Safe and Timely Delivery of Essential Surgical Care for Skin Cancer in a Pandemic
Abstract
1. Introduction
2. Materials and Methods
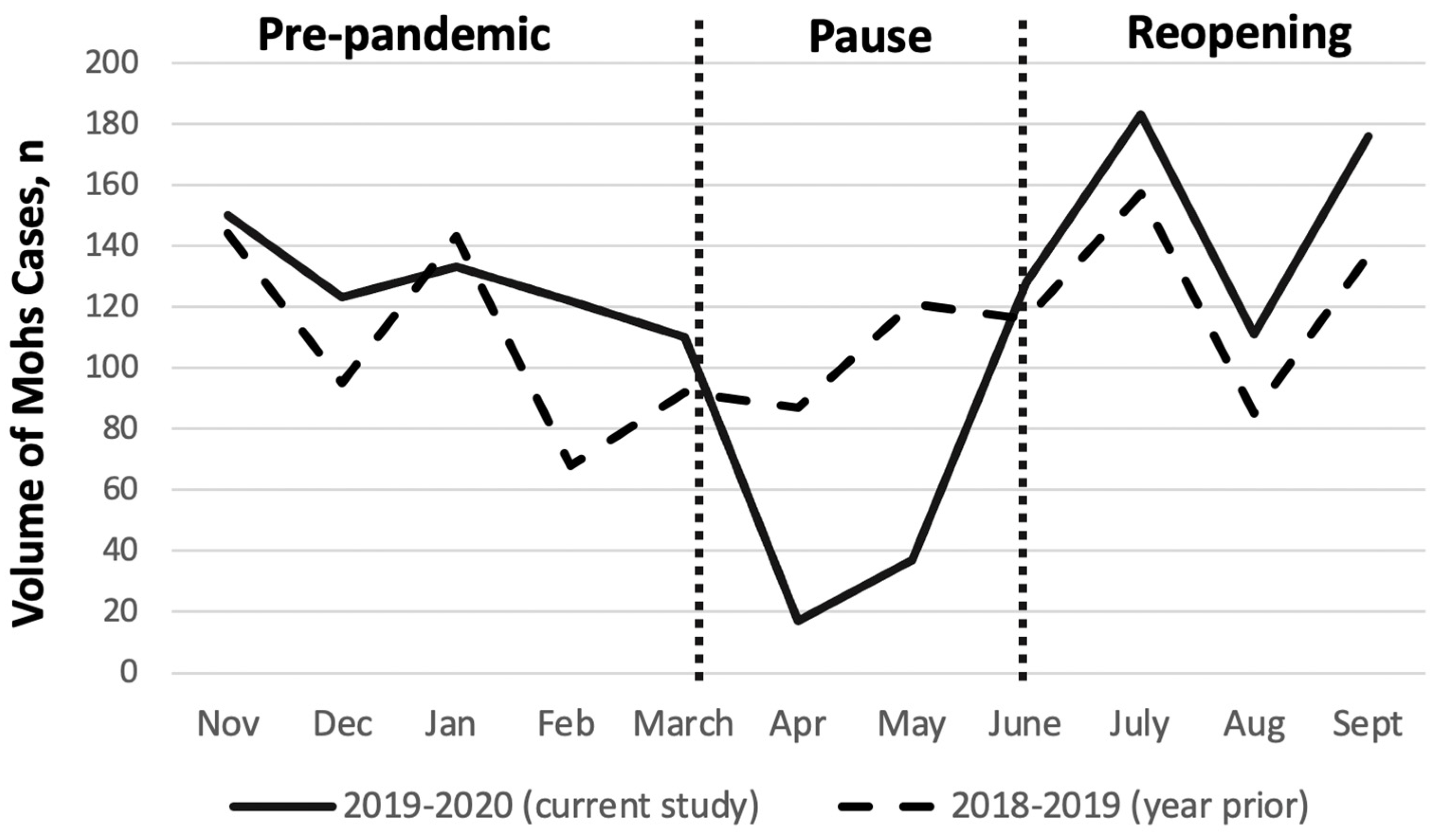
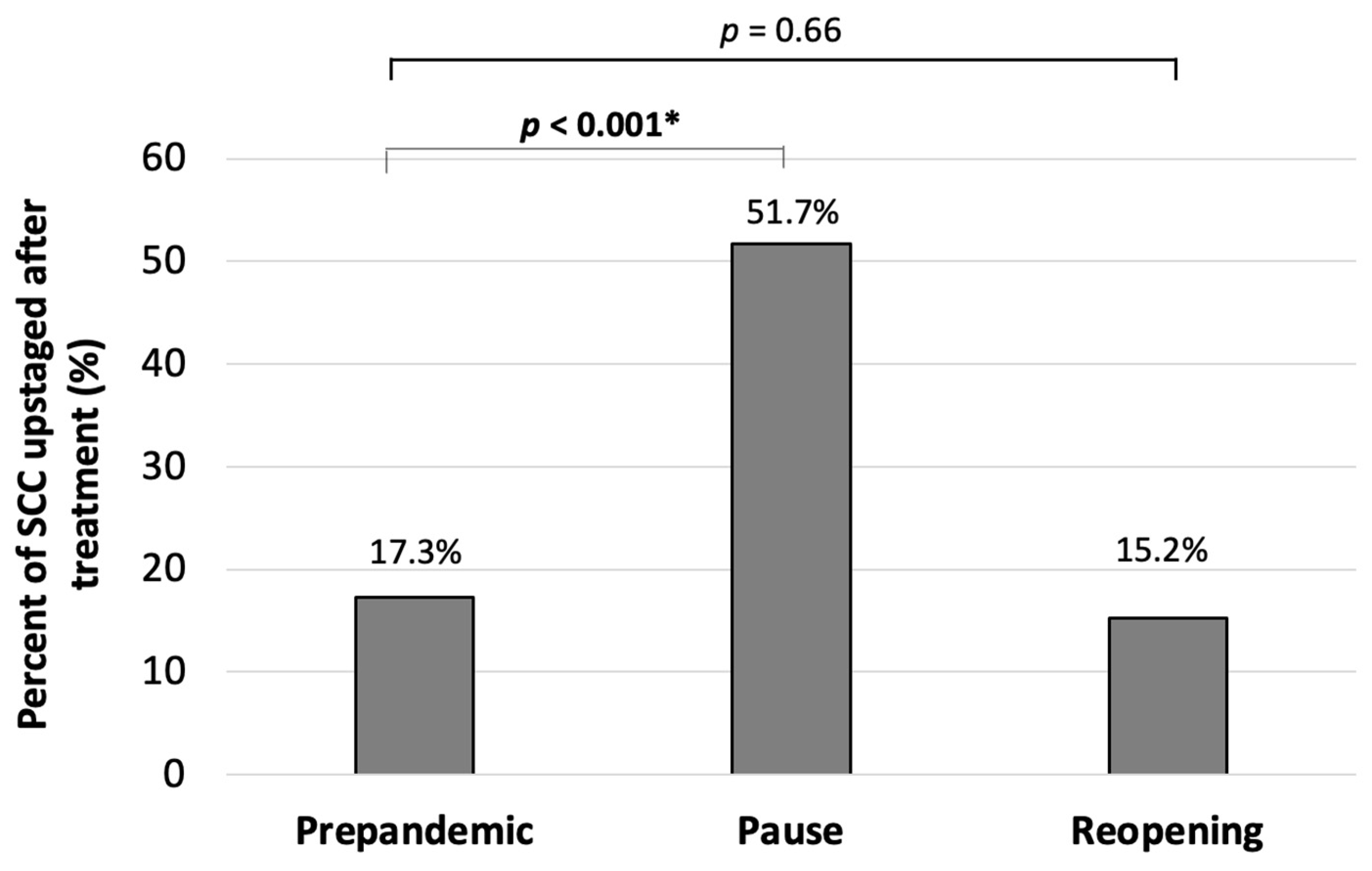
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Meredith, J.W.; High, K.P.; Freischlag, J.A. Preserving Elective Surgeries in the COVID-19 Pandemic and the Future. JAMA 2020, 324, 1725. [Google Scholar] [CrossRef]
- Dinmohamed, A.G.; Visser, O.; Verhoeven, R.H.A.; Louwman, M.W.J.; van Nederveen, F.H.; Willems, S.M.; Merkx, M.A.W.; Lemmens, V.E.P.P.; Nagtegaal, I.D.; Siesling, S. Fewer cancer diagnoses during the COVID-19 epidemic in the Netherlands. Lancet Oncol. 2020, 21, 750–751. [Google Scholar] [CrossRef]
- Ricci, F.; Fania, L.; Paradisi, A.; Di Lella, G.; Pallotta, S.; Sobrino, L.; Panebianco, A.; Annessi, G.; Abeni, D. Delayed melanoma diagnosis in the COVID-19 era: Increased breslow thickness in primary melanomas seen after the COVID-19 lockdown. J. Eur. Acad. Dermatol. Venereol. 2020, 34, e778–e779. [Google Scholar] [CrossRef]
- Nolan, G.S.; Dunne, J.A.; Kiely, A.L.; Jones, R.O.P.; Gardiner, M.; Jain, A.; Abdelaty, M. The effect of the COVID-19 pandemic on skin cancer surgery in the United Kingdom: A national, multi-centre, prospective cohort study and survey of Plastic Surgeons. Br. J. Surg. 2020, 107, e598–e600. [Google Scholar]
- Andrew, T.W.; Alrawi, M.; Lovat, P. Reduction in skin cancer diagnoses in the UK during the COVID-19 pandemic. Clin. Exp. Dermatol. 2020, 46, 145–146. [Google Scholar] [CrossRef]
- Wehner, M.; Dalma, N.; Landefeld, C.; Pare-Anastasiadou, A.; Koutelidas, I.; Chren, M.; Aji, N.; Teng, C.; Koenig, B.; Tang, J.; et al. Natural history of lesions suspicious for basal cell carcinoma in older adults in Ikaria, Greece. Br. J. Dermatol. 2018, 179, 767–768. [Google Scholar] [CrossRef]
- Fields, R.C.; Busam, K.J.; Chou, J.F.; Panageas, K.S.; Pulitzer, M.P.; Allen, P.J.; Kraus, D.H.; Brady, M.S.; Coit, D.G. Five Hundred Patients with Merkel Cell Carcinoma Evaluated at a Single Institution. Ann. Surg. 2011, 254, 465–475. [Google Scholar] [CrossRef]
- Heenan, P.J.; Holman, C.D. Nodular malignant melanoma: A distinct entity or a common end stage? Am. J. Dermatopathol. 1982, 4, 477–478. [Google Scholar]
- Lattanzi, M.; Lee, Y.; Simpson, D.; Moran, U.; Darvishian, F.; Kim, R.; Hernando, E.; Polsky, D.; Hanniford, D.; Shapiro, R.; et al. Primary Melanoma Histologic Subtype: Impact on Survival and Response to Therapy. Gynecol. Oncol. 2018, 111, 180–188. [Google Scholar] [CrossRef]
- Eigentler, T.K.; Leiter, U.; Häfner, H.-M.; Garbe, C.; Röcken, M.; Breuninger, H. Survival of Patients with Cutaneous Squamous Cell Carcinoma: Results of a Prospective Cohort Study. J. Investig. Dermatol. 2017, 137, 2309–2315. [Google Scholar] [CrossRef]
- Thompson, A.K.; Kelley, B.F.; Prokop, L.J.; Murad, M.H.; Baum, C.L. Risk Factors for Cutaneous Squamous Cell Carcinoma Recurrence, Metastasis, and Disease-Specific Death: A Systematic Review and Meta-analysis. JAMA Dermatol. 2016, 152, 419–428. [Google Scholar] [CrossRef]
- Ruiz, E.S.; Karia, P.S.; Besaw, R.; Schmults, C.D. Performance of the American Joint Committee on Cancer Staging Manual, 8th Edition vs. the Brigham and Women’s Hospital Tumor Classification System for Cutaneous Squamous Cell Carcinoma. JAMA Dermatol. 2019, 155, 819–825. [Google Scholar] [CrossRef]
- Pavia, G.; Gargiulo, L.; Valenti, M.; Facheris, P.; Nucca, O.; Narcisi, A.; Borroni, R.G.; Costanzo, A. Skin cancers: How to balance the risks and benefits of surgery during COVID-19 pandemic (a Northern Italy single-center experience). Int. J. Dermatol. 2020, 59, 1287–1289. [Google Scholar] [CrossRef]
- NCCN. National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology: Advisory Statement for Non-Melanoma Skin Cancer Care during the COVID-19 Pandemic. 2020. Available online: https://www.nccn.org/covid-19/pdf/NCCN-NMSC.pdf (accessed on 1 June 2021).
- Surgery AcoM. American College of Mohs Surgery: COVID-19 (Coronavirus) Preparedness. 2020. Available online: https://www.mohscollege.org/UserFiles/AM20/Member%20Alert/COVIDAlert3March20.pdf (accessed on 1 June 2021).
- NCCN. National Comprehensive Cancer Network (NCCN): NCCN Short-Term Recommendations for Cutaneous Melanoma Management during COVID-19 Pandemic. 2020. Available online: https://www.nccn.org/covid-19/pdf/melanoma.pdf (accessed on 1 June 2021).
- British Association of Dermatologists BSfDS. COVID-19 Skin Cancer Surgery Guidance. 2020. Available online: http://www.bad.org.uk/healthcare-professionals/covid-19 (accessed on 1 June 2021).
- Tee, M.W.; Stewart, C.; Aliessa, S.; Polansky, M.; Shah, K.; Petukhova, T.; Rossi, A.; Lipner, S.R.; Minkis, K. Dermatologic surgery during the COVID-19 pandemic: Experience of a large academic center. J. Am. Acad. Dermatol. 2020, 84, 1094–1096. [Google Scholar] [CrossRef]
- Brochez, L.; Baurain, J.; Del Marmol, V.; Nikkels, A.; Kruse, V.; Sales, F.; Stas, M.; Van Laethem, A.; Garmyn, M. Recommendations for skin cancer consultation and surgery during COVID-19 pandemic. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 1876–1878. [Google Scholar] [CrossRef]
- Geskin, L.J.; Trager, M.H.; Aasi, S.Z.; Bickers, D.R.; Carvajal, R.D.; Nghiem, P.; Taback, B.; Zeitouni, N.C.; Samie, F.H. Perspectives on the recommendations for skin cancer management during the COVID-19 pandemic. J. Am. Acad. Dermatol. 2020, 83, 295–296. [Google Scholar] [CrossRef]
- Miranda, B.H.; Hughes, W.R.M.; Pinto-Lopes, R.; Mathur, B.S.; Ramakrishnan, V.V.; Sood, M.K. St Andrew’s COVID-19 surgery safety (StACS) study: Elective plastic surgery, trauma & burns. J. Plast. Reconstr. Aesthet. Surg. 2021, 74, 211–222. [Google Scholar]
- Alam, M. Struggling with COVID: Every cancer patient is unique and needs their own plan. J. Am. Acad. Dermatol. 2020, 83, 297–298. [Google Scholar] [CrossRef]
- Baumann, B.C.; MacArthur, K.M.; Brewer, J.D.; Mendenhall, W.M.; Barker, C.A.; Etzkorn, J.R.; Jellinek, N.J.; Scott, J.F.; Gay, H.A.; Baumann, J.C.; et al. Management of primary skin cancer during a pandemic: Multidisciplinary recommendations. Cancer 2020, 126, 3900–3906. [Google Scholar] [CrossRef]
- Rosenbaum, L. The Untold Toll—The Pandemic’s Effects on Patients without Covid-19. N. Engl. J. Med. 2020, 382, 2368–2371. [Google Scholar] [CrossRef]
- Rich, H.; Jones, B.; Malin, I.; Hemington-Gorse, S.J.; Cubitt, J.J. Plastic surgical management of skin cancer patients during the COVID-19 pandemic. J. Plast. Reconstr. Aesthet. Surg. 2021, 74, 666–668. [Google Scholar] [CrossRef] [PubMed]
- Rich, H.; O’Neill, T. Major fall in urgent skin cancer referrals during the COVID-19 outbreak. J. Plast. Reconstr. Aesthet. Surg. 2021, 74, 670–671. [Google Scholar] [CrossRef]
- Shannon, A.B.; Sharon, C.E.; Straker, R.J., 3rd; Miura, J.T.; Ming, M.E.; Chu, E.Y.; Karakousis, G.C. The impact of the COVID-19 pandemic on the presentation status of newly diagnosed melanoma: A single institution experience. J. Am. Acad. Dermatol. 2021, 84, 1096–1098. [Google Scholar] [CrossRef] [PubMed]
- Capitelli-McMahon, H.; Hurley, A.; Pinder, R.; Matteucci, P.; Totty, J. Characterising non-melanoma skin cancer undergoing surgical management during the COVID-19 pandemic. J. Plast. Reconstr. Aesthet. Surg. 2021, 74, 668–670. [Google Scholar] [CrossRef] [PubMed]
- Beyaert, R.; Beaugerie, L.; Van Assche, G.; Brochez, L.; Renauld, J.-C.; Viguier, M.; Cocquyt, V.; Jerusalem, G.; Machiels, J.-P.; Prenen, H.; et al. Cancer risk in immune-mediated inflammatory diseases (IMID). Mol. Cancer 2013, 12, 98. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.-W.; Fung, K.; Austin, P.C.; Kim, S.J.; Singer, L.G.; Baxter, N.N.; Alhusayen, R.; Rochon, P.A. Improved keratinocyte carcinoma outcomes with annual dermatology assessment after solid organ transplantation: Population-based cohort study. Am. J. Transplant. 2018, 19, 522–531. [Google Scholar] [CrossRef]
- Acuna, S.A.; Huang, J.W.; Scott, A.L.; Micic, S.; Daly, C.; Brezden-Masley, C.; Kim, S.J.; Baxter, N.N. Cancer Screening Recommendations for Solid Organ Transplant Recipients: A Systematic Review of Clinical Practice Guidelines. Am. J. Transplant. 2016, 17, 103–114. [Google Scholar] [CrossRef]
- Collins, L.; Quinn, A.; Stasko, T. Skin Cancer and Immunosuppression. Dermatol. Clin. 2018, 37, 83–94. [Google Scholar] [CrossRef]
| Pre-PANDEMIC | Pause | Reopening | Total | ||
|---|---|---|---|---|---|
| Tumors, n | 429 | 59 | 424 | 912 | |
| Patients, n | 353 | 45 | 348 | 691 | |
| Age, years | |||||
| Average | 70.1 | 69.6 | 72.1 | 70.9 | |
| Range | 26.5–100.7 | 25.9–89.1 | 22.4–100.8 | 22–101 | |
| Sex, n | |||||
| Female | 168 | 27 | 153 | 348 | |
| Male | 262 | 31 | 271 | 564 | |
| COVID-19 transmission, n | 0 | 0 | 0 | 0 | |
| Immunocompromising Condition, n (%) | Pre-pandemic vs. Pause | Pre-pandemic vs. Reopening | |||
| Immunosuppressive medication | 30 (7.0) | 10 (16.9) | 18 (4.2) | p = 0.03 * | p = 0.19 |
| Organ transplant recipient | 19 (4.4) | 5 (8.4) | 11 (2.5) | p = 0.19 | p = 0.82 |
| Other immune compromising condition | 19 (4.4) | 2 (3.3) | 21 (4.95) | p = 1.0 | p = 0.75 |
| Time to Treatment, days | |||||
| From Biopsy | 66 | 56 | 47 | p = 0.7 | p = 0.01 * |
| From Consultation | 28 | 8 | 8 | p = 0.002 * | p = 0.001 * |
| Tumor Type/Histologic Diagnosis, n (%) | |||||
| BCC | 216 (50.4) | 19 (32.8) | 210 (49.5) | p = 0.01 * | p = 0.84 |
| SCC | 170 (39.6) | 29 (50.0) | 189 (44.6) | p = 0.20 | p = 0.15 |
| MM | 18 (4.2) | 5 (8.6) | 15 (3.5) | p = 0.18 | p = 0.72 |
| Atypical Nevus | 17 (3.9) | 2 (3.4) | 5 (1.2) | p = 1.0 | p = 0.02 * |
| Other | 8 (1.9) | 3 (5.1) | 5 (1.2) | p = 0.14 | p = 0.58 |
| Same-day Frozen Biopsy, n | 0 | 6 | 4 | -- | -- |
| Treatment Modalities, n | Total | ||||
| Mohs | 370 | 49 | 384 | 804 (88.2) | |
| Standard Excision | 43 | 7 | 31 | 81 (8.9) | |
| Staged Excision | 5 | 2 | 3 | 10 (1.1) | |
| ED&C | 12 | 0 | 4 | 16 (1.75) | |
| Post-operative Defect Size, cm2 | Pre-pandemic vs. Pause vs. Reopening | ||||
| All surgical cases | 3.1 ± 4.2 | 3.5 ± 3.3 | 3.0 ± 3.9 | p = 0.54 | |
| Mohs | 3.2 ± 2.7 | 3.1 ± 2.0 | 2.9 ± 4.0 | p = 0.75 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jackson Cullison, S.R.; Celen, A.; Doudican, N.A.; Carucci, J.A.; Stevenson, M.L. Safe and Timely Delivery of Essential Surgical Care for Skin Cancer in a Pandemic. COVID 2023, 3, 144-150. https://doi.org/10.3390/covid3020009
Jackson Cullison SR, Celen A, Doudican NA, Carucci JA, Stevenson ML. Safe and Timely Delivery of Essential Surgical Care for Skin Cancer in a Pandemic. COVID. 2023; 3(2):144-150. https://doi.org/10.3390/covid3020009
Chicago/Turabian StyleJackson Cullison, Stephanie R., Arda Celen, Nicole A. Doudican, John A. Carucci, and Mary L. Stevenson. 2023. "Safe and Timely Delivery of Essential Surgical Care for Skin Cancer in a Pandemic" COVID 3, no. 2: 144-150. https://doi.org/10.3390/covid3020009
APA StyleJackson Cullison, S. R., Celen, A., Doudican, N. A., Carucci, J. A., & Stevenson, M. L. (2023). Safe and Timely Delivery of Essential Surgical Care for Skin Cancer in a Pandemic. COVID, 3(2), 144-150. https://doi.org/10.3390/covid3020009






