Antimicrobial Resistance in Petting Zoo Animals in the United Kingdom
Abstract
1. Introduction
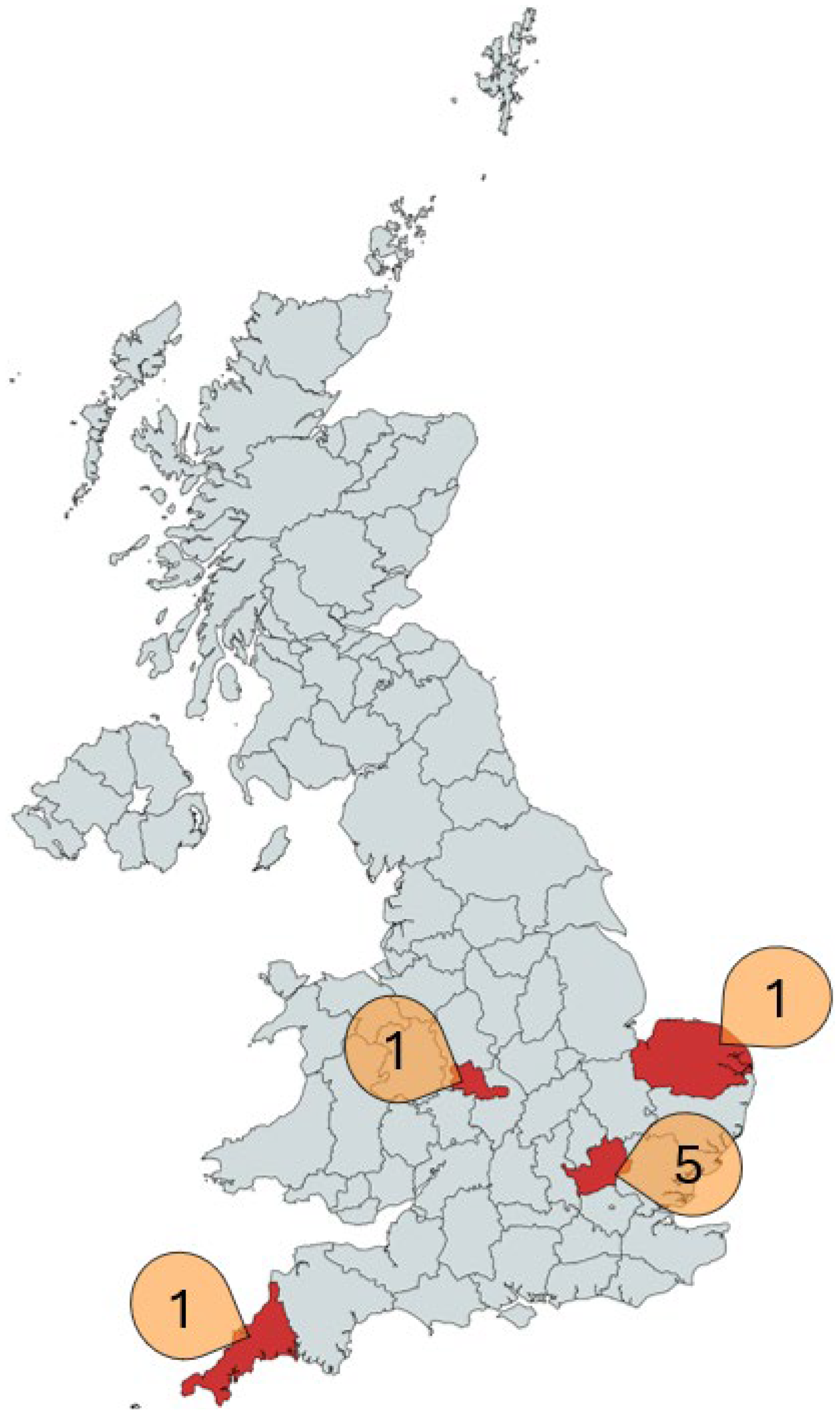
2. Materials and Methods
3. Results
3.1. Antimicrobial Resistance from Non-MDR-Selective Culture
3.2. ESBL-Producing E. coli, MRSA and MRSP
3.3. Effect of Centre and Animal Species on AMR and MDR in E. coli
3.4. Effect of Antimicrobial Use on AMR and MDR Prevalence in E. coli
4. Discussion
4.1. MDR E. coli: Prevalence, Diversity and Implications
4.2. Patterns of AMU and Resistance
4.3. Global Differences in Prevalence of ESBL-Producing E. coli
4.4. CoPS AMR and Recovery
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AmpC | Ampicillinase C |
| AMR | Antimicrobial resistance |
| AMU | Antimicrobial use |
| AST | Antimicrobial susceptibility testing |
| BIAZA | British and Irish Association of Zoos and Aquariums |
| CIA | Critically important antimicrobial |
| CLSI | Clinical and Laboratory Standards Institute |
| CoNS | Coagulase-negative staphylococci |
| CoPS | Coagulase-positive staphylococci |
| DNA | Deoxyribonucleic acid |
| ESBL | Extended-spectrum beta-lactamase |
| MALDI-TOF | Matrix assisted laser desorption/ionisation time-of-flight |
| MDR | Multidrug-resistant |
| MRSA | Methicillin-resistant Staphylococcus aureus |
| MRSP | Methicillin-resistant Staphylococcus pseudintermedius |
| MSA | Mannitol salt agar |
| MSA+ | Mannitol salt agar supplemented with 6 mg/L oxacillin |
| PCR | Polymerase chain reaction |
| PW | Peptone water |
| SIG | Staphylococcus intermedius group |
| TBX | Tryptone bile X-glucuronide agar |
| TMPS | Trimethoprim sulfamethoxazole |
| TSB+ | Tryptic soy broth supplemented with 10% Sodium Chloride |
| UK | United Kingdom |
References
- Velazquez-Meza, M.E.; Galarde-López, M.; Carrillo-Quiróz, B.; Alpuche-Aranda, C.M. Antimicrobial resistance: One Health approach. Vet. World 2022, 15, 743–749. [Google Scholar] [PubMed]
- De Witte, C.; Vereecke, N.; Theuns, S.; De Ruyck, C.; Vercammen, F.; Bouts, T.; Boyen, F.; Nauwynck, H.; Haesebrouck, F. Presence of Broad-Spectrum Beta-Lactamase-Producing Enterobacteriaceae in Zoo Mammals. Microorganisms 2021, 9, 834. [Google Scholar] [PubMed]
- Weese, J.S. Methicillin-resistant Staphylococcus aureus in animals. ILAR J. 2010, 51, 233–244. [Google Scholar] [CrossRef]
- Köck, R.; Herr, C.; Kreienbrock, L.; Schwarz, S.; Tenhagen, B.A.; Walther, B. Multiresistant Gram-Negative Pathogens—A Zoonotic Problem. Dtsch. Arztebl. Int. 2021, 118, 579–589. [Google Scholar]
- Bassiouny, M.; Neubauer, H.; Sprague, L.D. Gram-positive ESKAPE pathogens in Germany: A comprehensive analysis of occurrence and resistance development in animal, food, and environmental sources. One Health 2025, 20, 101099. [Google Scholar] [CrossRef]
- CDC. Antibiotic Resistance Threats in the United States, 2019; U.S. Department of Health and Human Services: Atlanta, GA, USA, 2019.
- Zogg, A.L.; Simmen, S.; Zurfluh, K.; Stephan, R.; Schmitt, S.N.; Nüesch-Inderbinen, M. High Prevalence of Extended-Spectrum β-Lactamase Producing Enterobacteriaceae Among Clinical Isolates from Cats and Dogs Admitted to a Veterinary Hospital in Switzerland. Front. Vet. Sci. 2018, 5, 62. [Google Scholar]
- Ortiz-Díez, G.; Mengíbar, R.L.; Turrientes, M.-C.; Artigao, M.-R.B.; Gallifa, R.L.; Tello, A.M.; Pérez, C.F.; Santiago, T.A. Prevalence, incidence and risk factors for acquisition and colonization of extended-spectrum beta-lactamase- and carbapenemase-producing Enterobacteriaceae from dogs attended at a veterinary hospital in Spain. Comp. Immunol. Microbiol. Infect. Dis. 2023, 92, 101922. [Google Scholar] [CrossRef]
- Mandujano-Hernández, A.; Martínez-Vázquez, A.V.; Paz-González, A.D.; Herrera-Mayorga, V.; Sánchez-Sánchez, M.; Lara-Ramírez, E.E.; Vázquez, K.; De Jesús De Luna-Santillana, E.; Bocanegra-García, V.; Rivera, G. The Global Rise of ESBL-Producing Escherichia coli in the Livestock Sector: A Five-Year Overview. Animals 2024, 14, 2490. [Google Scholar] [CrossRef]
- Rawat, D.; Nair, D. Extended-spectrum β-lactamases in Gram Negative Bacteria. J. Glob. Infect. Dis. 2010, 2, 263–274. [Google Scholar]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Gekenidis, M.-T.; Kläui, A.; Smalla, K.; Drissner, D. Transferable Extended-Spectrum β-Lactamase (ESBL) Plasmids in Enterobacteriaceae from Irrigation Water. Microorganisms 2020, 8, 978. [Google Scholar] [CrossRef]
- Carattoli, A. Plasmids and the spread of resistance. Int. J. Med. Microbiol. 2013, 303, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Conrad, C.C.; Stanford, K.; Narvaez-Bravo, C.; Callaway, T.; Mcallister, T. Farm Fairs and Petting Zoos: A Review of Animal Contact as a Source of Zoonotic Enteric Disease. Foodborne Pathog. Dis. 2016, 14, 59–73. [Google Scholar] [CrossRef] [PubMed]
- Wise, J. Outbreak of E. coli O157 is linked to Surrey open farm. BMJ 2009, 339, b3795. [Google Scholar] [CrossRef]
- Ljungquist, O.; Ljungquist, D.; Myrenås, M.; Rydén, C.; Finn, M.; Bengtsson, B. Evidence of household transfer of ESBL-/pAmpC-producing Enterobacteriaceae between humans and dogs—A pilot study. Infect. Ecol. Epidemiol. 2016, 6, 31514. [Google Scholar] [CrossRef]
- Van Hoek, A.; Dierikx, C.; Bosch, T.; Schouls, L.; Van Duijkeren, E.; Visser, M. Transmission of ESBL-producing Escherichia coli between broilers and humans on broiler farms. J. Antimicrob. Chemother. 2020, 75, 543–549. [Google Scholar] [CrossRef]
- Weese, J.S.; Mccarthy, L.; Mossop, M.; Martin, H.; Lefebvre, S. Observation of Practices at Petting Zoos and the Potential Impact on Zoonotic Disease Transmission. Clin. Infect. Dis. 2007, 45, 10–15. [Google Scholar] [CrossRef]
- Werden, K.E.B.; Paul, C. Compliance with Hygiene Recommendations for Human-animal Contact at Petting Zoos. Mich. J. Public Health 2008, 2, 19. [Google Scholar]
- Anderson, M.E.C.; Weese, J.S. Video observation of hand hygiene practices at a petting zoo and the impact of hand hygiene interventions. Epidemiol. Infect. 2012, 140, 182–190. [Google Scholar]
- Ihekweazu, C.; Carroll, K.; Adak, B.; Smith, G.; Pritchard, G.C.; Gillespie, I.A.; Verlander, N.Q.; Harvey-Vince, L.; Reacher, M.; Edeghere, O.; et al. Large outbreak of verocytotoxin-producing Escherichia coli O157 infection in visitors to a petting farm in South East England, 2009. Epidemiol. Infect. 2012, 140, 1400–1413. [Google Scholar] [CrossRef]
- Rowell, S.; King, C.; Jenkins, C.; Dallman, T.J.; Decraene, V.; Lamden, K.; Howard, A.; Featherstone, C.A.; Cleary, P. An outbreak of Shiga toxin-producing Escherichia coli serogroup O157 linked to a lamb-feeding event. Epidemiol. Infect. 2016, 144, 2494–2500. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.M.; Motoi, Y.; Sato, M.; Maruyama, A.; Watanabe, H.; Fukumoto, Y.; Shimamoto, T. Zoo animals as reservoirs of gram-negative bacteria harboring integrons and antimicrobial resistance genes. Appl. Environ. Microbiol. 2007, 73, 6686–6690. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, K.; Hosokawa, Y.; Makita, K.; Noda, J.; Ueno, H.; Muramatsu, Y.; Mukai, T.; Yamamoto, H.; Ito, M.; Tamura, Y. Factors associated with antimicrobial-resistant Escherichia coli in zoo animals. Res. Vet. Sci. 2012, 93, 574–580. [Google Scholar] [CrossRef]
- Wang, Y.; He, T.; Han, J.; Wang, J.; Foley, S.L.; Yang, G.; Wan, S.; Shen, J.; Wu, C. Prevalence of ESBLs and PMQR genes in fecal Escherichia coli isolated from the non-human primates in six zoos in China. Vet. Microbiol. 2012, 159, 53–59. [Google Scholar] [CrossRef]
- Dobiasova, H.; Dolejska, M.; Jamborova, I.; Brhelova, E.; Blazkova, L.; Papousek, I.; Kozlova, M.; Klimes, J.; Cizek, A.; Literak, I. Extended spectrum beta-lactamase and fluoroquinolone resistance genes and plasmids among Escherichia coli isolates from zoo animals, Czech Republic. FEMS Microbiol. Ecol. 2013, 85, 604–611. [Google Scholar] [CrossRef]
- Conrad, C.C.; Stanford, K.; Narvaez-Bravo, C.; Neumann, N.F.; Munns, K.; Tymensen, L.; Jokinen, C.; Mcallister, T.A. Zoonotic Fecal Pathogens and Antimicrobial Resistance in Canadian Petting Zoos. Microorganisms 2018, 6, 70. [Google Scholar] [CrossRef]
- Isler, M.; Wissmann, R.; Morach, M.; Zurfluh, K.; Stephan, R.; Nüesch-Inderbinen, M. Animal petting zoos as sources of Shiga toxin-producing Escherichia coli, Salmonella and extended-spectrum β-lactamase (ESBL)-producing Enterobacteriaceae. Zoonoses Public Health 2021, 68, 79–87. [Google Scholar] [CrossRef]
- Shnaiderman-Torban, A.; Steinman, A.; Meidan, G.; Paitan, Y.; Abu Ahmad, W.; Navon-Venezia, S. Petting Zoo Animals as an Emerging Reservoir of Extended-Spectrum β-Lactamase and AmpC-Producing Enterobacteriaceae. Front. Microbiol. 2019, 10, 2488. [Google Scholar] [CrossRef]
- Morris, D.O.; Loeffler, A.; Davis, M.F.; Guardabassi, L.; Weese, J.S. Recommendations for approaches to meticillin-resistant staphylococcal infections of small animals: Diagnosis, therapeutic considerations and preventative measures: Clinical Consensus Guidelines of the World Association for Veterinary Dermatology. Vet. Dermatol. 2017, 28, 304-e69. [Google Scholar] [CrossRef]
- Baptiste, K.E.; Williams, K.; Willams, N.J.; Wattret, A.; Clegg, P.D.; Dawson, S.; Corkill, J.E.; O’Neill, T.; Hart, C.A. Methicillin-resistant staphylococci in companion animals. Emerg. Infect. Dis. 2005, 11, 1942–1944. [Google Scholar] [CrossRef]
- Weese, J.S.; Van Duijkeren, E. Methicillin-resistant Staphylococcus aureus and Staphylococcus pseudintermedius in veterinary medicine. Vet. Microbiol. 2010, 140, 418–429. [Google Scholar] [CrossRef] [PubMed]
- Perreten, V.; Kadlec, K.; Schwarz, S.; Grönlund Andersson, U.; Finn, M.; Greko, C.; Moodley, A.; Kania, S.A.; Frank, L.A.; Bemis, D.A.; et al. Clonal spread of methicillin-resistant Staphylococcus pseudintermedius in Europe and North America: An international multicentre study. J. Antimicrob. Chemother. 2010, 65, 1145–1154. [Google Scholar] [CrossRef] [PubMed]
- Windahl, U.; Reimegård, E.; Holst, B.S.; Egenvall, A.; Fernström, L.; Fredriksson, M.; Trowald-Wigh, G.; Andersson, U.G. Carriage of methicillin-resistant Staphylococcus pseudintermedius in dogs-a longitudinal study. BMC Vet. Res. 2012, 8, 34. [Google Scholar] [CrossRef] [PubMed]
- Frosini, S.M.; Bond, R.; King, R.; Feudi, C.; Schwarz, S.; Loeffler, A. Effect of topical antimicrobial therapy and household cleaning on meticillin-resistant Staphylococcus pseudintermedius carriage in dogs. Vet. Rec. 2022, 190, e937. [Google Scholar]
- Sasaki, T.; Kikuchi, K.; Tanaka, Y.; Takahashi, N.; Kamata, S.; Hiramatsu, K. Reclassification of phenotypically identified Staphylococcus intermedius strains. J. Clin. Microbiol. 2007, 45, 2770–2778. [Google Scholar] [CrossRef]
- Frosini, S.M.; Bond, R.; McCarthy, A.J.; Feudi, C.; Schwarz, S.; Lindsay, J.A.; Loeffler, A. Genes on the Move: In Vitro Transduction of Antimicrobial Resistance Genes between Human and Canine Staphylococcal Pathogens. Microorganisms 2020, 8, 2031. [Google Scholar] [CrossRef]
- Graveland, H.; Duim, B.; Van Duijkeren, E.; Heederik, D.; Wagenaar, J.A. Livestock-associated methicillin-resistant Staphylococcus aureus in animals and humans. Int. J. Med. Microbiol. 2011, 301, 630–634. [Google Scholar]
- Graveland, H.; Wagenaar, J.A.; Heesterbeek, H.; Mevius, D.; Van Duijkeren, E.; Heederik, D. Methicillin Resistant Staphylococcus aureus ST398 in Veal Calf Farming: Human MRSA Carriage Related with Animal Antimicrobial Usage and Farm Hygiene. PLoS ONE 2010, 5, e10990. [Google Scholar]
- Moses, I.B.; Santos, F.F.; Gales, A.C. Human Colonization and Infection by Staphylococcus pseudintermedius: An Emerging and Underestimated Zoonotic Pathogen. Microorganisms 2023, 11, 581. [Google Scholar] [CrossRef]
- Göttling, J.; Heckel, J.-O.; Hotzel, H.; Fruth, A.; Pfeifer, Y.; Henning, K.; Kopp, P.; Mertens-Scholz, K.; Rietschel, W.; Pfeffer, M. Zoonotic bacteria in clinically healthy goats in petting zoo settings of zoological gardens in Germany. Zoonoses Public Health 2022, 69, 333–343. [Google Scholar] [CrossRef]
- Frosini, S.-M.; Bond, R.; King, R.H.; Loeffler, A. The nose is not enough: Multi-site sampling is best for MRSP detection in dogs and households. Vet. Dermatol. 2022, 33, 576–580. [Google Scholar] [CrossRef]
- Menezes, J.; Frosini, S.M.; Belas, A.; Marques, C.; Da Silva, J.M.; Amaral, A.J.; Loeffler, A.; Pomba, C. Longitudinal study of ESBL/AmpC-producing Enterobacterales strains sharing between cohabiting healthy companion animals and humans in Portugal and in the United Kingdom. Eur. J. Clin. Microbiol. Infect. Dis. 2023, 42, 1011–1024. [Google Scholar] [CrossRef]
- Stegger, M.; Andersen, P.S.; Kearns, A.; Pichon, B.; Holmes, M.A.; Edwards, G.; Laurent, F.; Teale, C.; Skov, R.; Larsen, A.R. Rapid detection, differentiation and typing of methicillin-resistant Staphylococcus aureus harbouring either mecA or the new mecA homologue mecALGA251. Clin. Microbiol. Infect. 2012, 18, 395–400. [Google Scholar] [CrossRef]
- Becker, K.; Von Eiff, C.; Keller, B.; Brück, M.; Etienne, J.; Peters, G. Thermonuclease gene as a target for specific identification of Staphylococcus intermedius isolates: Use of a PCR-DNA enzyme immunoassay. Diagn. Microbiol. Infect. Dis. 2005, 51, 237–244. [Google Scholar] [CrossRef] [PubMed]
- M02-A13; Performance Standards for Antimicrobial Disk Susceptibility Tests. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2024.
- VET01S-ED7; Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2024.
- M100-ED35; 2025 Performance Standards for Antimicrobial Susceptibility Testing. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2025.
- Gemeda, B.A.; Wieland, B.; Alemayehu, G.; Knight-Jones, T.J.D.; Wodajo, H.D.; Tefera, M.; Kumbe, A.; Olani, A.; Abera, S.; Amenu, K. Antimicrobial Resistance of Escherichia coli Isolates from Livestock and the Environment in Extensive Smallholder Livestock Production Systems in Ethiopia. Antibiotics 2023, 12, 941. [Google Scholar] [CrossRef] [PubMed]
- Garzon, A.; Portillo, R.; Habing, G.; Silva-Del-Rio, N.; Karle, B.M.; Pereira, R.V. Antimicrobial resistance of Escherichia coli from dairy farms participating in an antimicrobial stewardship educational program for farm employees. J. Dairy Sci. 2024, 107, 1645–1655. [Google Scholar] [CrossRef] [PubMed]
- UK-VARSS 2023; Veterinary Antibiotic Resistance and Sales Surveillance Report (UK-VARSS 2023). Veterinary Medicines Directorate: Addlestone, UK, 2023.
- Harada, K.; Asai, T. Role of Antimicrobial Selective Pressure and Secondary Factors on Antimicrobial Resistance Prevalence in Escherichia coli from Food-Producing Animals in Japan. BioMed Res. Int. 2010, 2010, 180682. [Google Scholar]
- Fletcher, S. Understanding the contribution of environmental factors in the spread of antimicrobial resistance. Environ. Health Prev. Med. 2015, 20, 243–252. [Google Scholar]
- Massé, J.; Lardé, H.; Fairbrother, J.M.; Roy, J.-P.; Francoz, D.; Dufour, S.; Archambault, M. Prevalence of Antimicrobial Resistance and Characteristics of Escherichia coli Isolates from Fecal and Manure Pit Samples on Dairy Farms in the Province of Québec, Canada. Front. Vet. Sci. 2021, 8, 654125. [Google Scholar]
- Ardakani, Z.; Aragrande, M.; Canali, M. Global antimicrobial use in livestock farming: An estimate for cattle, chickens, and pigs. Animal 2024, 18, 101060. [Google Scholar] [CrossRef]
- Alali, W.Q.; Scott, H.; Harvey, R.; Norby, B.; Lawhorn, D.; Pillai, S. Longitudinal study of antimicrobial resistance among Escherichia coli isolates from integrated multisite cohorts of humans and swine. Appl. Environ. Microbiol. 2008, 74, 3672–3681. [Google Scholar] [CrossRef]
- Liu, C.; Wang, P.; Dai, Y.; Liu, Y.; Song, Y.; Yu, L.; Feng, C.; Liu, M.; Xie, Z.; Shang, Y.; et al. Longitudinal monitoring of multidrug resistance in Escherichia coli on broiler chicken fattening farms in Shandong, China. Poult. Sci. 2021, 100, 100887. [Google Scholar] [CrossRef]
- Chantziaras, I.; Boyen, F.; Callens, B.; Dewulf, J. Correlation between veterinary antimicrobial use and antimicrobial resistance in food-producing animals: A report on seven countries. J. Antimicrob. Chemother. 2013, 69, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Dyar, O.J.; Huttner, B.; Schouten, J.; Pulcini, C. What is antimicrobial stewardship? Clin. Microbiol. Infect. 2017, 23, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Willems, E.; Cartuyvels, R.; Magerman, K.; Verhaegen, J. Evaluation of 3 different agar media for rapid detection of extended-spectrum β-lactamase–producing Enterobacteriaceae from surveillance samples. Diagn. Microbiol. Infect. Dis. 2013, 76, 16–19. [Google Scholar] [CrossRef] [PubMed]
- Blane, B.; Brodrick, H.J.; Gouliouris, T.; Ambridge, K.E.; Kidney, A.D.; Ludden, C.M.; Limmathurotsakul, D.; Török, M.E.; Peacock, S.J. Comparison of 2 chromogenic media for the detection of extended-spectrum β-lactamase producing Enterobacteriaceae stool carriage in nursing home residents. Diagn. Microbiol. Infect. Dis. 2016, 84, 181–183. [Google Scholar]
- Jazmati, T.; Hamprecht, A.; Jazmati, N. Comparison of stool samples and rectal swabs with and without pre-enrichment for the detection of third-generation cephalosporin-resistant Enterobacterales (3GCREB). Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 2431–2436. [Google Scholar]
- Woolhouse, M.E.; Ward, M.J. Sources of antimicrobial resistance. Science 2013, 341, 1460–1461. [Google Scholar] [CrossRef]
- Nüesch-Inderbinen, M.; Stephan, R. Epidemiology of Extended-Spectrum β-Lactamase-Producing Escherichia coli in the Human-Livestock Environment. Curr. Clin. Microbiol. Rep. 2016, 3, 1–9. [Google Scholar] [CrossRef]
- Nóbrega, D.B.; Brocchi, M. An overview of extended-spectrum beta-lactamases in veterinary medicine and their public health consequences. J. Infect. Dev. Ctries. 2014, 8, 954–960. [Google Scholar] [CrossRef]
- Husna, A.; Rahman, M.M.; Badruzzaman, A.T.M.; Sikder, M.H.; Islam, M.R.; Rahman, M.T.; Alam, J.; Ashour, H.M. Extended-Spectrum β-Lactamases (ESBL): Challenges and Opportunities. Biomedicines 2023, 11, 2937. [Google Scholar] [CrossRef] [PubMed]
- Vestergaard, M.; Frees, D.; Ingmer, H. Antibiotic Resistance and the MRSA Problem. Microbiol. Spectr. 2019, 7, 10.1128. [Google Scholar] [CrossRef] [PubMed]
- Delacour, H.; Van Cuyck, H.; Dubrous, P.; Soullié, B.; Leroy, P.; Koeck, J.L. Efficacy of a swab transport system in maintaining long-term viability of Staphylococcus aureus. Diagn. Microbiol. Infect. Dis. 2009, 65, 345–346. [Google Scholar] [CrossRef]
- Panisello Yagüe, D.; Mihaljevic, J.; Mbegbu, M.; Wood, C.V.; Hepp, C.; Kyman, S.; Hornstra, H.; Trotter, R.; Cope, E.; Pearson, T. Survival of Staphylococcus aureus on sampling swabs stored at different temperatures. J. Appl. Microbiol. 2021, 131, 1030–1038. [Google Scholar] [CrossRef]
- Iverson, S.A.; Brazil, A.M.; Ferguson, J.M.; Nelson, K.; Lautenbach, E.; Rankin, S.C.; Morris, D.O.; Davis, M.F. Anatomical patterns of colonization of pets with staphylococcal species in homes of people with methicillin-resistant Staphylococcus aureus (MRSA) skin or soft tissue infection (SSTI). Vet. Microbiol. 2015, 176, 202–208. [Google Scholar] [CrossRef]
- Verstappen, K.M.; Willems, E.; Fluit, A.C.; Duim, B.; Martens, M.; Wagenaar, J.A. Staphylococcus aureus Nasal Colonization Differs among Pig Lineages and Is Associated with the Presence of Other Staphylococcal Species. Front. Vet. Sci. 2017, 4, 97. [Google Scholar] [CrossRef]
- Bullone, M.; Bellato, A.; Robino, P.; Nebbia, P.; Morello, S.; Marchis, D.; Tarducci, A.; Ru, G. Prevalence and risk factors associated with nasal carriage of methicillin-resistant staphylococci in horses and their caregivers. Vet. Res. 2024, 55, 108. [Google Scholar] [CrossRef]
- Beça, N.; Bessa, L.J.; Mendes, Â.; Santos, J.; Leite-Martins, L.; Matos, A.J.F.; Da Costa, P.M. Coagulase-Positive Staphylococcus: Prevalence and Antimicrobial Resistance. J. Am. Anim. Hosp. Assoc. 2015, 51, 365–371. [Google Scholar] [CrossRef]
- Bean, D.; Wigmore, S. Carriage rate and antibiotic susceptibility of coagulase-positive staphylococci isolated from healthy dogs in Victoria, Australia. Aust. Vet. J. 2016, 94, 456–460. [Google Scholar] [CrossRef]
- Saputra, S.; Jordan, D.; Worthing, K.A.; Norris, J.M.; Wong, H.S.; Abraham, R.; Trott, D.J.; Abraham, S. Antimicrobial resistance in coagulase-positive staphylococci isolated from companion animals in Australia: A one year study. PLoS ONE 2017, 12, e0176379. [Google Scholar] [CrossRef]
- Elmoslemany, A.; Elsohaby, I.; Alorabi, M.; Alkafafy, M.; Al-Marri, T.; Aldoweriej, A.; Alaql, F.A.; Almubarak, A.; Fayez, M. Diversity and Risk Factors Associated with Multidrug and Methicillin-Resistant Staphylococci Isolated from Cats Admitted to a Veterinary Clinic in Eastern Province, Saudi Arabia. Antibiotics 2021, 10, 367. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.A.; Rodrigues Hoffmann, A.; Neufeld, J.D. The skin microbiome of vertebrates. Microbiome 2019, 7, 79. [Google Scholar] [CrossRef] [PubMed]
- Nakatsuji, T.; Chen, T.H.; Narala, S.; Chun, K.A.; Two, A.M.; Yun, T.; Shafiq, F.; Kotol, P.F.; Bouslimani, A.; Melnik, A.V.; et al. Antimicrobials from human skin commensal bacteria protect against Staphylococcus aureus and are deficient in atopic dermatitis. Sci. Transl. Med. 2017, 9, eaah4680. [Google Scholar] [CrossRef] [PubMed]
- Paharik, A.E.; Parlet, C.P.; Chung, N.; Todd, D.A.; Rodriguez, E.I.; Van Dyke, M.J.; Cech, N.B.; Horswill, A.R. Coagulase-Negative Staphylococcal Strain Prevents Staphylococcus aureus Colonization and Skin Infection by Blocking Quorum Sensing. Cell Host Microbe 2017, 22, 746–756.e5. [Google Scholar] [CrossRef]
- Tsubakishita, S.; Kuwahara-Arai, K.; Sasaki, T.; Hiramatsu, K. Origin and molecular evolution of the determinant of methicillin resistance in staphylococci. Antimicrob. Agents Chemother. 2010, 54, 4352–4359. [Google Scholar] [CrossRef]
- Warshawsky, B.; Gutmanis, I.; Henry, B.; Dow, J.; Reffle, J.; Pollett, G.; Ahmed, R.; Aldom, J.; Alves, D.; Chagla, A.; et al. Outbreak of Escherichia coli 0157:H7 related to animal contact at a petting zoo. Can. J. Infect. Dis. Med. Microbiol. 2002, 13, 175–181. [Google Scholar] [CrossRef]
- Schlager, S.; Lepuschitz, S.; Ruppitsch, W.; Ableitner, O.; Pietzka, A.; Neubauer, S.; Stöger, A.; Lassnig, H.; Mikula, C.; Springer, B.; et al. Petting zoos as sources of Shiga toxin-producing Escherichia coli (STEC) infections. Int. J. Med. Microbiol. 2018, 308, 927–932. [Google Scholar] [CrossRef]
- Dunn, J.R.; Behravesh, C.B.; Angulo, F.J. Diseases Transmitted by Domestic Livestock: Perils of the Petting Zoo. Microbiol. Spectr. 2015, 3. [Google Scholar] [CrossRef]
- Bloomfield, S.F.; Aiello, A.E.; Cookson, B.; O’boyle, C.; Larson, E.L. The effectiveness of hand hygiene procedures in reducing the risks of infections in home and community settings including handwashing and alcohol-based hand sanitizers. Am. J. Infect. Control 2007, 35, S27–S64. [Google Scholar]

| Animal Species | Common Name | Number Sampled | Number of Different Centres Represented |
|---|---|---|---|
| Bos taurus | Cattle | 31 | 2 |
| Capra hircus | Goat | 26 | 5 |
| Cavia porcellus | Guinea Pig | 6 | 3 |
| Equus asinus | Donkey | 7 | 4 |
| Equus caballus | Horse/Pony | 15 | 5 |
| Hydrochoerus hydrochaeris | Capybara | 3 | 1 |
| Mustela furo | Ferret | 6 | 2 |
| Oryctolagus cuniculus | Rabbit | 14 | 4 |
| Ovis aries | Sheep | 9 | 3 |
| Rangifer tarandus | Reindeer | 2 | 1 |
| Suricata suricatta | Meerkat | 7 | 1 |
| Sus domesticus | Pig | 12 | 5 |
| Varecia rubra | Red Ruffed Lemur | 3 | 1 |
| Vicugna pacos | Alpaca | 25 | 4 |
| Total | 166 |
| Number of E. coli (Total n = 223) | % | |
|---|---|---|
| No Resistance | 128 | 57.1 |
| Single Resistance | 54 | 24.1 |
| Ampicillin (AMP) | 42 | |
| Amoxicillin-clavulanic acid (AMC) | 1 | |
| Chloramphenicol (C) | 5 | |
| Gentamicin (CN) | 5 | |
| Enrofloxacin (ENR) | 0 | |
| Trimethoprim-sulfamethoxazole (SXT) | 0 | |
| Tetracycline (TE) | 1 | |
| 3rd Generation Cephalosporins (3GC) | 0 | |
| Double Resistance | 22 | 9.8 |
| AMP-AMC | 2 | |
| AMP-C | 4 | |
| AMP-CN | 2 | |
| AMP-ENR | 5 | |
| AMP-SXT | 3 | |
| AMP-TE | 2 | |
| C-ENR | 1 | |
| ENR-TE | 1 | |
| SXT-TE | 2 | |
| Multidrug resistance (MDR) | 19 | 8.5 |
| AMP-AMC-C | 1 | |
| AMP-AMC-CN | 3 | |
| AMP-AMC-ENR | 1 | |
| AMP-AMC-SXT | 1 | |
| AMP-C-ENR | 1 | |
| AMP-C-SXT | 1 | |
| AMP-C-TE | 1 | |
| AMP-ENR-TE | 1 | |
| AMC-CN-ENR | 1 | |
| AMP-AMC-C-CN | 1 | |
| AMP-AMC-C-3GC | 1 | |
| AMP-AMC-CN-ENR | 1 | |
| AMP-C-CN-ENR | 1 | |
| AMP-ENR-SXT-TE | 2 | |
| AMP-AMC-C-CN-ENR | 2 |
| Staphylococcus aureus | % | Staphylococcus intermedius Group (SIG) | % | |
|---|---|---|---|---|
| No Resistance | 51 | 72.9 | 11 | 84.6 |
| Single Resistance | 14 | 20.0 | 1 | 7.7 |
| Benzylpenicillin (P) | 1 | 1 | ||
| Cefoxitin (FOX) | 0 | N/A | ||
| Oxacillin (OX) | N/A | 0 | ||
| Enrofloxacin (ENR) | 3 | 0 | ||
| Erythromycin (E) | 2 | 0 | ||
| Chloramphenicol (C) | 0 | 0 | ||
| Gentamicin (CN) | 0 | 0 | ||
| Trimethoprim Sulfamethoxazole (SXT) | 0 | 0 | ||
| Tetracycline (TE) | 8 | 0 | ||
| Double Resistance | 5 | 7.1 | 0 | 0 |
| ENR-E | 3 | 0 | ||
| ENR-TE | 1 | 0 | ||
| E-TE | 1 | 0 | ||
| Multidrug Resistance (MDR) | 0 | 0 | 1 | 7.7 |
| ENR-E-TE | 0 | 1 |
| Animal Species Originating from | Centre | Ampicillin | Amoxicillin-Clavulanic Acid | 3rd Generation Cephalosporins | Chloramphenicol | Gentamicin | Enrofloxacin | Trimethoprim-Sulphamethoxazole | Tetracycline |
|---|---|---|---|---|---|---|---|---|---|
| Pig | A | R | S | R | S | S | R | S | S |
| A | R | S | R | S | S | R | S | S | |
| B | R | S | R | S | S | R | R | R | |
| Goat | C | R | S | R | S | R | S | S | S |
| C | R | R | R | S | R | S | S | S |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishigaki, A.; Arden, K.; Frosini, S.-M. Antimicrobial Resistance in Petting Zoo Animals in the United Kingdom. Appl. Microbiol. 2025, 5, 115. https://doi.org/10.3390/applmicrobiol5040115
Nishigaki A, Arden K, Frosini S-M. Antimicrobial Resistance in Petting Zoo Animals in the United Kingdom. Applied Microbiology. 2025; 5(4):115. https://doi.org/10.3390/applmicrobiol5040115
Chicago/Turabian StyleNishigaki, Alice, Kurt Arden, and Siân-Marie Frosini. 2025. "Antimicrobial Resistance in Petting Zoo Animals in the United Kingdom" Applied Microbiology 5, no. 4: 115. https://doi.org/10.3390/applmicrobiol5040115
APA StyleNishigaki, A., Arden, K., & Frosini, S.-M. (2025). Antimicrobial Resistance in Petting Zoo Animals in the United Kingdom. Applied Microbiology, 5(4), 115. https://doi.org/10.3390/applmicrobiol5040115





