accessed on 13 August 2023).
- Government of Mexico. COVID Tests (Translated from Spanish: Pruebas COVID). 2021. Available online: https://www.gob.mx/profeco/es/articulos/pruebas-covid?idiom=es (accessed on 13 August 2023).
- Health Secretariat Mexico. List of Molecular Tests RT-PCR Multiplex, Useful for the Diagnosis of SARS-CoV-2 during the COVID-19 Contingency in Mexico (Translated from Spanish: Listado de Pruebas Moleculares RT-PCR Multiplexado, Útiles para el Diagnóstico de SARS-CoV-2 Durante la Contingencia de COVID-19 en Mexico). 2022. Available online: https://www.gob.mx/cms/uploads/attachment/file/785143/Listado_de_pruebas_moleculares_por_RT-PCR_Multiplexado_evaluadas_para_el_diagn_stico_de_SARS-CoV-2.pdf (accessed on 13 August 2023).
- Ministry of Health Peru. National Multisector Health Policy 2030 (Translated from Spanish: Política Nacional Multisectorial de Salud al 2030). Lima. Available online: https://cdn.www.gob.pe/uploads/document/file/1272348/Pol%C3%ADtica%20Nacional%20Multisectorial%20de%20Salud%20al%202030.pdf (accessed on 13 August 2023).
- Ministry of Health Peru. National Plan for the Preparation and Response towards a Potential Influenza and Other Emerging Respiratory virus Pandemic and an Stational Influenza Increase 2014–2015 (Translated from Spanish: Plan Nacional de Preparación y Respuesta Frente a una Potencial Pandemia de Influenza u otros Virus Respiratorios Emergentes e Incremento Estacional de Influenza); Ministry of Health Peru: Lima, Peru, 2014. Available online: http://www.dge.gob.pe/portal/docs/tools/flu/RM747_2014.pdf (accessed on 13 August 2023).
- Ministry of Health Peru. Health Directive N061- MINSA/DGE V.01 for the Epidemiological Surveillance of the Acute Respiratory Infections (ARI) (Translated from Spanish: Directiva Sanitaria N061-MINSA/DGE V.01 para la Vigilancia Epidemiológica de las Infecciones Respiratorias Agudas (IRA). 2015. Available online: http://bvs.minsa.gob.pe/local/MINSA/3266.pdf (accessed on 13 August 2023).
- General Directorate of Medicines S and D (DIGEMID), P. Rapid Tests with Specificity and Sensitivity Authorized with Sanitary Registry 31/03/2023 (Translated from Spanish: Pruebas Rapidas con Especificidad y Sensibilidad Autorizadas con Registro Sanitario 31/03/2023). 2023. Available online: https://www.digemid.minsa.gob.pe/Archivos/PortalWeb/Informativo/Covid19/AutorizacionesExcepcionales/AE_PRUEBAS_RAPIDAS_.xlsx (accessed on 13 August 2023).
- Ministry of Health Costa Rica. Health Regulation-Bioequivalency (Translated from Spanish: Regulación de la Salud-Bioequivalencia). 2022. Available online: https://www.ministeriodesalud.go.cr/index.php/regulacion-de-la-salud/20-regulacion-de-la-salud/60-bioequivalencia-2 (accessed on 13 August 2023).
- Centers for Disease Control and Prevention (CDC). Information on Rapid Molecular Assays, RT-PCR, and other Molecular Assays for Diagnosis of Influenza Virus Infection. 2019. Available online: https://www.cdc.gov/flu/professionals/diagnosis/molecular-assays.htm (accessed on 13 August 2023).
- Ritchey, M.D.; Rosenblum, H.G.; Del Guercio, K.; Humbard, M.; Santos, S.; Hall, J.; Chaitram, J.; Salerno, R.M. COVID-19 Self-Test Data: Challenges and Opportunities—United States, 31 October 2021–11 June 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 1005–1010. [Google Scholar] [CrossRef]
- Hay, J.A.; Kennedy-Shaffer, L.; Kanjilal, S.; Lennon, N.J.; Gabriel, S.B.; Lipsitch, M.; Mina, M.J. Estimating Epidemiologic Dynamics from Cross-Sectional Viral Load Distributions. Science 2021, 373, eabh0635. [Google Scholar] [CrossRef]
- World Health Organization (WHO). COVID-19 Diagnostic Testing in the Context of International Travel-16 December 2020-19: Scientific Briefs. 2020. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-Sci_Brief-international_travel_testing-2020.1 (accessed on 13 August 2023).
- U.S. Food and Drug Administration (FDA). Antibody (Serology) Testing for COVID-19: Information for Patients and Consumers. 2023. Available online: https://www.fda.gov/medical-devices/coronavirus-covid-19-and-medical-devices/antibody-serology-testing-covid-19-information-patients-and-consumers (accessed on 13 August 2023).
- Kundu, D.; Gautam, P.; Dayanand, D.; Gunasekaran, K.; Manesh, A.; Sebastian, M.; Abhilash, K.P.P.; Zachariah, A.; George, T.; Sathyendra, S.; et al. The Role and Diagnostic Accuracy of Serology for COVID-19. BMC Infect. Dis. 2022, 22, 390. [Google Scholar] [CrossRef]
- Lai, C.K.C.; Lam, W. Laboratory Testing for the Diagnosis of COVID-19. Biochem. Biophys. Res. Commun. 2021, 538, 226–230. [Google Scholar] [CrossRef]
- Mitra, P.; Sharma, P. POCT in Developing Countries. EJIFCC 2021, 32, 195–199. [Google Scholar]
- Yadav, H.; Shah, D.; Sayed, S.; Horton, S.; Schroeder, L.F. Availability of Essential Diagnostics in Ten Low-Income and Middle-Income Countries: Results from National Health Facility Surveys. Lancet Glob. Health 2021, 9, e1553–e1560. [Google Scholar] [CrossRef]
- The World Bank. Current Health Expenditure (% of GDP)—Latin America & Caribbean. Available online: https://data.worldbank.org/indicator/SH.XPD.CHEX.GD.ZS?locations=ZJ&name_desc=true (accessed on 28 January 2024).
- Ortiz-Prado, E.; Henriquez-Trujillo, A.R.; Rivera-Olivero, I.A.; Freire-Paspuel, B.; Vallejo-Janeta, A.P.; Lozada, T.; Garcia-Bereguiain, M.A. Massive SARS-CoV-2 RT-PCR Testing on Rural Communities in Manabi Province (Ecuador) Reveals Severe COVID-19 Outbreaks. Am. J. Trop. Med. Hyg. 2021, 104, 1493–1494. [Google Scholar] [CrossRef] [PubMed]
- Nichols, J.H. Utilizing Point-of-Care Testing to Optimize Patient Care. EJIFCC 2021, 32, 140–144. [Google Scholar] [PubMed]
- Ibrahim, N.K. Epidemiologic Surveillance for Controlling Covid-19 Pandemic: Types, Challenges and Implications. J. Infect. Public Health 2020, 13, 1630–1638. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).