A Non-Electrolysis Bioelectric Effect for Gingivitis and Hygiene Contamination Biofilm Removal
Abstract
1. Introduction
2. Materials and Methods
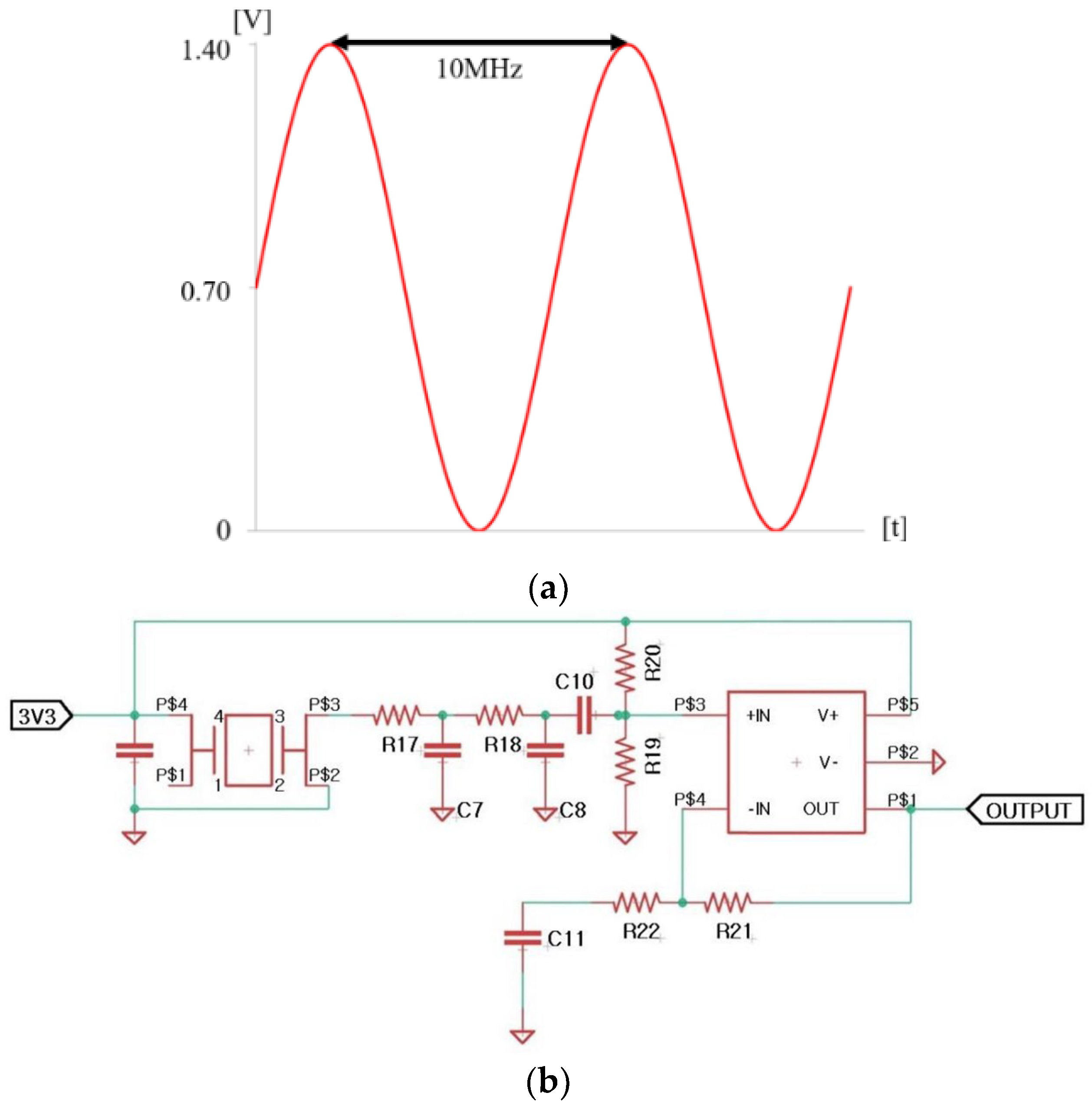
2.1. Description of Bioelectric Effect (BE) and Applied Systems
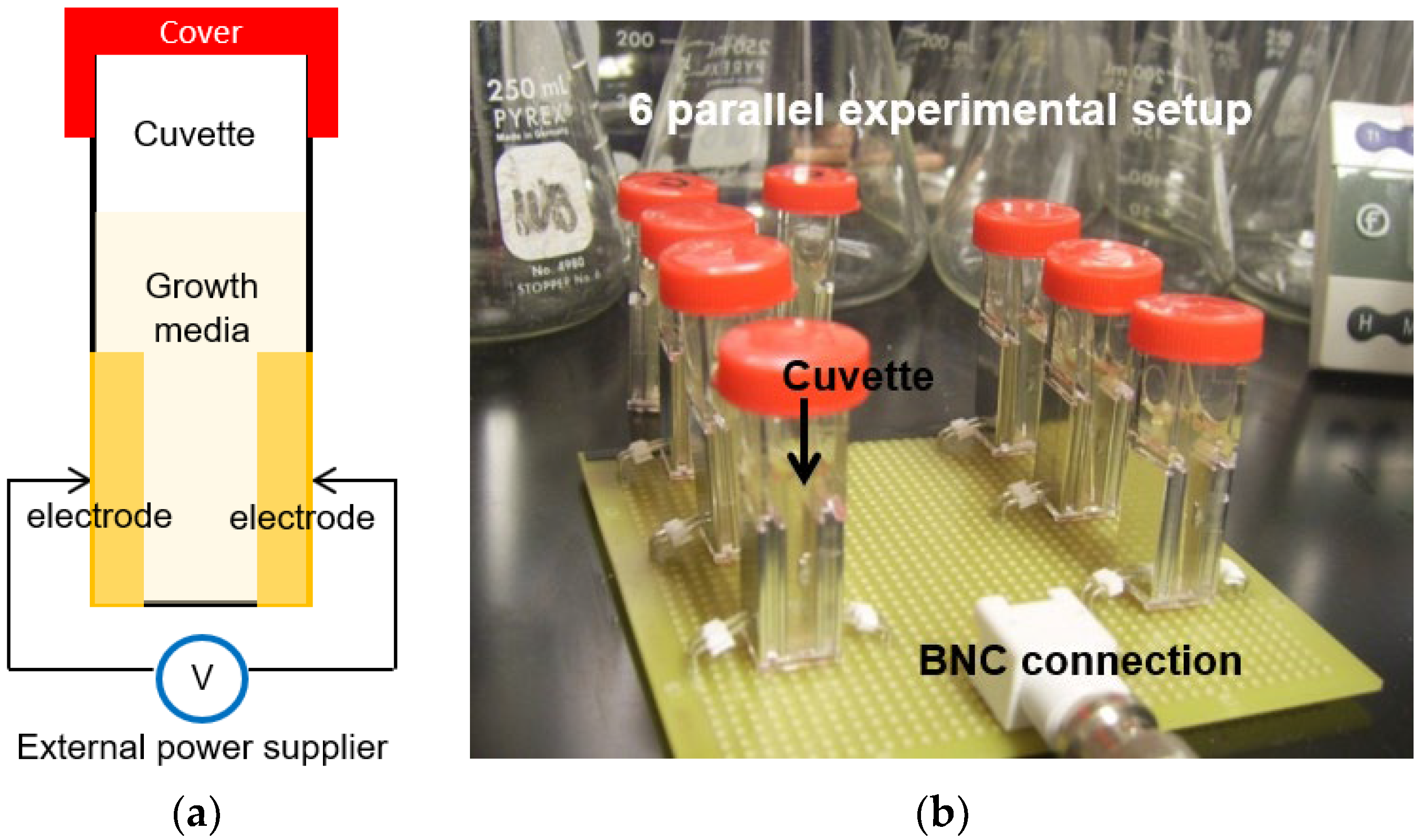
2.2. Quantification of Electrolysis Experiments
2.3. Investigation of Dental Gingivitis Using a BE Toothbrush
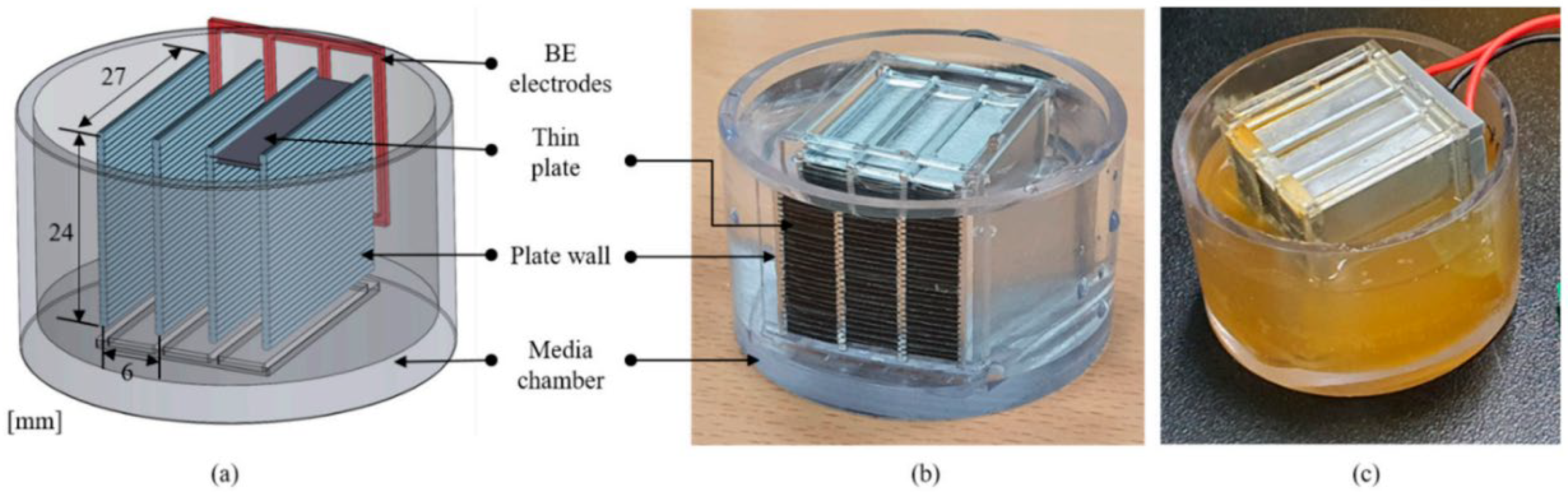
2.4. Investigation of Hygiene Biofilms Using a BE Application
3. Results
3.1. Quantification of Electrolysis
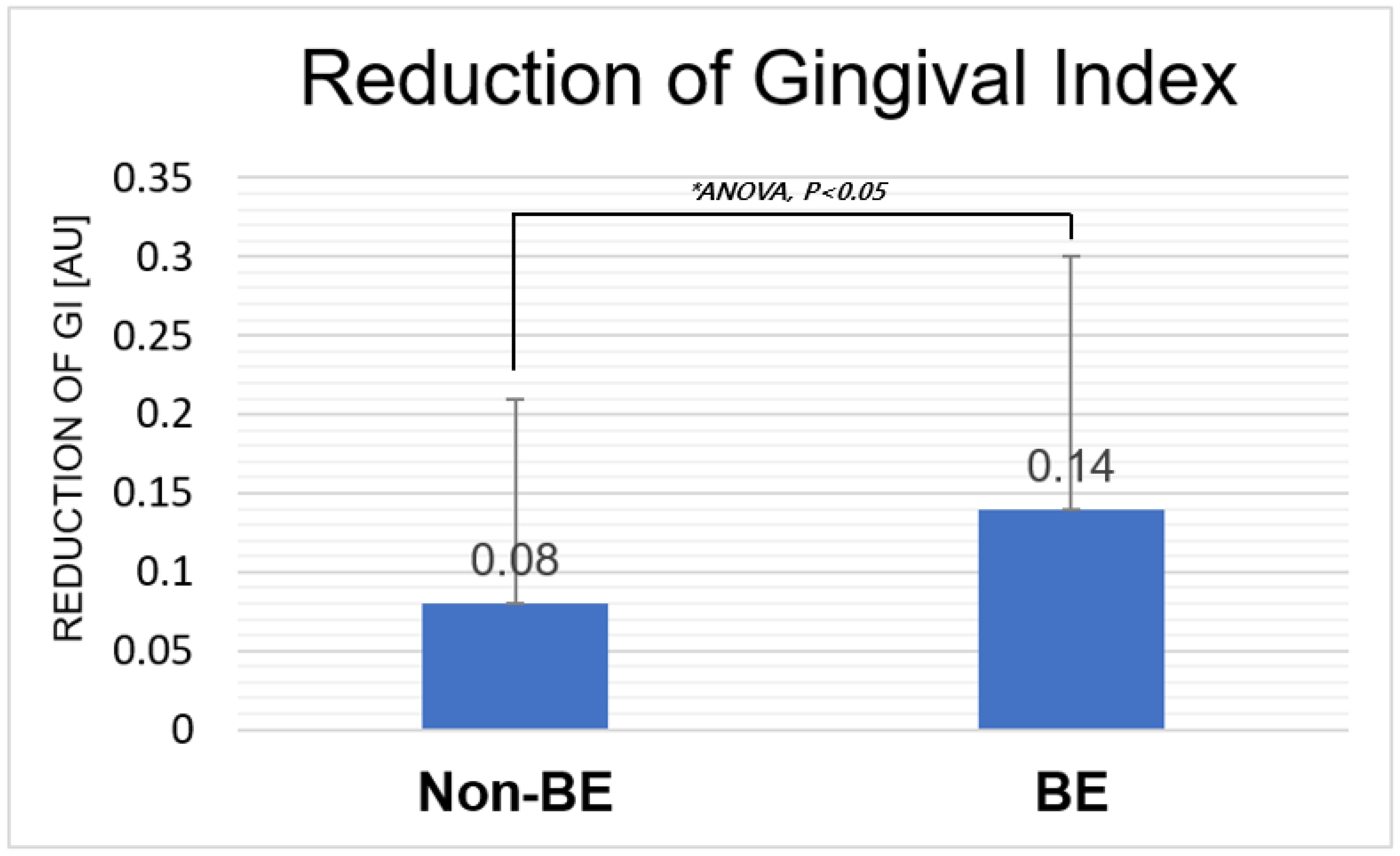
3.2. Reduction in Gingival Index
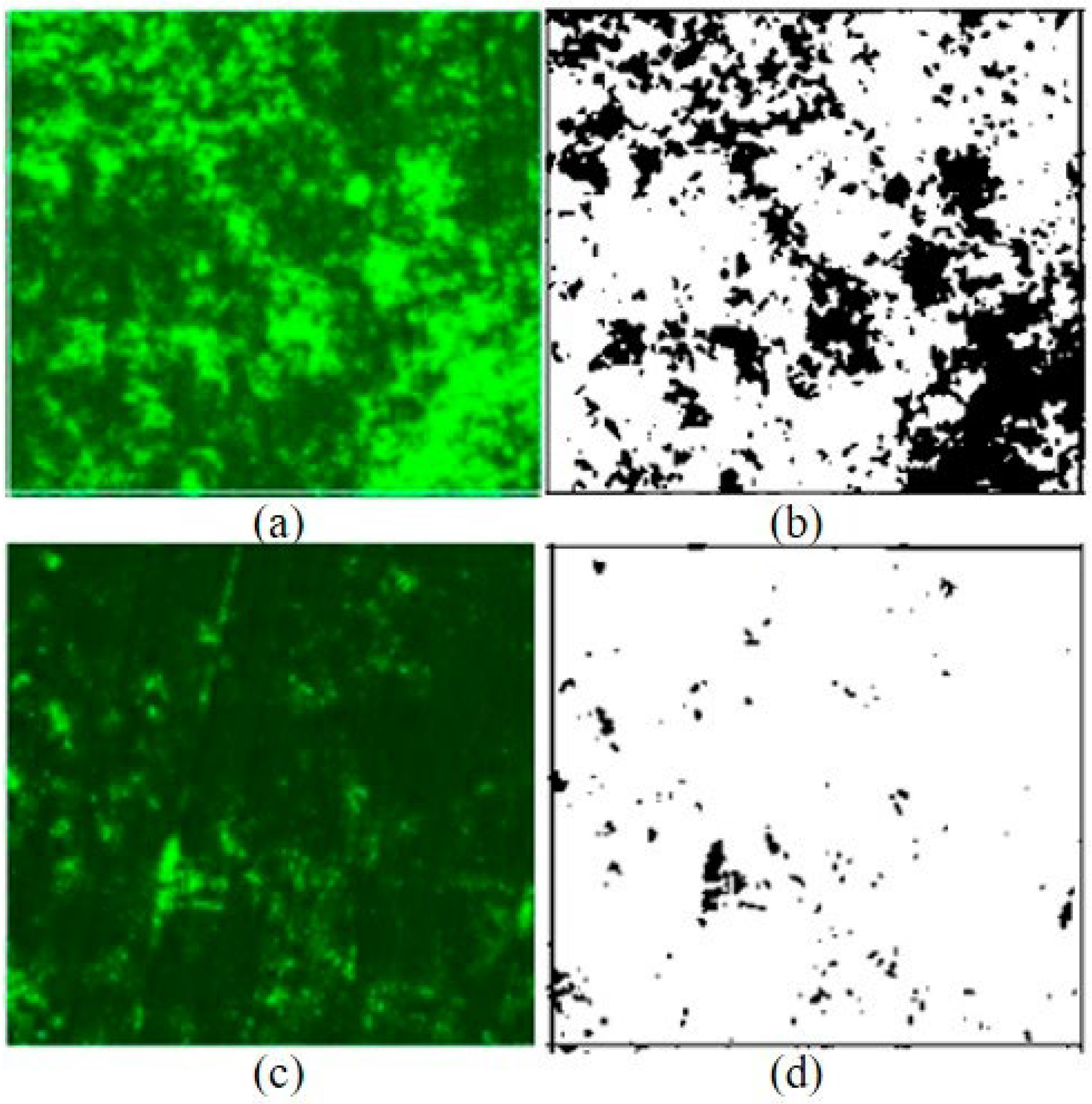
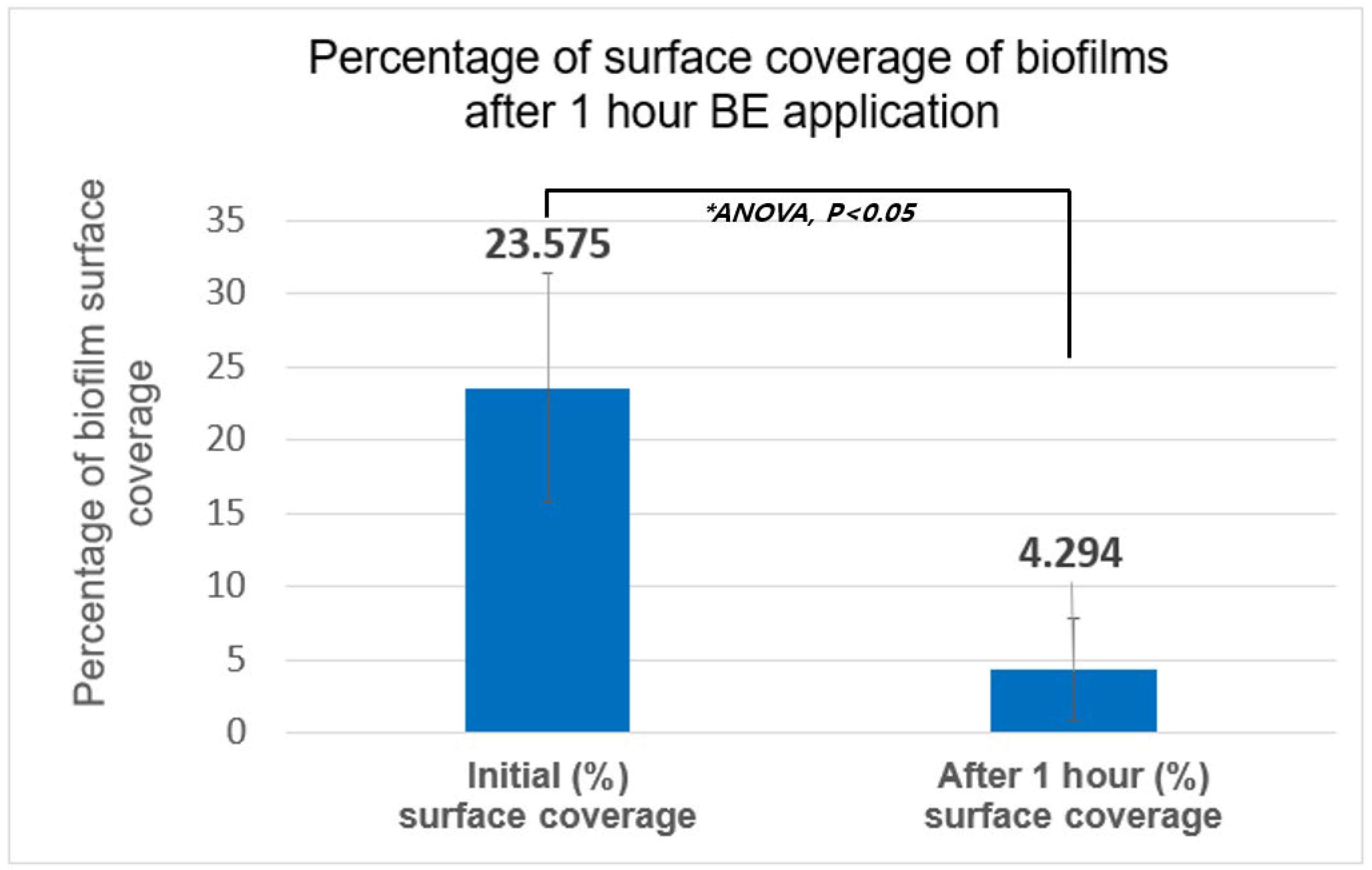
3.3. Reduction in HVAC Biofilms
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vestby, L.K.; Grønseth, T.; Simm, R.; Nesse, L.L. Bacterial Biofilm and its Role in the Pathogenesis of Disease. Antibiotics 2020, 9, 59. [Google Scholar] [CrossRef]
- Schulze, A.; Mitterer, F.; Pombo, J.P.; Schild, S. Biofilms by bacterial human pathogens: Clinical relevance–development, composition and regulation–therapeutical strategies. Microb. Cell 2021, 8, 28–56. [Google Scholar] [CrossRef] [PubMed]
- Okuda, K.; Nagahori, R.; Yamada, S.; Sugimoto, S.; Sato, C.; Sato, M.; Iwase, T.; Hashimoto, K.; Mizunoe, Y. The Composition and Structure of Biofilms Developed by Propionibacterium acnes Isolated from Cardiac Pacemaker Devices. Front. Microbiol. 2018, 9, 182. [Google Scholar] [CrossRef] [PubMed]
- Donlan, R.M. Biofilms: Microbial life on surfaces. Emerg. Infect. Dis. 2002, 8, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Høiby, N.; Bjarnsholt, T.; Givskov, M.; Molin, S.; Ciofu, O. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents 2010, 35, 322–332. [Google Scholar] [CrossRef]
- Petersen, P.E.; Baez, R.J.; Ogawa, H. Global application of oral disease prevention and health promotion as measured 10 years after the 2007 World Health Assembly statesment on oral health. Community Dent. Oral Epidemiol. 2020, 48, 338–348. [Google Scholar] [CrossRef]
- Abebe, G.M. Oral Biofilm and Its Impact on Oral Health, Psychological and Social Interaction. Int. J. Oral Dent. Health 2021, 7, 127. [Google Scholar]
- Alhadainy, H.A.; Keefe, T.; Abdel-Karim, A.H.; Abdulrab, S.; Halboub, E. Association between dental diseases and history of stroke in the United States. Clin. Exp. Dent. Res. 2021, 7, 845–851. [Google Scholar] [CrossRef]
- Albert, D.A.; Ward, A.; Allweiss, P. Diabetes and oral disease: Implications for health professionals. Ann. N. Y. Acad. Sci. 2012, 1255, 1–15. [Google Scholar] [CrossRef]
- Kotronia, E.; Brown, H.; Papacosta, A.O.; Lennon, L.T.; Weyant, R.J.; Whincup, P.H.; Wannamethee, S.G.; Ramsay, S.E. Oral health and all-cause, cardiovascular disease, and respiratory mortality in older people in the UK and USA. Sci. Rep. 2021, 11, 16452. [Google Scholar] [CrossRef]
- Ming, Y.; Hsu, S.W.; Yen, Y.; Lan, S.J. Association of oral health–related quality of life and Alzheimer disease: A systematic review. J. Prosthet. Dent. 2020, 124, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Nitschke, M.; Appleton, S.L.; Li, Q.; Tucker, G.R.; Shah, P.; Bi, P.; Pisaniello, D.L.; Adams, R.J. Lung Function Reductions Associated with Motor Vehicle Density in Chronic Obstructive Pulmonary Disease: A Cross-Sectional Study. Respir. Res. 2016, 17, 138. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.W.; Mosteller, M.P.; Subramanian, S.; Meyer, M.T.; Bentley, W.E.; Ghodssi, R. An Optical Microfluidic Platform for Spatiotemporal Biofilm Treatment Monitoring. J. Micromech. Microeng. 2015, 26, 015013. [Google Scholar] [CrossRef]
- Ciarolla, A.A.; Lapin, N.; Williams, D.; Chopra, R.; Greenberg, D.E. Physical Approaches to Prevent and Treat Bacterial Biofilm. Antibiotics 2023, 12, 54. [Google Scholar] [CrossRef]
- Cruz, A.; Condinho, M.; Carvalho, B.; Arraiano, C.M.; Pobre, V.; Pinto, S.N. The Two Weapons against Bacterial Biofilms: Detection and Treatment. Antibiotics 2021, 10, 1482. [Google Scholar] [CrossRef]
- Cogan, S.F.; Ludwig, K.A.; Welle, C.G.; Takmakov, P. Tissue damage thresholds during therapeutic electrical stimulation. J. Neural Eng. 2016, 13, 021001. [Google Scholar] [CrossRef]
- Del Pozo, J.L.; Rouse, M.S.; Patel, R. Bioelectric Effect and Bacterial Biofilms. A Systematic Review. Int. J. Artif. Organs 2008, 31, 786–795. [Google Scholar] [CrossRef]
- Nuccitelli, R.; Lui, K.; Kreis, M.; Athos, B.; Nuccitelli, P. Nanosecond pulsed electric field stimulation of reactive oxygen species in human pancreatic cancer cells is Ca2+-dependent. Biochem. Biophys. Res. Commun. 2013, 432, 580–585. [Google Scholar] [CrossRef]
- Lv, Y.; Zhang, Y.; Rubinsky, B. Molecular and histological study on the effects of electrolytic electroporation on the liver. Bioelectrochemistry 2019, 124, 79–89. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative stress: Harms and benefits for Human Health. Oxidative Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Kim, Y.; Subramanian, S.; Gerasopoulos, K. Effect of electrical energy on the efficacy of biofilm treatment using the bioelectric effect. NPJ Biofilms Microbiomes 2015, 1, 15016. [Google Scholar] [CrossRef]
- Subramanian, S.; Gerasopoulos, K.; Guo, M. Autoinducer-2 analogs and electric fields—An antibiotic-free bacterial biofilm combination treatment. Biomed. Microdevices 2016, 18, 95. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, S.; Huiszoon, R.C.; Chu, S.W.; Bentley, W.E.; Ghodssi, R. Microsystems for biofilm characterization and sensing—A review. Biofilm 2020, 2, 100015. [Google Scholar] [CrossRef] [PubMed]
- Huiszoon, R.C.; Subramanian, S.; Rajasekaran, P.R.; Beardslee, L.A.; Bentley, W.E.; Ghodssi, R. Flexible Platform for In Situ Impedimetric Detection and Bioelectric Effect Treatment of Escherichia coli Biofilms. IEEE Trans. Biomed. Eng. 2019, 66, 1337–1345. [Google Scholar] [CrossRef]
- Chen, B.K.; Wang, C.K. Electrolyzed Water and Its Pharmacological Activities: A Mini-Review. Molecules 2022, 27, 1222. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.W.; Lee, J.; Lee, T.H. Bioelectric effect utilized a healthcare device for effective management of dental biofilms and gingivitis. Med. Eng. Phys. 2022, 104, 103804. [Google Scholar] [CrossRef]
- Han, S.K.; Kim, Y.W.; Koo, B.-S.; Choi, H.W.; Lee, S. Developing an Electrical System toward the Prevention of Heat Ventilation Air Conditioning Contamination. Appl. Sci. 2022, 12, 11842. [Google Scholar] [CrossRef]
- Schwartz, S.B.; Christensen, J.R.; Fields, H. 31-Examination, Diagnosis, and Treatment Planning. In Wells, Pediatric Dentistry, 6th ed.; Arthur, J.N., John, R.C., Tad, R.M., Janice, A.T., Martha, H., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 419–454. [Google Scholar]
- Cámara, M.; Green, W.; MacPhee, C.E. Economic significance of biofilms: A multidisciplinary and cross-sectoral challenge. NPJ Biofilms Microbiomes 2022, 8, 42. [Google Scholar] [CrossRef]
- Xue, Y.; Zhao, J.; Qiu, R.; Zheng, J.; Lin, C.; Ma, B.; Wang, P. In situ glass antifouling using Pt nanoparticle coating for periodic electrolysis of seawater. Appl. Surf. Sci. 2015, 357, 60–68. [Google Scholar] [CrossRef]
- Gordon, V.D.; Davis-Fields, M.; Kovach, K.; Rodesney, C.A. Biofilms and Mechanics: A Review of Experimental Techniques and Findings. J. Phys. D Appl. Phys. 2017, 50, 223002. [Google Scholar] [CrossRef]
- Beech, I.B. Corrosion of technical materials in the presence of biofilms—Current understanding and state-of-the art methods of study. Int. Biodeterior. Biodegrad. 2004, 53, 177–183. [Google Scholar] [CrossRef]
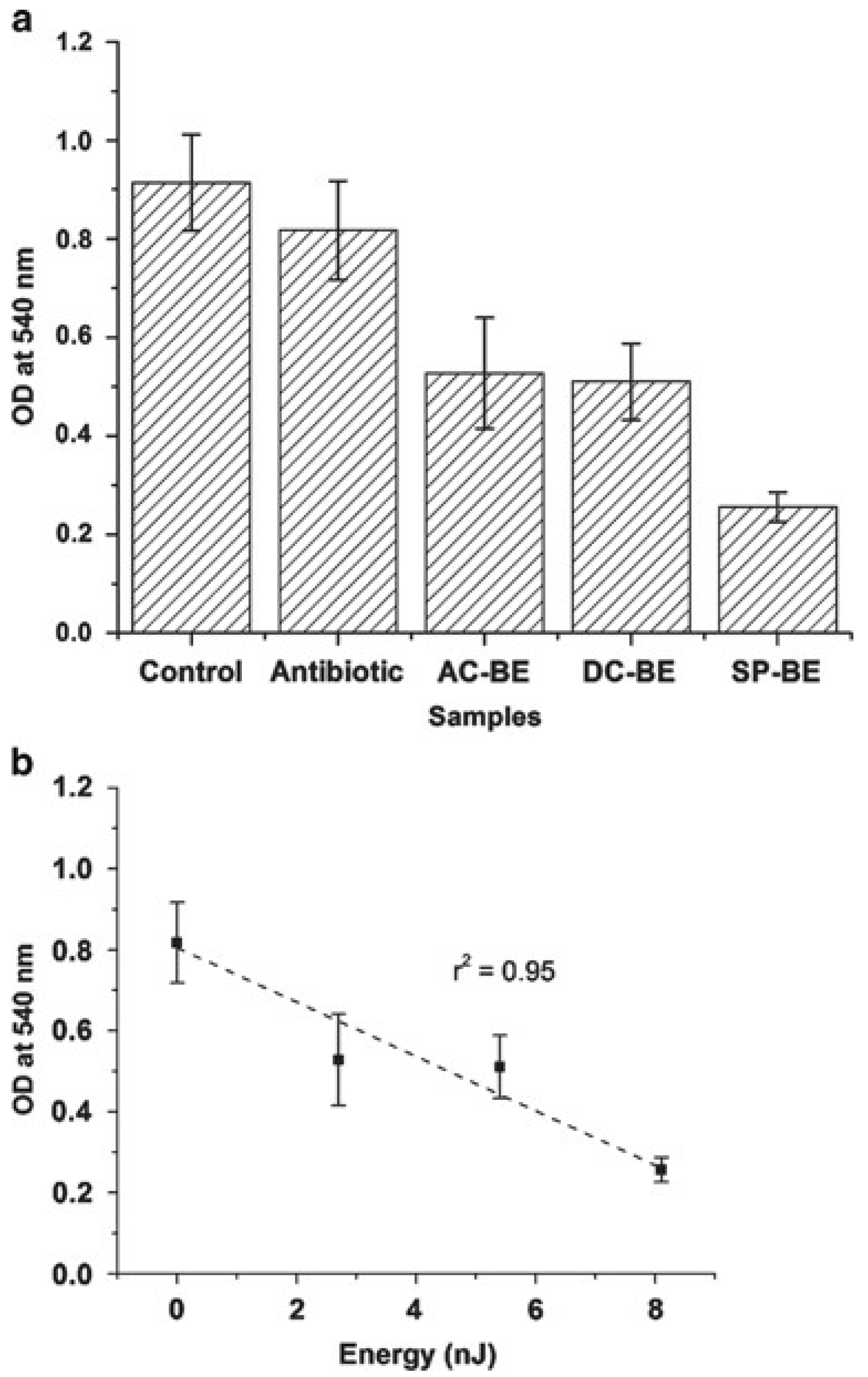


| OD616 | Control | BE | 0.82 V |
|---|---|---|---|
| Average | 0.007 | 0.006 | 0.360 |
| Stdev | 0.045 | 0.012 | 0.250 |
| p-value | NA | >0.05 | <0.05 |
| Reduction in Gingival Index | Non-BE | BE |
|---|---|---|
| Average (Stdev) | 0.08 (0.13) | 0.14 (0.16) |
| p-value (between non-bioelectric effect and bioelectric effect) | NA | 0.034 (p < 0.05) |
| Number of Samples | Initial (%) Surface Coverage | After 1 h BE (%) Surface Coverage | Reduction (%) (After-Initial)/(Initial) |
|---|---|---|---|
| 1 | 20.275 | 1.708 | −91.6% |
| 2 | 34.489 | 1.721 | −95.0% |
| 3 | 19.136 | 2.337 | −87.8% |
| 4 | 31.733 | 2.822 | −91.1% |
| 5 | 14.499 | 10.026 | −30.9% |
| 6 | 21.320 | 7.148 | −66.5% |
| Average | 23.575 | 4.294 | −81.8% |
| Stdev (p-value) | 7.794 | 3.473 | p < 0.05 (p = 0.00025) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.W.; Lee, J.; Han, S.K.; Koo, B.-S.; Park, T.; Park, H.M.; Lee, B. A Non-Electrolysis Bioelectric Effect for Gingivitis and Hygiene Contamination Biofilm Removal. Appl. Microbiol. 2023, 3, 675-686. https://doi.org/10.3390/applmicrobiol3030046
Kim YW, Lee J, Han SK, Koo B-S, Park T, Park HM, Lee B. A Non-Electrolysis Bioelectric Effect for Gingivitis and Hygiene Contamination Biofilm Removal. Applied Microbiology. 2023; 3(3):675-686. https://doi.org/10.3390/applmicrobiol3030046
Chicago/Turabian StyleKim, Young Wook, Jihyun Lee, Sang Kuy Han, Bon-Sang Koo, Taeguen Park, Hyun Mok Park, and Byoungdoo Lee. 2023. "A Non-Electrolysis Bioelectric Effect for Gingivitis and Hygiene Contamination Biofilm Removal" Applied Microbiology 3, no. 3: 675-686. https://doi.org/10.3390/applmicrobiol3030046
APA StyleKim, Y. W., Lee, J., Han, S. K., Koo, B.-S., Park, T., Park, H. M., & Lee, B. (2023). A Non-Electrolysis Bioelectric Effect for Gingivitis and Hygiene Contamination Biofilm Removal. Applied Microbiology, 3(3), 675-686. https://doi.org/10.3390/applmicrobiol3030046






