Three Years of COVID-19 Pandemic—Is the Heart Skipping a Beat?
Abstract
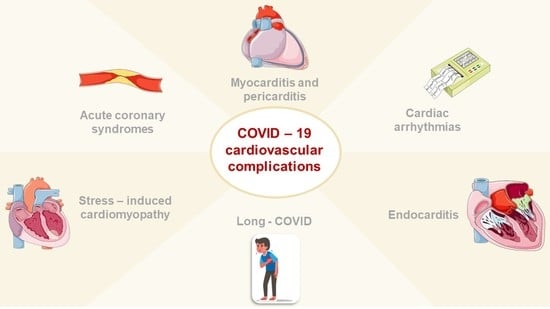
1. Introduction
2. Acute and Delayed Myocardial Injury
3. Myocarditis in COVID-19 Pneumonia
4. Pericarditis in COVID-19 Pneumonia
5. Stress-Induced Cardiomyopathy and COVID-19 Pneumonia
6. Acute Coronary Syndromes in COVID-19 Pneumonia
7. Endocarditis and COVID-19 Pneumonia
8. Cardiac Arrhythmias and COVID-19 Pneumonia
9. Long COVID and Cardiovascular Diseases
10. Postural Orthostatic Tachycardia Syndrome in COVID-19 Patients
11. Chronic Fatigue Syndrome and Long COVID
12. Controversial and Unresolved Issues
13. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alene, M.; Yismaw, L.; Assemie, M.A.; Ketema, D.B.; Mengist, B.; Kassie, B.; Birhan, T.Y. Magnitude of asymptomatic COVID-19 cases throughout the course of infection: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0249090. [Google Scholar] [CrossRef] [PubMed]
- Van Goethem, N.; Chung, P.Y.; Meurisse, M.; Vandromme, M.; De Mot, L.; Brondeel, R.; Stouten, V.; Klamer, S.; Cuypers, L.; Braeye, T.; et al. Clinical Severity of SARS-CoV-2 Omicron Variant Compared with Delta among Hospitalized COVID-19 Patients in Belgium during Autumn and Winter Season 2021–2022. Viruses 2022, 14, 1297. [Google Scholar] [CrossRef] [PubMed]
- Sabatino, J.; De Rosa, S.; Di Salvo, G.; Indolfi, C. Impact of cardiovascular risk profile on COVID-19 outcome. A meta-analysis. PLoS ONE 2020, 15, e0237131. [Google Scholar]
- Shi, S.; Qin, M.; Cai, Y.; Liu, T.; Shen, B.; Yang, F.; Cao, S.; Liu, X.; Xiang, Y.; Zhao, Q.; et al. Characteristics and clinical significance of myocardial injury in patients with severe coronavirus disease 2019. Eur. Heart J. 2020, 41, 2070–2079. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Shi, S.; Shi, S.; Qin, M.; Cai, Y.; Liu, T.; Liu, T.; Liu, T.; Shen, B.; Shen, B.; et al. Cardiovascular Implications of Fatal Outcomes of Patients with Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Luchian, M.-L.; Motoc, A.I.; Lochy, S.; Magne, J.; Roosens, B.; Belsack, D.; Bussche, K.V.D.; Von Kemp, B.; Galloo, X.; François, C.; et al. Troponin T in COVID-19 hospitalized patients: Kinetics matter. Cardiol. J. 2021, 28, 807–815. [Google Scholar] [CrossRef]
- Cosyns, B.; Lochy, S.; Luchian, M.L.; Gimelli, A.; Pontone, G.; Allard, S.D.; De Mey, J.; Rosseel, P.; Dweck, M.; Petersen, S.E.; et al. The role of cardiovascular imaging for myocardial injury in hospitalized COVID-19 patients. Eur. Heart J.-Cardiovasc. Imaging 2020, 21, 709–714. [Google Scholar] [CrossRef]
- Santoso, A.; Pranata, R.; Wibowo, A.; Al-Farabi, M.J.; Huang, I.; Antariksa, B. Cardiac injury is associated with mortality and critically ill pneumonia in COVID-19: A meta-analysis. Am. J. Emerg. Med. 2020, 44, 352–357. [Google Scholar] [CrossRef]
- Li, J.-W.; Han, T.-W.; Woodward, M.; Anderson, C.S.; Zhou, H.; Chen, Y.-D.; Neal, B. The impact of 2019 novel coronavirus on heart injury: A Systematic review and Meta-analysis. Prog. Cardiovasc. Dis. 2020, 63, 518–524. [Google Scholar] [CrossRef]
- Hanson, P.J.; Liu-Fei, F.; Ng, C.; Minato, T.A.; Lai, C.; Hossain, A.R.; Chan, R.; Grewal, B.; Singhera, G.; Rai, H.; et al. Characterization of COVID-19-associated cardiac injury: Evidence for a multifactorial disease in an autopsy cohort. Lab. Investig. 2022, 102, 814–825. [Google Scholar] [CrossRef]
- Fu, L.; Liu, X.; Su, Y.; Ma, J.; Hong, K. Prevalence and impact of cardiac injury onCOVID-19: A systematic review and meta-analysis. Clin. Cardiol. 2020, 44, 276–283. [Google Scholar] [CrossRef]
- Case, B.C.; Shea, C.; Rappaport, H.; Cellamare, M.; Zhang, C.; Zhu, M.; Medranda, G.A.; Satler, L.F.; Ben-Dor, I.; Hashim, H.; et al. The Evolving Impact of Myocardial Injury in Patients with COVID-19 Amid the Omicron Wave of the Pandemic. Am. J. Cardiol. 2022, 190, 54–60. [Google Scholar] [CrossRef]
- Escher, F.; Pietsch, H.; Aleshcheva, G.; Bock, T.; Baumeier, C.; Elsaesser, A.; Wenzel, P.; Hamm, C.; Westenfeld, R.; Schultheiss, M.; et al. Detection of viral SARS-CoV-2 genomes and histopathological changes in endomyocardial biopsies. ESC Heart Fail. 2020, 7, 2440–2447. [Google Scholar] [CrossRef]
- Khan, M.S.; Shahid, I.; Anker, S.D.; Solomon, S.D.; Vardeny, O.; Michos, E.D.; Fonarow, G.C.; Butler, J. Cardiovascular implications of COVID-19 versus influenza infection: A review. BMC Med. 2020, 18, 403. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Xu, E.; Bowe, B.; Al-Aly, Z. Long-term cardiovascular outcomes of COVID-19. Nat. Med. 2022, 28, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Bois, M.C.; Boire, N.A.; Layman, A.J.; Aubry, M.-C.; Alexander, M.P.; Roden, A.C.; Hagen, C.E.; Quinton, R.A.; Larsen, C.; Erben, Y.; et al. COVID-19–Associated Nonocclusive Fibrin Microthrombi in the Heart. Circulation 2021, 143, 230–243. [Google Scholar] [CrossRef] [PubMed]
- Basso, C.; Leone, O.; Rizzo, S.; De Gaspari, M.; Van Der Wal, A.C.; Aubry, M.-C.; Bois, M.C.; Lin, P.T.; Maleszewski, J.J.; Stone, J.R. Pathological features of COVID-19-associated myocardial injury: A multicentre cardiovascular pathology study. Eur. Heart J. 2020, 41, 3827–3835. [Google Scholar] [CrossRef]
- Shukla, A.K.; Banerjee, M. Angiotensin-Converting-Enzyme 2 and Renin-Angiotensin System Inhibitors in COVID-19: An Update. High Blood Press. Cardiovasc. Prev. 2021, 28, 129–139. [Google Scholar] [CrossRef]
- Castiello, T.; Georgiopoulos, G.; Finocchiaro, G.; Claudia, M.; Gianatti, A.; Delialis, D.; Aimo, A.; Prasad, S. COVID-19 and myocarditis: A systematic review and overview of current challenges. Heart Fail. Rev. 2021, 27, 251–261. [Google Scholar] [CrossRef]
- Orbach, A.; Ghugre, N.R.; Biswas, L.; Connelly, K.A.; Chan, A.; Strauss, B.H.; Wright, G.A.; Roifman, I. Low Prevalence of Late Myocardial Injury on Cardiac MRI Following COVID-19 Infection. J. Magn. Reson. Imaging, 2023; online ahead of print. [Google Scholar] [CrossRef]
- Blagova, O.; Lutokhina, Y.; Kogan, E.; Kukleva, A.; Ainetdinova, D.; Novosadov, V.; Rud’, R.; Savina, P.; Zaitsev, A.; Fomin, V. Chronic biopsy proven post-COVID myoendocarditis with SARS-CoV-2 persistence and high level of antiheart antibodies. Clin. Cardiol. 2022, 45, 952–959. [Google Scholar] [CrossRef]
- Parhizgar, P.; Yazdankhah, N.; Rzepka, A.M.; Chung, K.Y.C.; Ali, I.; Fur, R.L.F.; Russell, V.; Cheung, A.M. Beyond Acute COVID-19: A Review of Long-term Cardiovascular Outcomes. Can. J. Cardiol. 2023; online ahead of print. [Google Scholar] [CrossRef]
- Brawner, C.A.; Ehrman, J.K.; Bole, S.; Kerrigan, D.J.; Parikh, S.S.; Lewis, B.K.; Gindi, R.M.; Keteyian, C.; Abdul-Nour, K.; Keteyian, S.J. Inverse Relationship of Maximal Exercise Capacity to Hospitalization Secondary to Coronavirus Disease 2019. Mayo Clin. Proc. 2021, 96, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Zbinden-Foncea, H.; Francaux, M.; Deldicque, L.; Hawley, J.A. Does High Cardiorespiratory Fitness Confer Some Protection Against Proinflammatory Responses After Infection by SARS-CoV-2? Obesity 2020, 28, 1378–1381. [Google Scholar] [CrossRef] [PubMed]
- Goergen, J.; Bavishi, A.; Eimer, M.; Zielinski, A.R. COVID-19: The Risk to Athletes. Curr. Treat. Options Cardiovasc. Med. 2021, 23, 68. [Google Scholar] [CrossRef] [PubMed]
- Bauer, P.; Kraushaar, L.; Dörr, O.; Keranov, S.; Nef, H.; Hamm, C.W.; Most, A. Vascular alterations among male elite athletes recovering from SARS-CoV-2 infection. Sci. Rep. 2022, 12, 8655. [Google Scholar] [CrossRef]
- Alosaimi, B.; AlFayyad, I.; Alshuaibi, S.; Almutairi, G.; Alshaebi, N.; Alayyaf, A.; Alturaiki, W.; Shah, M.A. Cardiovascular complications and outcomes among athletes with COVID-19 disease: A systematic review. BMC Sports Sci. Med. Rehabil. 2022, 14, 74. [Google Scholar] [CrossRef]
- Mele, D.; Flamigni, F.; Rapezzi, C.; Ferrari, R. Myocarditis in COVID-19 patients: Current problems. Intern. Emerg. Med. 2021, 16, 1123–1129. [Google Scholar] [CrossRef]
- Kesici, S.; Aykan, H.H.; Orhan, D.; Bayrakci, B. Fulminant COVID-19-related myocarditis in an infant. Eur. Heart J. 2020, 41, 3021. [Google Scholar] [CrossRef]
- Rajpal, S.; Kahwash, R.; Tong, M.S.; Paschke, K.; Satoskar, A.A.; Foreman, B.; Allen, L.A.; Bhave, N.M.; Gluckman, T.J.; Fuster, V. Fulminant Myocarditis Following SARS-CoV-2 Infection: JACC Patient Care Pathways. J. Am. Coll. Cardiol. 2022, 79, 2144–2152. [Google Scholar] [CrossRef]
- Meyerowitz-Katz, G.; Merone, L. A systematic review and meta-analysis of published research data on COVID-19 infection fatality rates. Int. J. Infect. Dis. 2020, 101, 138–148. [Google Scholar] [CrossRef]
- Siripanthong, B.; Nazarian, S.; Muser, D.; Deo, R.; Santangeli, P.; Khanji, M.Y.; Cooper Jr, L.T.; Chahal, C.A.A. Recognizing COVID-19-related myocarditis: The possible pathophysiology and proposed guideline for diagnosis and management. Heart Rhythm 2020, 17, 1463–1471. [Google Scholar] [CrossRef]
- Boehmer, T.; Kompaniyets, L.; Lavery, A.; Hsu, J.; Ko, J.; Yusuf, H.; Romano, S.; Gundlapalli, A.; Oster, M.; Harris, A. Association Between COVID-19 and Myocarditis Using Hospital-Based Administrative Data—United States, March 2020–January 2021. MMWR. Morb. Mortal. Wkly. Rep. 2021, 70, 1228–1232. [Google Scholar] [CrossRef] [PubMed]
- Szarpak, L.; Pruc, M.; Filipiak, K.J.; Popieluch, J.; Bielski, A.; Jaguszewski, M.J.; Gilis-Malinowska, N.; Chirico, F.; Rafique, Z.; Peacock, F.W. Myocarditis: A complication of COVID-19 and long-COVID-19 syndrome as a serious threat in modern cardiology. Cardiol. J. 2022, 29, 178–179. [Google Scholar] [CrossRef] [PubMed]
- Ammirati, E.; Lupi, L.; Palazzini, M.; Hendren, N.S.; Grodin, J.L.; Cannistraci, C.V.; Schmidt, M.; Hekimian, G.; Peretto, G.; Bochaton, T.; et al. Prevalence, Characteristics, and Outcomes of COVID-19–Associated Acute Myocarditis. Circulation 2022, 145, 1123–1139. [Google Scholar] [CrossRef] [PubMed]
- Vidula, M.K.; Rajewska-Tabor, J.; Cao, J.J.; Kang, Y.; Craft, J.; Mei, W.; Chandrasekaran, P.S.; Clark, D.E.; Poenar, A.-M.; Gorecka, M.; et al. Myocardial Injury on CMR in Patients with COVID-19 and Suspected Cardiac Involvement. JACC Cardiovasc. Imaging, 2023; online ahead of print. [Google Scholar] [CrossRef]
- Daniels, C.J.; Rajpal, S.; Greenshields, J.T.; Rosenthal, G.L.; Chung, E.H.; Terrin, M.; Jeudy, J.; Mattson, S.E.; Law, I.H.; Borchers, J.; et al. Prevalence of Clinical and Subclinical Myocarditis in Competitive Athletes with Recent SARS-CoV-2 Infection: Results from the Big Ten COVID-19 Cardiac Registry. JAMA Cardiol. 2021, 6, 1078–1087. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M.W.; Tucker, A.M.; Bloom, O.J.; Green, G.; DiFiori, J.P.; Solomon, G.; Phelan, D.; Kim, J.H.; Meeuwisse, W.; Sills, A.K.; et al. Prevalence of Inflammatory Heart Disease Among Professional Athletes with Prior COVID-19 Infection Who Received Systematic Return-to-Play Cardiac Screening. JAMA Cardiol. 2021, 6, 745. [Google Scholar] [CrossRef]
- Starekova, J.; Bluemke, D.A.; Bradham, W.S.; Eckhardt, L.L.; Grist, T.M.; Kusmirek, J.E.; Purtell, C.S.; Schiebler, M.L.; Reeder, S.B. Evaluation for Myocarditis in Competitive Student Athletes Recovering from Coronavirus Disease 2019 with Cardiac Magnetic Resonance Imaging. JAMA Cardiol. 2021, 6, 945–950. [Google Scholar] [CrossRef]
- Rajpal, S.; Tong, M.S.; Borchers, J.; Zareba, K.M.; Obarski, T.P.; Simonetti, O.P.; Daniels, C.J. Cardiovascular Magnetic Resonance Findings in Competitive Athletes Recovering from COVID-19 Infection. JAMA Cardiol. 2020, 6, 116–118. [Google Scholar] [CrossRef]
- Clark, D.E.; Parikh, A.; Dendy, J.M.; Diamond, A.B.; George-Durrett, K.; Fish, F.A.; Slaughter, J.C.; Fitch, W.; Hughes, S.G.; Soslow, J.H. COVID-19 Myocardial Pathology Evaluation in Athletes with Cardiac Magnetic Resonance (COMPETE CMR). Circulation 2021, 143, 609–612. [Google Scholar] [CrossRef]
- Artico, J.; Shiwani, H.; Moon, J.C.; Gorecka, M.; McCann, G.P.; Roditi, G.; Morrow, A.; Mangion, K.; Lukaschuk, E.; Shanmuganathan, M.; et al. Myocardial Involvement After Hospitalization for COVID-19 Complicated by Troponin Elevation: A Prospective, Multicenter, Observational Study. Circulation 2023, 147, 364–374. [Google Scholar] [CrossRef]
- Haussner, W.; DeRosa, A.P.; Haussner, D.; Tran, J.; Torres-Lavoro, J.; Kamler, J.; Shah, K. COVID-19 associated myocarditis: A systematic review. Am. J. Emerg. Med. 2021, 51, 150–155. [Google Scholar] [CrossRef]
- Kariyanna, P.T.; Sabih, A.; Sutarjono, B.; Shah, K.; Peláez, A.V.; Lewis, J.; Yu, R.; Grewal, E.S.; Jayarangaiah, A.; Das, S.; et al. A Systematic Review of COVID-19 and Pericarditis. Cureus 2022, 14, e27948. [Google Scholar] [CrossRef] [PubMed]
- Chiabrando, J.G.; Bonaventura, A.; Vecchié, A.; Wohlford, G.F.; Mauro, A.G.; Jordan, J.H.; Grizzard, J.D.; Montecucco, F.; Berrocal, D.H.; Brucato, A.; et al. Management of Acute and Recurrent Pericarditis: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 75, 76–92. [Google Scholar] [CrossRef] [PubMed]
- Imazio, M.; Spodick, D.H.; Brucato, A.; Trinchero, R.; Adler, Y. Controversial Issues in the Management of Pericardial Diseases. Circulation 2010, 121, 916–928. [Google Scholar] [CrossRef] [PubMed]
- Linschoten, M.; Peters, S.; van Smeden, M.; Jewbali, L.S.; Schaap, J.; Siebelink, H.-M.; Smits, P.C.; Tieleman, R.G.; van der Harst, P.; van Gilst, W.H.; et al. Cardiac complications in patients hospitalised with COVID-19. Eur. Heart J. Acute Cardiovasc. Care 2020, 9, 817–823. [Google Scholar] [CrossRef]
- Bao, C.; Liu, X.; Zhang, H.; Li, Y.; Liu, J. Coronavirus Disease 2019 (COVID-19) CT Findings: A Systematic Review and Meta-analysis. J. Am. Coll. Radiol. 2020, 17, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Brito, D.; Meester, S.; Yanamala, N.; Patel, H.B.; Balcik, B.J.; Casaclang-Verzosa, G.; Seetharam, K.; Riveros, D.; Beto, R.J.; Balla, S.; et al. High Prevalence of Pericardial Involvement in College Student Athletes Recovering from COVID-19. JACC Cardiovasc. Imaging 2021, 14, 541–555. [Google Scholar] [CrossRef]
- Puntmann, V.O.; Carerj, M.L.; Wieters, I.; Fahim, M.; Arendt, C.; Hoffmann, J.; Shchendrygina, A.; Escher, F.; Vasa-Nicotera, M.; Zeiher, A.M.; et al. Outcomes of Cardiovascular Magnetic Resonance Imaging in Patients Recently Recovered from Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 1265. [Google Scholar] [CrossRef]
- Diaz-Arocutipa, C.; Saucedo-Chinchay, J.; Imazio, M. Pericarditis in patients with COVID-19: A systematic review. J. Cardiovasc. Med. 2021, 22, 693–700. [Google Scholar] [CrossRef]
- Citro, R.; Okura, H.; Ghadri, J.R.; Izumi, C.; Meimoun, P.; Izumo, M.; Dawson, D.; Kaji, S.; Eitel, I.; Kagiyama, N.; et al. Multimodality imaging in takotsubo syndrome: A joint consensus document of the European Association of Cardiovascular Imaging (EACVI) and the Japanese Society of Echocardiography (JSE). Eur. Heart J.-Cardiovasc. Imaging 2020, 21, 1184–1207. [Google Scholar] [CrossRef]
- Angelini, P.; Postalian, A.; Hernandez-Vila, E.; Uribe, C.; Costello, B. COVID-19 and the Heart: Could Transient Takotsubo Cardiomyopathy Be Related to the Pandemic by Incidence and Mechanisms? Front. Cardiovasc. Med. 2022, 9, 919715. [Google Scholar] [CrossRef]
- Salah, H.M.; Mehta, J.L. Takotsubo cardiomyopathy and COVID-19 infection. Eur. Heart J.-Cardiovasc. Imaging 2020, 21, 1299–1300. [Google Scholar] [CrossRef]
- Shah, R.M.; Shah, M.; Shah, S.; Li, A.; Jauhar, S. Takotsubo Syndrome and COVID-19: Associations and Implications. Curr. Probl. Cardiol. 2020, 46, 100763. [Google Scholar] [CrossRef] [PubMed]
- Scally, C.; Rudd, A.; Mezincescu, A.; Wilson, H.M.; Srivanasan, J.; Horgan, G.; Broadhurst, P.; Newby, D.E.; Henning, A.; Dawson, D. Persistent Long-Term Structural, Functional, and Metabolic Changes After Stress-Induced (Takotsubo) Cardiomyopathy. Circulation 2018, 137, 1039–1048. [Google Scholar] [CrossRef] [PubMed]
- Esposito, L.; Cancro, F.P.; Silverio, A.; Di Maio, M.; Iannece, P.; Damato, A.; Alfano, C.; De Luca, G.; Vecchione, C.; Galasso, G. COVID-19 and Acute Coronary Syndromes: From Pathophysiology to Clinical Perspectives. Oxidative Med. Cell. Longev. 2021, 2021, 4936571. [Google Scholar] [CrossRef] [PubMed]
- Quadri, G.; Rognoni, A.; Cerrato, E.; Baralis, G.; Boccuzzi, G.; Brscic, E.; Conrotto, F.; De Benedictis, M.; De Martino, L.; Di Leo, A.; et al. Catheterization laboratory activity before and during COVID-19 spread: A comparative analysis in Piedmont, Italy, by the Italian Society of Interventional Cardiology (GISE). Int. J. Cardiol. 2020, 323, 288–291. [Google Scholar] [CrossRef]
- Tam, C.-C.F.; Siu, D.; Tse, H.F. COVID-19 and Acute Coronary Syndrome: Lessons for Everyone. Lancet Reg. Health-West. Pac. 2021, 19, 100346. [Google Scholar] [CrossRef]
- Kite, T.A.; Ludman, P.F.; Gale, C.P.; Jianhua, W.; Adriano, C.; Jacques, M.; Manel, S.; Pilar, J.-Q.; Luciano, C.; Parham, S.; et al. International Prospective Registry of Acute Coronary Syndromes in Patients with COVID-19. J. Am. Coll. Cardiol. 2021, 77, 2466–2476. [Google Scholar] [CrossRef]
- Acharya, P.; Ranka, S.; Sethi, P.; Bharati, R.; Hu, J.; Noheria, A.; Nallamothu, B.K.; Hayek, S.S.; Gupta, K. Incidence, Predictors, and Outcomes of In-Hospital Cardiac Arrest in COVID-19 Patients Admitted to Intensive and Non-Intensive Care Units: Insights from the AHA COVID-19 CVD Registry. J. Am. Heart Assoc. 2021, 10, e021204. [Google Scholar] [CrossRef]
- Marijon, E.; Karam, N.; Jost, D.; Perrot, D.; Frattini, B.; Derkenne, C.; Sharifzadehgan, A.; Waldmann, V.; Beganton, F.; Narayanan, K.; et al. Out-of-hospital cardiac arrest during the COVID-19 pandemic in Paris, France: A population-based, observational study. Lancet Public Health 2020, 5, e437–e443. [Google Scholar] [CrossRef]
- Sultanian, P.; Lundgren, P.; Strömsöe, A.; Aune, S.; Bergström, G.; Hagberg, E.; Hollenberg, J.; Lindqvist, J.; Djärv, T.; Castelheim, A.; et al. Cardiac arrest in COVID-19: Characteristics and outcomes of in- and out-of-hospital cardiac arrest. A report from the Swedish Registry for Cardiopulmonary Resuscitation. Eur. Heart J. 2021, 42, 1094–1106. [Google Scholar] [CrossRef]
- Baldi, E.; Sechi, G.M.; Mare, C.; Canevari, F.; Brancaglione, A.; Primi, R.; Klersy, C.; Palo, A.; Contri, E.; Ronchi, V.; et al. Out-of-Hospital Cardiac Arrest during the COVID-19 Outbreak in Italy. N. Engl. J. Med. 2020, 383, 496–498. [Google Scholar] [CrossRef] [PubMed]
- Bilaloglu, S.; Aphinyanaphongs, Y.; Jones, S.; Iturrate, E.; Hochman, J.; Berger, J.S. Thrombosis in Hospitalized Patients with COVID-19 in a New York City Health System. JAMA 2020, 324, 799. [Google Scholar] [CrossRef] [PubMed]
- Price, L.C.; McCabe, C.; Garfield, B.; Wort, S.J. Thrombosis and COVID-19 pneumonia: The clot thickens! Eur. Respir. J. 2020, 56, 2001608. [Google Scholar] [CrossRef] [PubMed]
- Malas, M.B.; Naazie, I.N.; Elsayed, N.; Mathlouthi, A.; Marmor, R.; Clary, B. Thromboembolism risk of COVID-19 is high and associated with a higher risk of mortality: A systematic review and meta-analysis. Eclinicalmedicine 2020, 29–30, 100639. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Chi, J.; Gao, Q. Prevalence and risk factors of thrombotic events on patients with COVID-19: A systematic review and meta-analysis. Thromb. J. 2021, 19, 32. [Google Scholar] [CrossRef]
- Liao, S.-C.; Shao, S.-C.; Chen, Y.-T.; Chen, Y.-C.; Hung, M.-J. Incidence and mortality of pulmonary embolism in COVID-19: A systematic review and meta-analysis. Crit. Care 2020, 24, 464. [Google Scholar] [CrossRef]
- George, A.; Alampoondi Venkataramanan, S.V.; John, K.J.; Mishra, A.K. Infective endocarditis and COVID-19 coinfection: An updated review. Acta Biomed. 2022, 93, e2022030. [Google Scholar]
- Cosyns, B.; Motoc, A.; Arregle, F.; Habib, G. A Plea Not to Forget Infective Endocarditis in COVID-19 Era. JACC Cardiovasc. Imaging 2020, 13, 2470–2471. [Google Scholar] [CrossRef]
- Quintero-Martinez, J.A.; Hindy, J.-R.; Mahmood, M.; Gerberi, D.J.; DeSimone, D.C.; Baddour, L.M. A clinical profile of infective endocarditis in patients with recent COVID-19: A systematic review. Am. J. Med. Sci. 2022, 364, 16–22. [Google Scholar] [CrossRef]
- Bhatla, A.; Mayer, M.M.; Adusumalli, S.; Hyman, M.C.; Oh, E.; Tierney, A.; Moss, J.; Chahal, A.A.; Anesi, G.; Denduluri, S.; et al. COVID-19 and cardiac arrhythmias. Heart Rhythm 2020, 17, 1439–1444. [Google Scholar] [CrossRef]
- Garcia-Zamora, S.; Lee, S.; Haseeb, S.; Bazoukis, G.; Tse, G.; Alvarez-Garcia, J.; Gul, E.E.; Çinier, G.; Alexander, B.; Pinto-Filho, M.M.; et al. Arrhythmias and electrocardiographic findings in Coronavirus disease 2019: A systematic review and meta-analysis. Pacing Clin. Electrophysiol. 2021, 44, 1062–1074. [Google Scholar] [CrossRef] [PubMed]
- Wollborn, J.; Karamnov, S.; Fields, K.G.; Yeh, T.; Muehlschlegel, J.D. COVID-19 increases the risk for the onset of atrial fibrillation in hospitalized patients. Sci. Rep. 2022, 12, 12014. [Google Scholar] [CrossRef] [PubMed]
- Rosenblatt, A.G.; Ayers, C.R.; Rao, A.; Howell, S.J.; Hendren, N.S.; Zadikany, R.H.; Ebinger, J.E.; Daniels, J.D.; Link, M.S.; de Lemos, J.A.; et al. New-Onset Atrial Fibrillation in Patients Hospitalized with COVID-19: Results from the American Heart Association COVID-19 Cardiovascular Registry. Circ. Arrhythmia Electrophysiol. 2022, 15, e010666. [Google Scholar] [CrossRef] [PubMed]
- Parinita, D.; Joshua, L.; Pierre, Q.; Blake, O.; Sauer, W.H.; Bruce, K.; Usha, T. Arrhythmias and COVID-19. JACC Clin. Electrophysiol. 2020, 6, 1193–1204. [Google Scholar]
- Mercuro, N.J.; Yen, C.F.; Shim, D.J.; Maher, T.R.; McCoy, C.M.; Zimetbaum, P.J.; Gold, H.S. Risk of QT Interval Prolongation Associated with Use of Hydroxychloroquine with or Without Concomitant Azithromycin Among Hospitalized Patients Testing Positive for Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 1036. [Google Scholar] [CrossRef]
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef]
- Sukocheva, O.A.; Maksoud, R.; Beeraka, N.M.; Madhunapantula, S.V.; Sinelnikov, M.; Nikolenko, V.N.; Neganova, M.E.; Klochkov, S.G.; Kamal, M.A.; Staines, D.R.; et al. Analysis of post COVID-19 condition and its overlap with myalgic encephalomyelitis/chronic fatigue syndrome. J. Adv. Res. 2021, 40, 179–196. [Google Scholar] [CrossRef]
- Luchian, M.-L.; Motoc, A.; Lochy, S.; Magne, J.; Belsack, D.; De Mey, J.; Roosens, B.; Van den Bussche, K.; Boeckstaens, S.; Chameleva, H.; et al. Subclinical Myocardial Dysfunction in Patients with Persistent Dyspnea One Year after COVID-19. Diagnostics 2022, 12, 57. [Google Scholar] [CrossRef]
- Parry, A.H.; Wani, A.H.; Shah, N.N.; Jehangir, M. Medium-term chest computed tomography (CT) follow-up of COVID-19 pneumonia patients after recovery to assess the rate of resolution and determine the potential predictors of persistent lung changes. Egypt. J. Radiol. Nucl. Med. 2021, 52, 55. [Google Scholar] [CrossRef]
- Guan, C.-S.; Wei, L.-G.; Xie, R.-M.; Lv, Z.-B.; Yan, S.; Zhang, Z.-X.; Chen, B.-D.; Work, C.-S.G.A.L.-G.W.C.E.T.T. CT findings of COVID-19 in follow-up: Comparison between progression and recovery. Diagn. Interv. Radiol. 2020, 26, 301–307. [Google Scholar] [CrossRef]
- Havervall, S.; Rosell, A.; Phillipson, M.; Mangsbo, S.M.; Nilsson, P.; Hober, S.; Thålin, C. Symptoms and Functional Impairment Assessed 8 Months After Mild COVID-19 Among Health Care Workers. JAMA 2021, 325, 2015. [Google Scholar] [CrossRef] [PubMed]
- Salah, H.M.; Fudim, M.; O’neil, S.T.; Manna, A.; Chute, C.G.; Caughey, M.C. Post-recovery COVID-19 and incident heart failure in the National COVID Cohort Collaborative (N3C) study. Nat. Commun. 2022, 13, 4117. [Google Scholar] [CrossRef] [PubMed]
- Urmeneta Ulloa, J.; Martínez de Vega, V.; Salvador Montañés, O.; Álvarez Vázquez, A.; Sánchez-Enrique, C.; Hernández Jiménez, S.; Sancho García, F.D.; López Ruiz, L.; Recio Rodríguez, M.; Pizarro, G.; et al. Cardiac magnetic resonance in recovering COVID-19 patients. Feature tracking and mapping analysis to detect persistent myocardial involvement. IJC Heart Vasc. 2021, 36, 100854. [Google Scholar] [CrossRef] [PubMed]
- Puntmann, V.O.; Martin, S.; Shchendrygina, A.; Hoffmann, J.; Ka, M.M.; Giokoglu, E.; Vanchin, B.; Holm, N.; Karyou, A.; Laux, G.S.; et al. Long-term cardiac pathology in individuals with mild initial COVID-19 illness. Nat. Med. 2022, 28, 2117–2123. [Google Scholar] [CrossRef]
- Raisi-Estabragh, Z.; Cooper, J.; Salih, A.; Raman, B.; Lee, A.M.; Neubauer, S.; Harvey, N.C.; Petersen, S.E. Cardiovascular disease and mortality sequelae of COVID-19 in the UK Biobank. Heart 2022, 109, 119–126. [Google Scholar] [CrossRef]
- Satterfield, B.A.; Bhatt, D.L.; Gersh, B.J. Cardiac involvement in the long-term implications of COVID-19. Nat. Rev. Cardiol. 2021, 19, 332–341. [Google Scholar] [CrossRef]
- Zhao, S.; Tran, V.H. Postural Orthostatic Tachycardia Syndrome; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Ormiston, C.K.; Świątkiewicz, I.; Taub, P.R. Postural orthostatic tachycardia syndrome as a sequela of COVID-19. Heart Rhythm. 2022, 19, 1880–1889. [Google Scholar] [CrossRef]
- Lau, S.-T.; Yu, W.-C.; Mok, N.-S.; Tsui, P.-T.; Tong, W.-L.; Stella, W.C. Tachycardia amongst subjects recovering from severe acute respiratory syndrome (SARS). Int. J. Cardiol. 2005, 100, 167–169. [Google Scholar] [CrossRef]
- Brodin, P. Immune determinants of COVID-19 disease presentation and severity. Nat. Med. 2021, 27, 28–33. [Google Scholar] [CrossRef]
- Huang, C.; Huang, L.; Wang, Y.; Li, X.; Ren, L.; Gu, X.; Kang, L.; Guo, L.; Liu, M.; Zhou, X.; et al. 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet 2021, 397, 220–232. [Google Scholar] [CrossRef]
- Kedor, C.; Freitag, H.; Meyer-Arndt, L.; Wittke, K.; Hanitsch, L.G.; Zoller, T.; Steinbeis, F.; Haffke, M.; Rudolf, G.; Heidecker, B.; et al. A prospective observational study of post-COVID-19 chronic fatigue syndrome following the first pandemic wave in Germany and biomarkers associated with symptom severity. Nat. Commun. 2022, 13, 5104. [Google Scholar] [CrossRef] [PubMed]
- Salari, N.; Khodayari, Y.; Hosseinian-Far, A.; Zarei, H.; Rasoulpoor, S.; Akbari, H.; Mohammadi, M. Global prevalence of chronic fatigue syndrome among long COVID-19 patients: A systematic review and meta-analysis. Biopsychosoc. Med. 2022, 16, 21. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luchian, M.-L.; Demeure, F.; Higny, J.; Berners, Y.; Henry, J.; Guedes, A.; Laurence, G.; Saidane, L.; Höcher, A.; Roosens, B.; et al. Three Years of COVID-19 Pandemic—Is the Heart Skipping a Beat? COVID 2023, 3, 715-727. https://doi.org/10.3390/covid3050053
Luchian M-L, Demeure F, Higny J, Berners Y, Henry J, Guedes A, Laurence G, Saidane L, Höcher A, Roosens B, et al. Three Years of COVID-19 Pandemic—Is the Heart Skipping a Beat? COVID. 2023; 3(5):715-727. https://doi.org/10.3390/covid3050053
Chicago/Turabian StyleLuchian, Maria-Luiza, Fabian Demeure, Julien Higny, Yannick Berners, Jean Henry, Antoine Guedes, Gabriel Laurence, Lara Saidane, Alexandra Höcher, Bram Roosens, and et al. 2023. "Three Years of COVID-19 Pandemic—Is the Heart Skipping a Beat?" COVID 3, no. 5: 715-727. https://doi.org/10.3390/covid3050053
APA StyleLuchian, M.-L., Demeure, F., Higny, J., Berners, Y., Henry, J., Guedes, A., Laurence, G., Saidane, L., Höcher, A., Roosens, B., Droogmans, S., Cosyns, B., & Motoc, A. (2023). Three Years of COVID-19 Pandemic—Is the Heart Skipping a Beat? COVID, 3(5), 715-727. https://doi.org/10.3390/covid3050053