Abstract
The development of extensive antibiotic resistance has created an urgent demand to identify novel sources of antimicrobial agents. Interest in actinomycetes has become prevalent around the world because of their ability to generate several beneficial bioactive metabolites. In the present study, 32 marine soil samples were collected from Tyre City Beach, Lebanon, in different seasons. A total of 10 actinomycetes species were identified and characterized depending on their microscopic features. All isolates were tested for their potential to exert antimicrobial activities against varied microorganisms using cross-streak and agar well diffusion methods. All isolates displayed significant antimicrobial activities against the tested indicator microorganisms. Similarly, all 10 isolates of marine actinomycetes exhibited antifungal activity in cross-streak tests against Candida albicans, Aspergillus niger, and Aspergillus flavus. Moreover, the optimum conditions used to enhance the production of antimicrobial secondary metabolites against Bacillus cereus were tested for the three isolates Kocuria rosea, Micrococcus luteus, and Streptomyces longisporoflavus. Our results indicate that actinomycetes isolated from Tyre City Beach, Lebanon, represent a promising source of antimicrobial bioactive substances.
1. Introduction
Actinomycetes are filamentous Gram-positive bacteria with fungal morphology [1]. Among the diverse species of actinobacteria, Streptomyces spp. are mainly recognized as factories for a prominent number of effective biologically active compounds such as antibacterial, antifungals, and antivirals [2,3,4,5,6]. However, the emergence of multi-drug-resistant microorganisms has triggered the necessity to test new antimicrobial compounds derived from natural resources [7,8]. Indeed, marine actinomycetes serve as a great source of promising antimicrobial chemical compounds, enzymatic inhibitors, and anticancer compounds [9,10,11,12,13,14].
Today, actinomycetes play a vital role in nanotechnology and bioremediation, particularly in the synthesis of various nanoparticles utilized in sustainable agricultural processes, such as nanofertilizers and biocontrol agents [15]. A terrestrial actinomycete bacterial strain capable of producing selenium nanoparticles (Se NPs) was identified, followed by its purification and an evaluation of the biogenic Se NPs’ photocatalytic degradation efficiency compared to that of selenium dioxide [16]. Certain actinomycete genera such as Streptomyces and Micromonospora are capable of degrading organochlorines (lindane, chlordane, or methoxychlor) [17]. Streptomyces species have also been studied for removal of chlorpyrifos and pentachlorophenol [18]. It was reported that chlorpyrifos was effectively removed by Streptomyces sp. M7 (99.2%).
The soil reservoir in the Lebanese marine environment is poorly examined, and very few studies have been conducted to investigate it [19,20,21,22]. Despite the global search for novel actinomycetes, Lebanese marine soil remains largely unexplored, representing a significant research gap in microbial biodiversity studies. This under-researched habitat, shaped by unique environmental conditions and rich biodiversity, is influenced by unique ecological factors such as high salinity, diverse sediment types, and variable temperatures among the four different seasons. This may drive the evolution and discovery of new actinomycetes with valuable new bioactive compounds. Addressing this gap is critical, particularly in light of rising antibiotic resistance trends, which drive the urgent need for new antimicrobial agents.
The soil reservoir might serve as a significant context for Actinobacteria. In this regard, the current research attempted to isolate and distinguish multiple actinomycetes from marine soil in Tyre City Beach, Lebanon, and investigate the antimicrobial activities of their active metabolites.
2. Materials and Methods
2.1. Isolation of Different Actinomycetes from Different Areas of Marine Soil in Tyre City Beach, Lebanon
2.1.1. Sample Collection
A total of 32 marine soil samples were collected from various locations in Tyre City Beach (33°15′38.7″ N; 35°12′34.164″ E) in the four different seasons. At a depth of 15.0 cm below the surface of the sandy beach, a total of 16 samples were collected and labeled as A1, B1, C1, and D1, and the remaining samples were collected at a depth of 2.0 m under the sea surface, marked as A2, B2, C2, and D2. The parameters of soil collection are presented in Table 1 and Figure 1.

Table 1.
Different sampling sites in Tyre City Beach.
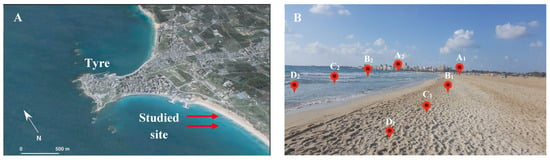
Figure 1.
Satellite images (Google Earth, 2006) and study sites. (A) Tyre City, Tyre Beach, and the studied site; (B) sampling sites from Tyre Beach marked as A1, A2, B1, B2, C1, C2, D1, and D2.
2.1.2. Isolation of Actinomycetes
Following the collection phase, 1 g of each sediment sample was suspended in 9 mL of sterile distilled water and then serially diluted ten-fold [23]. Starch casein agar (SCA) plates containing filter-sterilized cycloheximide (100 μg/mL) and nalidixic acid (30 μg/mL) were used to spread aliquots of 100 μL [24]. Afterward, the inoculated plates were incubated at 28 °C for 7 to 21 days.
2.2. Identification of Marine Actinomycetes
The spore arrangement of actinomycetes was microscopically evaluated. Starch casein agar was used to culture the isolated marine actinomycetes, and their morphological features, including shape, size, elevation, configuration, margin, and pigmentation, were examined [25,26]. Moreover, cell morphology was assessed using Gram staining. Furthermore, the isolates were further identified using the VITEK 2® Gp automated system (manufactured by bioMérieux, Marcy-l’Étoile, France).
Additionally, the identification of the isolates was conducted through established biochemical methods. Briefly, the development of oxygen bubbles with 3% hydrogen peroxide solution and the oxidation of TMPD (tetramethyl-phenylenediamine dihydrochloride, in the form of discs), respectively facilitated the recognition of catalase and oxidase activities. Citrate utilization was identified when a change in the color of the citrate agar medium appeared. Urease production was recognized by the presence of a bright pink color on the urea slant. Nitrate reduction was determined by the development of red color in the medium containing nitrate [26].
2.3. Screening of Marine Actinomycetes for Their Potential to Produce Antimicrobial Active Metabolites
2.3.1. Primary Screening for Antimicrobial Activity by Cross-Streak Method
Screening was performed on the isolated marine actinomycetes to assess their antimicrobial activity. The cross-streak method was used, and an assessment of the antimicrobial activity was conducted against bacterial and fungal pathogens using an antagonistic method. The actinomycetes were streaked on Mueller–Hinton agar plates (MHA) and incubated at room temperature for 24 h. To carry out antifungal screening, starch casein potato dextrose agar (SC-PDA) was used, and samples were incubated at room temperature for 2 to 7 days [27].
2.3.2. Secondary Screening for the Secretion of Antibacterial Metabolites by Agar Well Diffusion
A screening process of the chosen marine isolates was conducted using an agar well diffusion assay for environmental samples and bacterial pathogens with sterile Mueller–Hinton agar at 37 °C for 8 h. Plates were incubated at 37 °C, with the measurement of the zone of inhibition performed after 24 h of incubation. The screening was performed against nine clinically and environmentally relevant bacterial strains: Salmonella typhi, Escherichia coli, Staphylococcus aureus, Klebsiella pneumonia, Acinetobacter baumanni, Pseudomonas aeruginosa, Bacillus cereus, Staphylococcus aureus, Staphylococcus pseudintermedius, and Staphylococcus heamolyticus. Three fungal pathogenic indicators isolated from clinical samples were also used: Candida albicans, Aspergillus niger, and Aspergillus flavus. Thereafter, the most potent isolates having the most prominent antibacterial and antifungal activities were selected for further investigations.
2.4. Optimization of Antimicrobial Production
Bacteria were incubated with various sources of carbon—starch, fructose, glucose, mannitol, and sucrose (10 g/L)—in basal SCA medium to optimize the production of secondary metabolites [28]. In addition, the effect of different nitrogen sources—KNO3, (NH4)2SO4, NH4NO3, NaNO3, and Ca(NO3)2 (2 g/L)—in the basal SCA was investigated. Moreover, the impacts of culture conditions such as different incubation times (2–14 days), varying starting pH values (5, 6, 7, 8, 9, 10, and 11), and multiple starting inoculum concentrations (0.01, 0.1, 2, 5, 10, and 15%) were also examined [29]. Antimicrobial activity against bacterial indicators was performed using the well diffusion method following every experiment. The zones of inhibition were measured after incubation at 37 °C for 24 h.
2.5. Fermentation and Extraction of Secondary Metabolites
In order to obtain a crude extract from the studied actinomycetes, 1.5 mL of stock suspension of the actinomycetes associated with the highest antimicrobial activities was poured onto 200 mL of SCB and incubated at 30 °C at 200 rpm for 10 days. The supernatant was then collected and supplemented with an equal volume of ethyl acetate. Following centrifugation, the upper layer containing secondary metabolites was collected and evaporated using a rotary evaporator at 40 °C [30,31].
2.6. Identification of the Minimal Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) of the Isolated Strains
2.6.1. Minimal Inhibitory Concentration (MIC) Determination
Three concentrations of the secondary metabolites extracted from the 3 isolated strains—Kocuria rosea, Micrococcus luteus, and Streptomyces longisporoflavus—were used (125 mg/mL, 625 mg/mL, and 250 mg/mL) at bacterial concentrations equivalent to 0.5 McFarland’s standard to identify the MIC in nutrient broth, using erythromycin as a standard reference antibiotic. The control consisted of the inoculated broth only and was incubated for 24 h at 37 °C. The MIC endpoint was the lowest concentration of the extracted secondary metabolites where no visible growth was seen in the tubes. Before and after incubation, the visual turbidity of the tubes was assessed in order to validate the MIC value [31].
2.6.2. Minimum Bactericidal Concentration (MBC) Determination
After the MIC determination, aliquots of 50 µL from all the tubes with no visible bacterial growth were seeded on MHA plates and incubated for 24 h at 37 °C. MBC was represented by the lowest concentration at which 99.9% of the bacterial sample population was killed [32]. All the experiments were repeated at least three times.
2.7. Statistical Analysis
The statistical evaluation of the data and experimental design was performed in Excel® software 2016 MSO (16.0.4266.1001) 32 bit and GraphPad Prism (9.5.1). Statistical significance was identified by t-test and one-way ANOVA. Differences with a p-value < 0.05 were considered statistically significant.
3. Results
3.1. Sample Collection and Actinomycete Isolation
A total of 32 marine soil samples was gathered during the four seasons from different sites. There were 16 samples collected from the upper 15 cm layer of a sandy beach (marked as A1, B1, C1, and D1) and another 16 samples collected at 2 m depth under the sea surface (marked as A2, B2, C2, and D2). Among the isolates from the 32 collected marine soil samples, colonies exhibited various morphologies with a tough or powdery texture and a dry or folded appearance; the colonies were characterized by branching filaments with or without aerial mycelia.
3.2. Identification of Marine Actinomycetes
The isolated colonies initially exhibited a smooth outer surface. However, they later evolved into aerial mycelia that emerged to look either floccose, granular, powdery, or velvety. The color of the substrate and aerial mycelia varied, ranging from white or yellow–white to violet, green, or pink. Moreover, 80 isolates were identified as Kocuria kristinae (7), Kocuria rosea (18), Kocuria rhizophila (13), Kocuria varians (3), Kytococccus sedentarius (14), Dermacoccus nishinomiyaensis (10), Micrococcus luteus (6), Micrococcus laylae (3), Streptomyces longisporoflavus (3), and Streptomyces griseus (3).
In addition, the quantity of colony forming units (CFUs) per gram of soil (in CFU/mL) at the different sampling sites was measured and is tabulated in Table 2. Furthermore, the quantity of colony forming units differed among the four studied seasons and between the different sampling sites in Tyre City Beach, as shown in Figure 2.

Table 2.
Isolated strains from the different sampling sites with the quantity of colony forming units (CFUs) per gram of soil (in CFU/mL) from Tyre City Beach.
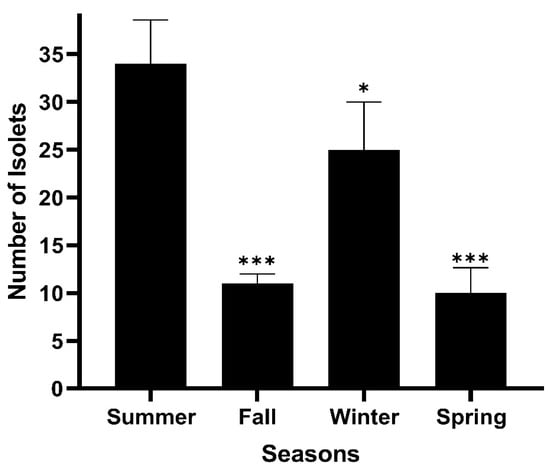
Figure 2.
Variation in the number of actinomycetes among the different studied seasons. p-values were calculated such that * p < 0.05, *** p < 0.001.
3.3. Biochemical and Physiological Characterization of the Isolated Actinomycetes from Tyre City Beach
The findings on the microscopic aspects of the 10 isolates are represented in Table 3 and Figure A1 (Appendix A). All the isolates successfully established well-grown colonies (3–7 mm) after 7 days of incubation at 28 °C. After this, the observation of the colonies as smooth with aerial and substrate mycelia of different colors and complete margins was reported. Overall, the isolates were Gram positive and exhibited branched filaments, thereby generating single oval sports or short to long chains.

Table 3.
Microscopic features of the isolated actinomycetes from Tyre City beach.
In addition, the biochemical and physiological characteristics of the 10 isolates identified and their production of different enzymes are illustrated in Table 4.

Table 4.
Biochemical characteristics result of Kocuria spp., Dermacoccus nishinomiyaensis, Kytococccus sedentarius, Micrococcus spp., and Streptomyces spp.
3.4. Screening of Marine Actinomycetes for Their Potential to Generate Antimicrobial Active Metabolites
All of the 10 isolates of marine actinomycetes showed promising antibacterial effects, as shown in Table 5. All isolates were effective against Bacillus cereus, whereas 30% isolates were effective against Escherichia coli, 50% actinomycetes were active against Staphylococcus aureus, 90% against Staphylococcus pseudintermedius, 10% against Staphylococcus heamolyticus, 60% against Pseudomonas aeruginosa, 40% against Klebsiella pneumonia, 30% against Salmonella typhi, and 40% against Acinetobacter baumanni.

Table 5.
Agar well diffusion results of the 10 isolated strains against the studied bacterial indicators after 24 h of incubation.
Similarly, all 10 isolates of marine actinomycetes exhibited antifungal activity by the cross-streak method, 70% of which were active against Candida albicans and 50% against Aspergillus nigar and Aspergillus flavus.
3.5. Optimization of the Production of Antimicrobial Secondary Metabolites
The finest conditions for the growth and creation of antimicrobials were determined using Kocuria rosea, Micrococcus luteus, and Streptomyces longisporoflavus against B. cereus, and the results are detailed in Table 6. A summary of the optimal conditions for secondary metabolite production for each selected isolate is presented in Table 7.

Table 6.
Different growth conditions of Kocuria rosea, Micrococcus luteus, and Streptomyces longisporoflavus and their antibacterial activity as determined by inhibition zones against B. cereus.

Table 7.
Summary of the optimal conditions for the three selected isolates, Kocuria rosea, Micrococcus luteus, and Streptomyces longisporoflavus, for secondary metabolite production in comparison with the normal (basal) conditions.
For Kocuria rosea, we showed that the maximum antimicrobial activity was achieved using ammonium sulfate and mannitol at concentrations of 0.2% and 2.5%, respectively. In addition, factors such as pH 7.0, a starting inoculum of 2%, and an incubation time of 11 days at 30 °C yielded high amounts of antimicrobial substances, as shown in Figure A2 (Appendix A).
On the other hand, our results showed that for Micrococcus luteus, the efficiency was achieved using glucose and peptone at concentrations of 0.5 and 1%, respectively. Different factors such as pH 7.0, a starting inoculum of 2%, and an incubation time of 7 days at 30 °C led to significant antimicrobial activity, as shown in Figure A3 (Appendix A).
Furthermore, for Streptomyces longisporoflavus, we showed that the optimal efficiency in production was accomplished through the use of starch and peptone at concentrations of 1%. Additional elements such as pH 7.0, an initiating inoculum of 2%, and an incubation time of 8 days at 30 °C yielded the highest antimicrobial activity, as shown in Figure A4 (Appendix A).
3.6. MIC and MBC Determination
The active metabolites of different concentrations extracted from the three isolated actinomycetes, Kocuria rosea, Micrococcus luteus, and Streptomyces longisporoflavus, were tested against Staphylococcus aureus, Bacillus cereus, Escherichia coli, and Salmonella typhi. The obtained results of the agar well diffusion method are illustrated in Table 8.

Table 8.
Agar well diffusion results of the secondary metabolites isolated from the three isolated strains against the studied bacterial indicators after 24 h of incubation.
The findings revealed that the lowest MIC value was recorded against Staphylococcus aureus and Bacillus cereus (93.75 µg/mL) by using the secondary metabolites extracted from Micrococcus luteus and Streptomyces longisporoflavus, while the largest MIC was recorded against Staphylococcus aureus, Escherichia coli, and Bacillus cereus (187.5 µg/mL) by using the secondary metabolites extracted from the three tested isolates.
Concerning MBC, the lowest MBC was recorded against Staphylococcus aureus, Escherichia coli, and Bacillus cereus (500 µg/mL). However, the largest MBC was verified against Staphylococcus aureus and Salmonella typhi (1000 µg/mL) by using the secondary metabolites extracted from Micrococcus luteus and Streptomyces longisporoflavus. The MIC and MBC findings are presented in Table 9.

Table 9.
MIC and MBC against the four indicator microorganisms determined by the broth micro-dilution method.
The antibacterial activity tests of the crude extracts against the four bacterial indicators, as illustrated in Table 9, showed that Kocuria rosea crude extract exhibited a strong bactericidal activity against S. aureus and B. cereus, while Micrococcus luteus crude extract showed strong bactericidal activity against E. coli and strong bacteriostatic activity against B. cereus and S. typhi. On the other hand, Streptomyces longisporoflavus crude extract showed strong bactericidal activity against E. coli and strong bacteriostatic activity against S. aureus, B. cereus, and S. typhi.
4. Discussion
Marine actinomycetes are prospective producers of beneficial secondary metabolites, and they are characterized as having prominent and diverse biological activities. Soil niches are considered rich in a variety of actinomycetes [33,34,35,36]. Marine actinomycetes in Lebanon are not fully characterized; therefore, additional studies are needed to unravel their distribution and role.
In the current study, 32 marine soil samples were collected from Tyre City Beach, specifically from the sandy beach and sea floor, in four different seasons.
The calculation of the quantity of isolates and the number of bacterial colonies (CFU/mL) in the different sampling sites among the four seasons revealed an increased number of bacterial loads during summer. This might be due to a correlation with the level of human activities, since during the summer, beaches often experience an influx of tourists and recreational activities. This increased human presence leads to more organic waste, such as food scraps and human waste, entering the coastal waters. Additionally, the improper disposal of waste and sewage discharge from nearby urban areas can further enrich the nutrient content in these environments. Actinomycetes thrive in nutrient-rich environments, and the added organic matter coupled with warmer temperatures provides a substantial source of nutrients, promoting their proliferation. In addition, activities like walking, playing, and construction on beaches can disturb natural sediments, releasing actinomycetes from the seabed into the water column. In addition, summer often brings more rainfall, leading to increased runoff from urban areas; this runoff can carry soil, organic matter, and pollutants, including fertilizers, pesticides, and organic waste, which can introduce or promote the growth of actinomycetes in coastal waters. Similarly, Goodfellow et al. discussed the impact of pollution, nutrient enrichment, and sediment disturbance on the growth of actinomycetes, offering a comprehensive view of how human actions contribute to their high load on beaches [37]. Das et al. examined the impact of coastal pollution on microbial populations, including actinomycetes, highlighting how human activities contribute to the distribution and abundance of these microorganisms [38]. Moreover, Pathom-aree et al. explained the diversity of actinomycetes in marine sediments and discussed how both natural and human-induced environmental factors, including pollution, influence these microbial communities [39]. A similar result was reported for actinomycete counts in sediment samples, where the highest counts of actinomycetes in seawater were detected in dry seasons, showing that temperature is very likely to be considered the most important ecological factor influencing population sizes [13].
Another factor could be the environmental conditions during summer, such as the temperature and pH of the soil. Notably, the pH is frequently lower during the summer (6 to 8) and early fall. Then, the pH experiences an increase as the soil moisture becomes higher. A decline in soil pH during this time is commonly based on soil drying, root, bacterial activity, and the nitrification of nitrogen fertilizers [40,41]. Furthermore, fertilizers and other chemicals can leach into the sand, where they undergo nitrification, a process that produces nitric acid and lowers pH; also, the decomposition of organic matter by microorganisms produces CO2, which forms carbonic acid when it dissolves in water within the sand, lowering the pH. Increased microbial activity also results in higher concentrations of metabolic byproducts like organic acids, which can further decrease the pH [42]. The most effective and favorable growth conditions for most actinomycetes are a temperature between 25 and 30 °C, neutral pH (6 to 8), and low moisture content [43,44]. According to McCarthy et al., organic matter, salinity, relative moisture, temperature, and pH are essential factors that control the frequency and proliferation of actinomycetes in soil [45]. Furthermore, Atlas et al. revealed that many environmental factors impact the providence of microbes in sand, and this involves abiotic factors, including moisture, temperature, sunlight, human activities, and nutrients. This explains the increase in the microbial load in the summer [46].
On the other hand, actinomycetes can produce several biologically active substances, including antimicrobial, antioxidant, antitumor, herbicidal, and pesticidal compounds. Thus, they serve as a source for various valuable industrial bioactive compounds [47]. In this study, all 10 of the isolated actinomycetes demonstrated different activities against the studied bacterial and fungal indicators. The crude extracts revealed significant antimicrobial activities against the tested organisms. The findings demonstrate that the extracted compounds were considered more active against Gram-positive than Gram-negative bacteria, while 70% of the isolates were active against Candida albicans and 50% against Aspergillus niger and Aspergillus flavus. Similarly, Elbendary et al. reported that four isolates exhibited activity against Candida albicans, two against A. niger, and two against A. flavus [47]. Actinomycetes examined by Rahman et al. exhibited antimicrobial activity against the test microorganisms [48]. In a similar way, Dehnad et al. examined the bioactivity of Streptomyces isolates from Iran [49]. Arifuzzaman et al. documented 20 isolates that showed activity against the test bacteria [50]. Among 287 actinomycetes, 166 isolates were perceived as antagonistic to Candida albicans, while 164, 134, and 132 actinomycetes revealed antagonistic features against A. niger, M. gypseum, and Trichophyton sp., respectively [51]. An actinomycete isolated by Dharumaduari et al. exhibited antimicrobial activity [52]. Furthermore, Ouhdouch et al. demonstrated that 32 out of 320 actinomycete strains isolated from several Moroccan habitats exhibited significant activity against yeast, molds, and bacteria [53]. In one study, Ramasamy et al. isolated 192 morphologically different actinomycete strains from the nearshore marine environment of Point Colimere, on the east coast of India, including 68 strains that displayed a maximum zone of inhibition against B. subtilis (16 mm), E. coli (15 mm), and also against the fungal pathogen Candida albicans (16 mm) [54]. Saurav and Kannabiran isolated 164 actinobacterial strains from samples of 39 sediment items gathered from the Bay of Bengal near the coast of Puducherry and Marakkanam, India. Among them, the selected isolates exhibited antibacterial activity against Bacillus subtilis (18 mm) and antifungal activity against Aspergillus niger (17 mm) [55].
Other results demonstrated that among 52 isolates, 43 isolates possessed antibacterial activity, of which 30 involved isolates exhibited activity against Escherichia coli, 35 isolates against Salmonella typhi, 12 isolates against Bacillus cereus, and 8 isolates against Klebsiella pneumonia, while 9 isolates demonstrated no activity against any human bacterial pathogens [34]. Similarly, Sathiyaseelan et al. performed an isolation process on five marine actinomycetes, out of which one isolate was active against the human pathogens Escherichia coli, Klebsiella pneumonia, Salmonella typhi, and Vibrio cholera [56]. Additionally, Shantikumar et al. measured 37 actinomycetes from lake sediments, out of which 21 isolates demonstrated antimicrobial activity, and only 12 active isolates showed good antifungal activity [57]. Lastly, Kathiresan et al. isolated 160 marine actinomycetes, and 31% of them demonstrated high efficacy and activity against Rhizoctonia solani [58].
Parameters such as temperature, initial pH, and others demonstrate a significant impact on the production of bioactive metabolites. A convenient initial pH is critical for an effective fermentation process; nevertheless, it can vary from one strain to another. Therefore, optimum growth conditions to ensure higher yields and more efficient production of antimicrobial substances from three different isolated species, Kocuria rosea, Micrococcus luteus, and Streptomyces longisporoflavus, were investigated. The results revealed that, for Kocuria rosea, the maximum effectiveness in production was accomplished after using mannitol and ammonium sulfate at concentrations of 0.2 and 2.5%, respectively. Additional factors such as pH 7.0, a starting inoculum of 2%, and incubation for 11 days at 30 °C contributed to a high production rate of antimicrobial substances. On the other hand, it was found that for Micrococcus luteus, maximum productivity was achieved after the use of glucose and peptone at concentrations of 0.5 and 1%, respectively. Extra factors such as pH 7.0, a starting inoculum of 2%, and incubation for 7 days at 30 °C contributed to the extensive generation of antimicrobial substances. Furthermore, for Streptomyces longisporoflavus, the most effective productivity rate was observed after the use of starch and peptone at concentrations of 1%. In other cases, a pH 7.0, a starting inoculum of 2%, and incubation for 8 days at 30 °C were considered contributors to the production a great amount of antimicrobial substances. In the presence of high concentrations of carbon and nitrogen sources, actinomycetes may allocate more energy and resources to primary metabolic processes (such as cell division and growth) rather than secondary metabolism. The increased availability of carbon and nitrogen sources can lead to an abundance of ATP and precursor metabolites, driving the cell to prioritize growth over the production of secondary metabolites. Also, high concentrations of carbon and nitrogen sources, by signaling a nutrient-rich environment, can suppress the activation of the regulatory pathways responsible for the production of secondary metabolites, leading to reduced expression of secondary metabolite biosynthetic genes [59,60,61,62].
Glucose and ammonium nitrate in synthetic media were the ideal carbon and nitrogen sources to ensure a high production rate of antibiotic metabolites [63]. In addition, arabinose and dextrose were the paramount carbon sources, and L-asparagine was the most notable nitrogen source in one investigation [29]. In their study, Pandey et al. experimented with a number of carbon and nitrogen compounds, emphasizing their impact on the production of an antibacterial antibiotic by Streptomyces kanamyceticus M27. It was revealed that dextrose constituted a highly convenient carbon source; however, maltose, sucrose, and soluble starch produced moderate results. (NH4)H2PO4 and yeast extract were considered suitable nitrogen sources with respect to the production of the antibiotic. It was established that media with an alkaline pH supported high antibiotic production [64].
MIC values ranged from 93.75 to 187.5 µg/mL. The lowest MIC was recorded against Bacillus cereus and Staphylococcus aureus (93.75 µg/mL) in the presence of secondary metabolites extracted from Micrococcus luteus and Streptomyces longisporoflavus, while largest MIC was recorded against Staphylococcus aureus, Escherichia coli, Bacillus cereus, and Salmonella typhi in the presence of secondary metabolites extracted from Kocuria rosea, Micrococcus luteus, and Streptomyces longisporoflavus (187.5 µg/mL). In other studies, MIC values, which ranged from 0.77 to 50 µg/mL, were examined in experiments with all indicator microorganisms using the broth micro-dilution method. Notably, the lowermost MIC was verified against Sarcina lutea (6.25 µg/mL), while the highest MIC was recorded against Candida albicans and Escherichia coli ATCC 8739 (25 µg/mL). Similarly, the recorded MIC was 12.5 µg/mL for Staphylococcus aureus ATCC 6538 and Bacillus subtilis [65].
MBC values ranged from 500 to 1000 µg/mL. The lowest MBC was recorded against Bacillus cereus, Staphylococcus aureus, and Escherichia coli (500 µg/mL) in the presence of secondary metabolites extracted from Kocuria rosea, Micrococcus luteus, and Streptomyces longisporoflavus, while largest MBC was recorded against Staphylococcus aureus and Salmonella typhi in the presence of secondary metabolites extracted from Micrococcus luteus and Streptomyces longisporoflavus (1000 µg/mL). Similarly, Sharmin et al. reported that the MIC values of an extract isolated from Streptomyces against Bacillus cereus, Streptococcus agalactiae, Pseudomonas aeruginosa, and Escherichia coli were 1, 1, 1, and 2 µg/mL, respectively, while the MBC values were 4, 4, 4, and 8 µg/mL, respectively. Furthermore, the crude extract was also active against Candida albicans and Aspergillus flavus, and the minimum antifungal concentration values were 64 and 8 µg/mL, respectively [66]. In addition, Saravanakumar, assessing the ethyl acetate extract of the soil actinobacterium Streptomyces lavendulae (SCA5), observed antimicrobial activity, with an MIC value of 31.25 mg/mL [67].
Bactericidal compounds destroy microorganisms, whereas bacteriostatic compounds prevent the division of cells. If the MIC index is ≤4, the compound will be regarded as bactericidal, but if it is >4 and <32, the compound will be considered bacteriostatic [68]. Results from this study reveal that the crude extracts have strong bactericidal and bacteriostatic activities against the bacterial indicators. Kocuria rosea crude extract showed significant bactericidal activity against S. aureus and B. cereus, while Micrococcus luteus crude extract showed strong bactericidal activity against E. coli and strong bacteriostatic activity against B. cereus and S. typhi. On the other hand, Streptomyces longisporoflavus crude extract showed strong bactericidal activity against E. coli and strong bacteriostatic activity against S. aureus, B. cereus, and S. typhi. Similarly, according to Pudi et al., the findings of their study showed that a crude alkaloid extract had significant bactericidal activity against S. aureus [68].
Thus, the current findings demonstrate that the isolated crude extracts can serve as a potential source of antibiotic compounds.
5. Conclusions
Considering the necessary approach for new, secure, and more efficient and effective antimicrobial antibiotics in medical research, the current study emphasized the biotechnological potential of actinomycetes in the management of multiple bacteria and fungi that cause illnesses. Further, the fermentation conditions required for optimal antibiotic production are easily manageable with actinomycetes, which is here reported for the first time in context of Lebanon’s Tyre City Beach. Antimicrobial metabolites can be produced under optimal conditions, purified, and utilized as biocontrol agents for the management of bacterium- and fungus-triggered diseases. In addition, many nanomaterials can be used along with actinomycetes to enhance the remediation efficiency. Thus, microbial nanoremediation is the future for bioremediation applications such as the removal of heavy metals, poisonous gas sensing, textile coatings for wearable electronic devices, biomedical applications, and the aforementioned photocatalytic degradation of organic contaminants.
While this study focuses on isolating and characterizing actinomycetes with antimicrobial potential, future research aims to include field trials to assess the performance of these compounds in diverse environmental and clinical settings. Such trials will provide valuable data on the metabolites’ effectiveness, stability, and scalability, helping to bridge the gap between laboratory findings and practical applications in medicine and biotechnology.
Author Contributions
H.K.D., Methodology, Formal analysis, Investigation, Data curation, Writing—original draft. M.I.K., Conceptualization, Formal analysis, Validation, Writing—review & editing, Supervision. R.E.H., Conceptualization, Formal analysis, Validation, Writing—review & editing, Supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data generated and analyzed during this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
There are no conflicts of interest to declare.
Appendix A


Figure A1.
Gram stain results of the 10 isolated actinomycetes.

Figure A2.
Effect of (a). carbon sources, (b). nitrogen sources, (c). initial pH of medium, (d). inoculum size (e). incubation period and (f). incubation temperature on antibacterial activity of Kocuria rosea against B. cereus.
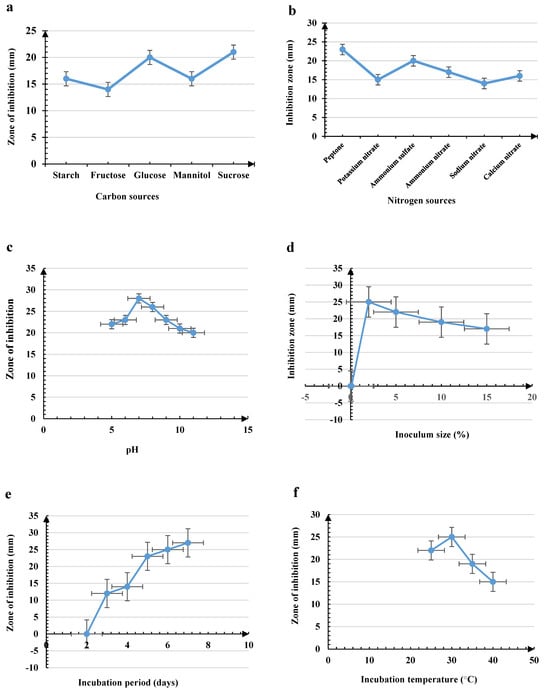
Figure A3.
Effect of (a). carbon sources, (b). nitrogen sources, (c). initial pH of medium, (d). inoculum size (e). incubation period and (f). incubation temperature on antibacterial activity of Micrococcus luteus against B. cereus.

Figure A4.
Effect of (a). carbon sources, (b). nitrogen sources, (c). initial pH of medium, (d). inoculum size (e). incubation period and (f). incubation temperature on antibacterial activity of Streptomyces longisporoflavus against B. cereus.

Table A1.
Calculated p-value and significance levels of the agar well diffusion results of the crude extracts from the three studied actinomycetes against the four bacterial indicators.
Table A1.
Calculated p-value and significance levels of the agar well diffusion results of the crude extracts from the three studied actinomycetes against the four bacterial indicators.
| No. | Spp. | Concentration of the Secondary Metabolites (Crude Extract) (mg/mL) | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Gram Positive Bacteria | Gram Negative Bacteria | |||||||||
| S. aureus | Significance | B. cereus | Significance | E. coli | Significance | S. typhi | Significance | |||
| 1 | Kocuria rosea | 0.0625 | ND | ND | ND | ND | ND | ND | ND | ND |
| 0.125 | ND | ND | ND | ND | ND | ND | ND | ND | ||
| 0.25 | 0.0006 | *** | 0.0002 | *** | ND | ND | ND | ND | ||
| 2 | Micrococcus luteus | 0.0625 | ND | ND | ND | ND | ND | ND | ND | ND |
| 0.125 | ND | ND | 0.001 | ** | ND | ND | ND | ND | ||
| 0.25 | ND | ND | 0.0006 | *** | 0.0002 | *** | 0.002 | ** | ||
| 3 | Streptomyces longisporoflavus | 0.0625 | ND | ND | ND | ND | ND | ND | ND | ND |
| 0.125 | 0.001 | ** | 0.0006 | *** | ND | ND | ND | ND | ||
| 0.25 | 0.0001 | *** | 0.0004 | *** | 0.0001 | *** | 0.0001 | *** | ||
ND: Not determined, p-values were calculated such that: ** p < 0.01, *** p < 0.001.
References
- Dilip, C.V.; Mulaje, S.; Mohalkar, R. A review on actinomycetes and their biotechnological application. Int. J. Pharm. Sci. Res. 2013, 4, 1730–1742. [Google Scholar]
- Sacramento, D.R.; Coelho, R.R.R.; Wigg, M.D.; Toledo Luna Linhares, L.F.D.; Matos dos Santos, M.G.; Azevedo Soares Semêdo, L.T.D.; Ribeiro da Silva, A.J. Antimicrobial and antiviral activities of an actinomycete (Streptomyces sp.) isolated from a Brazilian tropical forest soil. World J. Microbiol. Biotechnol. 2004, 20, 225–229. [Google Scholar] [CrossRef]
- Atta, H.M. An antifungal agent produced by Streptomyces olivaceiscleroticus, AZ-SH514. World Appl. Sci. J. 2009, 6, 1495–1505. [Google Scholar]
- Fukuchi, N.; Futaki, F.; Kito, M.; Sato, S.; Kajiura, T.; Ono, Y. Substance with Antithrombotic Activity and Method for Detecting Glycokallidin. U.S. Patent 7,608,695, 27 October 2009. [Google Scholar]
- Olano, C.; Méndez, C.; Salas, J.A. Antitumor compounds from actinomycetes: From gene clusters to new derivatives by combinatorial biosynthesis. Nat. Prod. Rep. 2009, 26, 628–660. [Google Scholar] [CrossRef]
- Ser, H.L.; Palanisamy, U.D.; Yin, W.F.; Abd Malek, S.N.; Chan, K.G.; Goh, B.H.; Lee, L.H. Presence of antioxidative agent, Pyrrolo [1, 2-a] pyrazine-1, 4- dione, hexahydro-in newly isolated Streptomyces mangrovisoli sp. nov. Front. Microbiol. 2015, 6, 854. [Google Scholar] [CrossRef]
- Berdy, J. Bioactive microbial metabolites. J. Antibiot. 2005, 58, 1–26. [Google Scholar] [CrossRef]
- Riedlinger, J.; Reike, A.; Zahner, H.; Krismer, B.; Bull, A.T.; Maldonado, L.A.; Ward, A.C.; Goodfellow, M.; Bister, B.; Bischoff, D.; et al. Abyssomicins, Inhibitors of the Para-aminobenzoic Acid. J. Antibiot. 2004, 57, 271–279. [Google Scholar] [CrossRef]
- Fehling, R.H.; Buchanan, G.O.; Mincer, T.J.; Kauffman, C.A.; Jensen, P.R.; Fenical, W. Salinosporamide A: A Highly Cytotoxic Proteasome Inhibitor From A Novel Microbial Source, A Marine Bacterium of the New Genus Salinospora. Angew. Chem. Int. Ed. 2003, 42, 355–357. [Google Scholar] [CrossRef]
- Hughes, C.; Prieto Davo, A.; Jensen, P.R.; Fenical, W. The Marynopyrroles, Antibiotics of an Unprecedented Structure Class from a Marine Streptomyces sp. Org. Lett. 2008, 10, 629–631. [Google Scholar] [CrossRef]
- Maldonado, L.A.; Fenical, W.; Jensen, P.R.; Kauffman, C.A.; Mincer, T.J.; Ward, A.C.; Bull, A.T.; Goodfellow, M. Salinispora arenicola gen. nov., sp. nov. and Salinispora tropica sp. nov., Obligate Marine Actinomycetes Belonging to the Family Micromonosporaceae. Int. J. Syst. Evol. Microbiol. 2005, 55, 1759–1766. [Google Scholar] [CrossRef]
- Baltz, R.H. Renaissance in antibacterial discovery from actinomycetes. Curr. Opin. Pharmacol. 2008, 8, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Ghanem, N.B.; Sabry, S.A.; El-Sherif, Z.M.; Abu El-Ela, G.A. Isolation and enumeration of marine actinomycetes from seawater and sediments in Alexandria. J. Gen. Appl. Microbiol. 2000, 46, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Zeng, W.; Huang, Y.; Yang, Z.; Li, J.; Cai, H.; Su, W. Detection of antitumor and antimicrobial activities in marine organism associated actinomycetes isolated from the Taiwan Strait, China. FEMS Microbiol. Lett. 2000, 188, 87–91. [Google Scholar] [CrossRef]
- Gupta, A.; Singh, D.; Singh, S.K.; Singh, V.K.; Singh, A.V.; Kumar, A. Role of actinomycetes in bioactive and nanoparticle synthesis. In Role of Plant Growth Promoting Microorganisms in Sustainable Agriculture and Nanotechnology; Woodhead Publishing: Sawston, UK, 2019; pp. 163–182. [Google Scholar]
- Ameri, A.; Shakibaie, M.; Ameri, A.; Faramarzi, M.A.; Amir-Heidari, B.; Forootanfar, H. Photocatalytic decolorization of bromothymol blue using biogenic selenium nanoparticles synthesized by terrestrial actinomycete Streptomyces griseobrunneus strain FSHH12. Desalination Water Treat. 2016, 57, 21552–21563. [Google Scholar] [CrossRef]
- Fuentes, M.S.; Benimeli, C.S.; Cuozzo, S.A.; Amoroso, M.J. Isolation of pesticide-degrading actinomycetes from a contaminated site: Bacterial growth, removal and dechlorination of organochlorine pesticides. Int. Biodeterior. Biodegrad. 2010, 64, 434–441. [Google Scholar] [CrossRef]
- Fuentes, M.S.; Briceño, G.E.; Saez, J.M.; Benimeli, C.S.; Diez, M.C.; Amoroso, M.J. Enhanced removal of a pesticides mixture by single cultures and consortia of free and immobilized Streptomyces strains. BioMed Res. Int. 2013, 2013, 392573. [Google Scholar] [CrossRef]
- Abou Fayad, A.; Itani, D.; Miari, M.; Tanelian, A.; Iweir, S.; Matar, G.M. From bugs to drugs: Combating antimicrobial resistance by discovering novel antibiotics. J. Infect. Dev. Ctries. 2018, 12, 3S. [Google Scholar] [CrossRef]
- Araj, G.F.; Baba, O.Z.; Itani, L.Y.; Avedissian, A.Z.; Sobh, G.M. Non-tuberculous mycobacteria profiles and their anti-mycobacterial resistance at a major medical center in Lebanon. J. Infect. Dev. Ctries. 2019, 13, 612–618. [Google Scholar] [CrossRef]
- Rim, I.W. Novel Anti-Biolfilm Natural Products from Microorganisms Isolated from Lebanon; AUB Scholar Works; American University of Beirut: Beirut, Lebanon, 2009. [Google Scholar]
- Abou Fayad, A.; Itani, D.; Miari, M.; Tanelian, A.; Matar, G.M. Combating antimicrobial resistance by utilizing novel antibiotics from soil and marine microorganisms in Lebanon. J. Infect. Dev. Ctries. 2019, 79, 52. [Google Scholar]
- Velasco-Alzate, K.Y.; Bauermeister, A.; Tangerina, M.M.; Lotufo, T.M.; Ferreira, M.J.; Jimenez, P.C.; Padilla, G.; Lopes, N.P.; Costa-Lotufo, L.V. Marine bacteria from Rocas Atoll as a rich source of pharmacologically active compounds. Mar. Drugs 2019, 17, 671. [Google Scholar] [CrossRef]
- Kumar, S.; Kannabiran, K. Diversity and Optimization of Process Parameters for the Growth of Streptomyces VITSVK9 spp. Isolated from Bay of Bengal, India. J. Nat. Env. Sci. 2010, 1, 56–65. [Google Scholar]
- Aghamirian, M.R.; Ghiasian, S.A. Isolation and characterization of medically important aerobic actinomycetes in soil of iran (2006–2007). Open Microbiol. J. 2009, 3, 53–57. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Reddy, N.; Ramakrishna, D.; Raja Gopal, S. A morphological, physiological and biochemical studies of marine Streptomyces rochei (MTCC 10109) showing antagonistic activity against selective human pathogenic microorganisms. Asian J. Biol. Sci. 2011, 4, 1–14. [Google Scholar] [CrossRef][Green Version]
- Dundar, H.; Brede, D.A.; La Rosa, S.L.; El-Gendy, A.O.; Diep, D.B.; Nes, I.F. The fsr quorum-sensing system and cognate gelatinase orchestrate the expression and processing of proprotein EF_1097 into the Mature antimicrobial peptide enterocin O16. J. Bacteriol. 2015, 197, 2112–2121. [Google Scholar] [CrossRef]
- Selvin, J.; Shanmughapriya, S.; Gandhimathi, R.; Seghal Kiran, G.; Rajeetha Ravji, T.; Natarajaseenivasan, K.; Hema, T.A. Optimization and production of novel antimicrobial agents from sponge associated marine actinomycetes Nocardiopsis dassonvillei MAD08. Appl. Microbiol. Biotechnol. 2009, 83, 435–445. [Google Scholar] [CrossRef]
- Kadiri, S.K.; Yarla, N.S. Optimization of antimicrobial metabolites production by Streptomyces fradiae. Int. J. Pharm. Pharm. Sci. 2016, 7, 223–225. [Google Scholar]
- Selvameenal, L.; Radhakrishnan, M.; Balagurunathan, R. Antibiotic pigment from desert soil actinomycetes; biological activity, purification and chemical screening. Indian J. Pharm. Sci. 2009, 71, 499–504. [Google Scholar]
- Janardhan, A.; Kumar, A.P.; Viswanath, B.; Saigopal, D.V.R.; Narasimha, G. Production of bioactive compounds by actinomycetes and their antioxidant properties. Biotechnol. Res. Int. 2014, 20, 217030. [Google Scholar] [CrossRef]
- Parvekar, P.; Palaskar, J.; Metgud, S.; Maria, R.; Dutta, S. The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of silver nanoparticles against Staphylococcus aureus. Biomater. Investig. Dent. 2020, 7, 105–109. [Google Scholar] [CrossRef]
- Savic, M.; Bratic, I.; Vasiljevic, B. Streptomyces durmitorensis sp. nov., a producer of an FK506-like immunosuppressant. Int. J. Syst. Evol. Microbiol. 2007, 57, 2119–2124. [Google Scholar] [CrossRef]
- Tan, L.; Ser, H.; Yin, W.; Chan, K.; Lee, L.; Goh, B. Investigation of Antioxidative and Anticancer Potentials of Streptomyces sp. MUM256 Isolated from Malaysia Mangrove Soil. Front. Microbiol. 2015, 6, 1316. [Google Scholar] [CrossRef] [PubMed]
- Mohanraj, G.; Sekar, T. Isolation and Screening of Actinomycetes from Marine Sediments for their Potential to Produce Antimicrobials. Int. J. Life Sci. Pharm. Res. 2013, 2, 115–126. [Google Scholar]
- Kumar, S.; Kannabiran, K. Antifungal Activity of Streptomyces VITSVK5 spp. Against Drug Resistant Aspergillus Clinical Isolates from Pulmonary Tuberculosis Patients. J. Mycol. Medicale 2010, 20, 101–107. [Google Scholar] [CrossRef]
- Goodfellow, M.; Fiedler, H.-P. A guide to successful bioprospecting: Informed by actinobacterial systematics. Antonie Van Leeuwenhoek 2010, 98, 119–142. [Google Scholar] [CrossRef]
- Das, S.; Lyla, P.S.; Khan, S.A. Marine microbial diversity and the impact of the coastal environment on microbial populations, including actinomycetes. Curr. Sci. 2006, 90, 1175–1183. [Google Scholar]
- Pathom-aree, W.; Stach JE, M.; Ward, A.C.; Horikoshi, K.; Bull, A.T.; Goodfellow, M. Diversity of actinomycetes isolated from Challenger Deep sediment (10,898 m) from the Mariana Trench. Extremophiles 2006, 10, 181–189. [Google Scholar] [CrossRef]
- Brady, N.; Weil, R. The Nature and Properties of Soils; Pearson Education: Bengaluru, India, 1999; 359p. [Google Scholar]
- James, D.W.; Wells, K.L. Soil Testing and Plant Analysis; SSSA Book Series 3; Wiley: Hoboken, NJ, USA, 1990; Volume 3, p. 40. [Google Scholar]
- Doney, S.C.; Fabry, V.J.; Feely, R.A.; Kleypas, J.A. Ocean acidification: The other CO2 problem. Annu. Rev. Mar. Sci. 2009, 1, 169–192. [Google Scholar] [CrossRef]
- Barka, E.A.; Vetsa, P.; Sanchez, L.; Gaveau-Vaillant, N.; Jacquard, C.; Klenk, H.-P.; Clément, C.; Ouhdouch, Y.; Van Wezel, G.P. Taxonomy, physiology, and natural products of actinobacteria. Microbiol. Mol. Biol. Rev. 2015, 80, 1–43. [Google Scholar] [CrossRef]
- Bhatti, A.A.; Haq, S.; Bhat, R.A. Actinomycetes benefaction role in soil and plant health. Microb. Pathog. 2017, 111, 458–467. [Google Scholar] [CrossRef]
- McCarthy, A.J.; Williams, S.T. Actinomycetes as Agents of Biodegradation in the Environment—A review. Gene 1992, 115, 189–192. [Google Scholar] [CrossRef]
- Atlas, R.M.; Bartha, R. Microbial Ecology: Fundamentals and Applications; Benjamin-Cummings Publishing Co.: Menlo Park, CA, USA, 1997; pp. 309–313. [Google Scholar]
- Elbendary, A.A.; Hessain, A.M.; El-Hariri, M.D.; Seida, A.A.; Moussa, I.M.; Mubarak, A.S.; Kabli, S.A.; Hemeg, H.A.; El Jakee, J.K. Isolation of Antimicrobial Producing Actinobacteria from Soil samples. Saudi J. Biol. Sci. 2018, 25, 44–46. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Islam, M.Z.; Islam, A.U. Antibacterial Activities of Actinomycete Isolates Collected from Soils of Rajshahi, Bangladesh. Biotechnol. Res. Int. 2011, 6, 857925. [Google Scholar] [CrossRef] [PubMed]
- Dehnad, A.; Parsa, L.; Bakhshi, R.; Soofiani, S.A.; Mokhtarzadeh, A. Investigation Antibacterial Activity of Streptomycetes Isolates from Soil Samples, West of Iran. Afr. J. Microbiol. Res. 2010, 4, 1542–1549. [Google Scholar]
- Arifuzzaman, M.; Khatun, M.R.; Rahman, H. Isolation and Screening of Actinomycetes from Sundarbans Soil for Antibacterial Activity. Afri. J. Biotech. 2010, 9, 4615–4619. [Google Scholar]
- Jain, P.K.; Jain, P.C. Antifungal Activity of Some Actinomycetes Isolated from Various Habitats. Hindustan Antibiot. Bull. 2003, 45–46, 5–10. [Google Scholar]
- Dharumaduari, D.; Nooruddin, T.; Annamalai, P. An Antifungal Compound: 40 Phenyl-1-napthyl- phenyl Acetamide from Streptomyces Species Universitatis Series. Med. Biol. 2008, 15, 7–12. [Google Scholar]
- Ouhdouch, Y.; Barakate, M.; Finance, C. Actinomycetes of Moroccan habitats: Isolation and Screening for Antifungal Activities. Eur. J. Soil. Biol. 2001, 37, 69–74. [Google Scholar] [CrossRef]
- Ramasamy, V.; Subban, M.; Annamalai, P. Isolation, characterization and antimicrobial activity of Actinobacteria from point Calimere coastal region, east coast of India. Int. Res. J. Pharm. 2010, 1, 358–365. [Google Scholar]
- Saurav, K.; Kannabiran, K. Cytotoxicity and antioxidant activity of 5-(2,4- Dimethylbenzyl) Pyrrolidin-2-one extracted from marine Streptomyces VITSVK5 spp. Saudi J. Biol. Sci. 2012, 19, 81–86. [Google Scholar] [CrossRef]
- Sathiyaseelan, K.; Stella, D. Isolation, Identification and Antimicrobial Activity of Marine Actinomycetes Isolated from Parangipettai. Recent Res. Sci. Technol. 2011, 3, 74–77. [Google Scholar]
- Shantikumar Singh, L.; Indra baruah Bora, T.C. Actinomycetes of Loktak Habitat; Isolation and Screening for Antimicrobial Activitie. Biotechnology 2006, 5, 217–221. [Google Scholar] [CrossRef]
- Kathiresan, K.; Balagurunathan, R.; Masilamani Selvam, M. Fungicidal Activity of Marine Actinomycetes against Phytopathogemic fungi. Indian J. Biotechnol. 2005, 4, 271–276. [Google Scholar]
- Sánchez, S.; Demain, A.L. Metabolic regulation of fermentation processes. Enzym. Microb. Technol. 2008, 31, 895–906. [Google Scholar] [CrossRef]
- Sánchez, S.; Chávez, A.; Forero, A.; García-Huante, Y.; Romero, A.; Sánchez, M.; Rocha, D. Carbon source regulation of antibiotic production. J. Antibiot. 2010, 63, 442–459. [Google Scholar] [CrossRef]
- Martín, J.F.; Liras, P. Organization and expression of genes involved in the biosynthesis of antibiotics and other secondary metabolites. Annu. Rev. Microbiol. 1989, 43, 173–206. [Google Scholar] [CrossRef]
- Martín, J.F.; Demain, A.L. Control of antibiotic biosynthesis. Microbiol. Mol. Biol. Rev. 1980, 44, 230–251. [Google Scholar] [CrossRef]
- Sujatha, P.; Raju, K.B.; Ramana, T. Studies on a new Marine Streptomycete BT-408 Producing Polyketide Antibiotic SBR-22 Effective Against Methicillin Resistant Staphylococcus aureus. Microbiol. Res. 2005, 160, 119–126. [Google Scholar] [CrossRef]
- Pandey, A.; Shukla, A.; Majumdar, S. Utilization of Carbon and Nitrogen Sources by Streptomyces kanamyceticus M 27 for the Production of an Anti-Bacterial Antibiotic. Afr. J. Biotechnol. 2005, 4, 909–910. [Google Scholar]
- Ahmad, M.S.; El-Gendy, A.O.; Ahmed, R.R.; Hassan, H.M.; El-Kabbany, H.M.; Merdash, A.G. Exploring the Antimicrobial and Antitumor Potentials of Streptomyces sp. AGM12-1 Isolated from Egyptian Soil. Front. Microbiol. 2017, 8, 438. [Google Scholar] [CrossRef]
- Sharmin, T.; Rahman, M.d.-A.; Anisuzzaman AS-Md Ul-Islam, M.d.-A. Antimicrobial and Cytotoxic Activities of Secondary Metabolites Obtained from a Novel Species of Streptomyces. Bangladesh Pharm. J. 2013, 16, 15–19. [Google Scholar] [CrossRef]
- Saravanakumar, P. In-vitro antimicrobial, antioxidant and cytotoxic properties of Streptomyces lavendulae strain SCA5. Biomed. Microbiol. 2014, 14, 291. [Google Scholar]
- Pudi, N.; Varikuti, G.D.; Badana, A.K.; Gavara, M.M.; Kumari, S.; Malla, R. Studies on Optimization of Growth Parameters for Enhanced Production of Antibiotic Alkaloids by Isolated Marine actinomycetes. J. Appl. Pharm. Sci. 2016, 6, 181–188. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).