Microbial Organisms in the Lower Respiratory Tract Associated with SARS-CoV-2 Infection: A Cross-Sectional Study in Northern Ghana
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Study Area
2.3. Study Design and Sample Collection
2.4. Laboratory Methods
Bacteria Culture
2.5. Detection of SARS-CoV-2
2.6. Data Collection
2.7. Statistical Analysis
3. Results
3.1. Socio-Demographic and Clinical Characteristics of Study Participants
3.2. Clinical and Socio-Demographic Risk Factors Associated with SARS-CoV-2 Infection
3.3. Co-Infection/Microbial Isolates among Study Participants
3.4. Sputum Culture and Microbial Isolates as Potential Risk Factors of SARS-CoV-2 Infection
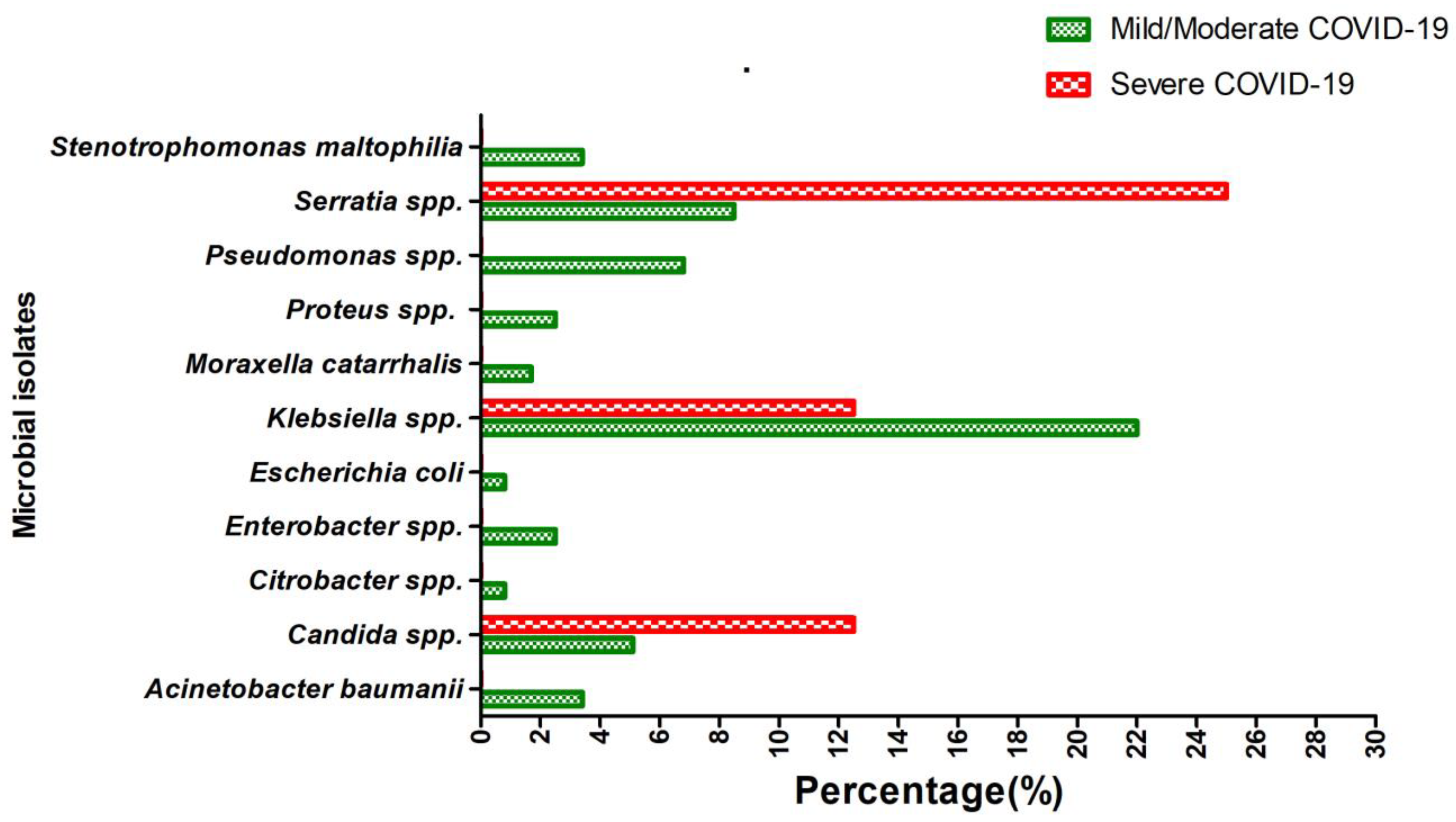
3.5. Distribution of Microbial Isolates According to the Disease Severity among COVID-19 Patients
3.6. Antimicrobial Resistance Pattern amongst Isolated Pathogens in SARS-CoV-2 Infections
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- He, C.; Qin, M.; Sun, X. Highly pathogenic coronaviruses: Thrusting vaccine development in the spotlight. Acta Pharm. Sin. B 2020, 10, 1175–1191. [Google Scholar] [CrossRef] [PubMed]
- Shereen, M.A.; Khan, S.; Kazmi, A.; Bashir, N.; Siddique, R. COVID-19 infection: Origin, transmission, and characteristics of human coronaviruses. J. Adv. Res. 2020, 24, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Cascella, M.; Rajnik, M.; Aleem, A.; Dulebohn, S.C.; Di Napoli, R. Features, evaluation and treatment coronavirus (COVID-19). In Statpearls [Internet]; StatPearls Publishing: Tampa, FL, USA, 2020. [Google Scholar]
- Chakraborty, I.; Maity, P. COVID-19 outbreak: Migration, effects on society, global environment and prevention. Sci. Total Environ. 2020, 728, 138882. [Google Scholar] [CrossRef] [PubMed]
- Ghana Health Service. COVID-19 Situation Dashboard, Ghana. Available online: https://ghs.gov.gh/covid19/dashboardm.php (accessed on 17 February 2023).
- Puthiyedath, R.; Kataria, S.; Payyappallimana, U.; Mangalath, P.; Nampoothiri, V.; Sharma, P.; Singh, M.K.; Kumar, K.; Trehan, N. Ayurvedic clinical profile of COVID-19—A preliminary report. J. Ayurveda Integr. Med. 2020, 13, 100326. [Google Scholar] [CrossRef] [PubMed]
- Kantarcioglu, B.; Iqbal, O.; Lewis, J.; Carter, C.A.; Singh, M.; Lievano, F.; Ligocki, M.; Jeske, W.; Adiguzel, C.; Gerotziafas, G.T.; et al. An Update on the Status of Vaccine Development for SARS-CoV-2 Including Variants. Practical Considerations for COVID-19 Special Populations. Clin. Appl. Thromb. Hemost. 2022, 28, 10760296211056648. [Google Scholar] [CrossRef] [PubMed]
- Paget, J.; Spreeuwenberg, P.; Charu, V.; Taylor, R.J.; Iuliano, A.D.; Bresee, J.; Simonsen, L.; Viboud, C.; Global Seasonal Influenza-Associated Mortality Collaborator Network and GLaMOR Collaborating Teams. Global mortality associated with seasonal influenza epidemics: New burden estimates and predictors from the GLaMOR Project. J. Glob. Health 2019, 9, 020421. [Google Scholar] [CrossRef]
- Mirzaei, R.; Goodarzi, P.; Asadi, M.; Soltani, A.; Aljanabi, H.A.A.; Jeda, A.S.; Dashtbin, S.; Jalalifar, S.; Mohammadzadeh, R.; Teimoori, A.; et al. Bacterial co-infections with SARS-CoV-2. IUBMB Life 2020, 72, 2097–2111. [Google Scholar] [CrossRef]
- Cao, Y.; Chen, M.; Dong, D.; Xie, S.; Liu, M. Environmental pollutants damage airway epithelial cell cilia: Implications for the prevention of obstructive lung diseases. Thorac. Cancer 2020, 11, 505–510. [Google Scholar] [CrossRef]
- Ghana Statistical Service, Population of Regions and Districts Report. Available online: https://statsghana.gov.gh/gssmain/fileUpload/pressrelease/2021%20PHC%20General%20Report%20Vol%203A_Population%20of%20Regions%20and%20Districts_181121.pdf (accessed on 23 March 2023).
- Sisó-Almirall, A.; Brito-Zerón, P.; Ferrín, L.C.; Kostov, B.; Moreno, A.M.; Mestres, J.; Sellarès, J.; Galindo, G.; Morera, R.; Basora, J.; et al. Long COVID-19: Proposed primary care clinical guidelines for diagnosis and disease management. Int. J. Environ. Res. Public Health 2021, 18, 4350. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing, 30th ed.; CLSI Supplement M100: Wayne, PA, USA, 2021. [Google Scholar]
- Owusu, M.; Sylverken, A.A.; Ankrah, S.T.; El-Duah, P.; Ayisi-Boateng, N.K.; Yeboah, R.; Gorman, R.; Asamoah, J.; Binger, T.; Acheampong, G.; et al. Epidemiological profile of SARS-CoV-2 among selected regions in Ghana: A cross-sectional retrospective study. PLoS ONE 2020, 15, e0243711. [Google Scholar] [CrossRef]
- Bao, L.; Zhang, C.; Dong, J.; Zhao, L.; Li, Y.; Sun, J. Oral microbiome and SARS-CoV-2: Beware of lung co-infection. Front. Microbiol. 2020, 11, 1840. [Google Scholar] [CrossRef]
- Ruxton, S.; Burrell, S. Masculinities and COVID-19: Making the Connections; Promundo: Washington, DC, USA, 2020. [Google Scholar]
- Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Faidah, H.; Al-Maiahy, T.J.; Cruz-Martins, N.; Batiha, G.E.S. The looming effects of estrogen in COVID-19: A Rocky Rollout. Front. Nutr. 2021, 8, 649128. [Google Scholar] [CrossRef] [PubMed]
- Whitaker, M.; Elliott, J.; Bodinier, B.; Barclay, W.; Ward, H.; Cooke, G.; Donnelly, C.A.; Chadeau-Hyam, M.; Elliott, P. Variant-specific symptoms of COVID-19 in a study of 1,542,510 adults in England. Nat. Commun. 2022, 13, 6856. [Google Scholar] [CrossRef]
- Lapostolle, F.; Schneider, E.; Vianu, I.; Dollet, G.; Roche, B.; Berdah, J.; Michel, J.; Goix, L.; Chanzy, E.; Petrovic, T.; et al. Clinical features of 1487 COVID-19 patients with outpatient management in the Greater Paris: The COVID-call study. Intern. Emerg. Med. 2020, 15, 813–817. [Google Scholar] [CrossRef]
- Abayomi, A.; Odukoya, O.; Osibogun, A.; Wright, O.; Adebayo, B.; Balogun, M.; Abdur-Razzaq, H. Presenting symptoms and predictors of poor outcomes among 2,184 patients with COVID-19 in Lagos State, Nigeria. Int. J. Infect. Dis. 2021, 102, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Uygun, Ö.; Ertaş, M.; Ekizoğlu, E.; Bolay, H.; Özge, A.; Kocasoy Orhan, E.; Çağatay, A.A.; Baykan, B. Headache characteristics in COVID-19 pandemic-a survey study. J. Headache Pain 2020, 21, 121. [Google Scholar] [CrossRef] [PubMed]
- Fernández-de-Las-Peñas, C.; Cuadrado, M.L.; Gómez-Mayordomo, V.; García-Azorín, D.; Arendt-Nielsen, L. Headache as a COVID-19 onset symptom or post-COVID symptom according to the SARS-CoV-2 variant. Expert Rev. Neurother. 2023, 23, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Westwood, D.; MacFadden, D.R.; Soucy, J.-P.R.; Daneman, N. Bacterial co-infection and secondary infection in patients with COVID-19: A living rapid review and meta-analysis. Clin. Microbiol. Infect. 2020, 26, 1622–1629. [Google Scholar] [CrossRef]
- Antinori, S.; Galimberti, L.; Milazzo, L.; Ridolfo, A.L. Bacterial and fungal infections among patients with SARS-CoV-2 pneumonia. Infez Med. 2020, 28 (Suppl. S1), 29–36. [Google Scholar]
- Contou, D.; Claudinon, A.; Pajot, O.; Micaëlo, M.; Longuet Flandre, P.; Dubert, M.; Cally, R.; Logre, E.; Fraissé, M.; Mentec, H.; et al. Bacterial and viral co-infections in patients with severe SARS-CoV-2 pneumonia admitted to a French ICU. Ann. Intensive Care 2020, 10, 119. [Google Scholar] [CrossRef]
- Sharifipour, E.; Shams, S.; Esmkhani, M.; Khodadadi, J.; Fotouhi-Ardakani, R.; Koohpaei, A.; Doosti, Z.; Golzari, S.E. Evaluation of bacterial co-infections of the respiratory tract in COVID-19 patients admitted to ICU. BMC Infect. Dis. 2020, 20, 646. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Ge, Y.; Wu, T.; Zhao, K.; Chen, Y.; Wu, B.; Zhu, F.; Zhu, B.; Cui, L. Co-infection with respiratory pathogens among COVID-2019 cases. Virus Res. 2020, 285, 198005. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, H.K.-A.; Mahmoud, M.A.; Aburahma, M.Z.; Elkhawaga, A.A.; El-Mokhtar, M.A.; Sayed, I.M.; Hosni, A.; Hassany, S.M.; Medhat, M.A. Predictors of severity and co-infection resistance profile in COVID-19 patients: First report from upper Egypt. Infect. Drug Resist. 2020, 13, 3409. [Google Scholar] [CrossRef]
- Lu, B.; Yan, Y.; Dong, L.; Han, L.; Liu, Y.; Yu, J.; Chen, J.; Yi, D.; Zhang, M.; Deng, X.; et al. Integrated characterization of SARS-CoV-2 genome, microbiome, antibiotic resistance and host response from single throat swabs. Cell Discov. 2021, 7, 19. [Google Scholar] [CrossRef] [PubMed]
- Feehan, A.K.; Rose, R.; Nolan, D.J.; Spitz, A.M.; Graubics, K.; Colwell, R.R.; Garcia-Diaz, J.; Lamers, S.L. Nasopharyngeal Microbiome Community Composition and Structure Is Associated with Severity of COVID-19 Disease and Breathing Treatment. Appl. Microbiol. 2021, 1, 177–188. [Google Scholar] [CrossRef]
- Deinhardt-Emmer, S.; Böttcher, S.; Häring, C.; Giebeler, L.; Henke, A.; Zell, R.; Jungwirth, J.; Jordan, P.M.; Werz, O.; Hornung, F.; et al. SARS-CoV-2 causes severe epithelial inflammation and barrier dysfunction. J. Virol. 2021, 95, e00110-21. [Google Scholar] [CrossRef]
- Ou, X.; Zhou, L.; Huang, H.; Lin, Y.; Pan, X.; Chen, D. A severe case with co-infection of SARS-CoV-2 and common respiratory pathogens. Travel Med. Infect. Dis. 2020, 35, 101672. [Google Scholar] [CrossRef]
- Peddu, V.; Shean, R.C.; Xie, H.; Shrestha, L.; Perchetti, G.A.; Minot, S.S.; Roychoudhury, P.; Huang, M.L.; Nalla, A.; Reddy, S.B.; et al. Metagenomic analysis reveals clinical SARS-CoV-2 infection and bacterial or viral superinfection and colonization. Clin. Chem. 2020, 66, 966–972. [Google Scholar] [CrossRef]
- Goel, N.; Ahmad, R.; Fatima, H.; Khare, S.K. New threatening of SARS-CoV-2 coinfection and strategies to fight the current pandemic. Med. Drug Discov. 2021, 10, 100089. [Google Scholar] [CrossRef]
- Davies-Bolorunduro, O.F.; Fowora, M.A.; Amoo, O.S.; Adeniji, E.; Osuolale, K.A.; Oladele, O.; Onuigbo, T.I.; Obi, J.C.; Oraegbu, J.; Ogundepo, O.; et al. Evaluation of respiratory tract bacterial co-infections in SARS-CoV-2 patients with mild or asymptomatic infection in Lagos, Nigeria. Bull. Natl. Res. Cent. 2022, 46, 115. [Google Scholar] [CrossRef]
- Chu, H.; Chan, J.F.W.; Wang, Y.; Yuen, T.T.T.; Chai, Y.; Hou, Y.; Shuai, H.; Yang, D.; Hu, B.; Huang, X.; et al. Comparative replication and immune activation profiles of SARS-CoV-2 and SARS-CoV in human lungs: An ex vivo study with implications for the pathogenesis of COVID-19. Clin. Infect. Dis. 2020, 71, 1400–1409. [Google Scholar] [CrossRef] [PubMed]
- Shah, V.M.; Brill, K.L.; Dhingra, G.; Kannan, S.G. Delayed recurrent spontaneous pneumothorax in a patient recovering from COVID-19 pneumonia. Korean J. Anesthesiol. 2021, 74, 183. [Google Scholar] [CrossRef] [PubMed]
- Asli, R.; Abdullah, M.S.; Chong, P.L.; Metussin, D.; Momin, R.N.; Mani, B.I.; Chong, V.H. Case report: Right bundle brunch block and QTc prolongation in a patient with COVID-19 treated with hydroxychloroquine. Am. J. Trop. Med. Hyg. 2020, 103, 79. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.-C.; Chen, S.-Y.; Ko, W.-C.; Hsueh, P.-R. Increased antimicrobial resistance during the COVID-19 pandemic. Int. J. Antimicrob. Agents 2021, 57, 106324. [Google Scholar] [CrossRef]
- Silva, D.; Lima, C.; Magalhães, V.; Baltazar, L.; Peres, N.; Caligiorne, R.; Moura, A.; Fereguetti, T.; Martins, J.; Rabelo, L.; et al. Fungal and bacterial coinfections increase mortality of severely ill COVID-19 patients. J. Hosp. Infect. 2021, 113, 145–154. [Google Scholar] [CrossRef]
- Egyir, B.; Obeng-Nkrumah, N.; Kyei, G.B. COVID-19 pandemic and antimicrobial resistance: Another call to strengthen laboratory diagnostic capacity in Africa. Afr. J. Lab. Med. 2020, 9, 4. [Google Scholar] [CrossRef] [PubMed]
- Kaynar, K.; Gul, S.; Ersoz, S.; Ozdemir, F.; Ulusoy, H.; Ulusoy, S. Amikacin-induced nephropathy: Is there any protective way? Ren. Fail. 2007, 29, 23–27. [Google Scholar] [CrossRef]
| Variable | All Participants n = 380 (%) | SARS-CoV-2 Negative n = 262 (%) | SARS-CoV-2 Positive n = 118 (%) | p-Value |
|---|---|---|---|---|
| Age group (years) | 0.699 | |||
| <20 | 26 (6.9) | 18 (6.9) | 8 (6.8) | |
| 21–30 | 112 (29.6) | 81 (31.2) | 31 (26.3) | |
| 31–40 | 102 (27.0) | 65 (25.0) | 37 (31.4) | |
| 41–50 | 48 (12.7) | 32 (12.3) | 16 (13.6) | |
| 51–94 | 90 (23.8) | 64 (24.6) | 26 (22.0) | |
| Gender | 0.821 | |||
| Female | 149 (39.2) | 104 (39.7) | 45 (38.1) | |
| Male | 231 (60.8) | 158 (60.3) | 73 (61.9) | |
| Clinical Symptoms | ||||
| Asymptomatic | 120 (31.6) | 60 (22.9) | 60 (50.8) | <0.001 |
| Symptomatic | 260 (68.4) | 202 (77.1) | 58 (49.2) | |
| Cough | <0.001 | |||
| No | 146 (38.6) | 69 (26.5) | 77 (65.3) | |
| Yes | 232 (61.4) | 191 (73.5) | 41 (34.7) | |
| Sore throat | 0.354 | |||
| No | 343 (90.3) | 239 (91.2) | 104 (88.1) | |
| Yes | 37 (9.7) | 23 (8.8) | 14 (11.9) | |
| Runny nose | 0.104 | |||
| No | 364 (95.8) | 254 (96.9) | 100 (93.2) | |
| Yes | 16 (4.2) | 8 (3.1) | 8 (6.8) | |
| Fever | 0.181 | |||
| No | 346 (91.1) | 242 (92.4) | 104 (88.1) | |
| Yes | 34 (8.9) | 20 (7.6) | 14 (11.9) | |
| Shortness of breath | 0.650 | |||
| No | 357 (93.9) | 247 (94.3) | 110 (93.2) | |
| Yes | 23 (6.1) | 15 (5.7) | 8 (6.8) | |
| Diarrhoea | 0.648 | |||
| No | 375 (98.7) | 259 (98.9) | 116 (98.3) | |
| Yes | 5 (1.3) | 3 (1.1) | 2 (1.7) | |
| Nausea/Vomiting | 0.886 | |||
| No | 373 (98.2) | 257 (98.1) | 116 (98.3) | |
| Yes | 7 (1.8) | 5 (1.9) | 2 (1.7) | |
| Headache | <0.001 | |||
| No | 365 (96.1) | 258 (98.5) | 107 (90.7) | |
| Yes | 15 (3.9) | 4 (1.5) | 11 (9.30) | |
| Irritability | ||||
| No | 380 (100.0) | 262 (100.0) | 118 (100.0) | 0.999 |
| Yes | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Pain | 0.127 | |||
| No | 345 (90.8) | 242 (92.4) | 103 (87.3) | |
| Yes | 35 (9.2) | 20 (7.6) | 15 (12.7) | |
| General weakness | 0.030 | |||
| No | 347 (91.3) | 245 (93.5) | 102 (86.4) | |
| Yes | 33 (8.7) | 17 (6.5) | 16 (13.6) |
| Variable | cOR (95% CI) | p-Value |
|---|---|---|
| Categories of age (years) | ||
| <20 | 1 (Ref) | |
| 21–30 | 0.86 (0.34–2.18) | 0.753 |
| 31–40 | 1.28 (0.51–3.23) | 0.6 |
| 41–50 | 1.13 (0.40–3.14) | 0.822 |
| 51–94 | 0.91 (0.35–2.36) | 0.853 |
| Gender | ||
| Female | 1 (Ref) | |
| Male | 1.07 (0.68–1.67) | 0.773 |
| Clinical Symptoms | ||
| Asymptomatic | 1 (Ref) | |
| Symptomatic | 0.29 (0.18–0.46) | <0.001 |
| Cough | ||
| No | 1 (Ref) | |
| Yes | 0.19 (0.12–0.31) | <0.001 |
| Sore throat | ||
| No | 1 (Ref) | |
| Yes | 1.40 (0.69–2.83) | 0.35 |
| Runny nose | ||
| No | 1 (Ref) | |
| Yes | 2.31 (0.85–6.31) | 0.103 |
| Fever | ||
| No | 1 (Ref) | |
| Yes | 1.63 (0.79–3.45) | 0.185 |
| Shortness of breath | ||
| No | 1 (Ref) | |
| Yes | 1.20 (0.49–2.91) | 0.69 |
| Diarrhea | ||
| No | 1 (Ref) | |
| Yes | 1.49 (0.25–9.03) | 0.665 |
| Nausea/Vomiting | ||
| No | 1 (Ref) | |
| Yes | 0.89 (0.17–4.64) | 0.886 |
| Headache | ||
| No | 1 (Ref) | |
| Yes | 6.63 (2.07–21.29) | 0.001 |
| Pain | ||
| No | 1 (Ref) | |
| Yes | 1.76 (0.87–3.58) | 0.117 |
| General weakness | ||
| No | 1 (Ref) | |
| Yes | 2.26 (1.10–4.65) | 0.027 |
| Microbial Isolate | All Participants n = 380 (%) | SARS-CoV-2 Negative n = 262 (%) | SARS-CoV-2 Positive n = 118 (%) | p-Value |
|---|---|---|---|---|
| Acinetobacter baumannii | 0.862 | |||
| No | 368 (96.8) | 254 (96.9) | 114 (96.6) | |
| Yes | 12 (3.2) | 8 (3.1) | 4 (3.4) | |
| Candida spp. | 0.733 | |||
| No | 355 (93.4) | 244 (93.1) | 111 (94.1) | |
| Yes | 25 (6.6) | 18 (6.9) | 7 (5.9) | |
| Citrobacter spp. | 0.932 | |||
| No | 377 (99.2) | 260 (99.2) | 117 (99.2) | |
| Yes | 3 (0.8) | 2 (0.8) | 1 (0.8) | |
| Enterobacter spp. | 0.496 | |||
| No | 373 (98.2) | 258 (98.5) | 115 (97.5) | |
| Yes | 7 (1.8) | 4 (1.5) | 3 (2.5) | |
| Escherichia coli | 0.084 | |||
| No | 368 (96.8) | 251 (95.8) | 117 (99.2) | |
| Yes | 12 (3.2) | 11 (4.2) | 1 (0.8) | |
| Klebsiella spp. | 0.446 | |||
| No | 302 (79.5) | 227 (86.6) | 95 (80.5) | |
| Yes | 78 (20.5) | 35 (13.4) | 23 (19.5) | |
| Moraxella catarrhalis | 0.002 | |||
| No | 350 (92.1) | 234 (89.3) | 116 (98.3) | |
| Yes | 30 (7.9) | 28 (10.7) | 2 (1.7) | |
| Proteus spp. | 0.312 | |||
| No | 374 (98.4) | 259 (98.9) | 115 (97.5) | |
| Yes | 6 (1.6) | 3 (1.1) | 3 (2.5) | |
| Pseudomonas spp. | 0.803 | |||
| No | 356 (93.7) | 246 (93.9) | 110 (93.2) | |
| Yes | 24 (6.3) | 16 (6.1) | 8 (6.8) | |
| Serratia spp. | <0.001 | |||
| No | 368 (96.8) | 262 (100.0) | 106 (89.8) | |
| Yes | 12 (3.2) | 0 (0.0) | 12 (10.2) | |
| Stenotrophomonas maltophilia | 0.017 | |||
| No | 375 (98.7) | 261 (99.6) | 114 (96.6) | |
| Yes | 5 (1.3) | 1 (0.4) | 4 (3.4) | |
| Streptococcus pneumoniae | 0.502 | |||
| No | 379 (99.7) | 261 (99.6) | 118 (100.0) | |
| Yes | 1 (0.3) | 1 (0.4) | 0 (0.0) |
| Microbial Isolate | cOR (95% CI) | p-Value | aOR (95% CI) | p-Value |
|---|---|---|---|---|
| Moraxella catarrhalis | 0.14 (0.03–0.62) | 0.009 | 0.15 (0.04–0.64) | 0.010 |
| Stenotrophomonas maltophilia | 9.16 (1.01–82.84) | 0.049 | 8.32 (0.92–75.32) | 0.059 |
| Antibiotic | Acinetobacter baumannii (n = 4) | Enterobacter spp. (n = 3) | Escherichia coli (n = 1) | Klebsiella spp. (n = 23) | Moraxella catarrhalis (n = 2) | Proteus spp. (n = 3) | Pseudomonas spp. (n = 8) | Serratia spp. (n = 12) | Stenotrophomonas maltophilia (n = 4) |
|---|---|---|---|---|---|---|---|---|---|
| CRO | 4 (100.0) | 1 (33.3) | 1 (100.0) | 12 (52.2) | - | 1 (33.3) | - | 3 (25.0) | 4 (100.0) |
| CTX | 4 (100.0) | 1 (33.3) | 1 (100.0) | 9 (39.1) | - | 1 (33.3) | - | 2 (16.7) | 4 (100.0) |
| AZM | 0 (0.0) | 1 (33.3) | 1(100.0) | 7 (30.4) | - | 2 (66.7) | - | 5 (41.7) | 0 (0.0) |
| AMC | 4 (100.0) | 3 (100.0) | 1 (100.0) | 15 (65.2) | - | 3 (100.0) | - | 12 (100.0) | 4 (100.0) |
| CIP | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (13.0) | 1 (50.0) | 1 (33.3) | 0 (0.0) | 2 (16.7) | 1 (25.0) |
| GEN | 1 (25.0) | 0 (0.0) | 1 (100.0) | 2 (8.7) | 0 (0.0) | 1 (33.3) | 3 (37.5) | 0 (0.0) | 1(25.0) |
| CAZ | 2 (50.0) | 0 (0.0) | 1 (100.0) | 9 (39.1) | 0 (0.0) | 0 (0.0) | 3(37.5) | 1 (8.3) | 2 (25.0) |
| AK | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (37.5) | 0 (0.0) | 0 (0.0) |
| MEM | - | - | - | - | 0 (0.0) | - | 4 (50.0) | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deberu, O.N.; Acheampong, G.; Nkrumah, B.; Ayisi-Boateng, N.K.; Afriyie, S.O.; Agyapong, F.O.; Owusu, D.O.; Mutocheluh, M.; Abdul-Karim, A.; El-Duah, P.; et al. Microbial Organisms in the Lower Respiratory Tract Associated with SARS-CoV-2 Infection: A Cross-Sectional Study in Northern Ghana. COVID 2023, 3, 440-451. https://doi.org/10.3390/covid3040033
Deberu ON, Acheampong G, Nkrumah B, Ayisi-Boateng NK, Afriyie SO, Agyapong FO, Owusu DO, Mutocheluh M, Abdul-Karim A, El-Duah P, et al. Microbial Organisms in the Lower Respiratory Tract Associated with SARS-CoV-2 Infection: A Cross-Sectional Study in Northern Ghana. COVID. 2023; 3(4):440-451. https://doi.org/10.3390/covid3040033
Chicago/Turabian StyleDeberu, Oliver Nangkuu, Godfred Acheampong, Bernard Nkrumah, Nana Kwame Ayisi-Boateng, Stephen Opoku Afriyie, Francis Opoku Agyapong, Dorcas Ohui Owusu, Mohamed Mutocheluh, Abass Abdul-Karim, Philip El-Duah, and et al. 2023. "Microbial Organisms in the Lower Respiratory Tract Associated with SARS-CoV-2 Infection: A Cross-Sectional Study in Northern Ghana" COVID 3, no. 4: 440-451. https://doi.org/10.3390/covid3040033
APA StyleDeberu, O. N., Acheampong, G., Nkrumah, B., Ayisi-Boateng, N. K., Afriyie, S. O., Agyapong, F. O., Owusu, D. O., Mutocheluh, M., Abdul-Karim, A., El-Duah, P., Sylverken, A. A., & Owusu, M. (2023). Microbial Organisms in the Lower Respiratory Tract Associated with SARS-CoV-2 Infection: A Cross-Sectional Study in Northern Ghana. COVID, 3(4), 440-451. https://doi.org/10.3390/covid3040033








