Surveillance of Bacterial Meningitis in the Italian Hospital of Desio: A Twenty-Year Retrospective Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Statistics
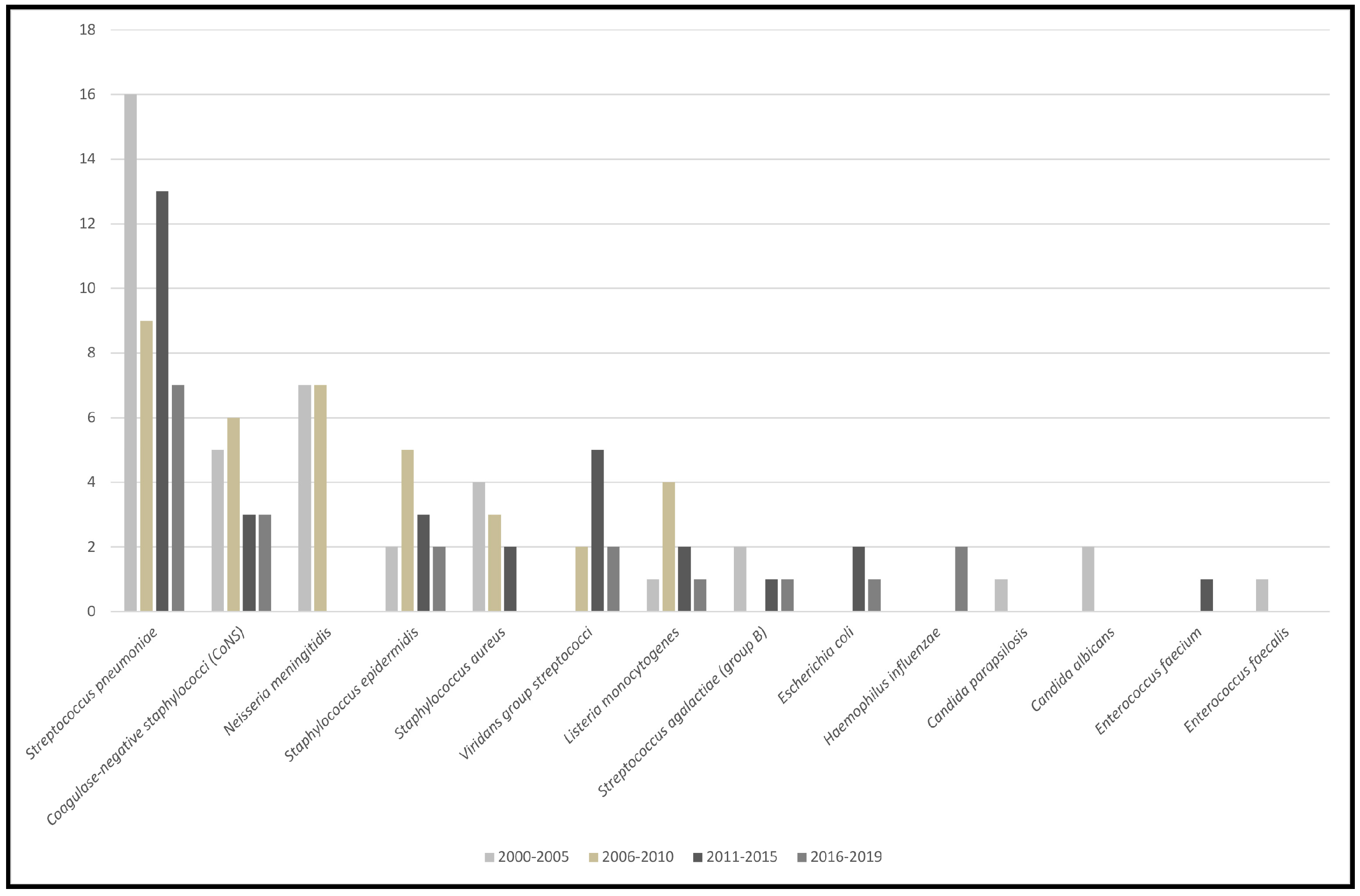
3. Results and Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hasbun, R. Progress and Challenges in Bacterial Meningitis: A Review. JAMA 2022, 328, 2147–2154. [Google Scholar] [CrossRef] [PubMed]
- Andrianou, X.D.; Riccardo, F.; Caporali, M.G.; Fazio, C.; Neri, A.; Vacca, P.; Ambrosio, L.; Pezzotti, P.; Stefanelli, P. Evaluation of the national surveillance system for invasive meningococcal disease, Italy, 2015–2018. PLoS ONE 2021, 16, e0244889. [Google Scholar] [CrossRef] [PubMed]
- Jayaraman, Y.; Veeraraghavan, B.; Kumar, C.G.; Sukumar, B.; Rajkumar, P.; Kangusamy, B.; Verghese, V.P.; Varghese, R.; Jayaraman, R.; Kapoor, A.N.; et al. Hospital-based sentinel surveillance for bacterial meningitis in under-five children prior to the introduction of the PCV13 in India. Vaccine 2021, 39, 3737–3744. [Google Scholar] [CrossRef] [PubMed]
- Giorgi Rossi, P.; Mantovani, J.; Ferroni, E.; Forcina, A.; Stanghellini, E.; Curtale, F.; Borgia, P. Incidence of bacterial meningitis (2001–2005) in Lazio, Italy: The results of a integrated surveillance system. BMC Infect. Dis. 2009, 9, 13. [Google Scholar] [CrossRef] [PubMed]
- Butsashvili, M.; Kandelaki, G.; Eloshvili, M.; Chlikadze, R.; Imnadze, P.; Avaliani, N. Surveillance of bacterial meningitis in the country of Georgia, 2006–2010. J. Community Health 2013, 38, 724–726. [Google Scholar] [CrossRef] [PubMed]
- Barichello, T.; Rocha Catalão, C.H.; Rohlwink, U.K.; Kuip, M.V.D.; Zaharie, D.; Solomons, R.S.; van Toorn, R.; Tutu van Furth, M.; Hasbun, R.; Iovino, F.; et al. Bacterial meningitis in Africa. Front. Neurol. 2023, 14, 822575. [Google Scholar] [CrossRef] [PubMed]
- Mwenda, J.M.; Soda, E.; Weldegebriel, G.; Katsande, R.; Biey, J.N.M.; Traore, T.; de Gouveia, L.; du Plessis, M.; von Gottberg, A.; Antonio, M.; et al. Pediatric Bacterial Meningitis Surveillance in the World Health Organization African Region Using the Invasive Bacterial Vaccine-Preventable Disease Surveillance Network, 2011–2016. Clin. Infect. Dis. 2019, 69 (Suppl. S2), S49–S57. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Cohen, A.L.; Schwartz, S.; Mwenda, J.M.; Weldegebriel, G.; Biey, J.N.; Katsande, R.; Ghoniem, A.; Fahmy, K.; Rahman, H.A.; et al. The Global Landscape of Pediatric Bacterial Meningitis Data Reported to the World Health Organization-Coordinated Invasive Bacterial Vaccine-Preventable Disease Surveillance Network, 2014–2019. J. Infect. Dis. 2021, 224 (Suppl. S2), S161–S173. [Google Scholar] [CrossRef] [PubMed]
- Ministero della Salute. National Immunization Prevention Plan 2017–2019; Italian Official Gazette: Rome, Italy, 2017; Available online: www.gazzettaufficiale.it/eli/id/2017/02/18/17A01195/sg (accessed on 1 January 2020).
- Subbarao, S.; Ribeiro, S.; Campbell, H.; Okike, I.; Ramsay, M.E.; Ladhani, S.N. Trends in laboratory-confirmed bacterial meningitis (2012–2019): National observational study, England. Lancet Reg. Health–Eur. 2023, 32, 100692. [Google Scholar] [CrossRef] [PubMed]
- Azimi, T.; Mirzadeh, M.; Sabour, S.; Nasser, A.; Fallah, F.; Pourmand, M.R. Coagulase-negative staphylococci (CoNS) meningitis: A narrative review of the literature from 2000 to 2020. New Microbes New Infect. 2020, 37, 100755. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Intra, J.; Carcione, D.; Sala, R.M.; Siracusa, C.; Brambilla, P.; Leoni, V. Surveillance of Bacterial Meningitis in the Italian Hospital of Desio: A Twenty-Year Retrospective Study. Appl. Microbiol. 2024, 4, 481-485. https://doi.org/10.3390/applmicrobiol4010033
Intra J, Carcione D, Sala RM, Siracusa C, Brambilla P, Leoni V. Surveillance of Bacterial Meningitis in the Italian Hospital of Desio: A Twenty-Year Retrospective Study. Applied Microbiology. 2024; 4(1):481-485. https://doi.org/10.3390/applmicrobiol4010033
Chicago/Turabian StyleIntra, Jari, Davide Carcione, Roberta Maria Sala, Claudia Siracusa, Paolo Brambilla, and Valerio Leoni. 2024. "Surveillance of Bacterial Meningitis in the Italian Hospital of Desio: A Twenty-Year Retrospective Study" Applied Microbiology 4, no. 1: 481-485. https://doi.org/10.3390/applmicrobiol4010033
APA StyleIntra, J., Carcione, D., Sala, R. M., Siracusa, C., Brambilla, P., & Leoni, V. (2024). Surveillance of Bacterial Meningitis in the Italian Hospital of Desio: A Twenty-Year Retrospective Study. Applied Microbiology, 4(1), 481-485. https://doi.org/10.3390/applmicrobiol4010033






