Abstract
The COVID-19 pandemic unnerved the global population in 2019 and has continued to evolve ever since. Throughout this time, investigations concerning the health of the groups most susceptible to this virus, including the elderly, those with compromised immunity or chronic diseases, and pregnant women, have taken place. Numerous articles have been formulated on the effects of COVID-19 infection on maternal, fetal, and neonatal health, but there are many controversies that still exist within the current literature. Even three years later, it is not fully understood how a maternal infection or vaccination of COVID-19 can impact pregnancy and the fetus, and these topics require further investigation and conclusive results. The aim of this article is to explain the risks for a mother and the neonate, during and after pregnancy, with the emergence of the COVID-19 pandemic. Additionally, this report presents the current state of the literature on whether vaccination during pregnancy is more beneficial or harmful. Finally, this review examines studies regarding the exacerbation of the effects of COVID-19 on pregnancies in various organ systems, particularly the cardiovascular system, in relevance to pre-existing and emerging conditions and the ethnicity of the mother.
1. Introduction
Pregnancy is a period in which the mother and fetus are significantly more vulnerable to infectious diseases and health conditions. This is a result of the several biomechanical and pathophysiological changes that occur within the mother’s cardiovascular and respiratory systems [1] and the immune system in order to accommodate the fetus [2,3]. Since 2019, a new obstacle for pregnant women has emanated with the spread of the coronavirus disease (COVID-19), an infectious disease caused by the SARS-CoV-2 virus [4]. When compared with uninfected mothers, pregnant women with a COVID-19 infection are at a higher risk for maternal mortality [5]. With the additional difficulties of the COVID-19 infection, pregnant persons also have higher hospitalization and ICU admission rates, increased risk of requiring ventilation, and elevated mortality rate than non-pregnant women who are infected, according to the Centers for Disease Control and Prevention (CDC) COVID-19 surveillance system [6]. Not only does COVID-19 infection cause immediate problems with maternal health, but it can also cause long-term adverse events such as difficulties with delivery and pre-term labor, compromised postpartum immunity and breastfeeding complications, and life-long effects on the neonate. These effects can be exacerbated by many factors, such as pre-existing cardiovascular issues, especially with the higher prevalence of heart disease in women. The infection attacks the compromised immune system of the pregnant individual, as well as the neonate, and socioeconomic factors, such as ethnicity, daily stress factors, and access to proper antenatal, perinatal, and postpartum care can worsen this effect as well.
Within the pregnant population, disparities especially occur among different ethnic groups, as there are several cultural and socioeconomic factors that may be involved in both the mother’s health and recovery, as well as proper fetal development [7]. The maternal mortality rate of Black women is 1.8 times the rate of the maternal mortality of Caucasian women [8]. According to data collected by the CDC from 2016–2018, 46.9% of pregnancy-related deaths were accounted for by cardiovascular conditions [9]. These are only a few considerations for what situations could have the ability to aggravate health concerns during pregnancy. The most common impediments to healthy and safe gestation and delivery are distributed between various direct or indirect causes and across body systems because of COVID-19’s tendency to affect several organ systems at once [10]. They include pre-term labor, stillbirth, miscarriage, pre-eclampsia, and other complications that occur in pregnancy. Vertical transmission appears to be rare, but in a few cases has resulted in impediments for the fetus [11,12].
Vaccination for COVID-19 became available to individuals above the age of 16 in December 2020 [13]. However, there was initial hesitation amongst researchers and the general population as to whether this vaccination is proven to be safe for pregnant and lactating women, leading to low vaccine coverage for pregnant individuals. Ethical concerns arose, and there was a consequent lack of clinical testing when considering vaccinating pregnant women [14]. Keeping more recent studies in mind, vaccines are reported with no significant increase in the risk of stillbirth, spontaneous abortions, or miscarriage events [15].
2. Ethnicity and Underlying Conditions Complicate COVID-19 Pregnancies
When examining COVID-19 and its effect on pregnant women, it is important to consider the situations that may make their pregnancies more difficult before the COVID-19 infection. Each individual pregnancy occurs under different circumstances. There are many factors that can make a pregnancy unique and even high-risk. These factors include pre-existing health conditions, the mother’s demographics, and lifestyle [16]. Current data points to the fact that health problems and ranging ethnicities can also influence the aftermath of the infection and of the pregnancy.
2.1. Ethnicity
Differences in how an individual can respond to and recover from infection have been proven over time to vary based on a plethora of factors [17]. Ethnicity plays a vital role in the risk factors of those experiencing COVID-19 [18]. Not only do different ethnicities have different immune system characteristics due to genetic makeup, but associated factors also play a role in maternal and fetal outcomes during and after pregnancy, as shown in Figure 1. There has been an excessive increase in deaths due to COVID-19 in minority groups when compared with white individuals [19]. This included a striking loss of life of those younger than 65 years of age, despite the smaller size of these minority groups in comparison with the non-Hispanic White group. Minority populations are at a disproportionate disadvantage concerning underlying comorbidities, such as diabetes and cardiovascular disease (CVD) [19]. A deeper investigation into the correlation between ethnicity and COVID-19 infection during pregnancy is needed to understand how to best care for minority individuals.
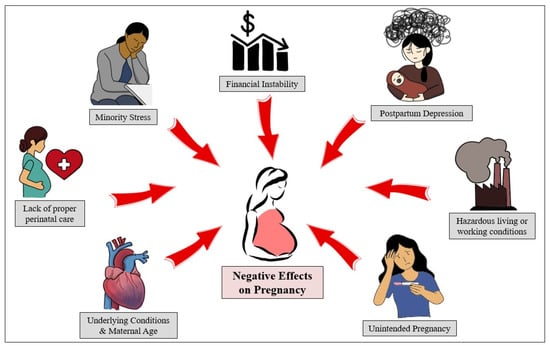
Figure 1.
Pregnancy is a complex process affected by various factors, some of which are beyond the scope of the mother’s medical or health-related concerns. With the COVID-19 pandemic, all of these elements have only worsened (i.e., postpartum depression [20], minority stress [21], and financial instability [22]).
Black women are three times more likely to die from pregnancy-related causes when compared to White women [23]. Due to many factors, such as structural racism and underlying chronic health problems that have a higher prevalence and less control, the risk of pregnancy-related mortality in Black women is significantly increased [8,24]. For example, Black women in the US are at a significantly higher risk for CVD, which can result in significant issues during fetal development [25]. Figure 1 demonstrates some of the socioeconomic factors that also play a role in this statistic, such as hazardous living conditions and financial instability.
Perinatal complications such as depression, pre-term labor, hypertensive disorders, cardiovascular disorders, and postpartum bleeding, are also more likely in Black patients who are pregnant [23]. Consequently, is there a higher concern for Black women regarding COVID-19 infection while pregnant? Without a doubt, the COVID-19 pandemic has exacerbated health disparities experienced by vulnerable populations, including the Black pregnant populations [23]. As data on COVID-19 has evolved, it appears that susceptibility rates mirror those of the general population and Black pregnant persons are more susceptible to infection [23]. According to the 2023 update on Heart Disease and Stroke Statistics by Circulation, peripartum cardiomyopathy is correlated with ethnicity; the highest risk is demonstrated in women with Black ancestry [26]. Pregnant Hispanic women are also exposed to a higher probability of being acutely infected by COVID-19 at delivery or being exposed during pregnancy than their non-Hispanic counterparts [27]. There are many speculations on why this is an occurrence, and social determinants of health are suspected to be contributing to this statistic. The proven disparities in both risks of infection and disease severity among minority pregnant women highlight the need to address and investigate the drivers of reverberations in these populations.
2.2. Underlying Conditions
Pregnant women with chronic diseases, such as epilepsy, kidney disease, diabetes, hypertension, and obesity, are at a higher risk of a range of adverse pregnancy outcomes [28,29]. Current research has found that maternal mortality correlated with a COVID-19 infection is higher in those with underlying conditions, especially mothers with CVD. Pregnancy has many effects on the cardiovascular system, such as an increase in cardiac output, arterial compliance, and extracellular fluid levels, as well as a decrease in blood pressure and total peripheral resistance [30]. These changes may also be unmasked underlying conditions or complications, such as pre-eclampsia, that may need to be monitored and treated appropriately, which makes it a challenge to both obstetricians and cardiologists [30]. Pre-existing CVD can also be worsened by gestational changes in the body [30]. Therefore, COVID-19, which has been shown to be associated with cardiovascular manifestations, presents a further challenge [31]. Patients with cardiac dysfunction are at risk for developing arrhythmias, pulmonary edema, and congestive heart failure because they do not tolerate the physiological changes of pregnancy well [32]. If the mother had a pregestational history of stroke, then pregnancies were associated with pre-term labor or pre-labor cesarean section [26]. It is important to understand how these conditions may play a role in a COVID-19 infection in pregnancy to find ways to combat negative effects.
A growing concern for the mother and her child during pregnancy is the relationship between COVID-19 and hypertensive disorders of pregnancy. Pregnant women diagnosed with COVID-19 have a higher likelihood of being diagnosed with pneumonia, hypertensive disorders of pregnancy, such as pre-eclampsia or eclampsia, and thromboembolic disease [33]. Another analysis found that pregnant women who contracted SARS-CoV-2 had notably higher odds of developing pre-eclampsia compared to those who did not have the infection [33]. A multicenter study conducted in Brazil found that among pregnant women who tested positive for COVID-19, those with pre-eclampsia had a higher rate of cesarean section delivery, and their newborns were more likely to require admission to the neonatal intensive care unit, likely due to complications related to preterm birth [34]. This is particularly concerning as hypertensive disorders affect 5% to 6% of all pregnant women and are the primary causes of maternal and fetal morbidity [35]. These findings indicate that pregnant women with both SARS-CoV-2 infection and pre-eclampsia experience a more severe clinical manifestation of the disease and are at higher risk of maternal and neonatal morbidity in comparison to those with either COVID-19 or pre-eclampsia alone.
Diabetes is another concern for an underlying condition that could make COVID-19 symptoms worse in pregnant women. An article has shown that pregnant women with COVID-19 who are of non-White ethnicity, older, overweight, and have pre-existing maternal conditions like chronic hypertension and diabetes, as well as pregnancy-related complications, such as gestational diabetes and pre-eclampsia, are at a higher risk for severe COVID-19, admission to an intensive care unit, invasive ventilation, and maternal death [36]. Additionally, women who had pre-existing diabetes mellitus were reported to have almost twice the possibility of contracting COVID-19 [37]. The increased risks for pregnant women with diabetes emphasize the importance of taking necessary precautions to prevent contraction and ensure a safe pregnancy.
There are many health concerns attributed to obesity that can lead to severe complexities during pregnancy, and in turn, during a COVID-19 infection. The immune system and mechanisms that defend the body are negatively affected by obesity. This is because obesity is associated with chronic activation of the immune system and inflammation, which can have negative effects on the immune system’s functionality [38]. Individuals with obesity have characteristics of a decrease in chest wall compliance, respiratory muscle strength and function, lung volumes, and peripheral airway size [39]. Therefore, decreased immune and respiratory function attributed to obesity can present specific risks to both COVID-19 and pregnancy.
It is apparent that pregnant women between the ages of 35 and 44 are at increased risk for severe disease, four times as likely to require ventilation, and twice as likely to die than nonpregnant women in the same age group [27]. Understanding these underlying conditions is crucial as it can allow for more personalized care plans to alleviate the increased risks associated with COVID-19 and pregnancy, which can improve both maternal and fetal outcomes.
3. Effects of COVID-19 Infection on Maternal and Fetal Health
Initially, it was unclear the impact a COVID-19 infection would have on pregnant individuals and their offspring. There has been evidence of past outbreaks of viral infections, specifically respiratory illnesses, such as the Middle East respiratory syndrome, ending poorly for both mother and baby [40]. There is also evidence to support that pregnant women are at an increased risk for more severe infections of the influenza virus, hepatitis E virus, herpes simplex virus, as well as malaria parasites [41]. At first, it was believed that because of the weakened immune system during pregnancy, they are more at risk for encountering COVID-19 [42]. However, it has been indicated that pregnancy does not raise the risk of contracting the disease, but it has the possibility to result in acute illness [27,43]. The CDC states that if an individual is pregnant or was recently pregnant, they are more likely to become severely ill from COVID-19 compared with those who are not pregnant, meaning that the individual may need hospitalization, admission into an ICU, and potentially a ventilator or other device to help them breathe [44,45,46].
Some earlier observations during the pandemic, including one published in November 2020, concluded that a COVID-19 infection in pregnancy was not associated with adverse maternal and fetal health outcomes [45]. Although, over the last three years, more data has been collected and published as the virus has evolved, that conclude that COVID-19 could very well be correlated with dire consequences in pregnancy. A recent meta-analysis published concluded that infection of SARS-CoV-2 in pregnancy at any time leads to an increased incidence of maternal mortality, maternal morbidities, and adverse newborn outcomes. It was found that those with an infection had a higher risk for several fatal effects, such as hypertensive disorders of pregnancy, pre-eclampsia or eclampsia, pre-term labor, and thromboembolic disease. The analysis included data from 12 studies and included 13,136 pregnant individuals from 12 different countries [5]. A systematic review also found that an increased risk of pre-eclampsia and pre-term birth is associated with a COVID-19 infection [46]. Pre-eclampsia has been associated with cardiovascular sequelae, such as hypertension and altered vascular function in the neonate. It is also a major cause of maternal morbidity and is connected to fetal health concerns [47]. More information on COVID-19 and pre-eclampsia is needed as it is a concern both for mother and baby, and COVID-19 infections could be contributing to increased incidence of developing the condition.
Furthermore, the gestational age can vary the turnout of COVID-19 infection in pregnancy. There are varying data on whether the incidence of adversities is higher in those at a lower gestational age or a higher one. A retrospective cohort study conveyed that a first- or second-trimester infection accounted for an increase in pre-term labor and stillbirth. The results concluded that regardless of the severity of the COVID-19 infection, pregnant people are at an increased risk for pre-term birth. The infected individuals were unvaccinated, highlighting a need for information on maternal infections in vaccinated mothers [48]. However, a different investigation concluded otherwise: that an infection in the first trimester showed no evidence of increased complications in the pregnancy [49]. The outcomes of COVID-19 in pregnancy should be further examined based on gestational age to understand how the two are correlated.
3.1. Risk of Stillbirth or Miscarriage
According to the CDC, a miscarriage is defined as the loss of a baby before 20 weeks of gestation or before viability, and stillbirth is defined as a loss of a fetus after 20 weeks of gestation [50]. Approximately 1 in 100 pregnancies at or after 20 weeks gestation is impacted by stillbirth [51,52]. There are some circumstances that can greatly higher the risk of an individual having a stillbirth, including being overweight or obese, being 35 years of age or older, and having pre-gestational and gestational medical disorders, such as hypertension and diabetes [53]. In total, 15.3% of pregnancies end in miscarriage, and risk factors for miscarriage include age, body mass index (BMI), and ethnicity [54]. Maternal infection in previous coronavirus epidemics has been linked with a higher incidence of pregnancy losses and miscarriage [55]. Furthermore, it has been a question since the beginning of the pandemic whether the same applies to COVID-19. As of today, COVID-19 has been proven to potentially have adverse effects on pregnant women and their fetuses, but there are still gaps in the literature to conclude whether infection with COVID-19 may or may not have a causal or correlational relationship with stillbirth or miscarriage.
Studies have been conducted to determine how infection of COVID-19 plays a role in the incidence of miscarriage, but the results are varied. COVID-19 appears to increase the risk of delivering a stillborn infant, according to the CDC [56,57]. However, a study published in early 2021 concluded that there was no difference in the cumulative incidence of miscarriage between those infected with the virus and the control group [58]. Eleven percent of the cases of infection ended in first-trimester pregnancy loss, and 9.6% of the control cases ended in a first-trimester loss and concluded that SARS-CoV-2 infection in the first trimester does not appear to cause early pregnancy loss as the difference was not considered significant [58]. Similarly, another article demonstrated that pregnant women with a self-reported COVID-19 infection had a 14% rate of miscarriage, compared to 5% in a group who were uncertain of their infection status and 8% in the presumed uninfected group [57]. The risk of early miscarriage did appear to be higher among women with a self-reported infection than those without an infection of COVID-19, but this data was not concluded to be statistically significant [57]. It was concluded by an analysis performed in 2021 that the miscarriage rates of pregnant people with COVID-19 seemed to be in the range of the rates in negative pregnant people. However, these results were limited by a small number of cases and lacked proper controls. It also concluded that the presence of symptoms during the acute phase of COVID-19, plasma viral load, severity, and obstetrical risk factors seem to increase the prevalence of miscarriage for women with a SARS-CoV-2 infection [58].
3.2. Postpartum Difficulties
Furthermore, investigating the effects of the infection during pregnancy in the postpartum period is important as well because although the pregnancy has ended, the mother may still be dealing with the ramifications of the infection. Because long-term outcomes of COVID-19 infections are not fully known at this point, as the pandemic began only three years ago, this makes determining the effects on the mother immediately after birth and forward more complex. Continued research, including long-term studies, is needed to determine the postpartum risks of a COVID-19 infection in pregnancy. Controversy has arisen as to whether COVID-19 is spreading through breast milk from mother to baby. However, it is not apparent that transmission of COVID-19 occurs through breast milk [59]. Having to stop breastfeeding abruptly can be a challenging and distressing experience for new mothers. While breastfed infants have tested positive for COVID-19, the mode of infection cannot be determined, and it is not recommended to interrupt breastfeeding.
There are other potential long-term complications for the mother after giving birth. Circulation by AHA found that women who had at least one pregnancy before menopause and those who experienced pre-eclampsia during their pregnancy were at a higher risk of stroke later in their lifetime [11,59]. With the prevalence of pre-eclampsia in COVID-19-infected mothers, these statistics raise a concern about the long-term effects of the infection on not just the neonate but also the mother.
3.3. Potential Adverse Effects on Fetus
There is little evidence to support whether mothers infected by COVID-19 transmit the virus to the fetus in utero. It has been demonstrated by the Boston University School of Medicine that Angiotensin-Converting-Enzyme 2 (ACE2) is found in lower concentrations in the placentas of those infected by COVID-19 compared to those pregnancies without infection [60]. It is currently known that the SARS-CoV-2 virus binds to ACE2 using a spike-like protein, acting as a cellular doorway for the virus [61,62]. Hence, because the placenta sheds these ACE2 receptors, it can be interpreted that the body performs this to protect the fetus from the virus. However, this phenomenon needs to be investigated deeper to form a solid conclusion.
Vertical transmission is defined as the possibility of an infection transmitting to the fetus from the mother before or during delivery or to the neonate after the delivery [57]. Furthermore, Figure 2 demonstrates the three possible ways that vertical transmission may occur. Vertical transmission is a rare incidence of COVID-19 infections, but there has been some evidence that it occurs in some cases, and the literature has no consensus on if it occurs and how it occurs. According to a systematic review conducted in 2021, vertical transmission of COVID-19 was found in 3.2% of the cases by infant nasopharyngeal swab testing. From this data, it is evident that vertical transmission is possible and does occur, albeit rarely [63]. This study highlighted the need for information on how the gestational age of the pregnancy plays a role in the incidence and severity of a case of vertical transmission. On reviewing the effect of infection on fetal health, vertical transmission was of high concern, especially during the beginning of the pandemic. Long-term investigations would be needed to explore the possible effects of infection during pregnancy.
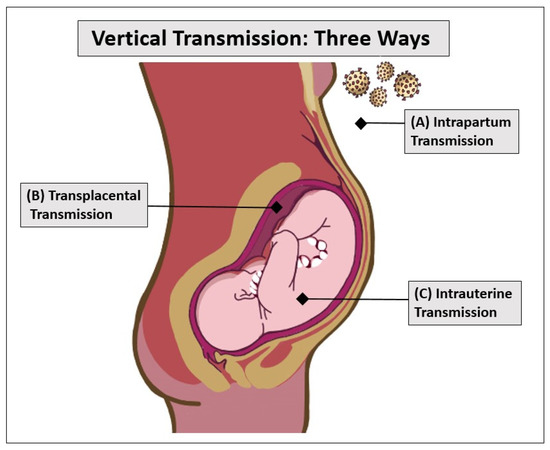
Figure 2.
With most infectious diseases, as well as COVID-19, vertical transmission can occur in three ways: intrapartum transmission (top), transplacental transmission (middle), and intrauterine transmission (bottom). Intrapartum transmission (A) happens when neonates are exposed to maternal blood or an environment containing the COVID-19 virus. Transplacental transmission (B) occurs when the virus crosses the protective placental barrier and infects the fetus. Intrauterine transmission (C) happens when the virus is swallowed by the fetus through amniotic fluid.
When assessing a group of COVID-19-positive mothers in 2020, 6.6% of neonates were found also to be COVID-19 positive; however, biological samples from the time of birth, such as cord blood, placental membrane, vaginal fluid, amniotic fluid, and peritoneal fluid from C-sections all tested positive, and so the study concluded that vertical transmission was not a possibility [64]. As mentioned in Figure 2, intrapartum transmission may have occurred after birth, where the babies may have been exposed to the virus after birth. However, there is no evidence of how the vertical transmission may have occurred. Conclusively, the neonates were discharged after two weeks with all routine immunizations, having shown no severe reactions to infection [64]. In yet another case, a baby tested positive 36 h after birth to an asymptomatic mother, and it was concluded that in utero transmission must have occurred [65].
While vertical transmission of the virus has not been observed in an overwhelming number of cases, there is still potential for maternal immune activation [66]. When maternal immune activation occurs, it has been stated that an impact on the developing fetal brain is likely. A case report published in April showed that two infants born to mothers who had COVID-19 during the second trimester were born with fatal brain injuries [67]. One infant was born prematurely and later died from an episode of asystole cardiac arrest. The other infant was born at full term and tested positive for SARS-CoV-2 at birth. The infant has suffered from clinical seizures, microcephaly, significant neurological delay, and multiple respiratory infections and is now in hospice [67]. Though cases like these are rare occurrences, they still raise a concern about the exact mechanisms under which they may have occurred at all. As discussed in earlier sections, the underlying conditions, ethnicity, associated factors, genetics, and vaccination status of the mother could also play a role since the mothers were of a minority ethnicity [63]. Another study concluded that among the offspring exposed to COVID-19 in utero, there was a higher risk for male offspring to have a neurodevelopmental diagnosis one year after birth. This is an association that needs to be further examined on a larger scale, as this was limited by a smaller cohort of infants [68]. Aside from neurological concerns, it has also been reported that COVID-19 infection in the third trimester could cause fetal kidney developmental injury [69]. This article included a limited number of newborns, and this conclusion needs to be investigated further on a larger scale.
In a contradictory observation, intrauterine vertical transmission was found to be a rare issue in COVID-19 during pregnancy which may lead to placental dysfunction. Upon assessing neonatal outcomes, the study concluded that the results were not severe [70]. Clinical observation also confirms this finding, where there were no serious effects on the neonate [71]. Comparing these cases above shows us two potential ramifications: one where vertical transmission has led to dire consequences for the baby and one where the effects are not grave. Most literature shows that vertical transmission of COVID-9 is rare [72], but if it is transpiring and has a possibility to affect future generations, then it should certainly be investigated. Despite these concerns, many studies show evidence that the transplacental transfer of antibodies occurs between the mother and the fetus, whether it is in the form of infection or vaccination [73,74,75]. Notably, the role of vertical transmission in fetal health is clearly undescribed and needs to be researched further.
4. COVID-19 Vaccination in Relevance to the Pregnant Populations
In the first few months of the rapid emergence of the COVID-19 virus, research was conducted on the adverse effects of the disease on every population, including men, women, the elderly, children, as well as pregnant women. Reviews performed in 2020 show that pregnant women infected with COVID-19 experienced a greater number of admissions to the ICU, cesarean deliveries, and an on-average higher rate of pre-term deliveries [76].
However, a lot of research funding was also directed toward creating a vaccine for the new disease. Factors that aided such a fast production and distribution of the vaccine included the past knowledge of coronaviruses, worldwide collaboration of scientists and leaders, funding, and especially mRNA technology [77]. Despite this feat of technology, it raised a significant concern when COVID-19 vaccines were first introduced to the pregnant population, where the dilemma concerned reducing the higher risks of mortality associated with COVID-19 infection during pregnancy but not without considering the ethics of testing and delivering vaccines on pregnant individuals.
This concern was proliferated by the fact that the vaccine-making process had been completed within a year, and the results of clinical trials on pregnant women had not been included [78,79,80]. Even a year after the emergence of the virus, a review of the COVID-19 vaccine clinical studies reports that clinical trials were performed on the vaccines ChAdOx1 nCoV-19, BNT162b2, mRNA-1273, and Ad26. COV2.5 and NVX-CoV2373 were all shown to have an efficacy of at least 70% in their respective tested populations, but results on the efficacy on pregnant and breastfeeding women were not tested [80]. A poll conducted in 16 different countries showed that even with 90% COVID-19 vaccine efficacy, only 52% of pregnant women were considering receiving the vaccine. The primary concerns were confidence in vaccine safety, COVID-19 itself, belief in the importance of vaccines in the specific country compliance to mask guidelines, and general trust of public health agencies [81,82,83]. To clear vaccination safety for pregnant individuals, substantial evidence was imperative. In April 2021, the CDC first highlighted new data regarding the safety of COVID-19 vaccination and concluded that it was safe for pregnant women to get the vaccine, and that would aid in countering any adverse effects on their pregnancy in the case of exposure or even infection [84]. Since then, researchers have been able to report on the effects of the vaccines on this vulnerable population.
4.1. Effects of COVID-19 Vaccination on Pregnancy
Studies have been reporting on some of the more common side effects of vaccination in pregnant women. This data is relatively recent, as vaccination for pregnant women was only cleared and recommended by the CDC in 2021 [84], and only afterward were researchers able to gain access to the specified population and any effects they may have had. Injection-site discomfort was the most common unpleasant response, noted to affect both pregnant and non-pregnant women who received vaccinations. Upon the second dose, the incidence of adverse reactions increased and included tiredness, migraines, shivers, malaise, rash, and vomiting. Even though these effects were noted, vaccination was not shown to have gestational or delivery impacts compared to unvaccinated pregnant women, with no significant differences in gestational hypertension or thrombosis, as well as incidence or premature birth, endometrial break, or unexpected ICU admission [83]. In a retrospective observational study, acute adverse events were noted up to 21 days after vaccination in pregnant women, namely lymphadenitis, acute myocardial infarction, appendicitis, Bell’s Palsy, cerebral venous sinus thrombosis, hemorrhagic and ischemic stroke, seizures, pulmonary embolism, and venous thromboembolism. These events did not occur with a significant difference between the vaccinated and unvaccinated groups but were of higher probability in the unvaccinated group [84].
There are concerns about whether vaccination of the mother in the first few weeks of pregnancy is related to spontaneous abortion of the fetus. One demonstrated that spontaneous abortions were the most reported adverse event in vaccinated mothers, with most cases occurring in the first trimester [85,86]. However, most literature shows that the risk of spontaneous abortion is not significantly increased in vaccinated mothers [87,88,89,90,91,92,93]. However, if there are cases in which vaccinations correlate with adverse effects on the fetus in the initial stages of pregnancy, then it should not be overlooked. There may be other aspects relating to the mothers’ health and stress conditions that may have contributed to these results and should be researched to prevent further occurrences.
4.2. COVID-19 Vaccination Timing in Pregnant Women
As differences in outcomes have been shown with the timing of infection of the mother during pregnancy, the timing of vaccinations was also examined in the literature for any correlations with effects on maternal and fetal health, either beneficial or harmful. An investigation compared pregnancy complications in pregnant women who were unvaccinated to pregnant women who had received at least one dose of the COVID-19 vaccine during the second and third trimesters [93]. The pregnant women that were vaccinated were at a similar risk for stillbirth, fetal abnormalities, postpartum hemorrhage, cesarean delivery, small gestational size of the neonate, maternal high-dependency unit for intensive care admission, or neonatal care unit admission. Intrapartum pyrexia, however, was increased in the vaccinated group, but this significance was lost after excluding women who were also infected with COVID-19 during their pregnancy. A case study found that a mother who was vaccinated during the third trimester of her pregnancy delivered a baby that obtained passive immunity [94]. Overall, the literature presents that vaccination is not associated with a higher risk of pregnancy difficulties and, for the time being, is a safe practice for pregnant women. Even with emerging variants of concern of the SARS-CoV-2 virus, such as Delta or Omicron, and their associated increased adverse maternal and fetal outcomes, vaccination has proven to be reducing severe maternal complications [95,96,97].
5. Conclusions and Future Directions
The COVID-19 pandemic presented a new obstacle for the entire world, especially for those groups who are most vulnerable. The most susceptible groups in this pandemic include people of ages 50 years or older, those with underlying health conditions and compromised immunity, and pregnant women [98]. The CDC also identifies that there are ethnic and socioeconomic disparities in populations prone to infection, and these discrepancies could be a great cause for concern in pregnant populations [98]. Moreover, pregnancy brings many physiological changes that have the potential to change the way the body fights an infection, such as COVID-19, as discussed previously. It is important to continue expanding on how COVID-19 can affect pregnant persons and their offspring to determine the best method of care and protection against the virus.
Preexisting conditions of the mother, especially CVD, can contribute to unfavorable events in pregnancy during a COVID-19 infection [99]. Ethnicity certainly plays a significant role, as even before the pandemic, non-Hispanic Black mothers faced a higher maternal mortality rate than their non-Hispanic White counterparts [100]. During pregnancy, Black women are more likely to experience difficulties, such as peripartum cardiomyopathy, than White women [101,102]. This is due to biological and psychological factors combined, including the stress of being a part of a minority group in the U.S. [7]. Furthermore, only a small number of reports have examined COVID-19-infected mothers that are of Hispanic descent, which is a concerning matter considering that this is a minority group highly susceptible to being infected and less likely to be vaccinated due to the various socioeconomic factors on their part.
COVID-19 has proven to be correlated with potential adverse outcomes for both mother and baby, including increased need for admittance to ICU, higher ventilation rate, CVD, preterm delivery, and altered neurodevelopment in offspring. Although there is some data to support these occurrences, more investigation is needed to confirm exactly what the risks are, especially as the virus has continued to appear in variants. The results of whether COVID-19 is correlated with a higher risk of miscarriage or stillbirth vary, but the literature shows that if there is a greater incidence, it is not a significant one. Long-term clinical trials will be needed to determine how the infection affects the mother and their offspring during and after the postpartum period. There are observations that focus on neurodevelopmental outcomes in offspring after a COVID-19 infection; however, they can only draw results from the first year or two of the life of the baby as infections only began three years ago [69,76]. Additional data is needed to investigate this, especially focusing on the age, ethnicity, and any underlying conditions in the pregnant person, as these categories have been previously overlooked due to ethical concerns and the lack of access to certain populations. Furthermore, vaccination is important for pregnant persons as it is the best way to protect against the virus. The overall protection the vaccine offers outweighs the slight risk of an adverse event. However, if negative effects are in place, then they should not be disregarded for the purposes of promotion of vaccination.
Since the pandemic began only three years ago, comprehensive data is inadequate for reliable presumptions about the effects of the pandemic on pregnancy. COVID-19 has influenced the world and especially those groups who are most vulnerable. It is important to evaluate COVID-19 infections in these groups to best prevent and mitigate an infection of COVID-19 during pregnancy for improved maternal and fetal health. While current literature highlights outcomes of COVID-19 in pregnancy, more research and data are needed to draw definite conclusions on the long-term, widespread impact of COVID-19 on pregnancy.
Author Contributions
The conceptualization, design, and execution of the concept were performed by L.C.K. and G.C.G. Initial literature collection, reading, and drafting of the review article material was conducted by G.C.G. and N.V. The figures were conceptualized and developed by N.V. The final draft writing, revision, and updating of the literature and figures was conducted by G.C.G., N.V., M.P.T., H.M.S. and L.C.K. G.C.G. and N.V. contributed equally to the literature search, writing, and organizing the manuscript. The final draft was revised by L.C.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
The authors would like to thank the ECU Brody School of Medicine Department of Physiology for their administrative support and facilities, as well as Pitt County Schools, the Health Sciences Academy, and the ECU-Pitt County Schools Honors Medical Research Program for allowing G.C.G. and H.M.S. to work with the lab.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Costantine, M.M. Physiologic and pharmacokinetic changes in pregnancy. Front. Pharmacol. 2014, 5, 65. [Google Scholar] [CrossRef] [PubMed]
- Abu-Raya, B.; Michalski, C.; Sadarangani, M.; Lavoie, P.M. Maternal Immunological Adaptation during Normal Pregnancy. Front. Immunol. 2020, 11, 575197. [Google Scholar] [CrossRef]
- Habel, J.R.; Chua, B.Y.; Kedzierski, L.; Selva, K.J.; Damelang, T.; Haycroft, E.R.; Nguyen, T.H.; Koay, H.-F.; Nicholson, S.; McQuilten, H.A.; et al. Immune profiling of SARS-CoV-2 infection during pregnancy reveals NK cell and γδ T cell perturbations. JCI Investig. 2023, 8, e167157. [Google Scholar] [CrossRef]
- World Health Organization. Coronavirus Disease (COVID-19). Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (accessed on 5 March 2023).
- Smith, E.R.; Oakley, E.; Grandner, G.W.; Ferguson, K.; Farooq, F.; Afshar, Y.; Ahlberg, M.; Ahmadzia, H.; Akelo, V.; Aldrovandi, G.; et al. Adverse maternal, fetal, and newborn outcomes among pregnant women with SARS-CoV-2 infection: An individual participant data meta-analysis. BMJ Glob. Health 2023, 8, e009495. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, D.J.; Rasmussen, S.A. An update on COVID-19 and pregnancy. Am. J. Obstet. Gynecol. 2021, 226, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Lee, S.M.; Bae, S.-H.; Kim, H.J.; Lim, N.G.; Yoon, S.-J.; Lee, J.Y.; Jo, M.-W. Socioeconomic status can affect pregnancy outcomes and complications, even with a universal healthcare system. Int. J. Equity Health 2018, 17, 2. [Google Scholar] [CrossRef]
- MacDorman, M.F.; Thoma, M.; Declcerq, E.; Howell, E.A. Racial and Ethnic Disparities in Maternal Mortality in the United States Using Enhanced Vital Records, 2016–2017. Am. J. Public Health 2021, 111, 1673–1681. [Google Scholar] [CrossRef]
- Pregnancy Mortality Surveillance System. Available online: https://www.cdc.gov/reproductivehealth/maternal-mortality/pregnancy-mortality-surveillance-system.htm (accessed on 5 March 2023).
- CDC. Long COVID or Post-COVID Conditions. 16 December 2022. Available online: https://www.cdc.gov/coronavirus/2019-ncov/long-term-effects/index.html (accessed on 5 March 2023).
- Peng, Z.; Zhang, J.; Shi, Y.; Yi, M. Research progress in vertical transmission of SARS-CoV-2 among infants born to mothers with COVID-19. Futur. Virol. 2022, 17, 211–214. [Google Scholar] [CrossRef]
- Kotlyar, A.M.; Grechukhina, O.; Chen, A.; Popkhadze, S.; Grimshaw, A.; Tal, O.; Taylor, H.S.; Tal, R. Vertical transmission of coronavirus disease 2019: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2020, 224, 35–53.e3. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. FDA Approves First COVID-19 Vaccine. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-covid-19-vaccine (accessed on 5 March 2023).
- Bianchi, F.P.; Stefanizzi, P.; Di Gioia, M.C.; Brescia, N.; Lattanzio, S.; Tafuri, S. COVID-19 vaccination hesitancy in pregnant and breastfeeding women and strategies to increase vaccination compliance: A systematic review and meta-analysis. Expert Rev. Vaccines 2022, 21, 1443–1454. [Google Scholar] [CrossRef]
- Rimmer, M.P.; Teh, J.J.; Mackenzie, S.C.; Al Wattar, B.H. The risk of miscarriage following COVID-19 vaccination: A systematic review and meta-analysis. Hum. Reprod. 2023, 38, 840–852. [Google Scholar] [CrossRef] [PubMed]
- What Are Some Factors That Make a Pregnancy High Risk—NICHD—Eunice Kennedy Shriver National Institute of Child Health and Human Development. Available online: https://www.nichd.nih.gov/health/topics/high-risk (accessed on 5 March 2023).
- Au, W.W. Life style factors and acquired susceptibility to environmental disease. Int. J. Hyg. Environ. Health 2001, 204, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.T.; Ricaldi, J.N.; Rose, C.E.; Fuld, J.; Parise, M.; Kang, G.J.; Driscoll, A.K.; Norris, T.; Wilson, N.; Rainisch, G.; et al. Disparities in Incidence of COVID-19 among Underrepresented Racial/Ethnic Groups in Counties Identified as Hotspots during June 5–18, 2020—22 States, February–June 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 1122–1126. [Google Scholar] [CrossRef]
- Hooper, M.W.; Nápoles, A.M.; Pérez-Stable, E.J. COVID-19 and Racial/Ethnic Disparities. JAMA J. Am. Med. Assoc. 2020, 323, 2466–2467. [Google Scholar] [CrossRef] [PubMed]
- Chang, O.; Layton, H.; Amani, B.; Merza, D.; Owais, S.; Van Lieshout, R.J. The Impact of the COVID-19 Pandemic on the Mental Health of Women Seeking Treatment for Postpartum Depression. J. Matern.-Fetal Neonatal Med. 2022, 35, 9086–9092. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.; Casement, M.D.; Cuellar, R.; Johnson, D.A.; Kalmbach, D.; Castelan, A.C.; Drake, C.L. Sleepless in COVID-19: Racial disparities during the pandemic as a consequence of structural inequity. Sleep 2021, 45, zsab242. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, M.R.; Brown, S.A.; Pelham, W.E.; Bodison, S.C.; McCabe, C.; Baker, F.C.; Baskin-Sommers, A.; Dick, A.S.; Dowling, G.J.; Gebreselassie, S.; et al. Family Well-Being During the COVID-19 Pandemic: The Risks of Financial Insecurity and Coping. J. Res. Adolesc. 2022, 33, 43–58. [Google Scholar] [CrossRef]
- McMillian-Bohler, J.; Bell, L.M. Considerations and Recommendations for Care of Black Pregnant Patients During COVID-19. Nurs. Clin. N. Am. 2022, 57, 443–452. [Google Scholar] [CrossRef]
- Petersen, E.E.; Davis, N.L.; Goodman, D.; Cox, S.; Syverson, C.; Seed, K.; Shapiro-Mendoza, C.; Callaghan, W.M.; Barfield, W. MMWR—Racial/Ethnic Disparities in Pregnancy-Related Deaths—United States, 2007–2016. 2007. Available online: https://www.cdc.gov/reproductivehealth/maternalinfanthealth/pqc.htm (accessed on 5 March 2023).
- Kalinowski, J.; Taylor, J.Y.; Spruill, T.M. Why Are Young Black Women at High Risk for Cardiovascular Disease? Circulation 2019, 139, 1003–1004. [Google Scholar] [CrossRef]
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Beaton, A.Z.; Boehme, A.K.; Buxton, A.E.; et al. Heart Disease and Stroke Statistics—2023 Update: A Report from the American Heart Association. Circulation 2023, 147, e93–e621. [Google Scholar] [CrossRef]
- Zambrano, L.D.; Ellington, S.; Strid, P.; Galang, R.R.; Galang, T.; Tong, V.T.; Woodworth, K.R.; Nahabedian, J.F., III; Azziz-Baumgartner, E.; Gilboa, S.M.; et al. Update: Characteristics of Symptomatic Women of Reproductive Age with Laboratory-Confirmed SARS-CoV-2 Infection by Pregnancy Status—United States, January 22–October 3, 2020. 2020. Available online: https://www.cdc.gov/mmwr/volumes/69/wr/mm6944e3.htm (accessed on 5 March 2023).
- Ralston, E.R.; Smith, P.; Chilcot, J.; Silverio, S.A.; Bramham, K. Perceptions of risk in pregnancy with chronic disease: A systematic review and thematic synthesis. PLoS ONE 2021, 16, e0254956. [Google Scholar] [CrossRef] [PubMed]
- Narayan, B.; Nelson-Piercy, C. Medical problems in pregnancy. Clin. Med. 2017, 17, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.E.; George, E.M.; Granger, J.P. The Heart during Pregnancy. Rev. Esp. Cardiol. 2011, 64, 1045–1050. [Google Scholar] [CrossRef] [PubMed]
- Magadum, A.; Kishore, R. Cardiovascular Manifestations of COVID-19 Infection. Cells 2020, 9, 2508. [Google Scholar] [CrossRef]
- Adam, K. Pregnancy in Women with Cardiovascular Diseases. Methodist DeBakey Cardiovasc. J. 2017, 13, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Conde-Agudelo, A.; Romero, R. SARS-CoV-2 infection during pregnancy and risk of preeclampsia: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2021, 226, 68–89.e3. [Google Scholar] [CrossRef] [PubMed]
- Guida, J.P.; Cecatti, J.G.; Souza, R.T.; Pacagnella, R.C.; Ribeiro-Do-Valle, C.C.; Luz, A.G.; Lajos, G.J.; Surita, F.G.; Nobrega, G.M.; Griggio, T.B.; et al. Preeclampsia among women with COVID-19 during pregnancy and its impact on maternal and perinatal outcomes: Results from a national multicenter study on COVID in Brazil, the REBRACO initiative. Pregnancy Hypertens. 2022, 28, 168–173. [Google Scholar] [CrossRef]
- Khedagi, A.M.; Bello, N.A. Hypertensive Disorders of Pregnancy. Cardiol. Clin. 2020, 39, 77–90. [Google Scholar] [CrossRef]
- Sliwa, K.; Hilfiker-Kleiner, D.; Petrie, M.C.; Mebazaa, A.; Pieske, B.; Buchmann, E.; Regitz-Zagrosek, V.; Schaufelberger, M.; Tavazzi, L.; van Veldhuisen, D.J.; et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of peripartum cardiomyopathy: A position statement from the Heart Failure Association of the European Society of Cardiology Working Group on peripartum cardiomyopathy. Eur. J. Heart Fail. 2010, 12, 767–778. [Google Scholar] [CrossRef]
- Gambahaya, E.T.; Minhas, A.S.; Sharma, G.; Vaught, A.J.; Adamo, L.; Zakaria, S.; Michos, E.D.; Hays, A.G. Racial Differences in Delivery Outcomes among Women with Peripartum Cardiomyopathy. CJC Open 2021, 4, 373–377. [Google Scholar] [CrossRef]
- Frasca, D.; McElhaney, J. Influence of Obesity on Pneumococcus Infection Risk in the Elderly. Front. Endocrinol. 2019, 10, 71. [Google Scholar] [CrossRef]
- McClean, K.M.; Kee, F.; Young, I.; Elborn, J.S. Obesity and the lung: 1 · Epidemiology. Thorax 2008, 63, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, S.A.; Kelley, C.F.; Horton, J.P.; Jamieson, D.J. Coronavirus Disease 2019 (COVID-19) Vaccines and Pregnancy. Obstet. Gynecol. 2021, 137, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Kourtis, A.P.; Read, J.S.; Jamieson, D.J. Pregnancy and Infection. N. Engl. J. Med. 2014, 370, 2211–2218. [Google Scholar] [CrossRef]
- Phoswa, W.N.; Khaliq, O.P. Is pregnancy a risk factor of COVID-19? Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 252, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Turan, O.; Hakim, A.; Dashraath, P.; Jeslyn, W.J.L.; Wright, A.; Abdul-Kadir, R. Clinical characteristics, prognostic factors, and maternal and neonatal outcomes of SARS-CoV-2 infection among hospitalized pregnant women: A systematic review. Int. J. Gynecol. Obstet. 2020, 151, 7–16. [Google Scholar] [CrossRef]
- CDC. People with Certain Medical Conditions; Center for Disease Control and Prevention: Atlanta, GA, USA, 2023.
- Capretti, M.G.; Marsico, C.; Gabrielli, L.; Vocale, C.; Arcuri, S.; Simonazzi, G.; Piccinini, A.R.; Brandolini, C.; Lazzarotto, T.; Corvaglia, L.T. Infants Born Following SARS-CoV-2 Infection in Pregnancy. Pediatrics 2022, 150, e2022056206. [Google Scholar] [CrossRef]
- Wei, S.Q.; Bilodeau-Bertrand, M.; Liu, S.; Auger, N. The impact of COVID-19 on pregnancy outcomes: A systematic review and meta-analysis. Can. Med. Assoc. J. 2021, 193, E540–E548. [Google Scholar] [CrossRef]
- Fox, R.; Kitt, J.; Leeson, P.; Aye, C.Y.; Lewandowski, A.J. Preeclampsia: Risk Factors, Diagnosis, Management, and the Cardiovascular Impact on the Offspring. J. Clin. Med. 2019, 8, 1625. [Google Scholar] [CrossRef]
- Piekos, S.N.; Roper, R.T.; Hwang, Y.M.; Sorensen, T.; Price, N.D.; Hood, L.; Hadlock, J.J. The effect of maternal SARS-CoV-2 infection timing on birth outcomes: A retrospective multicentre cohort study. Lancet Digit. Health 2022, 4, e95–e104. [Google Scholar] [CrossRef]
- Trilla, C.; Mora, J.; Crovetto, F.; Crispi, F.; Gratacós, E.; Llurba, E. First-Trimester SARS-CoV-2 Infection: Clinical Presentation, Inflammatory Markers, and Obstetric Outcomes. Fetal Diagn. Ther. 2022, 49, 67–76. [Google Scholar] [CrossRef]
- CDC. What Is Stillbirth? Center for Disease Control and Prevention: Atlanta, GA, USA, 2022.
- Centers for Disease Control and Prevention. Pregnancy and Infant Loss. Available online: https://www.cdc.gov/ncbddd/stillbirth/features/pregnancy-infant-loss.html (accessed on 5 March 2023).
- Quenby, S.; Gallos, I.D.; Dhillon-Smith, R.K.; Podesek, M.; Stephenson, M.D.; Fisher, J.; Brosens, J.J.; Brewin, J.; Ramhorst, R.; Lucas, E.S.; et al. Miscarriage matters: The epidemiological, physical, psychological, and economic costs of early pregnancy loss. Lancet 2021, 397, 1658–1667. [Google Scholar] [CrossRef]
- Flenady, V.; Koopmans, L.; Middleton, P.; Froen, J.F.; Smith, G.C.; Gibbons, K.; Coory, M.; Gordon, A.; Ellwood, D.; McIntyre, H.D.; et al. Major risk factors for stillbirth in high-income countries: A systematic review and meta-analysis. Lancet 2011, 377, 1331–1340. [Google Scholar] [CrossRef]
- Wong, S.F.; Chow, K.M.; Leung, T.N.; Ng, W.F.; Ng, T.K.; Shek, C.C.; Ng, P.C.; Lam, P.W.; Ho, L.C.; To, W.W.; et al. Pregnancy and perinatal outcomes of women with severe acute respiratory syndrome. Am. J. Obstet. Gynecol. 2004, 191, 292–297. [Google Scholar] [CrossRef]
- Desisto, C.L. Morbidity and Mortality Weekly Report Risk for Stillbirth among Women with and without COVID-19 at Delivery Hospitalization—United States, March 2020–September 2021. Available online: https://msdh.ms.gov/msdhsite/_static/23,23645,341.html (accessed on 5 March 2023).
- Cosma, S.; Carosso, A.R.; Cusato, J.; Borella, F.; Carosso, M.; Bovetti, M.; Filippini, C.; D’avolio, A.; Ghisetti, V.; Di Perri, G.; et al. Coronavirus disease 2019 and first-trimester spontaneous abortion: A case-control study of 225 pregnant patients. Am. J. Obstet. Gynecol. 2020, 224, 391.e1–391.e7. [Google Scholar] [CrossRef]
- Balachandren, N.; Davies, M.C.; A Hall, J.; Stephenson, J.M.; David, A.L.; Barrett, G.; O’neill, H.C.; Ploubidis, G.B.; Yasmin, E.; Mavrelos, D. SARS-CoV-2 infection in the first trimester and the risk of early miscarriage: A UK population-based prospective cohort study of 3041 pregnancies conceived during the pandemic. Hum. Reprod. 2022, 37, 1126–1133. [Google Scholar] [CrossRef]
- Cavalcante, M.B.; Cavalcante, C.T.D.M.B.; Cavalcante, A.N.M.; Sarno, M.; Barini, R.; Kwak-Kim, J. COVID-19 and miscarriage: From immunopathological mechanisms to actual clinical evidence. J. Reprod. Immunol. 2021, 148, 103382. [Google Scholar] [CrossRef]
- de Havenon, A.; Delic, A.; Stulberg, E.; Sheibani, N.; Stoddard, G.; Hanson, H.; Theilen, L. Association of Preeclampsia with Incident Stroke in Later Life among Women in the Framingham Heart Study. JAMA Netw. Open 2021, 4, e215077. [Google Scholar] [CrossRef]
- Bushnell, C.; Chireau, M. Preeclampsia and Stroke: Risks during and after Pregnancy. Stroke Res. Treat. 2011, 2011, 858134. [Google Scholar] [CrossRef]
- Taglauer, E.S.; Wachman, E.M.; Juttukonda, L.; Klouda, T.; Kim, J.; Wang, Q.; Ishiyama, A.; Hackam, D.J.; Yuan, K.; Jia, H. Acute SARS-CoV-2 infection in pregnancy is associated with placental ACE-2 shedding. bioRxiv 2021, preprint. [Google Scholar] [CrossRef]
- What Is the ACE2 Receptor, How Is It Connected to Coronavirus and Why Might It Be Key to Treating COVID-19? The Experts Explain. 2020. Available online: https://theconversation.com/what-is-the-ace2-receptor-how-is-it-connected-to-coronavirus-and-why-might-it-be-key-to-treating-covid-19-the-experts-explain-136928 (accessed on 5 March 2023).
- Katwa, L.C.; Mendoza, C.; Clements, M. CVD and COVID-19: Emerging Roles of Cardiac Fibroblasts and Myofibroblasts. Cells 2022, 11, 1316. [Google Scholar] [CrossRef] [PubMed]
- Benny, M.; Bandstra, E.S.; Saad, A.G.; Lopez-Alberola, R.; Saigal, G.; Paidas, M.J.; Jayakumar, A.R.; Duara, S. Maternal SARS-CoV-2, Placental Changes and Brain Injury in 2 Neonates. Pediatrics 2023, 151, e2022058271. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Seth, S.; Sharma, R.; Yadav, S.; Mishra, P.; Mukhopadhyay, S. Perinatal outcome and possible vertical transmission of coronavirus disease 2019: Experience from North India. Clin. Exp. Pediatr. 2021, 64, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Hernández, M.; de Rivera, I.H.-N.; Yoldi-Negrete, M.; Saviñon-Tejeda, P.; Franco-Cendejas, R.; López-Jácome, L.E.; Navarro-Castellanos, I. Probable Case of Vertical Transmission of SARS-CoV-2 in a Newborn in Mexico. Neonatology 2021, 118, 364–367. [Google Scholar] [CrossRef]
- Shook, L.L.; Sullivan, E.L.; Lo, J.O.; Perlis, R.H.; Edlow, A.G. COVID-19 in pregnancy: Implications for fetal brain development. Trends Mol. Med. 2022, 28, 319–330. [Google Scholar] [CrossRef]
- Edlow, A.G.; Castro, V.M.; Shook, L.L.; Kaimal, A.J.; Perlis, R.H. Neurodevelopmental Outcomes at 1 Year in Infants of Mothers Who Tested Positive for SARS-CoV-2 during Pregnancy. JAMA Netw. Open 2022, 5, e2215787. [Google Scholar] [CrossRef]
- Edlow, A.G.; Castro, V.M.; Shook, L.L.; Haneuse, S.; Kaimal, A.J.; Perlis, R.H. Sex-Specific Neurodevelopmental Outcomes among Offspring of Mothers with SARS-CoV-2 Infection during Pregnancy. JAMA Netw. Open 2023, 6, e234415. [Google Scholar] [CrossRef]
- He, Z.; Fang, Y.; Zuo, Q.; Huang, X.; Lei, Y.; Ren, X.; Liu, D. Vertical transmission and kidney damage in newborns whose mothers had coronavirus disease 2019 during pregnancy. Int. J. Antimicrob. Agents 2020, 57, 106260. [Google Scholar] [CrossRef]
- Zaigham, M.; Holmberg, A.; Karlberg, M.; Lindsjö, O.; Jokubkiene, L.; Sandblom, J.; Strand, A.; Andersson, O.; Hansson, S.; Nord, D.; et al. Intrauterine vertical SARS-CoV-2 infection: A case confirming transplacental transmission followed by divergence of the viral genome. BJOG Int. J. Obstet. Gynaecol. 2021, 128, 1388–1394. [Google Scholar] [CrossRef]
- Thapa, B.; Acharya, S.; Karki, S. Vertical Transmission of COVID-19: A Case Report and Review of Literature. J. Nepal Health Res. Counc. 2021, 19, 203–205. [Google Scholar] [CrossRef]
- Yuan, J.; Qian, H.; Cao, S.; Dong, B.; Yan, X.; Luo, S.; Zhou, M.; Zhou, S.; Ning, B.; Zhao, L. Is there possibility of vertical transmission of COVID-19: A systematic review. Transl. Pediatr. 2021, 10, 423–434. [Google Scholar] [CrossRef]
- Beharier, O.; Mayo, R.P.; Raz, T.; Sacks, K.N.; Schreiber, L.; Suissa-Cohen, Y.; Chen, R.; Gomez-Tolub, R.; Hadar, E.; Gabbay-Benziv, R.; et al. Efficient maternal to neonatal transfer of antibodies against SARS-CoV-2 and BNT162b2 mRNA COVID-19 vaccine. J. Clin. Investig. 2021, 131, e154834. [Google Scholar] [CrossRef]
- Flannery, D.D.; Gouma, S.; Dhudasia, M.B.; Mukhopadhyay, S.; Pfeifer, M.R.; Woodford, E.C.; Triebwasser, J.E.; Gerber, J.S.; Morris, J.S.; Weirick, M.E.; et al. Assessment of Maternal and Neonatal Cord Blood SARS-CoV-2 Antibodies and Placental Transfer Ratios. JAMA Pediatr. 2021, 175, 594–600. [Google Scholar] [CrossRef]
- Rottenstreich, A.; Zarbiv, G.; Oiknine-Djian, E.; Zigron, R.; Wolf, D.G.; Porat, S. Efficient maternofetal transplacental transfer of anti-SARS-CoV-2 spike antibodies after antenatal SARS-CoV-2 BNT162b2 mRNA vaccination. Clin. Infect. Dis. 2021, 73, 1909–1912. [Google Scholar] [CrossRef]
- Allotey, J.; Stallings, E.; Bonet, M.; Yap, M.; Chatterjee, S.; Kew, T.; Debenham, L.; Llavall, A.C.; Dixit, A.; Zhou, D.; et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: Living systematic review and meta-analysis. BMJ 2020, 370, m3320. [Google Scholar] [CrossRef]
- Druedahl, L.C.; Minssen, T.; Price, W.N. Collaboration in times of crisis: A study on COVID-19 vaccine R&D partnerships. Vaccine 2021, 39, 6291–6295. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Klein, S.L.; Creisher, P.S.; Burd, I. COVID-19 vaccine testing in pregnant females is necessary. J. Clin. Investig. 2021, 131, e147553. [Google Scholar] [CrossRef]
- Kwok, H.F. Review of COVID-19 vaccine clinical trials—A puzzle with missing pieces. Int. J. Biol. Sci. 2021, 17, 1461–1468. [Google Scholar] [CrossRef]
- Skjefte, M.; Ngirbabul, M.; Akeju, O.; Escudero, D.; Hernandez-Diaz, S.; Wyszynski, D.F.; Wu, J.W. COVID-19 vaccine acceptance among pregnant women and mothers of young children: Results of a survey in 16 countries. Eur. J. Epidemiol. 2021, 36, 197–211. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. COVID-19 Vaccine Pregnancy Registry. Available online: https://www.cdc.gov/vaccinesafety/ensuringsafety/monitoring/v-safe/covid-preg-reg.html (accessed on 5 March 2023).
- DeSilva, M.; Haapala, J.; Vazquez-Benitez, G.; Vesco, K.K.; Daley, M.F.; Getahun, D.; Zerbo, O.; Naleway, A.; Nelson, J.C.; Williams, J.T.; et al. Evaluation of Acute Adverse Events after COVID-19 Vaccination during Pregnancy. N. Engl. J. Med. 2022, 387, 187–189. [Google Scholar] [CrossRef] [PubMed]
- Leik, N.K.O.; Ahmedy, F.; Mac Guad, R.; Baharuddin, D.M.P. COVID-19 vaccine and its consequences in pregnancy: Brief review. Ann. Med. Surg. 2021, 72, 103103. [Google Scholar] [CrossRef] [PubMed]
- Shimabukuro, T.T.; Kim, S.Y.; Myers, T.R.; Moro, P.L.; Oduyebo, T.; Panagiotakopoulos, L.; Marquez, P.L.; Olson, C.K.; Liu, R.; Chang, K.T.; et al. Preliminary Findings of mRNA COVID-19 Vaccine Safety in Pregnant Persons. N. Engl. J. Med. 2021, 384, 2273–2282. [Google Scholar] [CrossRef] [PubMed]
- Wainstock, T.; Yoles, I.; Sergienko, R.; Sheiner, E. Prenatal maternal COVID-19 vaccination and pregnancy outcomes. Vaccine 2021, 39, 6037–6040. [Google Scholar] [CrossRef]
- Kadali, R.A.K.; Janagama, R.; Peruru, S.R.; Racherla, S.; Tirumala, R.; Madathala, R.R.; Gajula, V. Adverse effects of COVID-19 messenger RNA vaccines among pregnant women: A cross-sectional study on healthcare workers with detailed self-reported symptoms. Am. J. Obstet. Gynecol. 2021, 225, 458–460. [Google Scholar] [CrossRef]
- Kharbanda, E.O.; Haapala, J.; DeSilva, M.; Vazquez-Benitez, G.; Vesco, K.K.; Naleway, A.L.; Lipkind, H.S. Spontaneous Abortion Following COVID-19 Vaccination during Pregnancy. JAMA 2021, 326, 1629–1631. [Google Scholar] [CrossRef]
- Lipkind, H.S.; Vazquez-Benitez, G.; DeSilva, M.; Vesco, K.K. Receipt of COVID-19 Vaccine during Pregnancy and Preterm or Small-for-Gestational-Age at Birth-Eight Integrated Heaklth Care Organizations, United States, 15 December 2020–22 July 2021. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 26–30. [Google Scholar] [CrossRef]
- Peretz, S.B.; Regev, N.; Novick, L.; Nachshol, M.; Goffer, E.; Ben-David, A.; Asraf, K.; Doolman, R.; Levin, E.G.; Yochay, G.R.; et al. Short-term outcome of pregnant women vaccinated with BNT162b2 mRNA COVID-19 vaccine. Ultrasound Obstet. Gynecol. 2021, 58, 450–456. [Google Scholar] [CrossRef]
- Calvert, C.; Carruthers, J.; Denny, C.; Donaghy, J.; Hillman, S.; Hopcroft, L.E.M.; Hopkins, L.; Goulding, A.; Lindsay, L.; McLaughlin, T.; et al. A population-based matched cohort study of early pregnancy outcomes following COVID-19 vaccination and SARS-CoV-2 infection. Nat. Commun. 2022, 13, 6124. [Google Scholar] [CrossRef] [PubMed]
- Citu, I.M.; Citu, C.; Gorun, F.; Sas, I.; Bratosin, F.; Motoc, A.; Burlea, B.; Rosca, O.; Malita, D.; Gorun, O.M. The Risk of Spontaneous Abortion Does Not Increase Following First Trimester mRNA COVID-19 Vaccination. J. Clin. Med. 2022, 11, 1698. [Google Scholar] [CrossRef]
- Blakeway, H.; Prasad, S.; Kalafat, E.; Heath, P.T.; Ladhani, S.N.; Le Doare, K.; Magee, L.A.; O’brien, P.; Rezvani, A.; von Dadelszen, P.; et al. COVID-19 vaccination during pregnancy: Coverage and safety. Am. J. Obstet. Gynecol. 2021, 226, 236.e1–236.e14. [Google Scholar] [CrossRef]
- Menegali, B.T.; Schuelter-Trevisol, F.; Barbosa, A.N.; Izidoro, T.M.; Feurschuette, O.H.M.; Marcon, C.E.M.; Trevisol, D.J. Vertical transmission of maternal COVID-19 antibodies after CoronaVac vaccine: A case report. Rev. Soc. Bras. Med. Trop. 2021, 54, e0385. [Google Scholar] [CrossRef] [PubMed]
- Villar, J.; Conti, C.P.S.; Gunier, R.B.; Ariff, S.; Craik, R.; I Cavoretto, P.; Rauch, S.; Gandino, S.; Nieto, R.; Winsey, A.; et al. Pregnancy outcomes and vaccine effectiveness during the period of omicron as the variant of concern, INTERCOVID-2022: A multinational, observational study. Lancet 2023, 401, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Favre, G.; Maisonneuve, E.; Pomar, L.; Daire, C.; Poncelet, C.; Quibel, T.; Monod, C.; de Tejada, B.M.; Schäffer, L.; Papadia, A.; et al. Maternal and perinatal outcomes following pre-Delta, Delta, and Omicron SARS-CoV-2 variants infection among unvaccinated pregnant women in France and Switzerland: A prospective cohort study using the COVI-PREG registry. Lancet Reg. Health-Eur. 2023, 26, 100569. [Google Scholar] [CrossRef] [PubMed]
- Al Mamun, M.; Khan, M.R. COVID-19 Delta Variant-of-Concern: A Real Concern for Pregnant Women with Gestational Diabetes Mellitus. Front. Endocrinol. 2021, 12, 778911. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Underlying Medical Conditions Associated with Higher Risk for Severe COVID-19: Information for Healthcare Professionals. 9 February 2023. Available online: https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/underlyingconditions.html (accessed on 5 March 2023).
- Smith, E.R.; Oakley, E.; Grandner, G.W.; Rukundo, G.; Farooq, F.; Ferguson, K.; Baumann, S.; Waldorf, K.M.A.; Afshar, Y.; Ahlberg, M.; et al. Clinical risk factors of adverse outcomes among women with COVID-19 in the pregnancy and postpartum period: A sequential, prospective meta-analysis. Am. J. Obstet. Gynecol. 2022, 228, 161–177. [Google Scholar] [CrossRef]
- Crandall, K. Pregnancy-related death disparities in non-Hispanic Black women. Women’s Health 2021, 17, 17455065211019888. [Google Scholar] [CrossRef]
- Howell, E.A.M. Reducing Disparities in Severe Maternal Morbidity and Mortality. Clin. Obstet. Gynecol. 2018, 61, 387–399. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).