A Throat Lozenge with Fixed Combination of Cetylpyridinium Chloride and Benzydamine Hydrochloride Has Direct Virucidal Effect on SARS-CoV-2
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Preparation
2.2. Virus Isolation and Quantification
2.3. Preparation of Reagents
2.4. Quantitative Suspension Test for the Evaluation of Virucidal Activity
2.5. Control Experiments
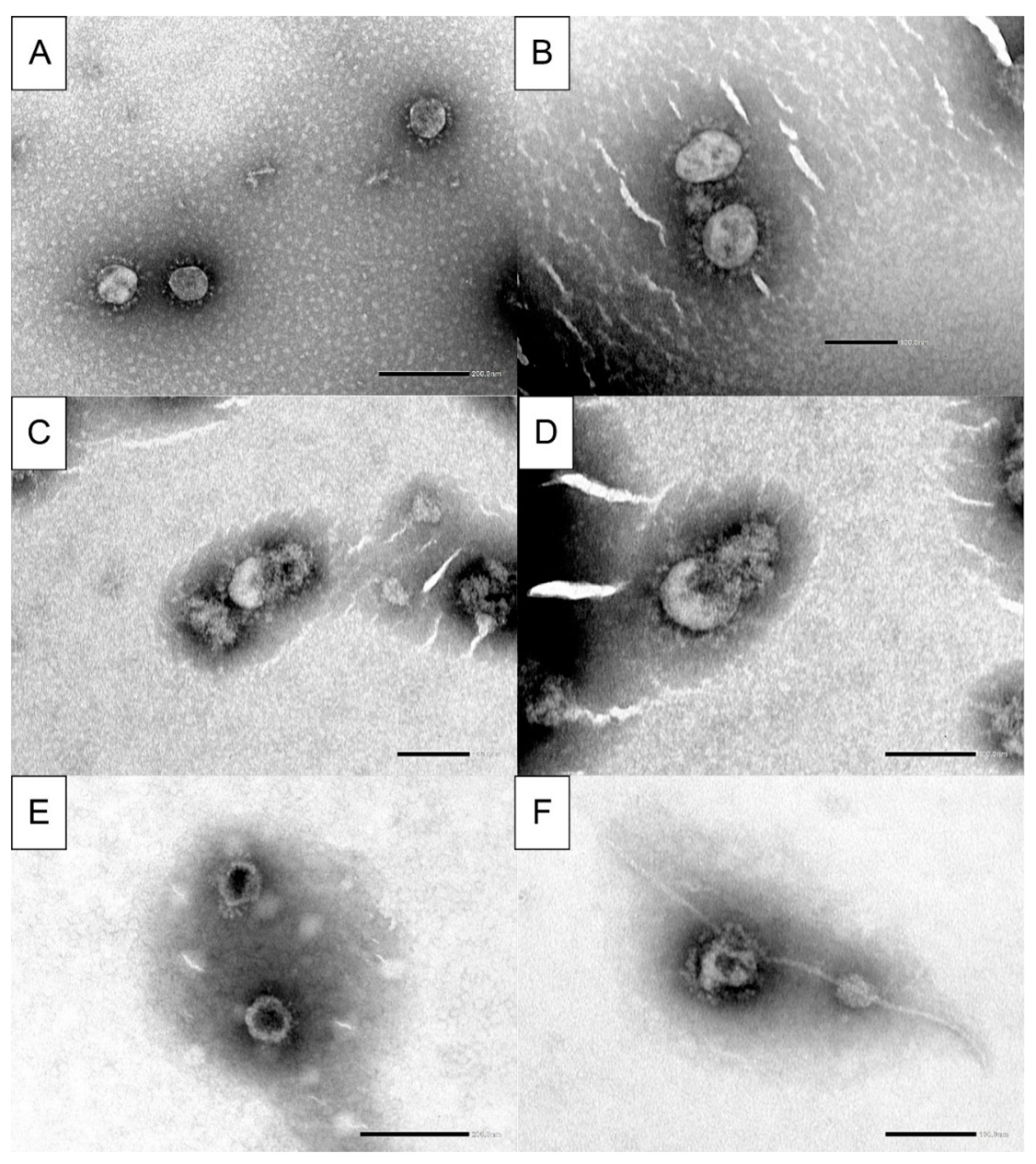
2.6. Electron Microscopy
2.7. Statistical Analysis
3. Results
3.1. Results of Control Procedures
3.1.1. Cells and Virus Control
3.1.2. Virus Control for the Total Contact Time of the Test
3.1.3. Test Product Activity Suppression in Ice-Cold Media
3.1.4. Interference Control—Control of Cell Susceptibility
3.1.5. Cytotoxic Effect
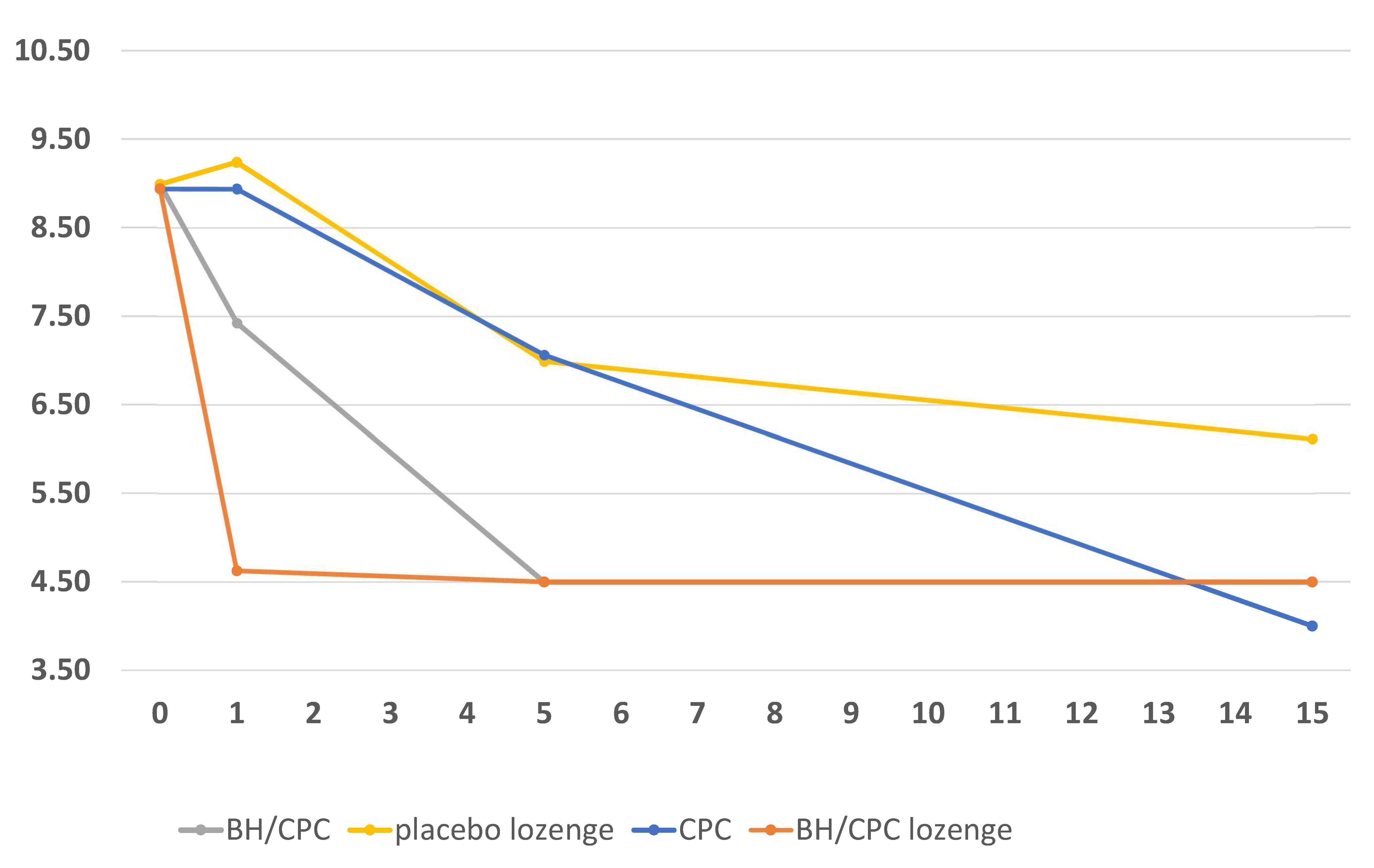
3.2. Results of Test Product Effectiveness on Virus Inactivation
3.2.1. Effects of Test Products in High Concentration
3.2.2. Effects of Test Products in Medium Concentration
3.2.3. Effects of Test Products in Low Concentration
3.3. Electron Microscopy Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morokutti-Kurz, M.; Graf, C.; Prieschl-Grassauer, E. Amylmetacresol/2,4-dichlorobenzyl alcohol, hexylresorcinol, or carrageenan lozenges as active treatments for sore throat. Int. J. Gen. Med. 2017, 10, 53–60. [Google Scholar] [CrossRef] [Green Version]
- Rey, F.A.; Lok, S.-M. Common Features of Enveloped Viruses and Implications for Immunogen Design for Next-Generation Vaccines. Cell 2018, 172, 1319–1334. [Google Scholar] [CrossRef]
- Eccles, R. Understanding the symptoms of the common cold and influenza. Lancet Infect. Dis. 2005, 5, 718–725. [Google Scholar] [CrossRef]
- Su, S.; Wong, G.; Shi, W.; Liu, J.; Lai, A.C.K.; Zhou, J.; Liu, W.; Bi, Y.; Gao, G.F. Epidemiology, Genetic Recombination, and Pathogenesis of Coronaviruses. Trends Microbiol. 2016, 24, 490–502. [Google Scholar] [CrossRef] [Green Version]
- de Wit, E.; van Doremalen, N.; Falzarano, D.; Munster, V.J. 2016 SARS and MERS: Recent insights into emerging coronaviruses. Nat. Rev. Microbiol. 2016, 14, 523–534. [Google Scholar] [CrossRef]
- Worldometer. Coronavirus Cases. 2021. Available online: https://www.worldometers.info/coronavirus/?zarsrc=130 (accessed on 22 April 2021).
- Wan, S.; Xiang, Y.; Fang, W.; Zheng, Y.; Li, B.; Hu, Y.; Lang, C.; Huang, D.; Sun, Q.; Xiong, Y.; et al. Clinical features and treatment of COVID-19 patients in northeast Chongqing. J. Med. Virol. 2020, 92, 797–806. [Google Scholar] [CrossRef]
- Hassan, S.A.; Sheikh, F.N.; Jamal, S.; Ezeh, J.K.; Akhtar, A. Coronavirus (COVID-19): A Review of Clinical Features, Diagnosis, and Treatment. Cureus 2020, 12, e7355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammacher, H. Selbstmedikation, Mund-und Rachentherapeutika; Deutscher Apotheker Verlag: Stuttgart, Germany, 1990; pp. 623–630. [Google Scholar]
- Kramer, A. Hagers Handbuch der Pharmazeutischen Praxis; Springer: Berlin/Heidelberg, Germany, 1990. [Google Scholar]
- Shirai, J.; Kanno, T.; Tsuchiya, Y.; Mitsubayashi, S.; Seki, R. Effects of Chlorine, Iodine, and Quaternary Ammonium Compound Disinfectants on Several Exotic Disease Viruses. J. Vet. Med. Sci. 2000, 62, 85–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popkin, D.L.; Zilka, S.; Dimaano, M.; Fujioka, H.; Rackley, C.; Salata, R.; Griffith, A.; Mukherjee, P.K.; Ghannoum, M.A.; Esper, F. Cetylpyridinium Chloride (CPC) Exhibits Potent, Rapid Activity Against Influenza Viruses in vitro and in vivo. Pathog. Immun. 2017, 2, 253–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Committee for Standardization. 2019. SIST EN 14476:2013+A2:2019 Chemical Disinfectants and Antiseptics—Quantitative Suspension Test for the Evaluation of Virucidal Activity in the Medical Area—Test Method and Requirements (Phase 2/Step 1). Available online: https://cdn.standards.iteh.ai/samples/69634/1b7c3643f04b45f1be4cdde6f2350c27/SIST-EN-14476-2013-A2-2019.pdf (accessed on 10 September 2020).
- Kärber, G. Beitrag zur kollektiven Behandlung pharmakologischer Reihenversuche. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1931, 162, 480–483. [Google Scholar] [CrossRef]
- Iorgulescu, G. Saliva between normal and pathological. Important factors in determining systemic and oral health. J. Med. Life 2010, 2, 303–307. [Google Scholar]
- Darnell, M.E.R.; Subbarao, K.; Feinstone, S.M.; Taylor, D.R. Inactivation of the coronavirus that induces severe acute respir-atory syndrome, SARS-CoV. J. Virol. Methods. 2004, 121, 85–91. [Google Scholar] [CrossRef]
- Scheller, C.; Krebs, F.; Minkner, R.; Astner, I.; Gil-Moles, M.; Wätzig, H. Physicochemical properties of SARS-CoV-2 for drug targeting, virus inactivation and attenuation, vaccine formulation and quality control. Electrophoresis 2020, 41, 1137–1151. [Google Scholar] [CrossRef]
- Li, Y.; Ren, B.; Peng, X.; Hu, T.; Li, J.; Gong, T.; Tang, B.; Xu, X.; Zhou, X. Saliva is a non-negligible factor in the spread of COVID-19. Mol. Oral Microbiol. 2020, 35, 141–145. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, D.; Yang, P.; Poon, L.L.M.; Wang, Q. Viral load of SARS-CoV-2 in clinical samples. Lancet Infect. Dis. 2020, 20, 411–412. [Google Scholar] [CrossRef]
- To, K.K.W.; Tsang, O.T.Y.; Leung, W.S.; Tam, A.R.; Wu, T.C.; Lung, D.C.; Yip, C.C.Y.; Cai, J.P.; Chan, J.M.C.; Chik, T.S.H.; et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: An observational cohort study. Lancet Infect. Dis. 2020, 20, 565–574. [Google Scholar] [CrossRef] [Green Version]
- Zou, L.; Ruan, F.; Huang, M.; Liang, L.; Huang, H.; Hong, Z.; Yu, J.; Kang, M.; Song, Y.; Xia, J.; et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N. Engl. J. Med. 2020, 382, 1177–1179. [Google Scholar] [CrossRef]
- Varia, M.; Wilson, S.; Sarwal, S.; McGeer, A.; Gournis, E.; Galanis, E. Investigation of a nosocomial outbreak of severe acute respiratory syndrome (SARS) in Toronto, Canada. CMAJ 2003, 169, 285–292. [Google Scholar]
- Yoon, J.G.; Yoon, J.; Song, J.Y.; Yoon, S.-Y.; Lim, C.S.; Seong, H.; Noh, J.Y.; Cheong, H.J.; Kim, W.J. Clinical Significance of a High SARS-CoV-2 Viral Load in the Saliva. J. Korean Med. Sci. 2020, 35, e195. [Google Scholar] [CrossRef]
- Lamas, L.M.; Dios, P.D.; Pérez-Rodríguez, M.T.; Pérez, V.D.C.; Alvargonzalez, J.J.C.; Domínguez, A.M.L.; Feijoo, J.F.; Diniz-Freitas, M.; Posse, J.L. Is povidone iodine mouthwash effective against SARS-CoV-2? First in vivo tests. Oral Dis. 2020. [Google Scholar] [CrossRef]
- Seneviratne, C.J.; Balan, P.; Ko, K.K.K.; Udawatte, N.S.; Lai, D.; Ng, D.H.L.; Venkatachalam, I.; Lim, K.S.; Ling, M.L.; Oon, L.; et al. Efficacy of commercial mouth-rinses on SARS-CoV-2 viral load in saliva: Randomized control trial in Singapore. Infection 2021, 49, 305–311. [Google Scholar] [CrossRef] [PubMed]
| Test Product | Test Temperature (°C) | Interfering Substance 2 | Contact Time |
|---|---|---|---|
| BH 1/CPC 2 lozenge Septolete total lozenge, containing 3 mg BH and 1 mg CPC dissolved in 4 mL, 20 mL and 30 mL of hard water Lozenge ingredients: BH, CPC, eucalyptus oil, levomenthol, anhydrous citric acid (E330), sucralose (E955), isomalt (E953), brilliant blue FCF (E133) | 37 | 3.0 g/L bovine albumin solution and 3.0 mL/L erythrocytes | 1, 5 and 15 min |
| Placebo lozenge for BH/CPC experiments Placebo lozenge dissolved in 4 mL, 20 mL and 30 mL of hard water lozenge ingredients: eucalyptus oil, levomenthol, anhydrous citric acid (E330), sucralose (E955), isomalt (E953), brilliant blue FCF (E133) | 37 | 3.0 g/L bovine albumin solution and 3.0 mL/L erythrocytes | 1, 5 and 15 min |
| CPC as free active substance 1 mg of CPC dissolved in 4 mL, 20 mL and 30 mL of hard water | 37 | 3.0 g/L bovine albumin solution and 3.0 mL/L erythrocytes | 1, 5 and 15 min |
| BH/CPC as active free substances 3 mg of BH and 1 mg of CPC dissolved in 4 mL, 20 mL and 30 mL of hard-water | 37 | 3.0 g/L bovine albumin solution and 3.0 mL/L erythrocytes | 1, 5 and 15 min |
| Test Virus | Test Product and Concentration | Titre at the Test Start (T = 0) log10(c) (TCID50/mL) ± 95% CI | Titre at the Test End (T = 15) log10(c) (TCID50/mL) ± 95% CI | Titre Difference |
|---|---|---|---|---|
| SARS-CoV-2 strain 751/20 | Virucidal activity 0.25 mg/mL CPC and one BH/CPC lozenge/4 mL | 8.94 ± 0.89 | 8.57 ± 0.62 | <1 |
| Virucidal activity 0.05 mg/mL CPC and one BH/CPC lozenge /20 mL | 8.50 ± 0.68 | 8.31 ± 0.69 | <1 | |
| Virucidal activity 0.033 mg/mL CPC and one BH/CPC lozenge/30 mL | 9.76 ± 0.79 | 9.00 ± 0.80 | <1 | |
| Virucidal activity 0.75 mg/mL + 0.25 mg/mL BH/CPC and one placebo lozenge/4 mL | 8.99 ± 0.67 | 9.12 ± 0.71 | <1 | |
| Virucidal activity 0.15 mg/mL + 0.05 mg/mL BH/CPC and one placebo lozenge/20 mL | 9.68 ± 0.67 | 9.78 ± 0.77 | <1 | |
| Virucidal activity 0.099 mg/mL + 0.033 mg/mL BH/CPC and one placebo lozenge/30 mL | 9.95 ± 0.67 | 8.93 ± 0.65 | 1.02 |
| Test Virus | Test Product Concentration | Titre at the Test Start (T = 0) log10(c) (TCID50/mL) ± 95% CI | Titre at the Test End (T = 15) log10(c) (TCID50/mL) ± 95% CI | Titre Difference |
|---|---|---|---|---|
| SARS-CoV-2 strain 751/20 | 0.25 mg/mL CPC as free active substance | 9.76 ± 0.79 | 9.75 ± 0.63 | <1 |
| 1 BH/CPC lozenge/4 mL | 9.76 ± 0.79 | 9.38 ± 0.76 | <1 | |
| 0.75 mg/mL BH + 0.25 mg/mL CPC as free active substance | 9.95 ± 0.67 | 10.25 ± 0.74 | <1 | |
| 1 placebo lozenge/4 mL | 10.46 ± 0.75 | 10.13 ± 0.85 | <1 |
| CPC as Free Active Substance | CPC/BH as Free Active Substance | BH/CPC Lozenge | Placebo Lozenge | |
|---|---|---|---|---|
| High concentration (disolved in 4 mL) | ||||
| pH value | 7.61 | 7.80 | 2.85 | 2.99 |
| Time (min) | ∆log10(c) (TCID50/mL) ± 95% CI | |||
| 1 | −0.00 ± 1.06 | −1.57 ± 0.86 * | −4.32 ± 0.82 * | +0.25 ± 1.01 |
| 5 | −1.88 ± 0.96 * | −4.49 ± 0.67 * | −4.44 ± 0.75 * | −2.00 ± 0.92 * |
| 15 | −4.94 ± 0.75 * | −4.49 ± 0.67 * | −4.44 ± 0.75 * | −2.88 ± 0.83 * |
| Medium concentration (disolved in 20 mL) | ||||
| pH value | 7.66 | 7.73 | 4.2 | 4.45 |
| Time (min) | ∆log10(c) (TCID50/mL) ± 95% CI | |||
| 1 | −0.06 ± 0.99 | −1.96 ± 0.95 * | −1.69 ± 0.96 * | −0.81 ± 0.92 |
| 5 | −0.75 ± 0.98 | −3.30 ± 0.96 * | −3.56 ± 0.86 * | −1.06 ± 0.98 |
| 15 | −2.44 ± 0.79 * | −5.36 ± 0.77 * | −5.00 ± 0.68 * | −0.65 ± 1.05 |
| Low concentration (disolved in 30 mL) | ||||
| pH value | 7.67 | 7.80 | 4.64 | 5.25 |
| Time (min) | ∆log10(c) (TCID50/mL) ± 95% CI | |||
| 1 | −0.32 ± 1.08 | −0.89 ± 1.03 | −0.88 ± 1.11 | +0.13 ± 1.06 |
| 5 | −0.70 ± 1.04 | −1.58 ± 0.98 * | −1.19 ± 1.03 | −0.46 ± 0.98 |
| 15 | −1.69 ± 1.03 * | −2.44 ± 0.94 * | −3.01 ± 1.05 * | −1.46 ± 0.97 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steyer, A.; Marušić, M.; Kolenc, M.; Triglav, T. A Throat Lozenge with Fixed Combination of Cetylpyridinium Chloride and Benzydamine Hydrochloride Has Direct Virucidal Effect on SARS-CoV-2. COVID 2021, 1, 435-446. https://doi.org/10.3390/covid1020037
Steyer A, Marušić M, Kolenc M, Triglav T. A Throat Lozenge with Fixed Combination of Cetylpyridinium Chloride and Benzydamine Hydrochloride Has Direct Virucidal Effect on SARS-CoV-2. COVID. 2021; 1(2):435-446. https://doi.org/10.3390/covid1020037
Chicago/Turabian StyleSteyer, Andrej, Miša Marušić, Marko Kolenc, and Tina Triglav. 2021. "A Throat Lozenge with Fixed Combination of Cetylpyridinium Chloride and Benzydamine Hydrochloride Has Direct Virucidal Effect on SARS-CoV-2" COVID 1, no. 2: 435-446. https://doi.org/10.3390/covid1020037
APA StyleSteyer, A., Marušić, M., Kolenc, M., & Triglav, T. (2021). A Throat Lozenge with Fixed Combination of Cetylpyridinium Chloride and Benzydamine Hydrochloride Has Direct Virucidal Effect on SARS-CoV-2. COVID, 1(2), 435-446. https://doi.org/10.3390/covid1020037






