LiCoO2/Graphite Cells with Localized High Concentration Carbonate Electrolytes for Higher Energy Density
Abstract
:1. Introduction
2. Materials and Methods
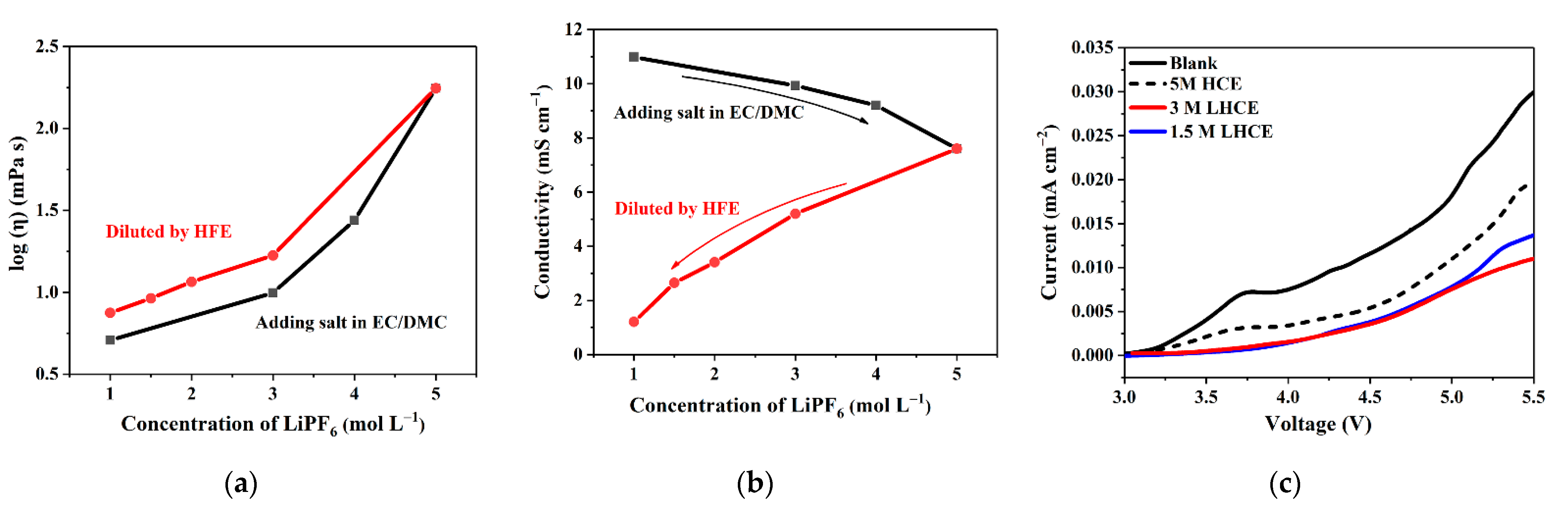
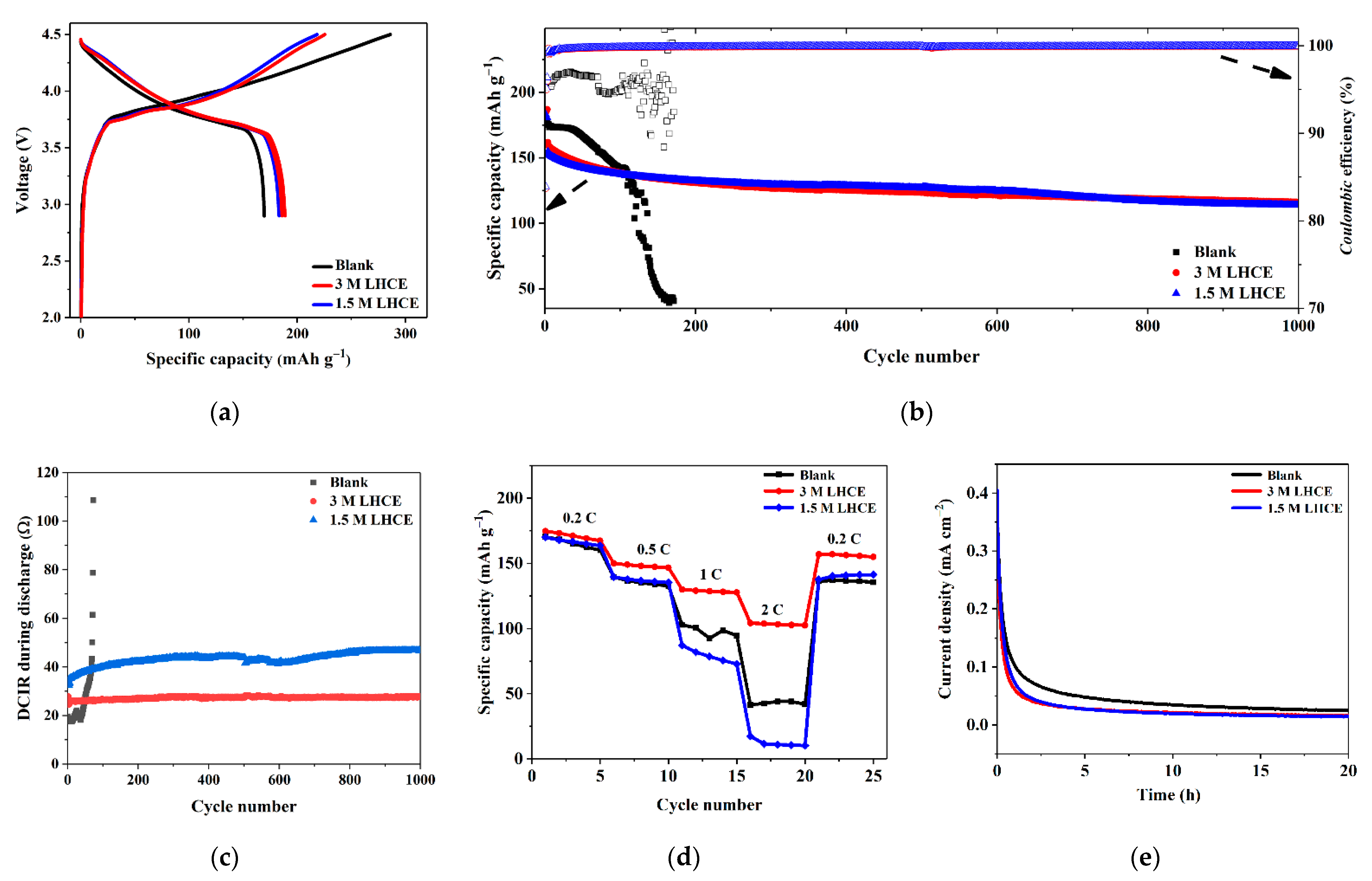
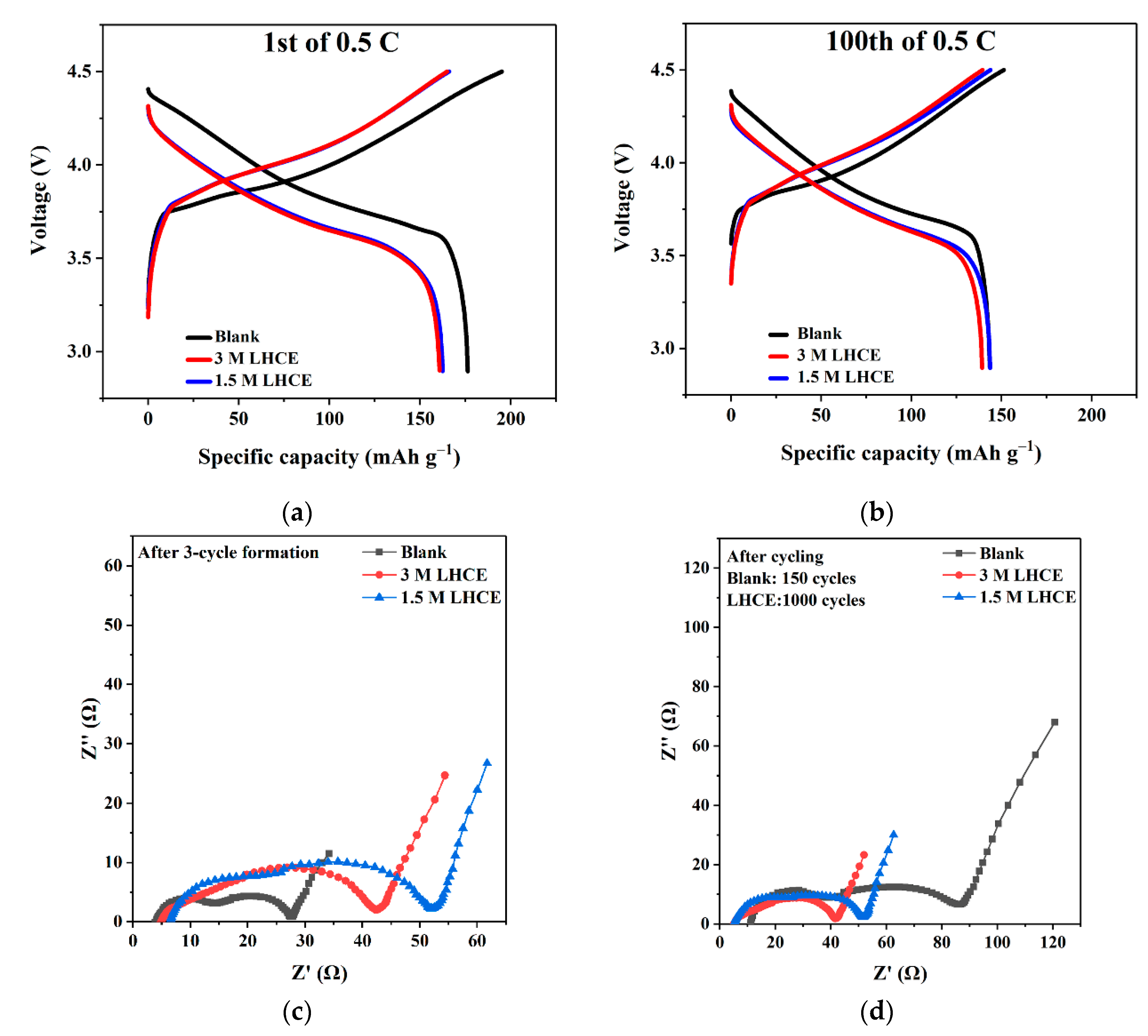
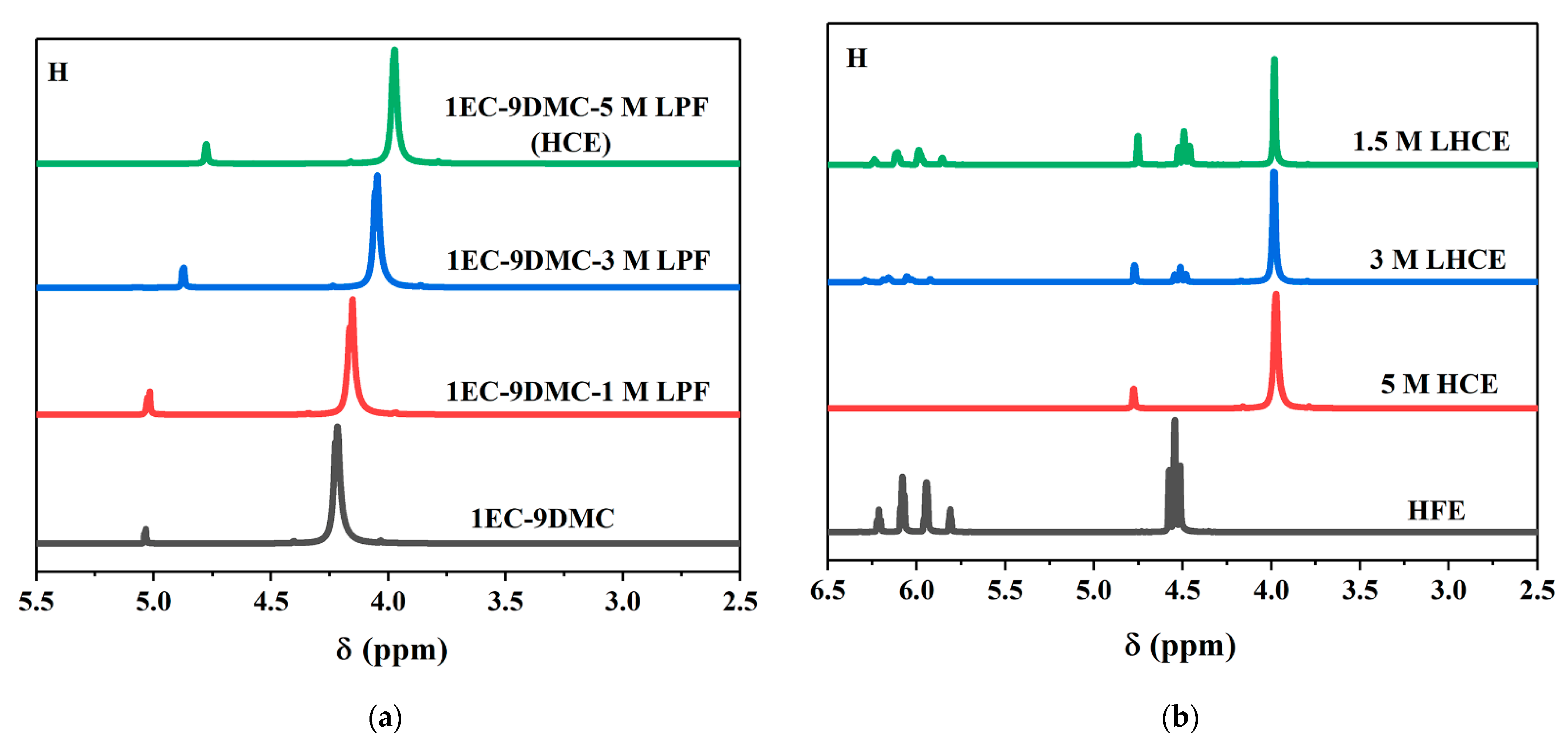
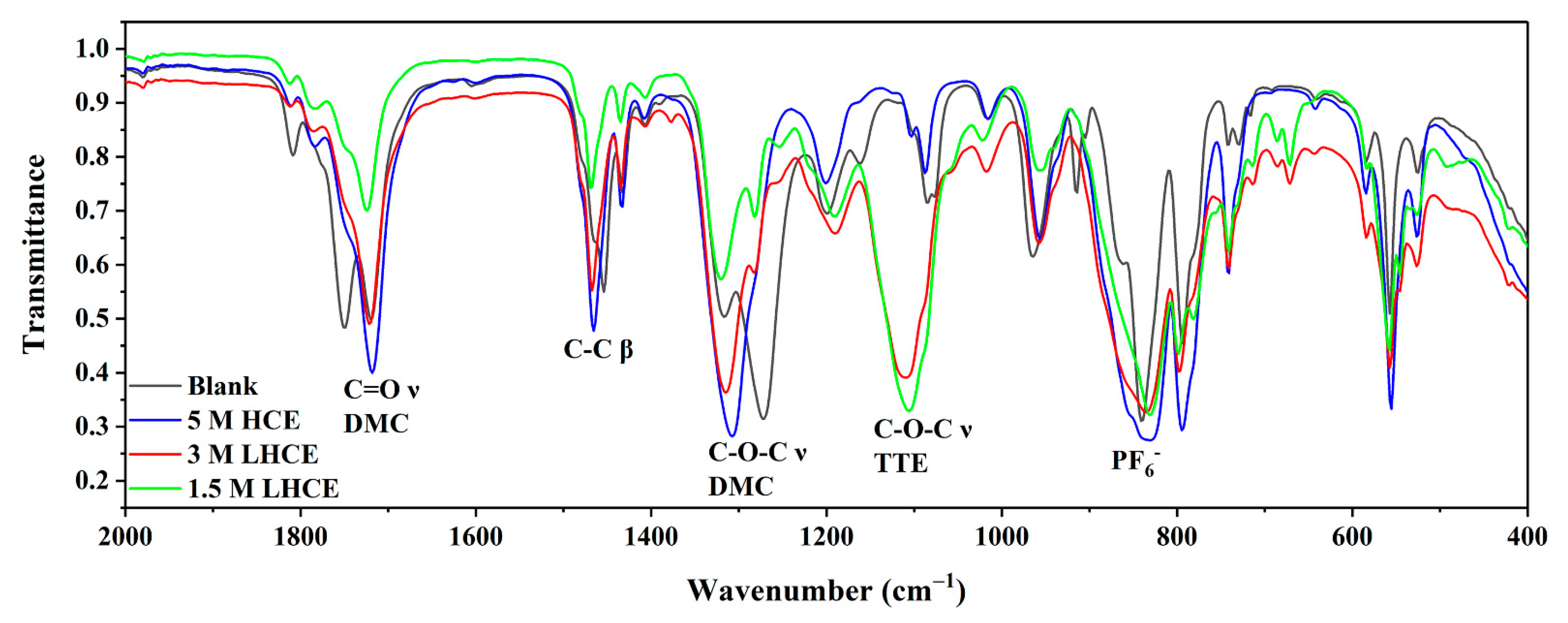
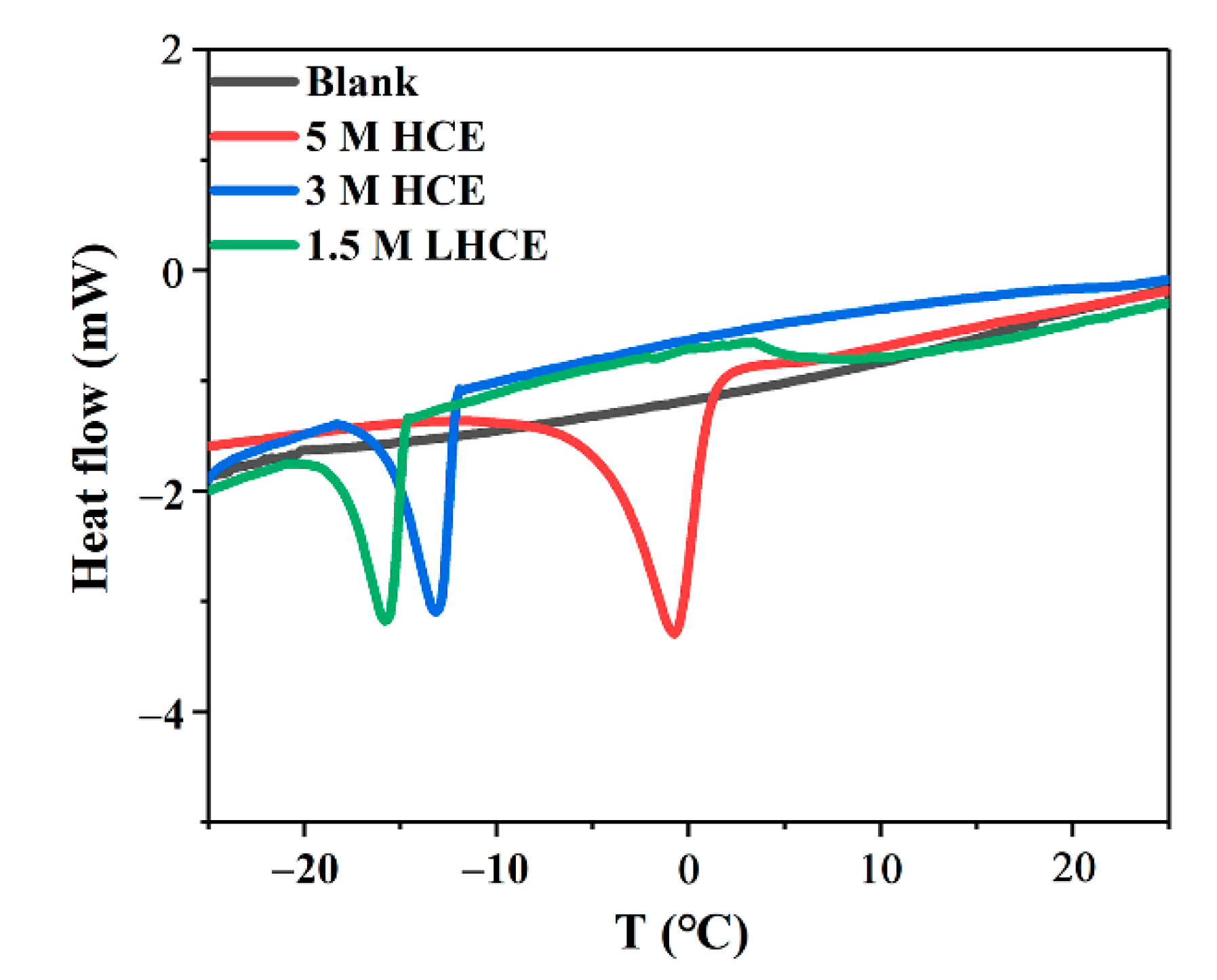
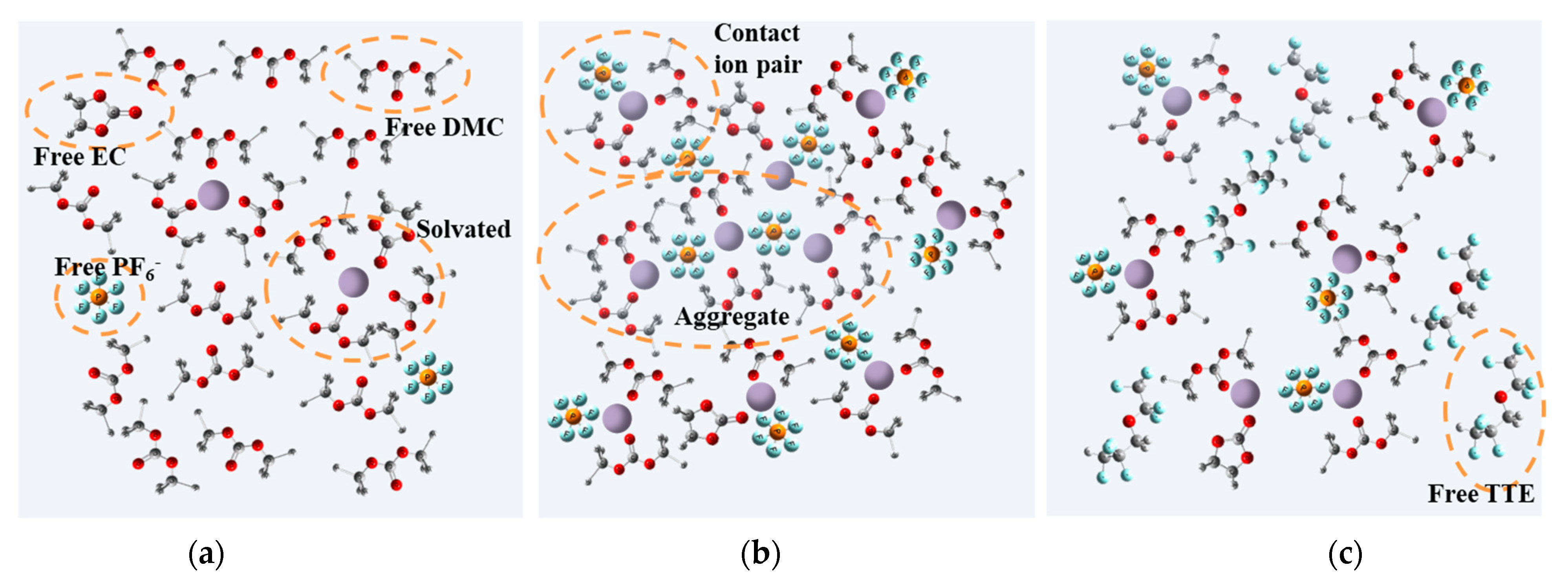
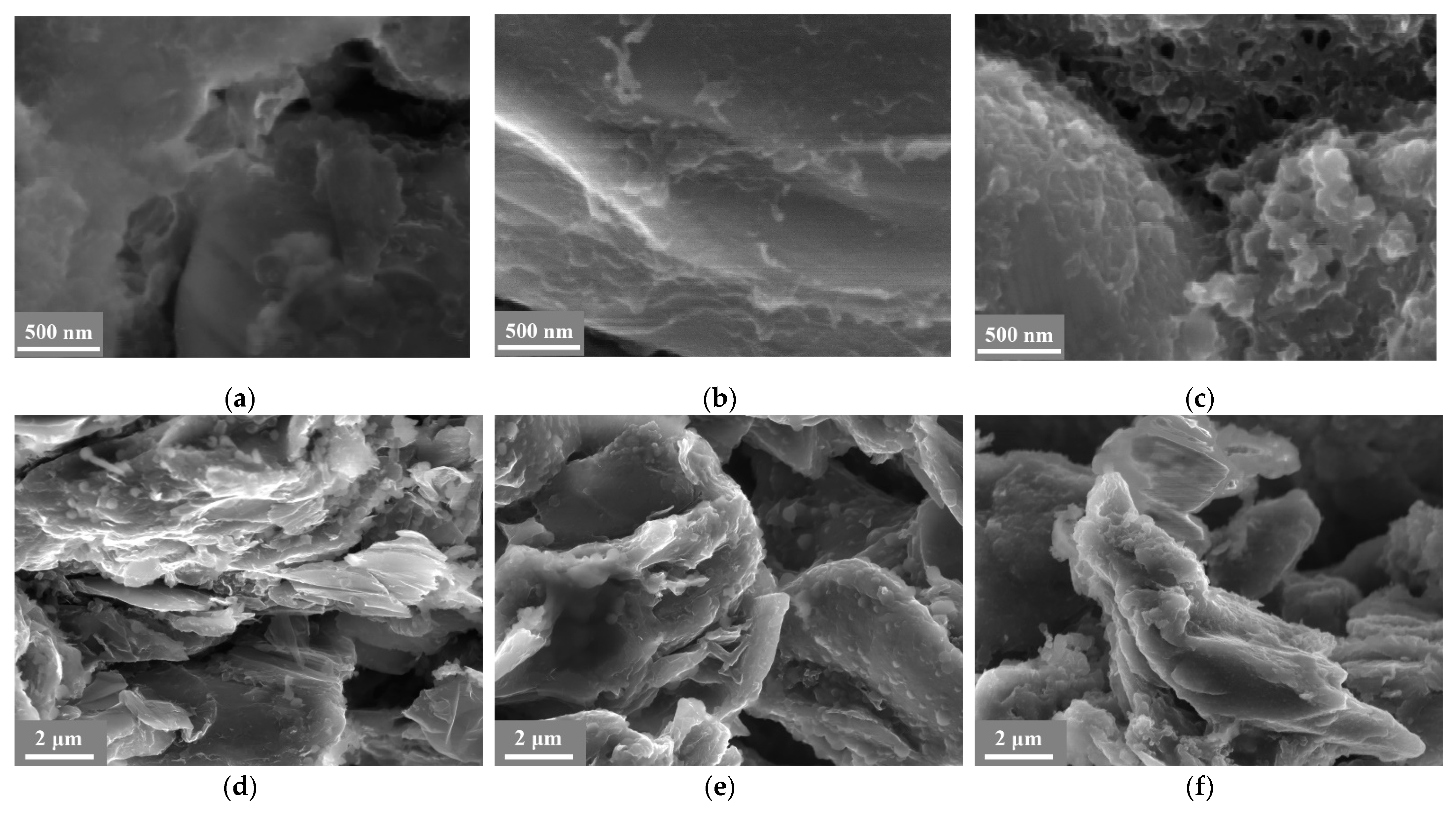
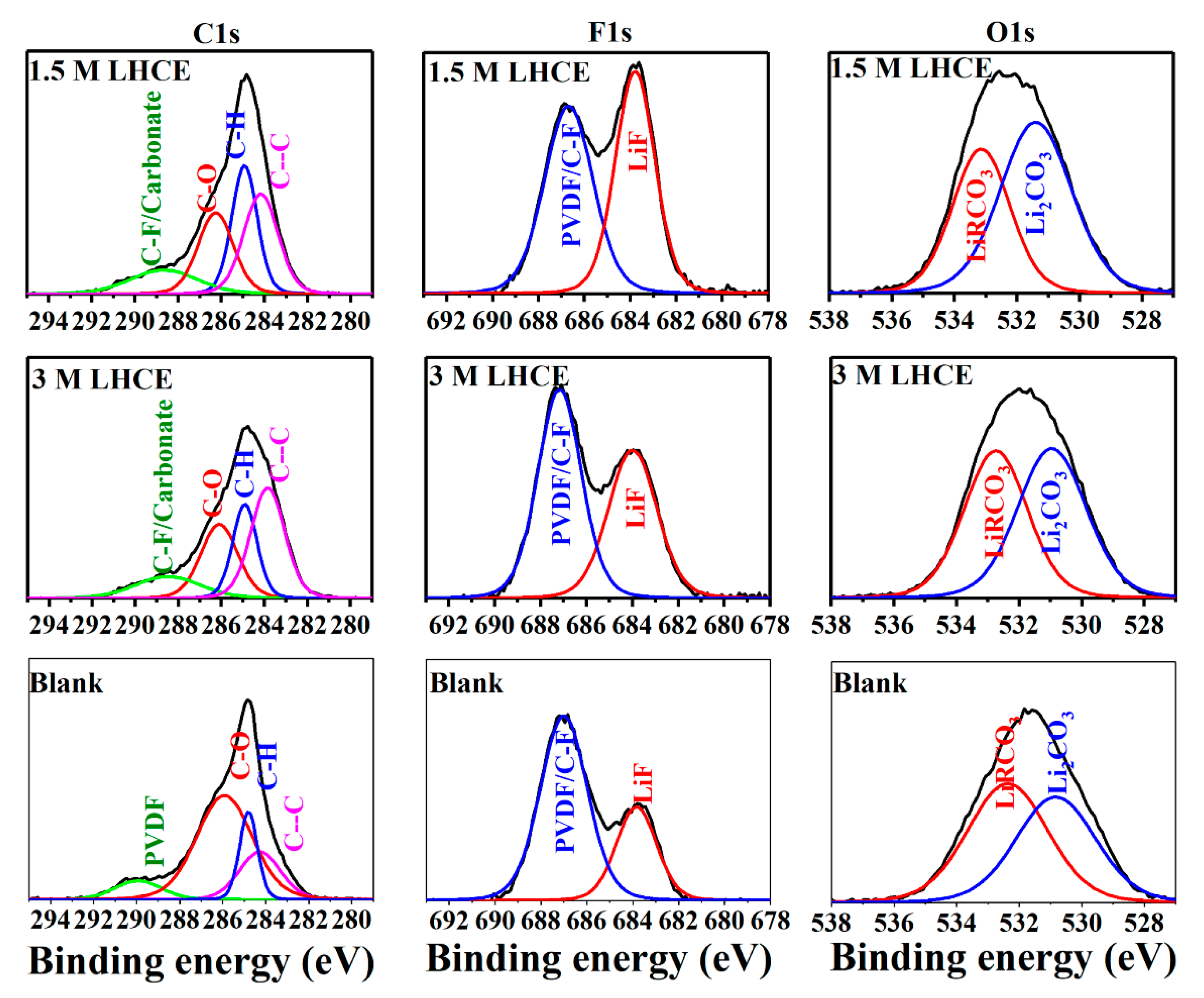
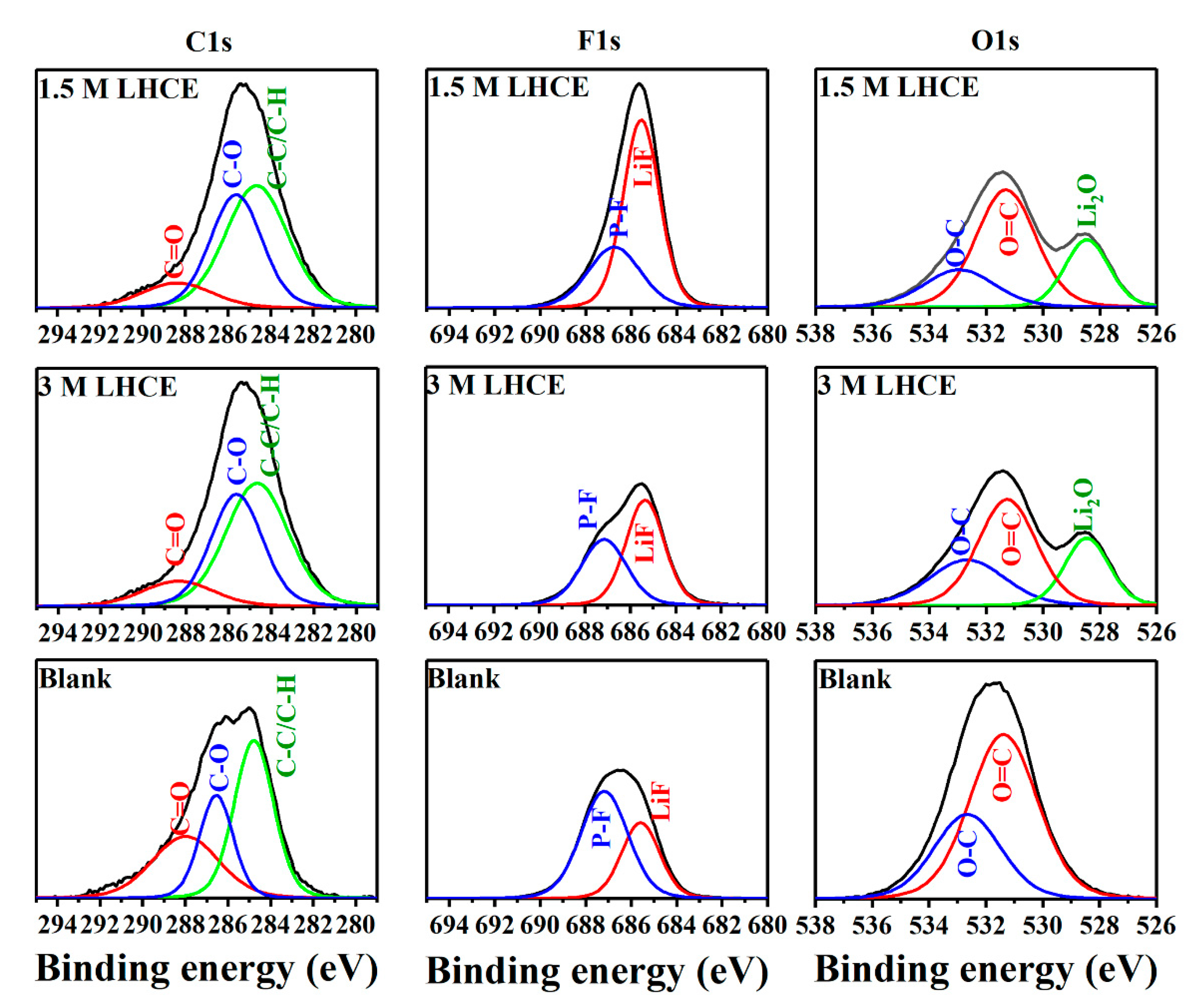
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Winter, M.; Barnett, B.; Xu, K. Before Li Ion Batteries. Chem. Rev. 2018, 118, 11433–11456. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, X.; He, X.; Senyshyn, A.; Wilken, A.; Zhou, D.; Fromm, O.; Niehoff, P.; Yan, B.; Li, J.; et al. Truncated Octahedral High-Voltage Spinel LiNi0.5Mn1.5O4 Cathode Materials for Lithium Ion Batteries: Positive Influences of Ni/Mn Disordering and Oxygen Vacancies. J. Electrochem. Soc. 2018, 165, A1886–A1896. [Google Scholar] [CrossRef]
- Liu, Y.; Hanai, K.; Yang, J.; Imanishi, N.; Hirano, A.; Takeda, Y. Silicon/carbon composites as anode materials for Li-ion batteries. Electrochem. Solid State Lett. 2004, 7, A369–A372. [Google Scholar] [CrossRef]
- Wong, D.P.; Tseng, H.-P.; Chen, Y.-T.; Hwang, B.-J.; Chen, L.-C.; Chen, K.-H. A stable silicon/graphene composite using solvent exchange method as anode material for lithium ion batteries. Carbon 2013, 63, 397–403. [Google Scholar] [CrossRef]
- Liu, Y.; Qin, L.; Liu, F.; Fan, Y.; Ruan, J.; Zhang, S. Interpenetrated 3D porous silicon as high stable anode material for Li-Ion battery. J. Power Sources 2018, 406, 167–175. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, M.; Yuan, Y.; Wu, Q.; Wang, H.; He, Y.; Lin, Z.; Zhou, F.; Ling, M.; Qian, C.; et al. Accommodation of Silicon in an Interconnected Copper Network for Robust Li-Ion Storage. Adv. Funct. Mater. 2020, 30, 1910249. [Google Scholar] [CrossRef]
- Zheng, G.; Wang, C.; Pei, A.; Lopez, J.; Shi, F.; Chen, Z.; Sendek, A.D.; Lee, H.-W.; Lu, Z.; Schneider, H.; et al. High-Performance Lithium Metal Negative Electrode with a Soft and Flowable Polymer Coating. ACS Energy Lett. 2016, 1, 1247–1255. [Google Scholar] [CrossRef]
- Han, B.; Feng, D.; Li, S.; Zhang, Z.; Zou, Y.; Gu, M.; Meng, H.; Wang, C.; Xu, K.; Zhao, Y.; et al. Self-Regulated Phenomenon of Inorganic Artificial Solid Electrolyte Interphase for Lithium Metal Batteries. Nano Lett. 2020, 20, 4029–4037. [Google Scholar] [CrossRef]
- Louli, A.J.; Eldesoky, A.; Weber, R.; Genovese, M.; Coon, M.; deGooyer, J.; Deng, Z.; White, R.T.; Lee, J.; Rodgers, T.; et al. Diagnosing and correcting anode-free cell failure via electrolyte and morphological analysis. Nat. Energy 2020, 5, 693–702. [Google Scholar]
- Nanda, S.; Gupta, A.; Manthiram, A. Anode-Free Full Cells: A Pathway to High-Energy Density Lithium-Metal Batteries. Adv. Energy Mater. 2021, 11, 2000804. [Google Scholar] [CrossRef]
- Zhao, H.; Qian, Y.; Hu, S.; Luo, G.; Nie, C.; Qiu, P.; Kang, Y.; Wang, H.; Chu, Y.; Wang, Q.; et al. Tale of Three Phosphate Additives for Stabilizing NCM811/Graphite Pouch Cells: Significance of Molecular Structure–Reactivity in Dictating Interphases and Cell Performance. ACS Appl. Mater. Interfaces 2021, 13, 29676–29690. [Google Scholar] [CrossRef]
- Yi, T.-F.; Mei, J.; Zhu, Y.-R. Key strategies for enhancing the cycling stability and rate capacity of LiNi0.5Mn1.5O4 as high-voltage cathode materials for high power lithium-ion batteries. J. Power Sources 2016, 316, 85–105. [Google Scholar] [CrossRef]
- Risthaus, T.; Wang, J.; Friesen, A.; Wilken, A.; Berghus, D.; Winter, M.; Li, J. Synthesis of spinel LiNi0.5Mn1.5O4 with secondary plate morphology as cathode material for lithium ion batteries. J. Power Sources 2015, 293, 137–142. [Google Scholar] [CrossRef]
- Wang, J.; He, X.; Kloepsch, R.; Wang, S.; Hoffmann, B.; Jeong, S.; Yang, Y.; Li, J. Increased Capacity of LiNi1/3Co1/3Mn1/3O2–Li[Li1/3Mn2/3]O2 Cathodes by MnOx-surface Modification for Lithium-Ion Batteries. Energy Technol. 2014, 2, 188–193. [Google Scholar] [CrossRef]
- Qiu, B.; Zhang, Q.; Hu, H.; Wang, J.; Liu, J.; Xia, Y.; Zeng, Y.; Wang, X.; Liu, Z. Electrochemical investigation of Li-excess layered oxide cathode materials/mesocarbon microbead in 18650 batteries. Electrochim. Acta 2014, 123, 317–324. [Google Scholar] [CrossRef]
- Ma, X.; Wang, M.; Qian, Y.; Feng, D.; Zhang, G.; Xu, D.; Kang, Y.; Liu, Z.; Hu, S.; Zheng, J.; et al. Poly (methyl vinyl ether-alt-maleic anhydride) as an ecofriendly electrolyte additive for high-voltage lithium-rich oxides with improved stability of interphase. Electrochim. Acta 2021, 400, 139467. [Google Scholar] [CrossRef]
- Di Lecce, D.; Gancitano, V.; Hassoun, J. Investigation of Mn and Fe Substitution Effects on the Characteristics of High-Voltage LiCo1–xMxPO4 (x = 0.1, 0.4) Cathodes Prepared by Sol–gel Route. ACS Sustain. Chem. Eng. 2020, 8, 278–289. [Google Scholar] [CrossRef]
- Luong, H.D.; Dinh, V.A.; Momida, H.; Oguchi, T. Tavorite-like orthorhombic AxVPO4F (A = Li, Na) for novel high-voltage cathodes in rechargeable batteries. J. Alloys Compd. 2021, 875, 159963. [Google Scholar] [CrossRef]
- Pagot, G.; Bertasi, F.; Nawn, G.; Negro, E.; Carraro, G.; Barreca, D.; Maccato, C.; Polizzi, S.; Di Noto, V. High-Performance Olivine for Lithium Batteries: Effects of Ni/Co Doping on the Properties of LiFeαNiβCoγPO4 Cathodes. Adv. Funct. Mater. 2015, 25, 4032–4037. [Google Scholar] [CrossRef]
- Pagot, G.; Bandiera, M.; Vezzù, K.; Migliori, A.; Bertoncello, R.; Negro, E.; Morandi, V.; Di Noto, V. High valence transition metal-doped olivine cathodes for superior energy and fast cycling lithium batteries. J. Mater. Chem. A 2020, 8, 25727–25738. [Google Scholar] [CrossRef]
- Xu, K. Nonaqueous liquid electrolytes for lithium-based rechargeable batteries. Chem. Rev. 2004, 104, 4303–4417. [Google Scholar] [CrossRef]
- Xu, K. Electrolytes and Interphases in Li-Ion Batteries and Beyond. Chem. Rev. 2014, 114, 11503–11618. [Google Scholar] [CrossRef]
- Zheng, X.; Huang, T.; Pan, Y.; Wang, W.; Fang, G.; Wu, M. High-voltage performance of LiNi1/3Co1/3Mn1/3O2/graphite batteries with di(methylsulfonyl) methane as a new sulfone-based electrolyte additive. J. Power Sources 2015, 293, 196–202. [Google Scholar] [CrossRef]
- Lan, J.; Zheng, Q.; Zhou, H.; Li, J.; Xing, L.; Xu, K.; Fan, W.; Yu, L.; Li, W. Stabilizing a High-Voltage Lithium-Rich Layered Oxide Cathode with a Novel Electrolyte Additive. ACS Appl. Mater. Interfaces 2019, 11, 28841–28850. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.J.; Soon, J.; Chae, S.; Ryu, J.H.; Oh, S.M. A Bifunctional Electrolyte Additive for High-Voltage LiNi0.5Mn1.5O4 Positive Electrodes. ACS Appl. Mater. Interfaces 2019, 11, 11306–11316. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Wang, J.; Du, L.; Liu, Z.; Zou, X.; Tang, X.; Cao, Z.; Wang, C.; Xiong, D.; Shi, Q.; et al. Overcharge Investigations of LiCoO2/Graphite Lithium Ion Batteries with Different Electrolytes. ACS Appl. Energy Mater. 2019, 2, 8615–8624. [Google Scholar] [CrossRef]
- Brutti, S.; Simonetti, E.; De Francesco, M.; Sarra, A.; Paolone, A.; Palumbo, O.; Fantini, S.; Lin, R.; Falgayrat, A.; Choi, H.; et al. Ionic liquid electrolytes for high-voltage, lithium-ion batteries. J. Power Sources 2020, 479, 228791. [Google Scholar] [CrossRef]
- Hu, Z.; Xian, F.; Guo, Z.; Lu, C.; Du, X.; Cheng, X.; Zhang, S.; Dong, S.; Cui, G.; Chen, L. Nonflammable Nitrile Deep Eutectic Electrolyte Enables High-Voltage Lithium Metal Batteries. Chem. Mater. 2020, 32, 3405–3413. [Google Scholar] [CrossRef]
- Doi, T.; Shimizu, Y.; Hashinokuchi, M.; Inaba, M. Dilution of Highly Concentrated LiBF4/Propylene Carbonate Electrolyte Solution with Fluoroalkyl Ethers for 5-V LiNi0.5Mn1.5O4 Positive Electrodes. J. Electrochem. Soc. 2017, 164, A6412–A6416. [Google Scholar] [CrossRef]
- Liu, Q.; Xu, H.; Wu, F.; Mu, D.; Shi, L.; Wang, L.; Bi, J.; Wu, B. Effects of a High-Concentration LiPF6-Based Carbonate Ester Electrolyte for the Electrochemical Performance of a High-Voltage Layered LiNi0.6Co0.2Mn0.2O2 Cathode. ACS Appl. Energy Mater. 2019, 2, 8878–8884. [Google Scholar] [CrossRef]
- McOwen, D.W.; Seo, D.M.; Borodin, O.; Vatamanu, J.; Boyle, P.D.; Henderson, W.A. Concentrated electrolytes: Decrypting electrolyte properties and reassessing Al corrosion mechanisms. Energy Environ. Sci. 2014, 7, 416–426. [Google Scholar] [CrossRef]
- Xia, L.; Yu, L.; Hu, D.; Chen, Z.G. Research Progress and Perspectives on High Voltage, Flame Retardant Electrolytes for Lithium-Ion Batteries. Acta Chim. Sin. 2017, 75, 1183–1195. [Google Scholar] [CrossRef]
- Zheng, J.; Chen, S.; Zhao, W.; Song, J.; Engelhard, M.H.; Zhang, J.-G. Extremely Stable Sodium Metal Batteries Enabled by Localized High-Concentration Electrolytes. ACS Energy Lett. 2018, 3, 315–321. [Google Scholar] [CrossRef]
- Chen, S.; Zheng, J.; Yu, L.; Ren, X.; Engelhard, M.H.; Niu, C.; Lee, H.; Xu, W.; Xiao, J.; Liu, J.; et al. High-Efficiency Lithium Metal Batteries with Fire-Retardant Electrolytes. Joule 2018, 2, 1548–1558. [Google Scholar] [CrossRef] [Green Version]
- Jie, Y.; Ren, X.; Cao, R.; Cai, W.; Jiao, S. Advanced Liquid Electrolytes for Rechargeable Li Metal Batteries. Adv. Funct. Mater. 2020, 30, 1910777. [Google Scholar] [CrossRef]
- Ren, X.; Zou, L.; Cao, X.; Engelhard, M.H.; Liu, W.; Burton, S.D.; Lee, H.; Niu, C.; Matthews, B.E.; Zhu, Z.; et al. Enabling High-Voltage Lithium-Metal Batteries under Practical Conditions. Joule 2019, 3, 1662–1676. [Google Scholar] [CrossRef]
- Ma, G.; Wang, L.; He, X.; Zhang, J.; Chen, H.; Xu, W.; Ding, Y. Pseudoconcentrated Electrolyte with High Ionic Conductivity and Stability Enables High-Voltage Lithium-Ion Battery Chemistry. ACS Appl. Energy Mater. 2018, 1, 5446–5452. [Google Scholar] [CrossRef]
- Chen, S.; Zheng, J.; Mei, D.; Han, K.S.; Engelhard, M.H.; Zhao, W.; Xu, W.; Liu, J.; Zhang, J.-G. High-Voltage Lithium-Metal Batteries Enabled by Localized High-Concentration Electrolytes. Adv. Mater. 2018, 30, 1706102. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Soto, F.A.; Ponce, V.; Seminario, J.M.; Cao, X.; Zhang, J.-G.; Balbuena, P.B. Localized high concentration electrolyte behavior near a lithium–metal anode surface. J. Mater. Chem. A 2019, 7, 25047–25055. [Google Scholar] [CrossRef]
- Cao, X.; Jia, H.; Xu, W.; Zhang, J.-G. Review—Localized High-Concentration Electrolytes for Lithium Batteries. J. Electrochem. Soc. 2021, 168, 010522. [Google Scholar] [CrossRef]
- Dai, W.; Dong, N.; Xia, Y.; Chen, S.; Luo, H.; Liu, Y.; Liu, Z. Localized concentrated high-concentration electrolyte enhanced stability and safety for high voltage Li-ion batteries. Electrochim. Acta 2019, 320, 134633. [Google Scholar] [CrossRef]
- Piao, N.; Ji, X.; Xu, H.; Fan, X.; Chen, L.; Liu, S.; Garaga, M.N.; Greenbaum, S.G.; Wang, L.; Wang, C.; et al. Countersolvent Electrolytes for Lithium-Metal Batteries. Adv. Energy Mater. 2020, 10, 1903568. [Google Scholar] [CrossRef]
- Su, C.C.; He, M.; Amine, R.; Amine, K. A Selection Rule for Hydrofluoroether Electrolyte Cosolvent: Establishing a Linear Free-Energy Relationship in Lithium-Sulfur Batteries. Angew. Chem. Int. Ed. 2019, 58, 10591–10595. [Google Scholar] [CrossRef]
- Ren, X.D.; Chen, S.R.; Lee, H.; Mei, D.H.; Engelhard, M.H.; Burton, S.D.; Zhao, W.G.; Zheng, J.M.; Li, Q.Y.; Ding, M.S.; et al. Localized High-Concentration Sulfone Electrolytes for High-Efficiency Lithium-Metal Batteries. Chem 2018, 4, 1877–1892. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.; Feng, D.; Xiao, Y.; Qian, Y.; Wang, Q.; Kang, Y.; Xu, D.; Zhao, H.; Xu, H.; Yi, H.; et al. Generating lithium fluoride-abundant interphase on layered lithium-rich oxide cathode with lithium 1,1,2,2,3,3-hexafluoropropane-1,3-disulfonimide. J. Power Sources 2021, 507, 230278. [Google Scholar] [CrossRef]
- Yamada, Y.; Chiang, C.H.; Sodeyama, K.; Wang, J.H.; Tateyama, Y.; Yamada, A. Corrosion Prevention Mechanism of Aluminum Metal in Superconcentrated Electrolytes. ChemElectroChem 2015, 2, 1687–1694. [Google Scholar] [CrossRef]
- Tan, S.; Zhang, Z.; Li, Y.; Li, Y.; Zheng, J.; Zhou, Z.; Yang, Y. Tris(hexafluoro-iso-propyl)phosphate as an SEI-Forming Additive on Improving the Electrochemical Performance of the Li[Li0.2Mn0.56Ni0.16Co0.08]O2Cathode Material. J. Electrochem. Soc. 2012, 160, A285–A292. [Google Scholar] [CrossRef]
- Pham, H.Q.; Nam, K.-M.; Hwang, E.-H.; Kwon, Y.-G.; Jung, H.M.; Song, S.-W. Performance Enhancement of 4.8 V Li1.2Mn0.525Ni0.175Co0.1O2Battery Cathode Using Fluorinated Linear Carbonate as a High-Voltage Additive. J. Electrochem. Soc. 2014, 161, A2002–A2011. [Google Scholar] [CrossRef]
- Li, J.; Xing, L.; Zhang, R.; Chen, M.; Wang, Z.; Xu, M.; Li, W. Tris(trimethylsilyl)borate as an electrolyte additive for improving interfacial stability of high voltage layered lithium-rich oxide cathode/carbonate-based electrolyte. J. Power Sources 2015, 285, 360–366. [Google Scholar] [CrossRef]
- Hong, P.; Xu, M.; Zheng, X.; Zhu, Y.; Liao, Y.; Xing, L.; Huang, Q.; Wan, H.; Yang, Y.; Li, W. Effect of ethylene glycol bis (propionitrile) ether (EGBE) on the performance and interfacial chemistry of lithium-rich layered oxide cathode. J. Power Sources 2016, 329, 216–224. [Google Scholar] [CrossRef]
- Wang, S.; Dai, A.; Cao, Y.; Yang, H.; Khalil, A.; Lu, J.; Li, H.; Ai, X. Enabling stable and high-rate cycling of a Ni-rich layered oxide cathode for lithium-ion batteries by modification with an artificial Li+-conducting cathode-electrolyte interphase. J. Mater. Chem. A 2021, 9, 11623–11631. [Google Scholar] [CrossRef]
- Xiao, Y.; Han, B.; Zeng, Y.; Chi, S.-S.; Zeng, X.; Zheng, Z.; Xu, K.; Deng, Y. New Lithium Salt Forms Interphases Suppressing Both Li Dendrite and Polysulfide Shuttling. Adv. Energy Mater. 2020, 10, 1903937. [Google Scholar] [CrossRef]
- Qian, Y.; Hu, S.; Zou, X.; Deng, Z.; Xu, Y.; Cao, Z.; Kang, Y.; Deng, Y.; Shi, Q.; Xu, K.; et al. How electrolyte additives work in Li-ion batteries. Energy Storage Mater. 2019, 20, 208–215. [Google Scholar] [CrossRef]
- Niehoff, P.; Passerini, S.; Winter, M. Interface Investigations of a Commercial Lithium Ion Battery Graphite Anode Material by Sputter Depth Profile X-ray Photoelectron Spectroscopy. Langmuir 2013, 29, 5806–5816. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Zou, L.; Gao, P.; Cao, X.; Zhao, W.; He, Y.; Engelhard, M.H.; Burton, S.D.; Wang, H.; Ren, X.; et al. High-Performance Silicon Anodes Enabled by Nonflammable Localized High-Concentration Electrolytes. Adv. Energy Mater. 2019, 9, 1900784. [Google Scholar] [CrossRef]
- Han, B.; Zou, Y.; Xu, G.; Hu, S.; Kang, Y.; Qian, Y.; Wu, J.; Ma, X.; Yao, J.; Li, T.; et al. Additive stabilization of SEI on graphite observed using cryo-electron microscopy. Energy Environ. Sci. 2021, 14, 4882–4889. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, X.; Zhang, P.; Zhao, H.; Wang, Q.; Zhang, G.; Chi, S.-S.; Liu, Z.; Qian, Y.; Wang, J.; Wang, C.; et al. LiCoO2/Graphite Cells with Localized High Concentration Carbonate Electrolytes for Higher Energy Density. Liquids 2021, 1, 60-74. https://doi.org/10.3390/liquids1010005
Ma X, Zhang P, Zhao H, Wang Q, Zhang G, Chi S-S, Liu Z, Qian Y, Wang J, Wang C, et al. LiCoO2/Graphite Cells with Localized High Concentration Carbonate Electrolytes for Higher Energy Density. Liquids. 2021; 1(1):60-74. https://doi.org/10.3390/liquids1010005
Chicago/Turabian StyleMa, Xin, Peng Zhang, Huajun Zhao, Qingrong Wang, Guangzhao Zhang, Shang-Sen Chi, Zhongbo Liu, Yunxian Qian, Jun Wang, Chaoyang Wang, and et al. 2021. "LiCoO2/Graphite Cells with Localized High Concentration Carbonate Electrolytes for Higher Energy Density" Liquids 1, no. 1: 60-74. https://doi.org/10.3390/liquids1010005
APA StyleMa, X., Zhang, P., Zhao, H., Wang, Q., Zhang, G., Chi, S.-S., Liu, Z., Qian, Y., Wang, J., Wang, C., & Deng, Y. (2021). LiCoO2/Graphite Cells with Localized High Concentration Carbonate Electrolytes for Higher Energy Density. Liquids, 1(1), 60-74. https://doi.org/10.3390/liquids1010005





