Abstract
Gut microbes are immunologically tolerated in the gastrointestinal tract but trigger aggressive immune responses upon translocation across the gut barrier. Although oral tolerance, a physiological process that dampens immune responses to food proteins and commensal microbiota, remains poorly defined, significant progress was made during and after the Human Immunodeficiency Virus epidemic in the 1980s and the discovery of regulatory T cells in 1995. Additional insight was gained after the discoveries of innate lymphoid cells in 2008 and the functional elucidation of mucosal mast cells. Prior to the historical discovery of human pathogens, the etiologies of most human diseases were considered unknown. The same was true about many genetic disorders prior to the Human Genome Project. Here, we hypothesize that many of the remaining idiopathic conditions, including autoimmune, fibroproliferative, and neuropsychiatric diseases as well as some cancers, can be considered microbial translocation disorders triggered by the host immune responses to extraintestinal gut microbes and/or their constituent parts. In addition to microbial translocation, we also discuss potential interventions for intestinal barrier rehabilitation, including antibodies against tumor necrosis factor-like ligand 1A and membrane lipid replacement supplements.
1. Introduction
Biological barriers, comprised primarily of epithelial and endothelial cells, separate the body compartments from each other and protect tissues and organs from external chemical, biological, and physical agents. Similarly, biological barriers, such as the blood-brain barrier (BBB) maintain the homeostasis of biomolecular transport into tissues and organs [1]. Although there are several biological barriers in the human body, here we focus primarily on the intestinal epithelial barrier that separates the luminal prokaryotes from eukaryotic host cells.
In the gastrointestinal (GI) system, gut barrier function is highly dependent on the integrity of intestinal epithelial cell (IEC) plasma membranes and the presence of tight junction (TJs) molecules that bind IECs tightly to each other. In addition, the adequate functioning of intestinal barriers requires an intact, gut-associated lymphoid tissue (GALT), that is comprised of B and T lymphocytes, macrophages, dendritic cells as well innate lymphoid cells (ILCs), that along with IECs can recognize gut antigens and bacteria [2]. Under pathological circumstances, damaged TJs and plasma membranes contribute to barrier dysfunction and microbial passage into host tissues [3].
Microbial translocation (MT) the term used throughout this article, refers to the “escape” of intestinal microorganisms or toxins into the host systemic circulation by passing through the intestinal barrier and lamina propria, reaching mesenteric lymph nodes and the circulatory system [4,5].
Different translocation pathways across the gut barrier have been described as paracellular or transcellular. The former facilitates the transport of solutes and nutrients, while the latter promotes the translocation of cells and particles, including granulocytes, microbes, and microbial components [4]. Such translocation can also involve specific cell types. For example, antigen processing by GALT requires transcytosis of microbes and toxins through the intestinal membranous (M) cells [5].
From a host perspective, the plasma membrane participates actively in immune defenses and contributes to microbiota tolerance by externalizing phosphatidylserine (PS), a global immunosuppressive marker [6]. Indeed, due to their short, 3–5 days lifespan, IECs can externalize PS en masse, extending tolerance to the gut microbial community [7]. Some gut microbes, including E. coli, contribute to the tolerogenic intestinal milieu by externalizing PS on their own surfaces, suppressing host immunity [8,9].
From the microbiota perspective, host tolerance can be promoted by increasing the levels of interleukin 10 (IL-10), a tolerogenic cytokine that upregulates regulatory T cells (T regs), dampening host immune responses to commensals [10,11]. For example, Faecalibacterium prausnitzii (F. prausnitzii), can induce host dendritic cells (DCs) to release IL-10 and other tolerogenic molecules, thus participating actively in the immune acceptance of gut flora [12]. Moreover, natural killer cells (NKCs) can promote gut tolerance by releasing interferon γ (IFN-γ), a cytokine that can convert tryptophan to kynurenine, causing upregulation of the key tolerogenic enzyme indoleamine 2,3-dioxygenase (IDO). In return, kynurenine can bind with aryl hydrocarbon receptor (AhR), suppressing immune responses to commensals [13,14,15,16].
The 1995 discovery of Tregs, an IL-2-expressing subgroup of T helper cells (Th), has contributed to a better understanding of the molecular underpinnings of oral and gut immune tolerance [17]. Mucosal mast cells (MCs) suppress T regs in the presence of gut pathogens, triggering the opposite drive, activation of effector T cells, and immunogenicity [18]. The relationship between Tregs and MCs maintains gut homeostasis by upholding the careful balance between commensal acceptance and pathogen rejection.
In addition, the finding that group-3 innate lymphoid cells (ILC3s) release IL-2, a Treg-activating cytokine, emphasizes further the molecular intricacies of gut immune tolerance [19] (Figure 1). Furthermore, the finding that IL-10, a primarily anti-inflammatory cytokine, enhances IL-2 secretion and suppresses proinflammatory IL-17, has shed additional light on the cellular and molecular underpinnings of the intestinal tolerogenic milieu [20,21]. Together, these novel findings have contributed to a better understanding of gut barrier homeostasis and MT.
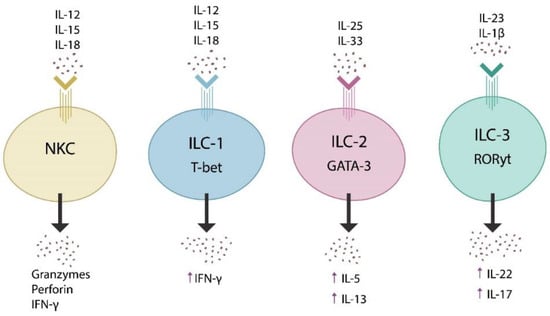
Figure 1.
Innate lymphoid cells are comprised of NKCs and innate lymphoid cells types 1, 2, and 3 (ILC1, ILC2, and ILC3). These cells are activated by various cytokines (top), release other cytokines (bottom), and express transcription factors, including T-bet, GATA-3, and RORγt. When dysregulated, these lymphoid systems may trigger autoimmunity, fibrotic and neuropsychiatric illnesses.
While increased immune tolerance can promote MT, an excessively immunogenic environment can be equally detrimental. For example, inflammatory bowel diseases (IBD), such as Chron’s disease or ulcerative colitis, has been associated with increased MT as evidenced by the elevated levels of translocation markers, such as lipopolysaccharide-binding protein (LBP) and soluble CD14 (sCD14), that have been reported in this pathology [22,23].
Numerous pathogens, including Human Immunodeficiency Virus (HIV), promote self-tolerance by exploiting the host tolerogenic systems, including PS, IL-2, IL-10, or IDO, and in the process facilitating MT and toxin passage [24]. Indeed, MT was poorly defined prior to the arrival of HIV, a virus capable of increasing intestinal permeability by depleting IL-22, the guardian of gut barrier function (Figure 2) [25,26,27,28,29].
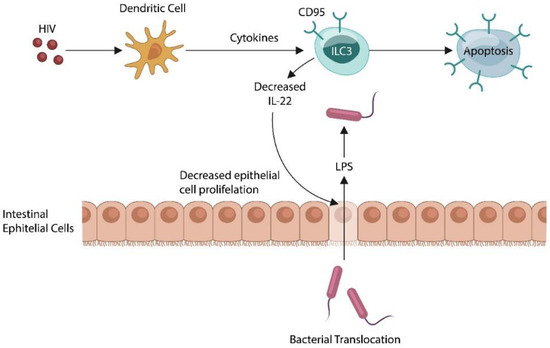
Figure 2.
HIV induces apoptotic loss of ILC3, lowering IL22, the guardian of the gut barrier. This, in turn, promotes the translocation of intestinal microbes and their molecules into the systemic circulation. Activated host immunity maintains a state of low-grade inflammation that characterizes many diseases of uncertain etiology.
In addition to HIV, several other pathogens can hijack the molecular pathways of intestinal tolerance, promoting MT. For example, bacteria and viruses can amplify infectivity by generating IL-10 orthologues or increasing IL-10 itself, inducing self-tolerance and averting host defenses [30,31]. Another mechanism utilized by enveloped viruses to induce tolerance is apoptotic mimicry, a Trojan horse maneuver of PS externalization to facilitate viral uptake by the phagocytes [32].
In 2002, a new family member of tumor necrosis factor α (TNFα) was discovered, the TNF-like cytokine 1A (TL1A) that over the past two decades has revolutionized the field of MT and very likely other disorders of uncertain etiology [33]. TL1A, a T cell co-stimulator, is a ligand of death receptor 3 (DR3) and participates actively in the pathogenesis of many cancers as well as other autoimmune, fibroproliferative diseases and neuropsychiatric disorders, including schizophrenia [34,35,36,37]. Indeed, anti-TL1A antibodies, currently in Phase 2 trials for ulcerative colitis, may prove beneficial for several idiopathic diseases [38,39,40] (NCT02840721).
Transcellular MT, a process of microbial passage through the IECs or M cells, is highly dependent on the integrity of the membrane lipid bilayer and its repair capacity [41]. Conversely, the replacement of damaged membrane lipids with undamaged glycerolphospholipids (GPL), using dietary supplements can repair cell membranes, lowering MT. Here, we propose that the concomitant treatment with TL1A antibody and membrane lipid replacement supplements may be a superior approach to achieve gut barrier repair than each modality applied individually [41].
2. The Microbiome and Innate Lymphoid Cells
The Human Microbiome Project, initiated in 2007, revealed that the microbial organ is comprised of more than 2000 bacterial species, belonging to 12 different phyla, more than 90% of which are related to Proteobacteria, Firmicutes, Actinobacteria, or Bacteroidetes species [42,43,44]. Aside from prokaryotic microbes, the gut microbiome also contains yeasts, fungi, and viruses that influence the barrier function by releasing signaling molecules and metabolites as well as by direct interaction with IECs [45,46]. For example, the human gastrointestinal (GI) tract virome (viral microbiome) contains a variety of RNA and DNA viruses, most of which are bacteriophages (phages) or bacteria-infecting viruses, that regulate the microbiome composition as well as the permeability of gut barrier [47]. In addition, phages can be disseminated throughout the body by the circulatory system and can interact directly with human cells [48,49]. Moreover, as phages ingress into microbial cells by lysing the cell wall, they often release microbial fragments, such as muramyl peptides, lipoteichoic acids, or LPS, triggering responses related to gut pathology [50].
Discovered in 2008, innate lymphoid cells (ILCs) are mucosa-anchored non-T, non-B lymphocytes, consisting of natural killer cells (NKCs) and ILC1, ILC2, and ILC3 cells [51]. These systems play a pivotal role in maintaining the homeostasis of the gut barrier, intestinal tolerance, and nutrient transport [52,53]. Unlike the B and T lymphocytes which express specific antigen receptors, ILCs respond to transcription factors and synthesize cytokines [54,55] (Figure 1). For example, ILC3 express retinoic acid receptor-related nuclear receptor γt (RORγt), are activated by IL-23 and IL-1β, while they release IL-22 and IL-17, cytokines involved in the integrity of the gut barrier as well as the blood-brain barrier (BBB) (Figure 1) [56,57].
MT was poorly defined prior to the HIV epidemic but was extensively studied in the context of this virus which was known for activating the immune system and increasing gut barrier permeability [58,59,60]. Indeed, HIV triggers ILC3 apoptosis, lowering IL-22, which in return alters the intestinal barrier, promoting MT [61] (Figure 2).
Under physiological conditions, IL-22, acts as a guardian of gut barrier function, while IL-17 is known for initiating inflammation and recruiting neutrophils, and clearing invading pathogens [62]. Dysfunctional IL-17 may trigger chronic inflammation, a characteristic of several disorders of unclear or unknown etiologies, including autoimmune diseases, pathological fibrosis, and neuropsychiatric disorders, such as schizophrenia [63,64,65]. Maintaining an appropriate balance between the tolerogenic IL-22 and proinflammatory IL-17 is necessary for enabling GALT to distinguish between gut commensals and pathogens [66]. For example, GALT dyshomeostasis, due to dysfunctional ILC3 and an impaired Tregs/Th17 ratio, was demonstrated to trigger food allergies and IBD [67,68,69].
Abundantly expressed in the intestinal mucosa, ILCs protect the gut barrier by releasing IFN-γ, a cytokine that activates tryptophan metabolism, promoting tolerance via kynurenine and IDO. On the other hand, the dysfunctional release of IFN-γ by NKCs or ILC1, may lead to pathological fibrosis, major depressive disorder (MDD), and systemic lupus erythematosus (SLE), further connecting ILCs with the disorders of uncertain etiology [70,71,72] (Figure 1). Indeed, impaired ILC2 and dysfunctional release of IL-5 and IL-13 were demonstrated in MDD and suicidal behavior, linking neuropathology to this lymphoid system. Along this line, a recent study has connected dysfunctional ILCs with the loss of Alloprevotella rava (A. rava), a, a salivary microbe whose absence was associated with suicidality [73,74]. Moreover, IL-5 and IL-13 have been known for their role in eliminating Helicobacter pylori (H. pylori), a pathogen previously implicated in MDD [75,76].
Taken together, these data show that ILCs are essential for maintaining the homeostasis of the intestinal barrier and the immune acceptance of gut microbes. Conversely, dysfunctional ILCs can trigger barrier dysfunction and MT, predisposing patients to disorders of uncertain etiology [77,78].
2.1. The Mast Cells of Intestinal Mucosa
The human gut harbors the largest body population of MCs, a system associated with ILCs, that regulates the gut barrier function and integrity [79]. Conversely, dysfunctional MCs with aberrant tryptase release were demonstrated in IBD, a disorder associated with systemic pathology and MT [80].
MCs have been known historically for their role in histamine release and allergic reactions however, these cells also participate in several other physiological and pathological processes, including immunity, inflammation, emotional regulation, olfaction, and cognition [81]. For example, Mastocytosis is a rare disease characterized by an increased number of MCs and a clinical picture marked by abdominal pain, enlarged lymph nodes as well as neuropathology, such as depression, anxiety, and cognitive disorders [82,83,84].
Cerebral MCs reside inside the BBB, including the thalamus, hypothalamus, and leptomeninges where they maintain CNS homeostasis by signaling with astrocytes, microglia, and endothelial cells (ECs) [85]. Regardless of their location, MCs respond to infection by forming extracellular traps (ET), also referred to as ETosis, or pathogen elimination by externalizing antimicrobial peptides (AMPs) and nuclear DNA [86]. ETosis may account for the elevated cell-free DNA (cfDNA) documented in many disorders of uncertain etiology, including autoimmunity, cancer, pulmonary fibrosis, and schizophrenia [87,88,89,90,91]. In this regard, as cfDNA and ETs have been associated with gut barrier breakdown and MCs may be the drivers of MT pathology [92].
Human MCs share some common characteristics with neurons as they are long living and capable of producing neurotransmitters, such as acetylcholine (ACh), gamma-aminobutyric acid (GABA), histamine, and serotonin (5-HT), suggesting their important role in maintaining CNS homeostasis [93]. Indeed, brain resident MCs produce approximately 50% of the CNS histamine, a biogenic amine implicated in the pathogenesis of schizophrenia, Parkinson’s disease (PD), and sleep disorders [94,95]. While neuronal histamine is produced in the hypothalamic tubero-mamillary nucleus, MCs are associated with meningeal vessels. Indeed, the dysfunctional release of histamine has been linked to migraine headaches [96]. Moreover, as histamine modulates the sleep-wake cycle, learning, and memory, dysfunctional histaminergic signaling may trigger certain neuropathology, including schizophrenia [97,98,99]. Indeed, histamine 3 (H3) receptor antagonists are currently in schizophrenia clinical trials [100].
Aside from schizophrenia, histamine has been found to be involved in other neuropsychiatric conditions, including Tourette’s syndrome, delirium, and postoperative cognitive impairment [101,102,103]. Moreover, histamine was demonstrated to activate microglia, suggesting that the dysfunctional release of this biogenic amine may trigger the elimination of healthy neurons and synapses by aberrant microglia [104,105,106].
Earlier studies have demonstrated that HIV can “hide” in MCs, utilizing them as reservoirs and thus preventing complete viral eradication. Since a subcategory of MCs can express chymase, an alternative SARS-CoV-2 entry portal, MCs may harbor this virus, maintaining a latent source of infection that could account for some cases of Long-COVID [107,108]. Interestingly, MCs express a viable renin angiotensin system (RAS), including angiotensin II (ANG II), a peptide implicated in the pathogenesis of several idiopathic diseases, including pulmonary fibrosis, autoimmune diseases, and neurodegenerative disorders [109,110,111].
Taken together, these data connect MCs with intestinal barrier disruption and MT, suggesting their involvement in the pathogenesis of idiopathic disorders.
2.2. Pathogen-Induced Genomic Disruption
Numerous studies have linked idiopathic disorders with both genomic damage and dysfunctional DNA damage repair (DDR) systems. However, this line of research is rarely considered when the etiopathogenesis of these conditions is discussed [112,113,114,115,116,117]. The COVID-19 pandemic has drawn the attention of researchers and clinicians to DNA damage as the SARS-CoV-2 virus can directly and indirectly alter the human genome [118,119]. This could be significant as the SARS-CoV-2 virus may exhibit bacteriophage behavior and trigger pathology similar to the E. coli-released colibactin, including IBD, colorectal carcinoma (CRC), and new onset psychosis [120,121].
Another gut commensal, Morganella morganii (M. morganii) was found to damage the host DNA by releasing indolimine, a recently identified genotoxic molecule [122]. Indeed, earlier studies have implicated E. coli and M. morganii in schizophrenia, further connecting dysfunctional DNA with disorders of uncertain etiology [123]. Moreover, colibactin and indolimine were demonstrated to increase the permeability of the gut barrier, facilitating MT, which likely promotes the pathogenesis of idiopathic illnesses [121,124,125,126,127]. On the other hand, MCs-released histamine, an established protector of DNA, was demonstrated to prevent physical and chemical genomic damage, while antihistaminic drugs may cause DNA insults [128,129]. This places MCs at the center of DDR, emphasizing the adaptive role of these cells in reversing genomic damage and promoting neuroplasticity. Indeed, an MC activator, IL-10 was associated with mild genotoxicity and enhanced DDR response, suggesting that limited genomic damage may be adaptive [130,131]. In this regard, novel studies have reported that mild to moderate DNA damage promotes DDR-mediated neuroplasticity, while extensive (beyond repair) genomic disruption triggers neuronal death, and eventually neurodegenerative disorders [132,133]. We feel that these observations are significant as dysfunctional MCs and IL-10 signaling have been implicated in psychiatric and neurocognitive disorders [134,135]. Interestingly, recent studies have found that neuronal DNA can differ from cell to cell, a phenomenon known as somatic mosaicism, and this condition may drive the pathogenesis of some neuropsychiatric disorders [136,137].
3. Autoimmune Disease as an MTD Phenomenon
Under physiological conditions, microbes and/or microbial components have been detected in many host tissues, including the circulatory system and the CNS [138,139]. Pathologically, microbial antigens, such as LPS, have been demonstrated in Alzheimer’s disease (AD) brains, cardiovascular disease (CVD), diabetes, and some cancers, suggesting that these conditions could be reconceptualized as microbial translocation disorders (MTDs) [140,141,142,143]. In addition, several autoimmune diseases, including SLE, were associated with MT, further linking these disorders to a dysfunctional GI tract barrier. Indeed, preclinical studies have implicated Enterococcus gallinarum (E. gallinarum) in SLE, and reports indicate that the elimination of this bacterium by the antibiotic treatment could alleviate many autoimmune effects [144,145].
3.1. MT in Neuropsychiatric Disorders
Microorganisms, including E. coli and H. pylori, express antigens resembling human glutamate receptors, suggesting that N-methyl-D-aspartate (NMDA) autoantibodies, documented in cases of schizophrenia, could be conventional immunoglobulins directed at translocated microbial proteins [146,147].
Bacteroides species and Pseudomonas fluorescens were shown to generate γ-aminobutyric acid (GABA), a neurotransmitter associated with neuropsychiatric pathology, including anxiety, schizophrenia, and epilepsy, and thus linking these conditions to MTDs [148,149]. For example, anti-GABA-B receptor encephalitis, an autoimmune disease mediated by antibodies against this protein, could be the result of translocated GABA-producing microbes [150]. Interestingly, autoantibodies against GABA-A receptor α1 were detected in patients with schizophrenia, further connecting neuropsychiatric conditions with MTDs [151].
Lactobacillus plantarum, Bacillus subtilis, and E. coli were demonstrated to synthesize acetylcholine (ACh) and express nicotinic or muscarinic receptors, and these can elicit the respective antibodies upon translocation [152,153]. For example, autoantibodies against cholinergic receptors, documented in myasthenia gravis, and Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS), may be conventional antibodies against translocated ACh-expressing microbes [154,155,156].
Furthermore, metabotropic glutamate receptors subtype 2 (mGluR2), an entry portal of both SARS-CoV-2 and rabies virus (RABV), suggests that “autoantibodies” against the virus/receptor complex may be conventional immunoglobulins against known pathogens [157,158]. Moreover, mGluR2 autoantibodies, documented in infections of Mycoplasma and Herpes viruses, may represent antibodies directed at these pathogens [159].
3.2. MT in Sleep Disorders and Neuropathy
Preclinical studies have reported that gut microbiota regulate neurological signaling associated with neuropathy and neuropathic pain, linking this disorder of unclear etiology to MT [160,161]. In addition, as histamine contributes to wakefulness, and several microbial species release histamine, insomnia may be linked to the translocation of histamine-releasing microbes, such as M. morganii [162,163,164]. Indeed, dysfunctional histamine signaling has been documented in infections of Trypanosoma brucei brucei, the etiologic agent of African sleeping sickness, suggesting that molecular mimicry with histaminergic receptors may drive this pathology [165]. Moreover, preclinical studies have linked intestinal dysbiosis to a dysfunctional sleep/wake cycle, further connecting insomnia with the GI tract microbes [166]. Interestingly, the hypnotic properties of microbial peptidoglycans and lipoteichoic acid (cell wall components of Gram-positive bacteria) have been known for decades [165,167]. For example, the sleep-promoting effects of a bacterial cell wall molecule, muramyl peptide, discovered in the 1970s, connects the microbiome with sleep [165].
Translocated muramyl peptide or lipoteichoic acid have been demonstrated to act as pathogen-associated molecular patterns (PAMPs) and trigger the release of IL1β and tumor necrosis factor alpha (TNFα), molecules that are involved in both sleep and autoimmune disorders [167,168,169]. Indeed, IL1β or TNFα antagonists, which are established rheumatoid arthritis (RA) treatments, were demonstrated to possess hypnotic properties, further linking insomnia to autoimmune inflammation [170,171]. Moreover, as muramyl peptide or lipoteichoic acid are bacteriophage entry portals, sleep disturbances may be associated with anti-phage antibodies [172,173,174]. Furthermore, the presence of influenza-induced narcolepsy, an autoimmune disease marked by autoantibodies against orexin-producing neurons, suggests that antiviral antibodies may cross-react with human proteins [175,176]. Moreover, encephalitis lethargica and post-viral parkinsonism have been associated with influenza virus cross-reactivity with basal ganglia proteins, suggesting that this virus in some instances may blend into the gut virome and remain in the body for longer periods of time [177,178]. Furthermore, microbe-generated N-formyl methionine-containing peptides were found to activate mammalian formyl peptide receptors (FPR), which are molecules involved in neuroinflammation and neurodegeneration, indicating their potential microbial involvement in these pathologies [179,180,181,182].
3.3. MT in Movement and Fatiguing Disorders
Yersinia enterocolitica and some E. coli species have been shown to express norepinephrine (NE) and dopamine (DA) receptors. Therefore, anti-dopamine D2 receptor (D2R) antibodies and antibodies against β adrenergic receptors (documented in movement disorders and ME/CFS respectively) may be directed at translocated NE and DA-generating microbes [183,184,185]. Furthermore, several gut species, including E. coli and Salmonella, express CpxA, a bacterial serotonin receptor that can trigger, upon translocation, antibodies against host serotonin (5HT), as demonstrated in fibromyalgia and panic disorder [186,187,188] (Table 1).

Table 1.
Gut microbes and viruses (column I) express biomolecules resembling human proteins (column II). When microbes translocate into the systemic circulation, the host mounts an antibody response against these antigens that can be misconstrued as autoantibodies (column III), resulting in human pathology (column IV).
Taken together, the line between conventional antibodies and autoantibodies has become blurred, especially after the discovery of the microbiome and MT, suggesting that many autoimmune disorders could, in fact, be reconceptualized as MTD.
4. Fibroproliferative Diseases Such as MTDs
Fibroproliferative diseases are idiopathic conditions associated with increased worldwide morbidity and mortality as well as a lack of effective therapies. They affect many organs, including the intestine, liver, kidney, heart, and lung, and are characterized by pathological scar formation [189]. Indeed, fibrogenesis is a physiological or pathological response to chemical, mechanical, or immune insults in which the healing process involves fibroblast-mediated reorganization of the extracellular matrix (ECM) [190,191]. Dysfunctional healing with excessive fibroblast activation and overproduction of ECM is believed to drive pathological fibrosis. In addition, fibroblast growth factors are established participants in gut barrier integrity and loss of fibroblast growth factor 9 (FGF9) has been associated with pulmonary fibrosis, further linking this pathology to MTD [192,193,194].
Transforming growth factor-β (TGF-β), a tolerogenic cytokine and master regulator of gut microbiota, was demonstrated to promote fibrosis by abnormally activating ILCs, Th17, NKCs, and B lymphocytes [195,196,197,198]. In addition, a subset of IL-10-producing NKCs was reported to promote fibrosis, an action independent of the elimination of defective cells [199,200]. Moreover, ILC2-released IL-13 was shown to drive collagen deposition, indicating that dysfunctional ILCs may promote pathological fibrosis [201] (Figure 1). Furthermore, microbiota-generated curli amyloid fibrils were reported to disrupt ILC3, IL-17, and IL-22, promoting both autoimmunity and intestinal fibrosis, highlighting the interconnectedness of these pathologies [202,203,204,205].
A variety of gut viruses and microbial species can drive excessive fibrosis as they express the integrin motif or arginine-glycine-aspartate (RGD), known for binding integrins αvβ6 and αvβ8, highlighting a mechanism of pathological fibroproliferation [206,207]. Interestingly, integrin αvβ6 is an activator of TGF-β, liking integrins to the master regulator of gut microbiota [208].
Considering the above data together, translocated microbes may have the ability to drive the pathogenesis of fibroproliferative disorders by disrupting the function of NKCs and ILCs. At the molecular level, ECM proliferation may be triggered by microbe-disrupted integrins and fibroblast growth factors.
5. Neuropathology as MTDs
Several recent studies have reported new onset psychosis after SARS-CoV-2 infection or even after the administration of mRNA vaccines, connecting viral antigens with this neuropsychiatric pathology [209,210,211,212,213,214]. As SARS-CoV-2 disrupts the intestinal barrier and tryptophan absorption, it enables MT and secondary bacterial infections. These observations have reawakened the interest in the infectious hypothesis of neuropsychiatric illness entertained by many researchers and clinicians both at present and in the past. For example, Emil Kraepelin hinted at the microbiome when hypothesizing that under pathological circumstances microbes from various body compartments could migrate into the brain and trigger neuropathology [215]. Along this line of thought, IBD and CRC, conditions characterized by increased intestinal permeability and MT appear to validate Kraepelin’s migration model [216,217,218]. The same can be stated about psychosis induced by urinary tract infections (UTI), a destabilizing condition in patients with psychiatric illness [219,220,221] (next subsection). Moreover, Kraepelin’s paradigm appears to be validated by the link between maternal influenza and offspring with schizophrenia, or childhood viral enteritis, and the development of psychiatric illness later in life [222,223,224,225]. Furthermore, the finding that some gut microbes express tryptophan decarboxylase and can synthesize endogenous hallucinogens, such as tryptamine or N, N-dimethyltryptamine (DMT), further suggests support for Kraepelin’s hypothesis of microbe-mediated neuropathology [226,227]. Indeed, new onset psychosis associated with COVID-19 may be explained by the selective upregulation of tryptophan decarboxylase-expressing gut microbes, such as M. morganii, a bacterium previously implicated in schizophrenia [123].
The 2001 discovery of trace amine-associated receptors (TAARs), proteins that can sense minute amounts of tryptamine, further substantiates Kraepelin’s hypothesis [228]. The recent finding that microbial metabolite 4-ethylphenyl sulfate (4EPS) disrupts oligodendrocyte maturation in multiple sclerosis (MS), has further connected the microbiome to this neuropathology [229,230,231]. Furthermore, in other studies, several gut microbial species were demonstrated to trigger celiac disease by synthesizing gliadin-mimicking peptides, molecules capable of altering the TJ protein, zonulin [232,233]. This appears to be significant as the systemic translocation of these microbes and their zonulin-like antigens may lead to the generation of anti-gliandin antibodies, further linking the microbiome to autoimmunity [234]. Interestingly, elevated IgA anti-gliadin antibodies were found in patients with schizophrenia, connecting this disorder to dysfunctional biological barriers [235].
Urinary Tract Microbiome and MTDs
Urinary tract infections (UTIs) are common conditions that affect over 150 million people worldwide each year [236,237]. UTI-associated bladder carcinoma is a serious complication connected to microbial genotoxins, including colibactin and indolimines secreted by E. coli and M. morganii respectively [238]. In addition, UTIs are often associated with acute psychosis or psychotic decompensation in stable patients with psychiatric illness [239,240,241]. Along this line, a novel study has taken a fresh look at the connection between UTI and psychosis, emphasizing the role of the previously underappreciated bladder microbiome [221,242]. A recent study, found that a large number of UTI patients were hospitalized with new onset psychosis or psychotic decompensation, suggesting a link between psychopathology and uropathogenic bacteria [239,240,241,242]. For example, UTI-associated E. coli and M. morganii, were found to activate local and CNS MCs, leading to excessive histamine release and exacerbation of both cystitis and psychosis [243,244,245,246,247]. Indeed, schizophrenia with negative symptoms, was associated with UTI caused by M. morganii, Hafnei alvei, Pseudomonas aeruginosa, Pseudomonas putida, and Klebsiella pneumoniae, further connecting the bladder microbiome with neuropathologies [248,249,250].
The urinary bladder expresses numerous receptors, including serotonergic 5-HT7 (5-HT7Rs) and 5-HT4Rs. These receptors are also present in the brain and were previously implicated in schizophrenia [251,252,253,254]. Despite the bladder’s anti-bacterial defenses, including the urothelial barrier, AMP-containing mucus, local ILCs, and MCs, uropathogenic bacteria can overcome these barriers by upregulating IL-10 [255]. Recent studies have found that IL-10 could also account for the absence of long-lasting antibodies in UTIs, and the frequent recurrences found in patients [236,256,257,258]. Moreover, dysfunctional ILCs were associated with both UTI and schizophrenia, connecting immunity to both pathologies [259,260]. Furthermore, dysfunctional MCs and histamine signaling have been documented in interstitial cystitis and psychosis, highlighting the key role of these cells in disorders of unclear etiology [261,262,263].
6. GI Tract Cancer as MTD
The microbiome can be related to cancer development through the induction of inflammation, as well as the production of toxins, in both cases creating favorable niche environments for tumor development.
Genotoxic molecules released by some intestinal microbial species have been shown to activate bacteriophages (phage) embedded in commensals’ DNA. Similarly, phages can eliminate susceptible microbes and increase the abundance of virus-resilient microorganisms, such as tryptophan carboxylase-expressing bacteria that synthesize endogenous serotonergic hallucinogens [264,265]. In addition, some strains of E. coli inhibit DNA mismatch repair proteins by synthesizing colibactin, a tumor-promoting toxin. Colibactin-associated IBD and CRC are more prevalent in patients with schizophrenia compared to the general population, linking this disorder to both DNA damage and MT [266,267,268,269]. The standard histopathological detection of gut inflammation is specified by the presence of inflammatory infiltrates. However, low-grade inflammation may be a much more common occurrence, defined by the elevated systemic expression of proinflammatory cytokines in the absence of the inflammatory infiltrate aggregates. Low-grade inflammation can be casually related to dietary habits, microbiome dysregulation, or both [270,271]. Thus, aside from genomic damage, some E. coli species have been known for maintaining a state of low-grade inflammation that can predispose patients to cancer [267]. The cancer risk is further increased by the inflammation caused by a colibactin-disrupted gut barrier and MT [266,268].
Alteration of the human gut phageome, especially bacteriophages infecting Fusobacterium nucleatum (F. nucleatum), Peptacetobacter hiranonis, and Parvimonas micra, has been associated with CRC, emphasizing the likely viral etiology of this type of tumor [272].
F. nucleatum is a common oral microbe which can reach the colon, accelerating the progression of CRC. F. nucleatum can cause transient bacteremia triggered by chewing, daily hygiene, or dental procedures, indicating several pathways for contributing to tumorigenesis [273]. Aside from Fusobacterium, Bacteriodes fragilis, especially via its B. fragilis toxin (BFT) can trigger chronic intestinal inflammation, predisposing to CRC [274]. The common microbial strains linked to CRC are Bacteroides fragilis, Escherichia coli, Enterococcus faecalis and Streptococcus gallolyticus. Other species identified in CRC patients’ tumor and fecal samples include F. nucleatum, Parvimonas, Peptostreptococcus, Porphyromonas and Prevotella [275].
Indeed, previous studies have connected F. nucleatum with carcinogenesis as well as the pathogenesis of poorly understood disorders, including CVD, preeclampsia, AD, and autoimmune disorders. These observations further link various microbes to these pathologies [273].
A growing body of evidence has shown that the SARS-CoV-2 virus can thrive in GI tract reservoirs, likely blending into the gut virome [274,275].
Indeed, as some gut microbes express angiotensin converting enzyme 2 (ACE-2), the SARS-CoV-2 cell membrane entry portal, they may harbor the virus, possibly accounting for some, if not many of the symptoms of long COVID [276]. It remains to be seen if a higher occurrence of GI-related oncogenesis will be positively correlated with Long-COVID
Microbe-induced, genomic damage can also reactivate dormant viruses, including herpes simplex virus type 1 (HSV1) and Epstein-Barr virus (EBV), pathogens previously associated with schizophrenia as well as with various malignancies [277,278,279,280]. Indeed, EBV is a well-characterized oncovirus associated with several cancers, including CRC [281]. In addition, novel studies have connected phages and fungi to CRC, indicating the possible multi-kingdom etiology of this tumor [282,283].
7. Interventions
In two previous studies, we have discussed several strategies for gut barrier restoration and these therapeutic approaches will not be repeated here in more detail [284,285]. Table 2. summarizes these interventions. In this article, we will focus primarily on the TL1A antibody, currently known as PF-06480605, as well as on membrane lipid replacement therapy (MLR) [286] (287A).

Table 2.
Quick reminder of gut barrier restoration strategies.
7.1. PF-06480605 as a Senolytic
Antibody PF-06480605 is currently in phase 2a clinical trials for ulcerative colitis (NCT02840721). This monoclonal antibody inhibits TL1A, lowering innate and adaptive immunity as well as the fibrotic pathways [296] (Figure 3). TL1A, a T cell co-stimulator, is encoded by the TNFSF15 gene and binds to DR3, encoded by the TNFRSF25 gene [297]. In addition to being a T cell co-stimulator, TL1A/DR3 signaling can activate many cell types, including fibroblasts, ILC2 and ILC3, cancer cells, and macrophages/microglia. DR3 is abundantly expressed in the brain and has been implicated in the pathogenesis of several neuropsychiatric disorders and neurodegenerative diseases [298,299,300].
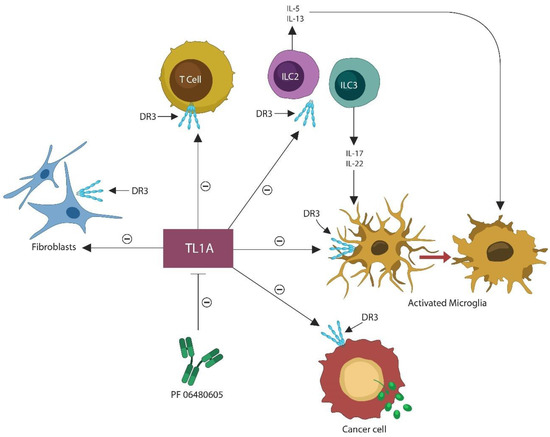
Figure 3.
TL1A, a T cell co-stimulator, also stimulates other DR3-expressing cells, including ILC2, ILC3, fibroblasts, cancer cells, and microphages, including the brain macrophages, and microglia. This places TL1A at the center of the disorders of uncertain etiology, while its inhibition likely diminishes this pathology. Activated microglia often engages in the elimination of viable neurons, contributing to neurodegeneration. Anti-TL1A antibody, PF-06480605 blocks TL1A, inhibiting the stimulatory effect on these systems, and thus decreasing the incidence of idiopathic disorders.
The TL1A and TNFSF15 gene have been involved in premature endothelial cell (EC) senescence, a cellular program of proliferation arrest with active metabolism and a detrimental, barrier-disrupting secretome, the senescence-associated secretory phenotype (SASP) [301,302].
Premature EC senescence and SASP have been associated with neuropsychiatric illness and pulmonary fibrosis, while in cancer, senescence can induce both tumorigenesis or tumor suppression [303,304,305]. Aside from T cells, TL1A also co-stimulates ILC2 and ILC3, promoting the release of IL-5, IL-13, IL-22, and IL-17, cytokines associated with premature cellular senescence. This suggests that PF-06480605 may possess senolytic properties [306,307,308,309,310].
7.2. Membrane Lipid Replacement Therapy
Cellular senescence, a term coined by Hayflick, denotes the arrest of cellular proliferation despite the presence of adequate nutrients and growth factors in the medium [311]. Due to their exit from the cell cycle, senescent cells may not undergo malignant transformation however, the long-term SASP presence in the microenvironment can promote tumorigenesis [312].
Senescent cells upregulate intracellular iron, often leading to plasma membrane damage by lipid peroxidation, a phenomenon we previously identified as ferosenescence [313]. In opposition to ferroptosis which is a nonapoptotic form of cell death, ferrosenescent cells remain alive and are characterized by upregulated cytosolic iron and damaged plasma membranes. In this regard, ferrosenescence bridges the gap between cellular senescence and senescence-associated lipid pathology (SALP) [314].
In addition, ferrosenescence is reversible, which is in line with the novel findings, showing that cellular senescence can be reversed by inhibiting 3-phosphoinositide-dependent protein kinase 1 (PDK1) [315,316].
Since PS, a glycerophospholipid, was demonstrated to maintain PDK1 in an inactive conformation, the efficacy of membrane lipid replacement therapy is likely based, in part, on PDK1-reversed cellular senescence (Figure 4) [317]. Therefore, membrane phospholipid supplementation with natural glycerolphospholipids and PDK1 inhibition, could lower the detrimental effects of PDK1 in cancer (especially breast and CRC), schizophrenia, excessive fibrosis, and autoimmune inflammation [318,319,320,321].
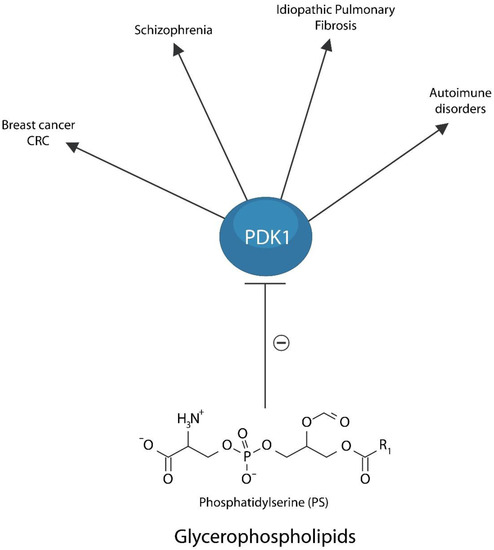
Figure 4.
Membrane Lipid Replacement therapy with dietary glycerophospholipids maintains PDK1 in an inactive conformation, preventing its detrimental action in breast cancer and CRC, neuropsychiatric illness, including schizophrenia, pulmonary fibrosis, and autoimmune disorders. This model suggests that membrane lipid replacement therapy is likely beneficial for disorders of unclear etiology.
Membrane lipid replacement is also important in maintaining cell membrane integrity and function and appropriate membrane barriers (287A). In patients with a variety of diseases and disorders, including idiopathic diseases and those involving MT, membrane lipid replacement improved neurological, GI, and other symptoms, without any adverse effects, suggesting that other idiopathic diseases would also benefit from its use [285,286].
7.3. Challenges and Limitations
The observations presented here on the microbiome and idiopathic diseases are mostly based on retrospective longitudinal cohorts. Some of these cohorts may not have been sufficiently large to investigate in detail the host-pathogen interactions. For example, lack of specimens or inability to link clinical records to the retrospective data (or both) comprises interpretative limitations and challenges. As such additional, studies based on prospective population cohorts are necessary for elucidating the complete role of gut microbial community in systemic disorders.
There is a growing body of literature on specific host-microbe interactions that are (of course) measurable, however, the MTD impact would be more likely akin to a network effect, involving different types, strengths, and microbes acting synergistically across biological thresholds. Unpicking this experimentally remains challenging. Moreover, a common underlying biological mechanism for MTDs does not equate to common clinical treatments, as these are likely to emerge on a disease-specific basis.
For this reason, what we hope to accomplish here is to raise the awareness of researchers and clinicians to a neglected pathology highlighted by HIV and COVID-19, that may account for many insufficiently understood illnesses.
8. Conclusions
Immunological tolerance vs. immunogenicity comprises a homeostatic continuum necessary for the immune acceptance of gut commensals and rejection of pathogens. This continuum is part of a larger adaptive system that ensures the perpetuation of species by extending immune tolerance to the microbiome, food, and fetal proteins.
Dietary tryptophan and microbial organ contribute to the biosynthesis of kynurenine and IDO, tolerogenic biomolecules capable of inhibiting immune system activation in response to selective foreign proteins, including food, microbiota, and the fetus. Disruption of tryptophan degradation and loss of beneficial commensals can break immune tolerance, triggering immunogenicity against beneficial gut flora as observed in IBD.
Disruption of the intestinal barrier promotes MT and subsequent immune activation in response to “escaped” microbes as tolerance is not extended outside the GI tract. Indeed, host responses to translocated microorganisms likely contribute to the pathogenesis of idiopathic illnesses, including autoimmune, fibroproliferative, malignant, and neuropsychiatric conditions as well as ME/CFS, fibromyalgia, and Gulf War Syndromes (GWSs). As these disorders are marked by premature cellular senescence, a defense mechanism against iron-induced membrane damage, restoring lipid homeostasis and repairing plasma membrane damage may reverse barrier dysfunction.
Several gut barrier rehabilitation strategies have been described and here we have focused on TL1A antibody and membrane lipid replacement. PF-06480605 and PDK1 inhibitors likely restore the homeostasis of plasma membranes, reversing ferrosenescence and rescuing IECs from ferroptosis, while membrane lipid replacement repairs and restores membrane barrier and other functions and reduces a variety of symptoms associated with loss of barrier function.
Author Contributions
Conceptualization: A.S. and Z.K.; data curation: S.H. and G.L.N.; writing: D.O.S., S.S. and L.R.; formal analysis: C.M.Z.-M.d.C., J.J.A. and C.V.A.; project administration: C.K. All authors have read and agreed to the published version of the manuscript.
Funding
This reaserch was funded by Institute for Molecular Medicine, and Nutritional Therapeutics, Inc.
Conflicts of Interest
Where authors are identified as personnel of the International Agency for Research on Cancer/WHO, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy or views of the International Agency for Research on Cancer/WHO. Garth L. Nicolson is a part-time consultant to Nutritional Therapeutics, Inc., New York and Naturally Plus Inc., Taiwan.
References
- Groschwitz, K.R.; Hogan, S.P. Intestinal barrier function: Molecular regulation and disease pathogenesis. J. Allergy Clin. Immunol. 2009, 124, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Pearson, C.; Uhlig, H.H.; Powrie, F. Lymphoid microenvironments and innate lymphoid cells in the gut. Trends Immunol. 2012, 33, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Assimakopoulos, S.F.; Triantos, C.; Maroulis, I.; Gogos, C. The Role of the Gut Barrier Function in Health and Disease. Gastroenterol. Res. 2018, 11, 261–263. [Google Scholar] [CrossRef] [PubMed]
- Pai, Y.C.; Weng, L.T.; Wei, S.C.; Wu, L.L.; Shih, D.Q.; Targan, S.R.; Turner, J.R.; Yu, L.C. Gut microbial transcytosis induced by tumor necrosis factor-like 1A-dependent activation of a myosin light chain kinase splice variant contributes to IBD. J. Crohns Colitis 2020, 15, 258–272. [Google Scholar] [CrossRef] [PubMed]
- Corr, S.C.; Gahan, C.C.; Hill, C. M-cells: Origin, morphology and role in mucosal immunity and microbial pathogenesis. FEMS Immunol. Med. Microbiol. 2008, 52, 2–12. [Google Scholar] [CrossRef]
- Birge, R.B.; Boeltz, S.; Kumar, S.; Carlson, J.; Wanderley, J.; Calianese, D.; Barcinski, M.; Brekken, R.A.; Huang, X.; Hutchins, J.T.; et al. Phosphatidylserine is a global immunosuppressive signal in efferocytosis, infectious disease, and cancer. Cell Death Differ. 2016, 23, 962–978. [Google Scholar] [CrossRef]
- Park, J.H.; Kotani, T.; Konno, T.; Setiawan, J.; Kitamura, Y.; Imada, S.; Usui, Y.; Hatano, N.; Shinohara, M.; Saito, Y.; et al. Promotion of Intestinal Epithelial Cell Turnover by Commensal Bacteria: Role of Short-Chain Fatty Acids. PLoS ONE 2016, 11, e0156334. [Google Scholar] [CrossRef]
- Matsumoto, K. Phosphatidylserine synthase from bacteria. Biochim. Biophys. Acta 1997, 1348, 214–227. [Google Scholar] [CrossRef]
- Langley, K.E.; Hawrot, E.; Kennedy, E.P. Membrane assembly: Movement of phosphatidylserine between the cytoplasmic and outer membranes of Escherichia coli. J. Bacteriol. 1982, 152, 1033–1041. [Google Scholar] [CrossRef]
- Mishima, Y.; Oka, A.; Liu, B.; Herzog, J.W.; Eun, C.S.; Fan, T.J.; Bulik-Sullivan, E.; Carroll, I.M.; Hansen, J.J.; Chen, L.; et al. Microbiota maintain colonic homeostasis by activating TLR2/MyD88/PI3K signaling in IL-10-producing regulatory B cells. J. Clin. Investig. 2019, 129, 3702–3716. [Google Scholar] [CrossRef]
- Harmon, A.; Cornelius, D.; Amaral, L.; Paige, A.; Herse, F.; Ibrahim, T.; Wallukat, G.; Faulkner, J.; Moseley, J.; Dechend, R.; et al. IL-10 supplementation increases Tregs and decreases hypertension in the RUPP rat model of preeclampsia. Hypertens. Pregnancy 2015, 34, 291–306. [Google Scholar] [CrossRef]
- Alameddine, J.; Godefroy, E.; Papargyris, L.; Sarrabayrouse, G.; Tabiasco, J.; Bridonneau, C.; Yazdanbakhsh, K.; Sokol, H.; Altare, F.; Jotereau, F. Faecalibacterium prausnitzii Skews Human DC to Prime IL10-Producing T Cells Through TLR2/6/JNK Signaling and IL-10, IL-27, CD39, and IDO-1 Induction. Front. Immunol. 2019, 10, 143. [Google Scholar] [CrossRef]
- Watcharanurak, K.; Zang, L.; Nishikawa, M.; Yoshinaga, K.; Yamamoto, Y.; Takahashi, Y.; Ando, M.; Saito, K.; Watanabe, Y.; Takakura, Y. Effects of upregulated indoleamine 2, 3-dioxygenase 1 by interferon γ gene transfer on interferon γ-mediated antitumor activity. Gene Ther. 2014, 21, 794–801. [Google Scholar] [CrossRef]
- Poggi, A.; Benelli, R.; Venè, R.; Costa, D.; Ferrari, N.; Tosetti, F.; Zocchi, M.R. Human Gut-Associated Natural Killer Cells in Health and Disease. Front. Immunol. 2019, 10, 961. [Google Scholar] [CrossRef]
- Bessede, A.; Gargaro, M.; Pallotta, M.T.; Matino, D.; Servillo, G.; Brunacci, C.; Bicciato, S.; Mazza, E.M.; Macchiarulo, A.; Vacca, C.; et al. Aryl hydrocarbon receptor control of a disease tolerance defence pathway. Nature 2014, 511, 184–190. [Google Scholar] [CrossRef]
- Wolochow, H.; Hildebrand, G.J.; Lamanna, C. Translocation of microorganisms across the intestinal wall of the rat: Effect of microbial size and concentration. J. Infect. Dis. 1966, 116, 523–528. [Google Scholar] [CrossRef]
- Sakaguchi, S.; Sakaguchi, N.; Asano, M.; Itoh, M.; Toda, M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 1995, 155, 1151–1164. [Google Scholar] [CrossRef]
- Piconese, S.; Gri, G.; Tripodo, C.; Musio, S.; Gorzanelli, A.; Frossi, B.; Pedotti, R.; Pucillo, C.E.; Colombo, M.P. Mast cells counteract regulatory T-cell suppression through interleukin-6 and OX40/OX40L axis toward Th17-cell differentiation. Blood 2009, 114, 2639–2648. [Google Scholar] [CrossRef]
- Zhou, L.; Chu, C.; Teng, F.; Bessman, N.J.; Goc, J.; Santosa, E.K.; Putzel, G.G.; Kabata, H.; Kelsen, J.R.; Baldassano, R.N.; et al. Innate lymphoid cells support regulatory T cells in the intestine through interleukin-2. Nature 2019, 568, 405–409. [Google Scholar] [CrossRef]
- Ebert, E.C. IL-10 enhances IL-2-induced proliferation and cytotoxicity by human intestinal lymphocytes. Clin. Exp. Immunol. 2000, 119, 426–432. [Google Scholar] [CrossRef]
- Chaudhry, A.; Samstein, R.M.; Treuting, P.; Liang, Y.; Pils, M.C.; Heinrich, J.M.; Jack, R.S.; Wunderlich, F.T.; Brüning, J.C.; Müller, W.; et al. Interleukin-10 signaling in regulatory T cells is required for suppression of Th17 cell-mediated inflammation. Immunity 2011, 34, 566–578. [Google Scholar] [CrossRef] [PubMed]
- Linares, R.; Francés, R.; Gutiérrez, A.; Juanola, O. Bacterial Translocation as Inflammatory Driver in Crohn’s Disease. Front. Cell Dev. Biol. 2021, 9, 703310. [Google Scholar] [CrossRef] [PubMed]
- Vrakas, S.; Mountzouris, K.C.; Michalopoulos, G.; Karamanolis, G.; Papatheodoridis, G.; Tzathas, C.; Gazouli, M. Intestinal Bacteria Composition and Translocation of Bacteria in Inflammatory Bowel Disease. PLoS ONE 2017, 12, e0170034. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, G.; Tincati, C.; Silvestri, G. Microbial translocation in the pathogenesis of HIV infection and AIDS. Clin. Microbiol. Rev. 2013, 26, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.X.; Wang, B.; Li, B. IL-10 and IL-22 in Mucosal Immunity: Driving Protection and Pathology. Front. Immunol. 2020, 11, 1315. [Google Scholar] [CrossRef] [PubMed]
- Keir, M.; Yi, Y.; Lu, T.; Ghilardi, N. The role of IL-22 in intestinal health and disease. J. Exp. Med. 2020, 217, e20192195. [Google Scholar] [CrossRef]
- Kløverpris, H.N.; Kazer, S.W.; Mjösberg, J.; Mabuka, J.M.; Wellmann, A.; Ndhlovu, Z.; Yadon, M.C.; Nhamoyebonde, S.; Muenchhoff, M.; Simoni, Y.; et al. Innate Lymphoid Cells Are Depleted Irreversibly during Acute HIV-1 Infection in the Absence of Viral Suppression. Immunity 2016, 44, 391–405. [Google Scholar] [CrossRef] [PubMed]
- Klase, Z.; Ortiz, A.; Deleage, C.; Mudd, J.C.; Quiñones, M.; Schwartzman, E.; Klatt, N.R.; Canary, L.; Estes, J.D.; Brenchley, J.M. Dysbiotic bacteria translocate in progressive SIV infection. Mucosal. Immunol. 2015, 8, 1009–1020. [Google Scholar] [CrossRef]
- Dillon, S.M.; Lee, E.J.; Kotter, C.V.; Austin, G.L.; Dong, Z.; Hecht, D.K.; Gianella, S.; Siewe, B.; Smith, D.M.; Landay, A.L.; et al. An altered intestinal mucosal microbiome in HIV-1 infection is associated with mucosal and systemic immune activation and endotoxemia. Mucosal Immunol. 2014, 7, 983–994. [Google Scholar] [CrossRef]
- Rojas, J.M.; Avia, M.; Martín, V.; Sevilla, N. IL-10, A Multifunctional Cytokine in Viral Infections. J. Immunol. Res. 2017, 2017, 6104054. [Google Scholar] [CrossRef]
- Ouyang, W.; Rutz, S.; Crellin, N.K.; Valdez, P.A.; Hymowitz, S.G. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu. Rev. Immunol. 2011, 29, 71–109. [Google Scholar] [CrossRef]
- Amara, A.; Mercer, J. Viral apoptotic mimicry. Nat. Rev. Microbiol. 2015, 13, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Migone, T.S.; Zhang, J.; Zhuang, L.; Chen, C.; Hu, B.; Hong, J.S.; Perry, J.W.; Chen, S.F.; Zhou, J.X.; Cho, Y.H. TL1A is a TNF-like ligand for DR3 and TR6/DcR3 and functions as a T cell costimulator. Immunity 2002, 16, 479–492. [Google Scholar] [CrossRef]
- Siakavellas, S.I.; Sfikakis, P.P.; Bamias, G. The TL1A/DR3/DcR3 pathway in autoimmune rheumatic diseases. Semin. Arthritis Rheum. 2015, 45, 1–8. [Google Scholar] [CrossRef]
- Jacob, N.; Kumagai, K.; Abraham, J.P.; Shimodaira, Y.; Ye, Y.; Luu, J.; Blackwood, A.Y.; Castanon, S.L.; Stamps, D.T.; Thomas, L.S.; et al. Direct signaling of TL1A-DR3 on fibroblasts induces intestinal fibrosis in vivo. Sci. Rep. 2020, 10, 18189. [Google Scholar] [CrossRef]
- Ge, Z.; Sanders, A.J.; Ye, L.; Mansel, R.E.; Jiang, W.G. Expression of death receptor-3 in human breast cancer and its functional effects on breast cancer cells in vitro. Oncol. Rep. 2013, 29, 1356–1364. [Google Scholar] [CrossRef]
- Lan, X.; Lan, X.; Chang, Y.; Zhang, X.; Liu, J.; Vikash, V.; Wang, W.; Huang, M.; Wang, X.; Zhou, F.; et al. Identification of Two Additional Susceptibility Loci for Inflammatory Bowel Disease in a Chinese Population. Cell Physiol. Biochem. 2017, 41, 2077–2090. [Google Scholar] [CrossRef]
- Hassan-Zahraee, M.; Ye, Z.; Xi, L.; Baniecki, M.L.; Li, X.; Hyde, C.L.; Zhang, J.; Raha, N.; Karlsson, F.; Quan, J.; et al. Antitumor Necrosis Factor-like Ligand 1A Therapy Targets Tissue Inflammation and Fibrosis Pathways and Reduces Gut Pathobionts in Ulcerative Colitis. Inflamm. Bowel. Dis. 2022, 28, 434–446. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.D.; Li, R.; Huang, A.F. Role of TL1A in Inflammatory Autoimmune Diseases: A Comprehensive Review. Front. Immunol. 2022, 13, 891328. [Google Scholar] [CrossRef] [PubMed]
- Herro, R.; Miki, H.; Sethi, G.S.; Mills, D.; Mehta, A.K.; Nguyen, X.X.; Feghali-Bostwick, C.; Miller, M.; Broide, D.H.; Soloff, R.; et al. TL1A Promotes Lung Tissue Fibrosis and Airway Remodeling. J. Immunol. 2020, 205, 2414–2422. [Google Scholar] [CrossRef] [PubMed]
- Nicolson, G.L.; Ash, M.E. Lipid Replacement Therapy: A natural medicine approach to replacing damaged lipids in cellular membranes and organelles and restoring function. Biochim. Biophys. Acta 2014, 1838, 1657–1679. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Ley, R.E.; Hamady, M.; Fraser-Liggett, C.M.; Knight, R.; Gordon, J.I. The human microbiome project. Nature 2007, 449, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Committee on Metagenomics. Challenges and Functional Applications, National Research Council. In The New Science of Metagenomics: Revealing the Secrets of Our Microbial Planet; The National Academies Press: Washington, DC, USA, 2007; p. 174. [Google Scholar]
- Hugon, P.; Dufour, J.C.; Colson, P.; Fournier, P.E.; Sallah, K.; Raoult, D. A comprehensive repertoire of prokaryotic species identified in human beings. Lancet Infect. Dis. 2015, 15, 1211–1219. [Google Scholar] [CrossRef] [PubMed]
- Scarpellini, E.; Ianiro, G.; Attili, F.; Bassanelli, C.; De Santis, A.; Gasbarrini, A. The human gut microbiota and virome: Potential therapeutic implications. Dig. Liver Dis. 2015, 47, 1007–1012. [Google Scholar] [CrossRef] [PubMed]
- Robinson, C.M.; Pfeiffer, J.K. Viruses and the Microbiota. Annu. Rev. Virol. 2014, 1, 55–69. [Google Scholar] [CrossRef]
- Shkoporov, A.N.; Turkington, C.J.; Hill, C. Mutualistic interplay between bacteriophages and bacteria in the human gut. Nat. Rev. Microbiol. 2022, 20, 737–749. [Google Scholar] [CrossRef]
- Barr, J.J. A bacteriophages journey through the human body. Immunol. Rev. 2017, 279, 106–122. [Google Scholar] [CrossRef]
- Górski, A.; Borysowski, J.; Miȩdzybrodzki, R. Bacteriophage Interactions With Epithelial Cells: Therapeutic Implications. Front. Microbiol. 2021, 11, 631161. [Google Scholar] [CrossRef]
- Stone, E.; Campbell, K.; Grant, I.; McAuliffe, O. Understanding and Exploiting Phage-Host Interactions. Viruses 2019, 11, 567. [Google Scholar] [CrossRef]
- Vivier, E.; Artis, D.; Colonna, M.; Diefenbach, A.; Di Santo, J.P.; Eberl, G.; Koyasu, S.; Locksley, R.M.; McKenzie, A.N.; Mebius, R.E.; et al. Innate Lymphoid Cells: 10 Years On. Cell 2018, 174, 1054–1066. [Google Scholar] [CrossRef]
- Artis, D.; Spits, H. The biology of innate lymphoid cells. Nature 2015, 517, 293–301. [Google Scholar] [CrossRef]
- Fan, H.; Wang, A.; Wang, Y.; Sun, Y.; Han, J.; Chen, W.; Wang, S.; Wu, Y.; Lu, Y. Innate Lymphoid Cells: Regulators of Gut Barrier Function and Immune Homeostasis. J. Immunol. Res. 2019, 2019, 2525984. [Google Scholar] [CrossRef] [PubMed]
- Crinier, A.; Viant, C.; Girard-Madoux, M.; Vivier, É. Les cellules lymphoïdes innées [Innate lymphoid cells]. Med. Sci. 2017, 33, 534–542. [Google Scholar] [CrossRef]
- Ochel, A.; Tiegs, G.; Neumann, K. Type 2 Innate Lymphoid Cells in Liver and Gut: From Current Knowledge to Future Perspectives. Int. J. Mol. Sci. 2019, 20, 1896. [Google Scholar] [CrossRef]
- Milovanovic, J.; Arsenijevic, A.; Stojanovic, B.; Kanjevac, T.; Arsenijevic, D.; Radosavljevic, G.; Milovanovic, M.; Arsenijevic, N. Interleukin-17 in Chronic Inflammatory Neurological Diseases. Front. Immunol. 2020, 11, 947. [Google Scholar] [CrossRef]
- Lee, D.; Jo, H.; Go, C.; Jang, Y.; Chu, N.; Bae, S.; Kang, D.; Kim, Y.; Kang, J.S. The Roles of IL-22 and Its Receptor in the Regulation of Inflammatory Responses in the Brain. Int. J. Mol. Sci. 2022, 23, 757. [Google Scholar] [CrossRef]
- Sandler, N.G.; Wand, H.; Roque, A.; Law, M.; Nason, M.C.; Nixon, D.E.; Pedersen, C.; Ruxrungtham, K.; Lewin, S.R.; Emery, S.; et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J. Infect. Dis. 2011, 203, 780–790. [Google Scholar] [CrossRef] [PubMed]
- Brenchley, J.M.; Price, D.A.; Schacker, T.W.; Asher, T.E.; Silvestri, G.; Rao, S.; Kazzaz, Z.; Bornstein, E.; Lambotte, O.; Altmann, D.; et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat. Med. 2006, 12, 1365–1371. [Google Scholar] [CrossRef]
- Cassol, E.; Malfeld, S.; Mahasha, P.; Van der Merwe, S.; Cassol, S.; Seebregts, C.; Alfano, M.; Poli, G.; Rossouw, T. Persistent microbial translocation immune activation in HIV-1-infected South Africans receiving combination antiretroviral therapy. J. Infect. Dis. 2010, 202, 723–733. [Google Scholar] [CrossRef]
- Kim, C.J.; Nazli, A.; Rojas, O.L.; Chege, D.; Alidina, Z.; Huibner, S.; Mujib, S.; Benko, E.; Kovacs, C.; Shin, L.Y.Y.; et al. A role for mucosal IL-22 production and Th22 cells in HIV-associated mucosal immunopathogenesis. Mucosal Immunol. 2012, 5, 670–680. [Google Scholar] [CrossRef]
- Mills, K.H.G. IL-17 and IL-17-producing cells in protection versus pathology. Nat. Rev. Immunol. 2022, 23, 38–54. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; De Salvo, C.; Pizarro, T.T. Central role of IL-17/Th17 immune responses and the gut microbiota in the pathogenesis of intestinal fibrosis. Curr. Opin. Gastroenterol. 2014, 30, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Regen, T.; Isaac, S.; Amorim, A.; Núñez, N.G.; Hauptmann, J.; Shanmugavadivu, A.; Klein, M.; Sankowski, R.; Mufazalov, I.A.; Yogev, N.; et al. IL-17 controls central nervous system autoimmunity through the intestinal microbiome. Sci. Immunol. 2021, 6, eaaz6563. [Google Scholar] [CrossRef] [PubMed]
- Chenniappan, R.; Nandeesha, H.; Kattimani, S.; Nanjaiah, N.D. Interleukin-17 and Interleukin-10 Association with Disease Progression in Schizophrenia. Ann. Neurosci. 2020, 27, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Biagioli, M.; Marchianò, S.; Carino, A.; Di Giorgio, C.; Santucci, L.; Distrutti, E.; Fiorucci, S. Bile Acids Activated Receptors in Inflammatory Bowel Disease. Cells 2021, 10, 1281. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liang, W.; Wang, Y.; Yi, R.; Luo, L.; Wang, W.; Sun, N.; Yu, M.; Xu, W.; Sheng, Q.; et al. Ontogeny of RORγt+ cells in the intestine of newborns and its role in the development of experimental necrotizing enterocolitis. Cell BioSci. 2022, 12, 3. [Google Scholar] [CrossRef]
- Bassolas-Molina, H.; Raymond, E.; Labadia, M.; Wahle, J.; Ferrer-Picón, E.; Panzenbeck, M.; Zheng, J.; Harcken, C.; Hughes, R.; Turner, M.; et al. An RORγt Oral Inhibitor Modulates IL-17 Responses in Peripheral Blood and Intestinal Mucosa of Crohn’s Disease Patients. Front. Immunol. 2018, 9, 2307. [Google Scholar] [CrossRef]
- Stephen-Victor, E.; Chatila, T.A. Regulation of oral immune tolerance by the microbiome in food allergy. Curr. Opin. Immunol. 2019, 60, 141–147. [Google Scholar] [CrossRef]
- Hu, Z.J.; Xu, J.; Yin, J.M.; Li, L.; Hou, W.; Zhang, L.L.; Zhou, Z.; Yu, Y.Z.; Li, H.J.; Feng, Y.M.; et al. Lower Circulating Interferon-Gamma Is a Risk Factor for Lung Fibrosis in COVID-19 Patients. Front. Immunol. 2020, 11, 585647. [Google Scholar] [CrossRef]
- Daria, S.; Proma, M.A.; Shahriar, M.; Islam, S.M.A.; Bhuiyan, M.A.; Islam, M.R. Serum interferon-gamma level is associated with drug-naïve major depressive disorder. SAGE Open Med. 2020, 8, 2050312120974169. [Google Scholar] [CrossRef]
- Liu, W.; Li, M.; Wang, Z.; Wang, J. IFN-γ Mediates the Development of Systemic Lupus Erythematosus. Biomed. Res. Int. 2020, 2020, 7176515. [Google Scholar] [CrossRef] [PubMed]
- Ahrens, A.P.; Sanchez-Padilla, D.E.; Drew, J.C.; Oli, M.W.; Roesch, L.F.W.; Triplett, E.W. Saliva microbiome, dietary, and genetic markers are associated with suicidal ideation in university students. Sci. Rep. 2022, 12, 14306. [Google Scholar] [CrossRef] [PubMed]
- Elomaa, A.P.; Niskanen, L.; Herzig, K.H.; Viinamäki, H.; Hintikka, J.; Koivumaa-Honkanen, H.; Honkalampi, K.; Valkonen-Korhonen, M.; Harvima, I.T.; Lehto, S.M. Elevated levels of serum IL-5 are associated with an increased likelihood of major depressive disorder. BMC Psychiatry 2012, 12, 2. [Google Scholar] [CrossRef] [PubMed]
- Mannon, P.; Reinisch, W. Interleukin 13 and its role in gut defence and inflammation. Gut 2012, 61, 1765–1773. [Google Scholar] [CrossRef]
- Al Quraan, A.M.; Beriwal, N.; Sangay, P.; Namgyal, T. The Psychotic Impact of Helicobacter pylori Gastritis and Functional Dyspepsia on Depression: A Systematic Review. Cureus 2019, 11, e5956. [Google Scholar] [CrossRef] [PubMed]
- Cairo, C.; Webb, T.J. Effective Barriers: The Role of NKT Cells and Innate Lymphoid Cells in the Gut. J. Immunol. 2022, 208, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Zaph, C.; Du, Y.; Saenz, S.A.; Nair, M.G.; Perrigoue, J.G.; Taylor, B.C.; Troy, A.E.; Kobuley, D.E.; Kastelein, R.A.; Cua, D.J.; et al. Commensal-dependent expression of IL-25 regulates the IL-23-IL-17 axis in the intestine. J. Exp. Med. 2008, 205, 2191–2198. [Google Scholar] [CrossRef]
- Albert-Bayo, M.; Paracuellos, I.; González-Castro, A.M.; Rodríguez-Urrutia, A.; Rodríguez-Lagunas, M.J.; Alonso-Cotoner, C.; Santos, J.; Vicario, M. Intestinal Mucosal Mast Cells: Key Modulators of Barrier Function and Homeostasis. Cells 2019, 8, 135. [Google Scholar] [CrossRef]
- Stoyanova, I.I.; Gulubova, M.V. Mast cells and inflammatory mediators in chronic ulcerative colitis. Acta Histochem. 2002, 104, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Theoharides, T.C.; Valent, P.; Akin, C. Mast Cells, Mastocytosis, and Related Disorders. N. Engl. J. Med. 2015, 373, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Carter, M.C.; Metcalfe, D.D.; Komarow, H.D. Mastocytosis. Immunol. Allergy Clin. N. Am. 2014, 34, 181–196. [Google Scholar] [CrossRef] [PubMed]
- Georgin-Lavialle, S.; Gaillard, R.; Moura, D.; Hermine, O. Mastocytosis in adulthood and neuropsychiatric disorders. Transl Res. 2016, 174, 77–85.e1. [Google Scholar] [CrossRef] [PubMed]
- Afrin, L.B.; Pöhlau, D.; Raithel, M.; Haenisch, B.; Dumoulin, F.L.; Homann, J.; Mauer, U.M.; Harzer, S.; Molderings, G.J. Mast cell activation disease: An underappreciated cause of neurologic and psychiatric symptoms and diseases. Brain Behav. Immun. 2015, 50, 314–321. [Google Scholar] [CrossRef]
- Dong, H.; Wang, Y.; Zhang, X.; Zhang, X.; Qian, Y.; Ding, H.; Zhang, S. Stabilization of Brain Mast Cells Alleviates LPS-Induced Neuroinflammation by Inhibiting Microglia Activation. Front. Cell Neurosci. 2019, 13, 191. [Google Scholar] [CrossRef]
- Guimarães-Costa, A.B.; Nascimento, M.T.; Wardini, A.B.; Pinto-da-Silva, L.H.; Saraiva, E.M. ETosis: A Microbicidal Mechanism beyond Cell Death. J. Parasitol. Res. 2012, 2012, 929743. [Google Scholar] [CrossRef] [PubMed]
- Duvvuri, B.; Lood, C. Cell-Free DNA as a Biomarker in Autoimmune Rheumatic Diseases. Front. Immunol. 2019, 10, 502. [Google Scholar] [CrossRef]
- Cisneros-Villanueva, M.; Hidalgo-Pérez, L.; Rios-Romero, M.; Cedro-Tanda, A.; Ruiz-Villavicencio, C.A.; Page, K.; Hastings, R.; Fernandez-Garcia, D.; Allsopp, R.; Fonseca-Montaño, M.A.; et al. Cell-free DNA analysis in current cancer clinical trials: A review. Br. J. Cancer 2022, 126, 391–400. [Google Scholar] [CrossRef]
- Whalen, W.; Buyukozkan, M.; Moore, B.; Moon, J.S.; Cruz, C.S.D.; Martinez, F.J.; Choi, A.M.; Krumsiek, J.; Stout-Delgado, H.; Cho, S.J. Association of circulating cell-free double-stranded DNA and metabolic derangements in idiopathic pulmonary fibrosis. Thorax 2022, 77, 186–190. [Google Scholar] [CrossRef]
- Lubotzky, A.; Pelov, I.; Teplitz, R.; Neiman, D.; Smadja, A.; Zemmour, H.; Piyanzin, S.; Ochana, B.L.; Spalding, K.L.; Glaser, B.; et al. Elevated brain-derived cell-free DNA among patients with first psychotic episode—A proof-of-concept study. Elife 2022, 11, e76391. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Liu, L.; Ma, X.; Cao, X.; Chen, Y.; Qu, X.; Ji, M.; Liu, H.; Liu, C.; Qin, X.; et al. The Role of DNA Damage and Repair in Idiopathic Pulmonary Fibrosis. Antioxidants 2022, 11, 2292. [Google Scholar] [CrossRef] [PubMed]
- Bednarska, O.; Walter, S.A.; Casado-Bedmar, M.; Ström, M.; Salvo-Romero, E.; Vicario, M.; Mayer, E.A.; Keita, Å.V. Vasoactive Intestinal Polypeptide and Mast Cells Regulate Increased Passage of Colonic Bacteria in Patients with Irritable Bowel Syndrome. Gastroenterology 2017, 153, 948–960.e3. [Google Scholar] [CrossRef] [PubMed]
- Babina, M.; Franke, K.; Bal, G. How “Neuronal” Are Human Mast Cells? Int. J. Mol. Sci. 2022, 23, 10871. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.S.; Heng, Y.; Yuan, Y.H.; Chen, N.H. Pathological alpha-synuclein exacerbates the progression of Parkinson’s disease through microglial activation. Toxicol. Lett. 2017, 265, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Arrang, J.M. Histamine and schizophrenia. Int. Rev. Neurobiol. 2007, 78, 247–287. [Google Scholar] [CrossRef]
- Koyuncu Irmak, D.; Kilinc, E.; Tore, F. Shared Fate of Meningeal Mast Cells and Sensory Neurons in Migraine. Front. Cell Neurosci. 2019, 13, 136. [Google Scholar] [CrossRef]
- Provensi, G.; Costa, A.; Izquierdo, I.; Blandina, P.; Passani, M.B. Brain histamine modulates recognition memory: Possible implications in major cognitive disorders. Br. J. Pharmacol. 2020, 177, 539–556. [Google Scholar] [CrossRef] [PubMed]
- Ito, C. The role of the central histaminergic system on schizophrenia. Drug News Perspect. 2004, 17, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Reichert, C.F.; Maire, M.; Schmidt, C.; Cajochen, C. Sleep-Wake Regulation and Its Impact on Working Memory Performance: The Role of Adenosine. Biology 2016, 5, 11. [Google Scholar] [CrossRef]
- Mahmood, D. Histamine H3 receptors and its antagonism as a novel mechanism for antipsychotic effect: A current preclinical clinical perspective. Int. J. Health Sci. 2016, 10, 564–575. [Google Scholar]
- Rapanelli, M.; Frick, L.; Pogorelov, V.; Ohtsu, H.; Bito, H.; Pittenger, C. Histamine H3R receptor activation in the dorsal striatum triggers stereotypies in a mouse model of tic disorders. Transl. Psychiatry 2017, 7, e1013. [Google Scholar] [CrossRef]
- Chazot, P.L.; Johnston, L.; Mcauley, E.; Bonner, S. Histamine and Delirium: Current Opinion. Front. Pharmacol. 2019, 10, 299. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Dong, H.; Li, N.; Zhang, S.; Sun, J.; Zhang, S.; Qian, Y. Activated brain mast cells contribute to postoperative cognitive dysfunction by evoking microglia activation and neuronal apoptosis. J. Neuroinflamm. 2016, 13, 127. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, X.; Zhang, Y.; Qu, C.; Zhou, X.; Zhang, S. Histamine Induces Microglia Activation and the Release of Proinflammatory Mediators in Rat Brain Via H1R or H4R. J. Neuroimmune Pharmacol. 2020, 15, 280–291. [Google Scholar] [CrossRef]
- Yanuck, S.F. Microglial Phagocytosis of Neurons: Diminishing Neuronal Loss in Traumatic, Infectious, Inflammatory, and Autoimmune CNS Disorders. Front. Psychiatry 2019, 10, 712. [Google Scholar] [CrossRef] [PubMed]
- Neher, J.J.; Neniskyte, U.; Brown, G.C. Primary phagocytosis of neurons by inflamed microglia: Potential roles in neurodegeneration. Front. Pharmacol. 2012, 3, 27. [Google Scholar] [CrossRef]
- Liu, S.; Suzuki, Y.; Takemasa, E.; Watanabe, R.; Mogi, M. Mast cells promote viral entry of SARS-CoV-2 via formation of chymase/spike protein complex. Eur. J. Pharmacol. 2022, 930, 175169. [Google Scholar] [CrossRef]
- Weinstock, L.B.; Brook, J.B.; Walters, A.S.; Goris, A.; Afrin, L.B.; Molderings, G.J. Mast cell activation symptoms are prevalent in Long-COVID. Int. J. Infect. Dis. 2021, 112, 217–226. [Google Scholar] [CrossRef]
- Budinger, G.R. Angiotensin II and pulmonary fibrosis, a new twist on an old story. Am. J. Physiol. Lung Cell Mol. Physiol. 2011, 301, L267–L268. [Google Scholar] [CrossRef]
- Chang, Y.; Wei, W. Angiotensin II in inflammation, immunity and rheumatoid arthritis. Clin. Exp. Immunol. 2015, 179, 137–145. [Google Scholar] [CrossRef]
- Abiodun, O.A.; Ola, M.S. Role of brain renin angiotensin system in neurodegeneration: An update. Saudi J. Biol. Sci. 2020, 27, 905–912. [Google Scholar] [CrossRef]
- Vlachogiannis, N.I.; Pappa, M.; Ntouros, P.A.; Nezos, A.; Mavragani, C.P.; Souliotis, V.L.; Sfikakis, P.P. Association Between DNA Damage Response, Fibrosis and Type I Interferon Signature in Systemic Sclerosis. Front. Immunol. 2020, 11, 582401. [Google Scholar] [CrossRef] [PubMed]
- Manolakou, T.; Nikolopoulos, D.; Gkikas, D.; Filia, A.; Samiotaki, M.; Stamatakis, G.; Fanouriakis, A.; Politis, P.; Banos, A.; Alissafi, T.; et al. ATR-mediated DNA damage responses underlie aberrant B cell activity in systemic lupus erythematosus. Sci. Adv. 2022, 8, eabo5840. [Google Scholar] [CrossRef]
- Napoli, E.; Wong, S.; Giulivi, C. Evidence of reactive oxygen species-mediated damage to mitochondrial DNA in children with typical autism. Mol. Autism 2013, 4, 2. [Google Scholar] [CrossRef] [PubMed]
- Saadat, M.; Pakyari, N.; Farrashbandi, H. Genetic polymorphism in the DNA repair gene XRCC1 and susceptibility to schizophrenia. Psychiatry Res. 2008, 157, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Psimadas, D.; Messini-Nikolaki, N.; Zafiropoulou, M.; Fortos, A.; Tsilimigaki, S.; Piperakis, S.M. DNA damage and repair efficiency in lymphocytes from schizophrenic patients. Cancer Lett. 2004, 204, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Raza, M.U.; Tufan, T.; Wang, Y.; Hill, C.; Zhu, M.Y. DNA Damage in Major Psychiatric Diseases. Neurotox Res. 2016, 30, 251–267. [Google Scholar] [CrossRef] [PubMed]
- Victor, J.; Jordan, T.; Lamkin, E.; Ikeh, K.; March, A.; Frere, J.; Crompton, A.; Allen, L.; Fanning, J.; Lim, W.Y.; et al. SARS-CoV-2 hijacks host cell genome instability pathways. Res. Sq. 2022. [Google Scholar] [CrossRef]
- Zhang, L.; Richards, A.; Barrasa, M.I.; Hughes, S.H.; Young, R.A.; Jaenisch, R. Reverse-transcribed SARS-CoV-2 RNA can integrate into the genome of cultured human cells and can be expressed in patient-derived tissues. Proc. Natl. Acad. Sci. USA 2021, 118, e2105968118. [Google Scholar] [CrossRef]
- Brogna, C.; Brogna, B.; Bisaccia, D.R.; Lauritano, F.; Marino, G.; Montano, L.; Cristoni, S.; Prisco, M.; Piscopo, M. Could SARS-CoV-2 Have Bacteriophage Behavior or Induce the Activity of Other Bacteriophages? Vaccines 2022, 10, 708. [Google Scholar] [CrossRef]
- Dziubańska-Kusibab, P.J.; Berger, H.; Battistini, F.; Bouwman, B.A.M.; Iftekhar, A.; Katainen, R.; Cajuso, T.; Crosetto, N.; Orozco, M.; Aaltonen, L.A.; et al. Colibactin DNA-damage signature indicates mutational impact in colorectal cancer. Nat. Med. 2020, 26, 1063–1069. [Google Scholar] [CrossRef]
- Liu, H.; Zhu, J.; Hu, Q.; Rao, X. Morganella morganii, a non-negligent opportunistic pathogen. Int. J. Infect. Dis. 2016, 50, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Kanchanatawan, B.; Sirivichayakul, S.; Carvalho, A.F. In Schizophrenia, Increased Plasma IgM/IgA Responses to Gut Commensal Bacteria Are Associated with Negative Symptoms, Neurocognitive Impairments, and the Deficit Phenotype. Neurotox Res. 2019, 35, 684–698. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Oh, J.; Xue, M.; Huh, W.J.; Wang, J.; Gonzalez-Hernandez, J.A.; Rice, T.A.; Martin, A.L.; Song, D.; Crawford, J.M.; et al. Commensal microbiota from patients with inflammatory bowel disease produce genotoxic metabolites. Science 2022, 378, eabm3233. [Google Scholar] [CrossRef] [PubMed]
- Markkanen, E.; Meyer, U.; Dianov, G.L. DNA Damage and Repair in Schizophrenia and Autism: Implications for Cancer Comorbidity and Beyond. Int. J. Mol. Sci. 2016, 17, 856. [Google Scholar] [CrossRef] [PubMed]
- Czarny, P.; Bialek, K.; Ziolkowska, S.; Strycharz, J.; Sliwinski, T. DNA damage and repair in neuropsychiatric disorders. What do we know and what are the future perspectives? Mutagenesis 2020, 35, 79–106. [Google Scholar] [CrossRef]
- Topak, O.Z.; Ozdel, O.; Dodurga, Y.; Secme, M. An evaluation of the differences in DNA damage in lymphocytes and repair efficiencies in patients with schizophrenia and schizoaffective disorder. Schizophr. Res. 2018, 202, 99–105. [Google Scholar] [CrossRef]
- Martinel Lamas, D.J.; Carabajal, E.; Prestifilippo, J.P.; Rossi, L.; Elverdin, J.C.; Merani, S.; Bergoc, R.M.; Rivera, E.S.; Medina, V.A. Protection of radiation-induced damage to the hematopoietic system, small intestine and salivary glands in rats by JNJ7777120 compound, a histamine H4 ligand. PLoS ONE 2013, 8, e69106. [Google Scholar] [CrossRef]
- Jangi, S.M.; Díaz-Pérez, J.L.; Ochoa-Lizarralde, B.; Martín-Ruiz, I.; Asumendi, A.; Pérez-Yarza, G.; Gardeazabal, J.; Díaz-Ramón, J.L.; Boyano, M.D. H1 histamine receptor antagonists induce genotoxic and caspase-2-dependent apoptosis in human melanoma cells. Carcinogenesis 2006, 27, 1787–1796. [Google Scholar] [CrossRef]
- Sanchez-Molina, P.; Almolda, B.; Giménez-Llort, L.; González, B.; Castellano, B. Chronic IL-10 overproduction disrupts microglia-neuron dialogue similar to aging, resulting in impaired hippocampal neurogenesis and spatial memory. Brain Behav. Immun. 2022, 101, 231–245. [Google Scholar] [CrossRef]
- Nishigori, C.; Yarosh, D.B.; Ullrich, S.E.; Vink, A.A.; Bucana, C.D.; Roza, L.; Kripke, M.L. Evidence that DNA damage triggers interleukin 10 cytokine production in UV-irradiated murine keratinocytes. Proc. Natl. Acad. Sci. USA 1996, 93, 10354–10359. [Google Scholar] [CrossRef]
- Konopka, A.; Atkin, J.D. The Role of DNA Damage in Neural Plasticity in Physiology and Neurodegeneration. Front. Cell Neurosci. 2022, 16, 836885. [Google Scholar] [CrossRef] [PubMed]
- Merlo, D.; Cuchillo-Ibañez, I.; Parlato, R.; Rammes, G. DNA Damage, Neurodegeneration, and Synaptic Plasticity. Neural. Plast. 2016, 2016, 1206840. [Google Scholar] [CrossRef] [PubMed]
- Al-Asmary, S.M.; Kadasah, S.; Arfin, M.; Tariq, M.; Al-Asmari, A. Genetic Variants of Interleukin-10 Gene Promoter are Associated with Schizophrenia in Saudi Patients: A Case-Control Study. N. Am. J. Med. Sci. 2014, 6, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Fu, G.; Zhang, W.; Dai, J.; Liu, J.; Li, F.; Wu, D.; Xiao, Y.; Shah, C.; Sweeney, J.A.; Wu, M.; et al. Increased Peripheral Interleukin 10 Relate to White Matter Integrity in Schizophrenia. Front. Neurosci. 2019, 13, 52. [Google Scholar] [CrossRef] [PubMed]
- Paquola, A.C.M.; Erwin, J.A.; Gage, F.H. Insights into the role of somatic mosaicism in the brain. Curr. Opin. Syst. Biol. 2017, 1, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Jourdon, A.; Fasching, L.; Scuderi, S.; Abyzov, A.; Vaccarino, F.M. The role of somatic mosaicism in brain disease. Curr. Opin. Genet. Dev. 2020, 65, 84–90. [Google Scholar] [CrossRef] [PubMed]
- D’Aquila, P.; Giacconi, R.; Malavolta, M.; Piacenza, F.; Bürkle, A.; Villanueva, M.M.; Dollé, M.E.T.; Jansen, E.; Grune, T.; Gonos, E.S.; et al. Microbiome in Blood Samples from the General Population Recruited in the MARK-AGE Project: A Pilot Study. Front. Microbiol. 2021, 12, 707515. [Google Scholar] [CrossRef]
- Branton, W.G.; Ellestad, K.K.; Maingat, F.; Wheatley, B.M.; Rud, E.; Warren, R.L.; Holt, R.A.; Surette, M.G.; Power, C. Brain microbial populations in HIV/AIDS: Alpha-proteobacteria predominate independent of host immune status. PLoS ONE 2013, 8, e54673. [Google Scholar] [CrossRef]
- Poore, G.D.; Kopylova, E.; Zhu, Q.; Carpenter, C.; Fraraccio, S.; Wandro, S.; Kosciolek, T.; Janssen, S.; Metcalf, J.; Song, S.J.; et al. Microbiome analyses of blood and tissues suggest cancer diagnostic approach. Nature 2020, 579, 567–574. [Google Scholar] [CrossRef]
- Velmurugan, G.; Dinakaran, V.; Rajendhran, J.; Swaminathan, K. Blood Microbiota and Circulating Microbial Metabolites in Diabetes and Cardiovascular Disease. Trends Endocrinol. Metab. 2020, 31, 835–847. [Google Scholar] [CrossRef]
- Dominy, S.S.; Lynch, C.; Ermini, F.; Benedyk, M.; Marczyk, A.; Konradi, A.; Nguyen, M.; Haditsch, U.; Raha, D.; Griffin, C.; et al. Porphyromonas gingivalis in Alzheimer’s disease brains: Evidence for disease causation and treatment with small-molecule inhibitors. Sci. Adv. 2019, 5, eaau3333. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.; Stamova, B.; Jin, L.W.; DeCarli, C.; Phinney, B.; Sharp, F.R. Gram-negative bacterial molecules associate with Alzheimer disease pathology. Neurology 2016, 87, 2324–2332. [Google Scholar] [CrossRef] [PubMed]
- Manfredo Vieira, S.; Hiltensperger, M.; Kumar, V.; Zegarra-Ruiz, D.; Dehner, C.; Khan, N.; Costa, F.R.C.; Tiniakou, E.; Greiling, T.; Ruff, W.; et al. Translocation of a gut pathobiont drives autoimmunity in mice and humans. Science 2018, 359, 1156–1161. [Google Scholar] [CrossRef] [PubMed]
- Park, C.S.; Kim, S.H.; Lee, C.K. Immunotherapy of Autoimmune Diseases with Nonantibiotic Properties of Tetracyclines. Immune Netw. 2020, 20, e47. [Google Scholar] [CrossRef] [PubMed]
- Käbisch, R.; Semper, R.P.; Wüstner, S.; Gerhard, M.; Mejías-Luque, R. Helicobacter pylori γ-Glutamyltranspeptidase Induces Tolerogenic Human Dendritic Cells by Activation of Glutamate Receptors. J. Immunol. 2016, 196, 4246–4252. [Google Scholar] [CrossRef] [PubMed]
- Arvola, M.; Keinänen, K. Characterization of the ligand-binding domains of glutamate receptor (GluR)-B and GluR-D subunits expressed in Escherichia coli as periplasmic proteins. J. Biol. Chem. 1996, 271, 15527–15532. [Google Scholar] [CrossRef]
- Dagorn, A.; Chapalain, A.; Mijouin, L.; Hillion, M.; Duclairoir-Poc, C.; Chevalier, S.; Taupin, L.; Orange, N.; Feuilloley, M.G. Effect of GABA, a bacterial metabolite, on Pseudomonas fluorescens surface properties and cytotoxicity. Int. J. Mol. Sci. 2013, 14, 12186–12204. [Google Scholar] [CrossRef]
- Wouters, T.; Braegger, C.; Lacroix, C.; Pugin, B. GABA Production by Human Intestinal Bacteroides spp.: Prevalence, Regulation, and Role in Acid Stress Tolerance. Front. Microbiol. 2021, 12, 656895. [Google Scholar] [CrossRef]
- Zhu, F.; Shan, W.; Lv, R.; Li, Z.; Wang, Q. Clinical Characteristics of Anti-GABA-B Receptor Encephalitis. Front. Neurol. 2020, 11, 403. [Google Scholar] [CrossRef]
- Shiwaku, H.; Nakano, Y.; Kato, M.; Takahashi, H. Detection of autoantibodies against GABAARα1 in patients with schizophrenia. Schizophr. Res. 2020, 216, 543–546. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, J.; Chen, Y. Regulation of Neurotransmitters by the Gut Microbiota and Effects on Cognition in Neurological Disorders. Nutrients 2021, 13, 2099. [Google Scholar] [CrossRef] [PubMed]
- Wessler, I.K.; Kirkpatrick, C.J. Non-neuronal acetylcholine involved in reproduction in mammals and honeybees. J. Neurochem. 2017, 142 (Suppl. 2), 144–150. [Google Scholar] [CrossRef]
- Iwasa, K.; Yoshikawa, H.; Hamaguchi, T.; Sakai, K.; Shinohara-Noguchi, M.; Samuraki, M.; Takahashi, K.; Yanase, D.; Ono, K.; Ishida, C.; et al. Time-series analysis: Variation of anti-acetylcholine receptor antibody titer in myasthenia gravis is related to incidence of Mycoplasma pneumoniae and influenza virus infections. Neurol. Res. 2018, 40, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Bynke, A.; Julin, P.; Gottfries, C.G.; Heidecke, H.; Scheibenbogen, C.; Bergquist, J. Autoantibodies to beta-adrenergic and muscarinic cholinergic receptors in Myalgic Encephalomyelitis (ME) patients—A validation study in plasma and cerebrospinal fluid from two Swedish cohorts. Brain Behav. Immun. Health 2020, 7, 100107. [Google Scholar] [CrossRef]
- Yoshikawa, H.; Lennon, V.A. ACh receptor protein drives primary and memory autoantibody responses in chimeric human-SCID mice. Clin. Immunol. 2002, 104, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, G.; Wang, X.; Wen, Z.; Shuai, L.; Luo, J.; Wang, C.; Sun, Z.; Liu, R.; Ge, J.; et al. SARS-CoV-2 uses metabotropic glutamate receptor subtype 2 as an internalization factor to infect cells. Cell Discov. 2021, 7, 119. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, Z.; Liu, R.; Shuai, L.; Wang, X.; Luo, J.; Wang, C.; Chen, W.; Wang, X.; Ge, J.; et al. Metabotropic glutamate receptor subtype 2 is a cellular receptor for rabies virus. PLoS Pathog. 2018, 14, e1007189. [Google Scholar] [CrossRef]
- Ruiz-García, R.; Martínez-Hernández, E.; Joubert, B.; Petit-Pedrol, M.; Pajarón-Boix, E.; Fernández, V.; Salais, L.; Del Pozo, M.; Armangué, T.; Sabater, L.; et al. Paraneoplastic cerebellar ataxia and antibodies to metabotropic glutamate receptor 2. Neurol. NeuroImmunol. Neuroinflamm. 2019, 7, e658. [Google Scholar] [CrossRef]
- Obara, I.; Telezhkin, V.; Alrashdi, I.; Chazot, P.L. Histamine, histamine receptors, and neuropathic pain relief. Br. J. Pharmacol. 2020, 177, 580–599. [Google Scholar] [CrossRef]
- De Palma, G.; Shimbori, C.; Reed, D.E.; Yu, Y.; Rabbia, V.; Lu, J.; Jimenez-Vargas, N.; Sessenwein, J.; Lopez-Lopez, C.; Pigrau, M.; et al. Histamine production by the gut microbiota induces visceral hyperalgesia through histamine 4 receptor signaling in mice. Sci. Transl. Med. 2022, 14, eabj1895. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, M.M. Histamine in the regulation of wakefulness. Sleep Med. Rev. 2011, 15, 65–74. [Google Scholar] [CrossRef]
- Zhang, M.; Venable, J.D.; Thurmond, R.L. The histamine H4 receptor in autoimmune disease. Expert Opin. Investig. Drugs. 2006, 15, 1443–1452. [Google Scholar] [CrossRef] [PubMed]
- Jutel, M.; Watanabe, T.; Klunker, S.; Akdis, M.; Thomet, O.A.R.; Malolepszy, J.; Zak-Nejmark, T.; Koga, R.; Kobayashi, T.; Blaser, K.; et al. Histamine regulates T-cell and antibody responses by differential expression of H1 and H2 receptors. Nature 2001, 413, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Krueger, J.M.; Rosenthal, R.S.; Martin, S.A.; Walter, J.; Davenne, D.; Shoham, S.; Kubillus, S.L.; Biemann, K. Bacterial peptidoglycans as modulators of sleep. I. Anhydro forms of muramyl peptides enhance somnogenic potency. Brain Res. 1987, 403, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, Y.; Miyoshi, C.; Obana, N.; Yajima, K.; Hotta-Hirashima, N.; Ikkyu, A.; Kanno, S.; Soga, T.; Fukuda, S.; Yanagisawa, M. Gut microbiota depletion by chronic antibiotic treatment alters the sleep/wake architecture and sleep EEG power spectra in mice. Sci. Rep. 2020, 10, 19554. [Google Scholar] [CrossRef]
- Szentirmai, É.; Massie, A.R.; Kapás, L. Lipoteichoic acid, a cell wall component of Gram-positive bacteria, induces sleep and fever and suppresses feeding. Brain Behav. Immun. 2021, 92, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Rockstrom, M.D.; Chen, L.; Taishi, P.; Nguyen, J.T.; Gibbons, C.M.; Veasey, S.C.; Krueger, J.M. Tumor necrosis factor alpha in sleep regulation. Sleep Med. Rev. 2018, 40, 69–78. [Google Scholar] [CrossRef]
- Jewett, K.A.; Krueger, J.M. Humoral sleep regulation; interleukin-1 and tumor necrosis factor. Vitam. Horm. 2012, 89, 241–257. [Google Scholar] [CrossRef] [PubMed]
- Karatas, G.; Bal, A.; Yuceege, M.; Firat, H.; Gurcay, E.; Ardic, S.; Cakci, F.A. Evaluation of sleep quality in patients with ankylosing spondylitis and efficacy of anti-TNF-α therapy on sleep problems: A polisomnographic study. Int. J. Rheum Dis. 2018, 21, 1263–1269. [Google Scholar] [CrossRef]
- Schmidt, E.M.; Linz, B.; Diekelmann, S.; Besedovsky, L.; Lange, T.; Born, J. Effects of an interleukin-1 receptor antagonist on human sleep, sleep-associated memory consolidation, and blood monocytes. Brain Behav. Immun. 2015, 47, 178–185. [Google Scholar] [CrossRef]
- Eugster, M.R.; Loessner, M.J. Wall teichoic acids restrict access of bacteriophage endolysin Ply118, Ply511, and PlyP40 cell wall binding domains to the Listeria monocytogenes peptidoglycan. J. Bacteriol. 2012, 194, 6498–6506. [Google Scholar] [CrossRef] [PubMed]
- Räisänen, L.; Schubert, K.; Jaakonsaari, T.; Alatossava, T. Characterization of lipoteichoic acids as Lactobacillus delbrueckii phage receptor components. J. Bacteriol. 2004, 186, 5529–5532. [Google Scholar] [CrossRef]
- Guryanova, S.V. Regulation of Immune Homeostasis via Muramyl Peptides-Low Molecular Weight Bioregulators of Bacterial Origin. Microorganisms 2022, 10, 1526. [Google Scholar] [CrossRef] [PubMed]
- Sarkanen, T.O.; Alakuijala, A.P.E.; Dauvilliers, Y.A.; Partinen, M.M. Incidence of narcolepsy after H1N1 influenza and vaccinations: Systematic review and meta-analysis. Sleep Med. Rev. 2018, 38, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Tesoriero, C.; Codita, A.; Zhang, M.D.; Cherninsky, A.; Karlsson, H.; Grassi-Zucconi, G.; Bertini, G.; Harkany, T.; Ljungberg, K.; Liljeström, P.; et al. H1N1 influenza virus induces narcolepsy-like sleep disruption and targets sleep-wake regulatory neurons in mice. Proc. Natl. Acad. Sci. USA 2016, 113, E368–E377. [Google Scholar] [CrossRef]
- Dale, R.C.; Church, A.J.; Surtees, R.A.; Lees, A.J.; Adcock, J.E.; Harding, B.; Neville, B.G.; Giovannoni, G. Encephalitis lethargica syndrome: 20 new cases and evidence of basal ganglia autoimmunity. Brain 2004, 127 Pt 1, 21–33. [Google Scholar] [CrossRef]
- Leta, V.; Urso, D.; Batzu, L.; Lau, Y.H.; Mathew, D.; Boura, I.; Raeder, V.; Falup-Pecurariu, C.; van Wamelen, D.; Ray Chaudhuri, K. Viruses, parkinsonism and Parkinson’s disease: The past, present and future. J. Neural Transm. 2022, 129, 1119–1132. [Google Scholar] [CrossRef]
- Weiß, E.; Kretschmer, D. Formyl-Peptide Receptors in Infection, Inflammation, and Cancer. Trends Immunol. 2018, 39, 815–829. [Google Scholar] [CrossRef]
- Matei, D.E.; Menon, M.; Alber, D.G.; Smith, A.M.; Nedjat-Shokouhi, B.; Fasano, A.; Magill, L.; Duhlin, A.; Bitoun, S.; Gleizes, A.; et al. Intestinal barrier dysfunction plays an integral role in arthritis pathology and can be targeted to ameliorate disease. Med 2021, 2, 864–883.e9. [Google Scholar] [CrossRef]
- Mormile, I.; Rossi, F.W.; Prevete, N.; Granata, F.; Pucino, V.; de Paulis, A. The N-Formyl Peptide Receptors and Rheumatoid Arthritis: A Dangerous Liaison or Confusing Relationship? Front. Immunol. 2021, 12, 685214. [Google Scholar] [CrossRef]
- He, H.Q.; Ye, R.D. The Formyl Peptide Receptors: Diversity of Ligands and Mechanism for Recognition. Molecules 2017, 22, 455. [Google Scholar] [CrossRef] [PubMed]
- Addabbo, F.; Baglioni, V.; Schrag, A.; Schwarz, M.J.; Dietrich, A.; Hoekstra, P.J.; Martino, D.; Buttiglione, M. Anti-dopamine D2 receptor antibodies in chronic tic disorders. Dev. Med. Child. Neurol. 2020, 62, 1205–1212. [Google Scholar] [CrossRef] [PubMed]
- Freestone, P.P.; Haigh, R.D.; Lyte, M. Specificity of catecholamine-induced growth in Escherichia coli O157, H7, Salmonella enterica and Yersinia enterocolitica. FEMS Microbiol. Lett. 2007, 269, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Loebel, M.; Grabowski, P.; Heidecke, H.; Bauer, S.; Hanitsch, L.G.; Wittke, K.; Meisel, C.; Reinke, P.; Volk, H.-D.; Fluge, Ø; et al. Antibodies to β adrenergic and muscarinic cholinergic receptors in patients with Chronic Fatigue Syndrome. Brain Behav. Immun. 2016, 52, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.; Bänsch, M.; Berg, P.A. Clinical relevance of antibodies against serotonin and gangliosides in patients with primary fibromyalgia syndrome. Psychoneuroendocrinology 1992, 17, 593–598. [Google Scholar] [CrossRef]
- Coplan, J.D.; Tamir, H.; Calaprice, D.; DeJesus, M.; de la Nuez, M.; Pine, D.; Papp, L.A.; Klein, D.F.; Gorman, J.M. Plasma anti-serotonin and serotonin anti-idiotypic antibodies are elevated in panic disorder. Neuropsychopharmacology 1999, 20, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Russell, R.M.; Pifer, R.; Menezes-Garcia, Z.; Cuesta, S.; Narayanan, S.; MacMillan, J.B.; Sperandio, V. The Serotonin Neurotransmitter Modulates Virulence of Enteric Pathogens. Cell Host Microbe 2020, 28, 41–53.e8. [Google Scholar] [CrossRef] [PubMed]
- Henderson, N.C.; Rieder, F.; Wynn, T.A. Fibrosis: From mechanisms to medicines. Nature 2020, 587, 555–566. [Google Scholar] [CrossRef]
- Moretti, L.; Stalfort, J.; Barker, T.H.; Abebayehu, D. The interplay of fibroblasts, the extracellular matrix, and inflammation in scar formation. J. Biol. Chem. 2022, 298, 101530. [Google Scholar] [CrossRef] [PubMed]
- Shaw, T.J.; Rognoni, E. Dissecting Fibroblast Heterogeneity in Health and Fibrotic Disease. Curr. Rheumatol. Rep. 2020, 22, 33. [Google Scholar] [CrossRef]
- Joannes, A.; Brayer, S.; Besnard, V.; Marchal-Sommé, J.; Jaillet, M.; Mordant, P.; Mal, H.; Borie, R.; Crestani, B.; Mailleux, A.A. FGF9 and FGF18 in idiopathic pulmonary fibrosis promote survival and migration and inhibit myofibroblast differentiation of human lung fibroblasts in vitro. Am. J. Physiol. Lung Cell Mol. Physiol. 2016, 310, L615–L629. [Google Scholar] [CrossRef]
- Coffey, E.; Newman, D.R.; Sannes, P.L. Expression of fibroblast growth factor 9 in normal human lung and idiopathic pulmonary fibrosis. J. Histochem. Cytochem. 2013, 61, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Danopoulos, S.; Schlieve, C.R.; Grikscheit, T.C.; Al Alam, D. Fibroblast Growth Factors in the Gastrointestinal Tract: Twists and Turns. Dev. Dyn. 2017, 246, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Bottino, C.; Walzer, T.; Santoni, A.; Castriconi, R. Editorial: TGF-β as a Key Regulator of NK and ILCs Development and Functions. Front. Immunol. 2021, 11, 631712. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N. Transforming growth factor-β in tissue fibrosis. J. Exp. Med. 2020, 217, e20190103. [Google Scholar] [CrossRef]
- Horsburgh, S.; Todryk, S.; Ramming, A.; Distler, J.H.W.; O’Reilly, S. Innate lymphoid cells and fibrotic regulation. Immunol. Lett. 2018, 195, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Bauché, D.; Marie, J.C. Transforming growth factor β: A master regulator of the gut microbiota and immune cell interactions. Clin. Transl. Immunol. 2017, 6, e136. [Google Scholar] [CrossRef]
- Sobecki, M.; Krzywinska, E.; Nagarajan, S.; Audigé, A.; Huỳnh, K.; Zacharjasz, J.; Debbache, J.; Kerdiles, Y.; Gotthardt, D.; Takeda, N.; et al. NK cells in hypoxic skin mediate a trade-off between wound healing and antibacterial defence. Nat. Commun. 2021, 12, 4700. [Google Scholar] [CrossRef]
- Shi, F.-D.; Ljunggren, H.-G.; La Cava, A.; Van Kaer, L. Organ-specific features of natural killer cells. Nat. Rev. Immunol. 2011, 11, 658–671. [Google Scholar] [CrossRef]
- Hams, E.; Armstrong, M.E.; Barlow, J.L.; Saunders, S.P.; Schwartz, C.; Cooke, G.; Fahy, R.J.; Crotty, T.B.; Hirani, N.; Flynn, R.J.; et al. IL-25 and type 2 innate lymphoid cells induce pulmonary fibrosis. Proc. Natl. Acad. Sci. USA 2014, 111, 367–372. [Google Scholar] [CrossRef]
- Nicastro, L.K.; de Anda, J.; Jain, N.; Grando, K.C.M.; Miller, A.L.; Bessho, S.; Gallucci, S.; Wong, G.C.L.; Tükel, Ç. Assembly of ordered DNA-curli fibril complexes during Salmonella biofilm formation correlates with strengths of the type I interferon and autoimmune responses. PLoS Pathog. 2022, 18, e1010742. [Google Scholar] [CrossRef]
- Ramani, K.; Biswas, P.S. Interleukin-17: Friend or foe in organ fibrosis. Cytokine 2019, 120, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Hoyne, G.F.; Elliott, H.; Mutsaers, S.E.; Prêle, C.M. Idiopathic pulmonary fibrosis and a role for autoimmunity. Immunol. Cell Biol. 2017, 95, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Nishimori, J.H.; Newman, T.N.; Oppong, G.O.; Rapsinski, G.J.; Yen, J.-H.; Biesecker, S.G.; Wilson, R.P.; Butler, B.P.; Winter, M.G.; Tsolis, R.M.; et al. Microbial amyloids induce interleukin 17A (IL-17A) and IL-22 responses via Toll-like receptor 2 activation in the intestinal mucosa. Infect. Immun. 2012, 80, 4398–4408. [Google Scholar] [CrossRef] [PubMed]
- Reed, N.I.; Jo, H.; Chen, C.; Tsujino, K.; Arnold, T.D.; DeGrado, W.F.; Sheppard, D. The αvβ1 integrin plays a critical in vivo role in tissue fibrosis. Sci. Transl. Med. 2015, 7, 288. [Google Scholar] [CrossRef]
- Ulanova, M.; Gravelle, S.; Barnes, R. The role of epithelial integrin receptors in recognition of pulmonary pathogens. J. Innate Immun. 2009, 1, 4–17. [Google Scholar] [CrossRef]
- Peng, C.; Zou, X.; Xia, W.; Gao, H.; Li, Z.; Liu, N.; Xu, Z.; Gao, C.; He, Z.; Niu, W.; et al. Integrin αvβ6 plays a bi-directional regulation role between colon cancer cells and cancer-associated fibroblasts. BioSci. Rep. 2018, 38, BSR20180243. [Google Scholar] [CrossRef]
- Desai, S.; Sheikh, B.; Belzie, L. New-Onset Psychosis Following COVID-19 Infection. Cureus 2021, 13, e17904. [Google Scholar] [CrossRef]
- Kozato, N.; Mishra, M.; Firdosi, M. New-onset psychosis due to COVID-19. BMJ. Case Rep. 2021, 14, e242538. [Google Scholar] [CrossRef]
- Segev, A.; Hirsch-Klein, E.; Kotz, G.; Kamhi-Nesher, S.; Halimi, S.; Qashu, K.; Schreiber, E.; Krivoy, A. Trends of new-onset psychosis or mania in psychiatric emergency departments during the COVID19 pandemic: A longitudinal comparative study. Sci. Rep. 2021, 11, 21002. [Google Scholar] [CrossRef]
- Grover, S.; Rani, S.; Kohat, K.; Kathiravan, S.; Patel, G.; Sahoo, S.; Mehra, A.; Singh, S.; Bhadada, S. First episode psychosis following receipt of first dose of COVID-19 vaccine: A case report. Schizophr. Res. 2022, 241, 70–71. [Google Scholar] [CrossRef] [PubMed]
- Reinfeld, S.; Cáceda, R.; Gil, R.; Strom, H.; Chacko, M. Can new onset psychosis occur after mRNA based COVID-19 vaccine administration? A case report. Psychiatry Res. 2021, 304, 114165. [Google Scholar] [CrossRef] [PubMed]
- Aljeshi, A.A.; Abdelrahim, A.S.I.; Aljeshi, M.A. Psychosis Associated With COVID-19 Vaccination. Prim. Care Companion CNS Disord. 2022, 24, 21cr03160. [Google Scholar] [CrossRef] [PubMed]
- Noll, R. Kraepelin’s ‘lost biological psychiatry’? Autointoxication, organotherapy and surgery for dementia praecox. Hist Psychiatry 2007, 18 Pt 3, 301–320. [Google Scholar] [CrossRef]
- Quaglio, A.E.V.; Grillo, T.G.; De Oliveira, E.C.S.; Di Stasi, L.C.; Sassaki, L.Y. Gut microbiota, inflammatory bowel disease and colorectal cancer. World J. Gastroenterol. 2022, 28, 4053–4060. [Google Scholar] [CrossRef]
- Luchetti, M.M.; Ciccia, F.; Avellini, C.; Benfaremo, D.; Rizzo, A.; Spadoni, T.; Svegliati, S.; Marzioni, D.; Santinelli, A.; Costantini, A.; et al. Gut epithelial impairment, microbial translocation and immune system activation in inflammatory bowel disease-associated spondyloarthritis. Rheumatology 2021, 60, 92–102. [Google Scholar] [CrossRef]
- Kouzu, K.; Tsujimoto, H.; Kishi, Y.; Ueno, H.; Shinomiya, N. Bacterial Translocation in Gastrointestinal Cancers and Cancer Treatment. Biomedicines 2022, 10, 380. [Google Scholar] [CrossRef]
- Graham, K.L.; Carson, C.M.; Ezeoke, A.; Buckley, P.F.; Miller, B.J. Urinary tract infections in acute psychosis. J. Clin. Psychiatry 2014, 75, 379–385. [Google Scholar] [CrossRef]
- Lee, P.; Oleszak, F.; Nihalani, A.; Velayudhan, V.; McFarlane, I.M. Acute Psychosis Precipitated by Urinary Tract Infection in a Patient with Gliosis of the Basal Ganglia. Am. J. Med. Case Rep. 2019, 7, 329–333. [Google Scholar] [CrossRef]
- Ketcham, E.; Miller, B.J. Recurrent antimicrobial exposure and acute psychosis. Ann. Clin. Psychiatry 2022, 34, 221–226. [Google Scholar] [CrossRef]
- Moreno, J.L.; Kurita, M.; Holloway, T.; López, J.; Cadagan, R.; Martínez-Sobrido, L.; García-Sastre, A.; González-Maeso, J. Maternal influenza viral infection causes schizophrenia-like alterations of 5-HT2A and mGlu2 receptors in the adult offspring. J. Neurosci. 2011, 31, 1863–1872. [Google Scholar] [CrossRef] [PubMed]
- Severance, E.G.; Dickerson, F.B.; Viscidi, R.P.; Bossis, I.; Stallings, C.R.; Origoni, A.E.; Sullens, A.; Yolken, R.H. Coronavirus immunoreactivity in individuals with a recent onset of psychotic symptoms. Schizophr. Bull. 2011, 37, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Khandaker, G.M.; Zimbron, J.; Dalman, C.; Lewis, G.; Jones, P.B. Childhood infection and adult schizophrenia: A meta-analysis of population-based studies. Schizophr. Res. 2012, 139, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Colijn, M.A. The Co-occurrence of Gastrointestinal Symptoms and Psychosis: Diagnostic Considerations. Prim. Care Companion CNS Disord. 2022, 24, 22nr03236. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, Y.; Williams, B.B.; Battaglioli, E.J.; Whitaker, W.R.; Till, L.; Grover, M.; Linden, D.R.; Akiba, Y.; Kandimalla, K.K.; Zachos, N.C.; et al. Gut Microbiota-Produced Tryptamine Activates an Epithelial G-Protein-Coupled Receptor to Increase Colonic Secretion. Cell Host Microbe 2018, 23, 775–785.e5. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Bose, C.; Mande, S.S. Tryptophan Metabolism by Gut Microbiome and Gut-Brain-Axis: An in silico Analysis. Front. Neurosci. 2019, 13, 1365. [Google Scholar] [CrossRef] [PubMed]
- Liberles, S.D. Trace amine-associated receptors: Ligands, neural circuits, and behaviors. Curr. Opin. Neurobiol. 2015, 34, 1–7. [Google Scholar] [CrossRef]
- NeeNeedham, B.D.; Funabashi, M.; Adame, M.D.; Wang, Z.; Boktor, J.C.; Haney, J.; Wu, W.-L.; Rabut, C.; Ladinsky, M.S.; Hwang, S.-J.; et al. A gut-derived metabolite alters brain activity and anxiety behaviour in mice. Nature 2022, 602, 647–653. [Google Scholar] [CrossRef]
- Berer, K.; Mues, M.; Koutrolos, M.; Rasbi, Z.A.; Boziki, M.; Johner, C.; Wekerle, H. Krishnamoorthy G: Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature 2011, 479, 538–541. [Google Scholar] [CrossRef]
- Buscarinu, M.C.; Cerasoli, B.; Annibali, V.; Policano, C.; Lionetto, L.; Capi, M.; Mechelli, R.; Romano, S.; Fornasiero, A.; Mattei, G.; et al. Altered intestinal permeability in patients with relapsing-remitting multiple sclerosis: A pilot study. Mult. Scler. 2017, 23, 442–446. [Google Scholar] [CrossRef]
- Drago, S.; El Asmar, R.; Di Pierro, M.; Grazia Clemente, M.; Tripathi, A.; Sapone, A.; Thakar, M.; Iacono, G.; Carroccio, A.; D’Agate, C.; et al. Gliadin, zonulin and gut permeability: Effects on celiac and non-celiac intestinal mucosa and intestinal cell lines. Scand. J. Gastroenterol. 2006, 41, 408–419. [Google Scholar] [CrossRef] [PubMed]
- Petersen, J.; Ciacchi, L.; Tran, M.T.; Loh, K.L.; Kooy-Winkelaar, Y.; Croft, N.P.; Hardy, M.Y.; Chen, Z.; McCluskey, J.; Anderson, R.P.; et al. T cell receptor cross-reactivity between gliadin and bacterial peptides in celiac disease. Nat. Struct. Mol. Biol. 2020, 27, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Soltani, Z.; Baghdadi, A.; Nejadhosseinian, M.; Faezi, S.T.; Shahbazkhani, B.; Mousavi, S.A.; Kazemi, K. Celiac disease in patients with systemic lupus erythematosus. Reumatologia 2021, 59, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.Z.; Wu, N.; Xu, Q.; Zhang, X.; Ju, G.Z.; Law, M.H.; Wei, J. A study of circulating gliadin antibodies in schizophrenia among a Chinese population. Schizophr. Bull. 2012, 38, 514–518. [Google Scholar] [CrossRef] [PubMed]
- Flores-Mireles, A.L.; Walker, J.N.; Caparon, M.; Hultgren, S.J. Urinary tract infections: Epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 2015, 13, 269–284. [Google Scholar] [CrossRef]
- Stamm, W.E.; Norrby, S.R. Urinary tract infections: Disease panorama and challenges. J. Infect. Dis. 2001, 183 (Suppl. 1), S1–S4. [Google Scholar] [CrossRef]
- Salesse, L.; Lucas, C.; Hoang, M.H.T.; Sauvanet, P.; Rezard, A.; Rosenstiel, P.; Damon-Soubeyrand, C.; Barnich, N.; Godfraind, C.; Dalmasso, G.; et al. Colibactin-Producing Escherichia coli Induce the Formation of Invasive Carcinomas in a Chronic Inflammation-Associated Mouse Model. Cancers 2021, 13, 2060. [Google Scholar] [CrossRef]
- Minnullina, L.; Pudova, D.; Shagimardanova, E.; Shigapova, L.; Sharipova, M.; Mardanova, A. Comparative Genome Analysis of Uropathogenic Morganella morganii Strains. Front. Cell Infect. Microbiol. 2019, 9, 167. [Google Scholar] [CrossRef]
- Forsyth, V.S.; Armbruster, C.E.; Smith, S.N.; Pirani, A.; Springman, A.C.; Walters, M.S.; Nielubowicz, G.R.; Himpsl, S.D.; Snitkin, E.S.; Mobley, H.L.T. Rapid Growth of Uropathogenic Escherichia coli during Human Urinary Tract Infection. mBio 2018, 9, e00186-18. [Google Scholar] [CrossRef]
- Chagneau, C.V.; Massip, C.; Bossuet-Greif, N.; Fremez, C.; Motta, J.P.; Shima, A.; Besson, C.; Le Faouder, P.; Cénac, N.; Roth, M.P.; et al. Uropathogenic E. coli induces DNA damage in the bladder. PLoS Pathog. 2021, 17, e1009310. [Google Scholar] [CrossRef]
- Miller, B.J.; Graham, K.L.; Bodenheimer, C.M.; Culpepper, N.H.; Waller, J.L.; Buckley, P.F. A prevalence study of urinary tract infections in acute relapse of schizophrenia. J. Clin. Psychiatry 2013, 74, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, A.K.; Zhang, D.; Pouget, J.G.; Zai, C.C.; Chowdhury, N.I.; Brandl, E.J.; Qin, L.; Freeman, N.; Lieberman, J.A.; Meltzer, H.Y.; et al. Impact of histamine receptors H1 and H3 polymorphisms on antipsychotic-induced weight gain. World J. Biol. Psychiatry 2018, 19 (Suppl. 3), S97–S105. [Google Scholar] [CrossRef] [PubMed]
- Deutsch, S.I.; Rosse, R.B.; Schwartz, B.L. Histamine H2 Receptor Antagonists in Schizophrenia. CNS Drugs 1997, 8, 276–284. [Google Scholar] [CrossRef]
- Iwabuchi, K.; Ito, C.; Tashiro, M.; Kato, M.; Kano, M.; Itoh, M.; Iwata, R.; Matsuoka, H.; Sato, M.; Yanai, K. Histamine H1 receptors in schizophrenic patients measured by positron emission tomography. Eur. Neuropsychopharmacol. 2005, 15, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Rudick, C.N.; Bryce, P.J.; Guichelaar, L.A.; Berry, R.E.; Klumpp, D.J. Mast cell-derived histamine mediates cystitis pain. PLoS ONE 2008, 3, e2096. [Google Scholar] [CrossRef] [PubMed]
- Shan, H.; Zhang, E.W.; Zhang, P.; Zhang, X.D.; Zhang, N.; Du, P.; Yang, Y. Differential expression of histamine receptors in the bladder wall tissues of patients with bladder pain syndrome/interstitial cystitis—Significance in the responsiveness to antihistamine treatment and disease symptoms. BMC Urol. 2019, 19, 115. [Google Scholar] [CrossRef] [PubMed]
- Rudzki, L.; Szulc, A. “Immune Gate” of Psychopathology-The Role of Gut Derived Immune Activation in Major Psychiatric Disorders. Front. Psychiatry 2018, 9, 205. [Google Scholar] [CrossRef]
- Averina, O.V.; Zorkina, Y.A.; Yunes, R.A.; Kovtun, A.S.; Ushakova, V.M.; Morozova, A.Y.; Kostyuk, G.P.; Danilenko, V.N.; Chekhonin, V.P. Bacterial Metabolites of Human Gut Microbiota Correlating with Depression. Int. J. Mol. Sci. 2020, 21, 9234. [Google Scholar] [CrossRef]
- Simeonova, D.; Stoyanov, D.; Leunis, J.C.; Carvalho, A.F.; Kubera, M.; Murdjeva, M.; Maes, M. Increased Serum Immunoglobulin Responses to Gut Commensal Gram-Negative Bacteria in Unipolar Major Depression and Bipolar Disorder Type 1, Especially When Melancholia Is Present. Neurotox Res. 2020, 37, 338–348. [Google Scholar] [CrossRef]
- Ramage, A.G. The role of central 5-hydroxytryptamine (5-HT, serotonin) receptors in the control of micturition. Br. J. Pharmacol. 2006, 147 (Suppl. 2), S120–S131. [Google Scholar] [CrossRef]
- Khodaverdi, M.; Rahdar, M.; Davoudi, S.; Hajisoltani, R.; Tavassoli, Z.; Ghasemi, Z.; Amini, A.E.; Hosseinmardi, N.; Behzadi, G.; Janahmadi, M. 5-HT7 receptor activation rescues impaired synaptic plasticity in an autistic-like rat model induced by prenatal VPA exposure. Neurobiol. Learn Mem. 2021, 183, 107462. [Google Scholar] [CrossRef]
- Ford, A.P.D.W.; Kava, M.S. 5-HT4 Receptors in Lower Urinary Tract Tissues. In 5-HT4 Receptors in the Brain and Periphery; Eglen, R.M., Ed.; Biotechnology Intelligence Unit, Springer: Berlin/Heidelberg, Germany, 1998. [Google Scholar] [CrossRef]
- Rebholz, H.; Friedman, E.; Castello, J. Alterations of Expression of the Serotonin 5-HT4 Receptor in Brain Disorders. Int. J. Mol. Sci. 2018, 19, 3581. [Google Scholar] [CrossRef] [PubMed]
- Acharya, D.; Sullivan, M.J.; Duell, B.L.; Goh, K.G.K.; Katupitiya, L.; Gosling, D.; Chamoun, M.N.; Kakkanat, A.; Chattopadhyay, D.; Crowley, M.; et al. Rapid Bladder Interleukin-10 Synthesis in Response to Uropathogenic Escherichia coli Is Part of a Defense Strategy Triggered by the Major Bacterial Flagellar Filament FliC and Contingent on TLR5. mSphere 2019, 4, e00545-19. [Google Scholar] [CrossRef]
- Drage, L.K.L.; Robson, W.; Mowbray, C.; Ali, A.; Perry, J.D.; Walton, K.E.; Harding, C.; Pickard, R.; Hall, J.; Aldridge, P.D. Elevated urine IL-10 concentrations associate with Escherichia coli persistence in older patients susceptible to recurrent urinary tract infections. Immun. Ageing 2019, 16, 16. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Hill, W.G.; Li, F.; Shi, B.; Chai, T.C. Early Increased Urinary IL-2 and IL-10 Levels Were Associated With Development of Chronic UTI in a Murine Model. Urology 2020, 141, 188.e1–188.e6. [Google Scholar] [CrossRef] [PubMed]
- Messing, M.; Jan-Abu, S.C.; McNagny, K. Group 2 Innate Lymphoid Cells: Central Players in a Recurring Theme of Repair and Regeneration. Int. J. Mol. Sci. 2020, 21, 1350. [Google Scholar] [CrossRef]
- Barichello, T. The role of innate lymphoid cells (ILCs) in mental health. Discov. Ment. Health 2022, 2, 2. [Google Scholar] [CrossRef]
- Huang, J.; Fu, L.; Huang, J.; Zhao, J.; Zhang, X.; Wang, W.; Liu, Y.; Sun, B.; Qiu, J.; Hu, X.; et al. Group 3 Innate Lymphoid Cells Protect the Host from the Uropathogenic Escherichia coli Infection in the Bladder. Adv. Sci. 2022, 9, e2103303. [Google Scholar] [CrossRef]
- Sant, G.R.; Theoharides, T.C. The role of the mast cell in interstitial cystitis. Urol. Clin. N. Am. 1994, 21, 41–53. [Google Scholar] [CrossRef]
- Treviranus, G.R.S. Psychoses by Attacks from Subverted Mast Cells: A Role for Arterial Intramural Flow Badly Steered by the Nasal Ganglia? Psychiatr. Danub. 2020, 32 (Suppl. 1), 93–104. [Google Scholar]
- Cheng, L.; Xu, C.; Wang, L.; An, D.; Jiang, L.; Zheng, Y.; Xu, Y.; Wang, Y.; Wang, Y.; Zhang, K.; et al. Histamine H1 receptor deletion in cholinergic neurons induces sensorimotor gating ability deficit and social impairments in mice. Nat. Commun. 2021, 12, 1142. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.B.; Van Benschoten, A.H.; Cimermancic, P.; Donia, M.S.; Zimmermann, M.; Taketani, M.; Ishihara, A.; Kashyap, P.C.; Fraser, J.S.; Fischbach, M.A. Discovery and characterization of gut microbiota decarboxylases that can produce the neurotransmitter tryptamine. Cell Host Microbe 2014, 16, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Sun, S.; Wang, P.; Sun, Y.; Hu, Q.; Wang, X. The Mechanism of Secretion and Metabolism of Gut-Derived 5-Hydroxytryptamine. Int. J. Mol. Sci. 2021, 22, 7931. [Google Scholar] [CrossRef] [PubMed]
- Messaritakis, I.; Vogiatzoglou, K.; Tsantaki, K.; Ntretaki, A.; Sfakianaki, M.; Koulouridi, A.; Tsiaoussis, J.; Mavroudis, D.; Souglakos, J. The Prognostic Value of the Detection of Microbial Translocation in the Blood of Colorectal Cancer Patients. Cancers 2020, 12, 1058. [Google Scholar] [CrossRef] [PubMed]
- Nouri, R.; Hasani, A.; Shirazi, K.M.; Alivand, M.R.; Sepehri, B.; Sotoodeh, S.; Hemmati, F.; Rezaee, M.A. Escherichia coli and Colorectal Cancer: Unfolding the Enigmatic Relationship. Curr. Pharm. Biotechnol. 2022, 23, 1257–1268. [Google Scholar] [CrossRef] [PubMed]
- Sung, K.Y.; Zhang, B.; Wang, H.E.; Bai, Y.M.; Tsai, S.J.; Su, T.P.; Chen, T.J.; Hou, M.C.; Lu, C.L.; Wang, Y.P.; et al. Schizophrenia and risk of new-onset inflammatory bowel disease: A nationwide longitudinal study. Aliment Pharm. Ther. 2022, 55, 1192–1201. [Google Scholar] [CrossRef]
- Protani, M.M.; Jordan, S.J.; Kendall, B.J.; Siskind, D.; Lawrence, D.; Sara, G.; Brophy, L.; Kisely, S. Colorectal cancer Outcomes in people with Severe Mental Illness Cohort (COSMIC): A protocol for an Australian retrospective cohort using linked administrative data. BMJ Open 2021, 11, e044737. [Google Scholar] [CrossRef]
- Chassaing, B.; Koren, O.; Goodrich, J.K.; Poole, A.C.; Srinivasan, S.; Ley, R.E.; Gewirtz, A.T. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature 2015, 519, 92–96. [Google Scholar] [CrossRef]
- Chassaing, B.; Gewirtz, A.T. Gut microbiota, low-grade inflammation, and metabolic syndrome. Toxicol. Pathol. 2014, 42, 49–53. [Google Scholar] [CrossRef]
- Shen, S.; Huo, D.; Ma, C.; Jiang, S.; Zhang, J. Expanding the Colorectal Cancer Biomarkers Based on the Human Gut Phageome. Microbiol. Spectr. 2021, 9, e0009021. [Google Scholar] [CrossRef]
- Abed, J.; Maalouf, N.; Manson, A.L.; Earl, A.M.; Parhi, L.; Emgård, J.E.M.; Klutstein, M.; Tayeb, S.; Almogy, G.; Atlan, K.A.; et al. Colon Cancer-Associated Fusobacterium nucleatum May Originate from the Oral Cavity and Reach Colon Tumors via the Circulatory System. Front. Cell Infect. Microbiol. 2020, 10, 400. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.T.; Kantilal, H.K.; Davamani, F. The Mechanism of Bacteroides fragilis Toxin Contributes to Colon Cancer Formation. Malays. J. Med. Sci. 2020, 27, 9–21. [Google Scholar] [CrossRef]
- Rebersek, M. Gut microbiome and its role in colorectal cancer. BMC Cancer 2021, 21, 1325. [Google Scholar] [CrossRef]
- Verma, A.; Xu, K.; Du, T.; Zhu, P.; Liang, Z.; Liao, S.; Zhang, J.; Raizada, M.K.; Grant, M.B.; Li, Q. Expression of Human ACE2 in Lactobacillus and Beneficial Effects in Diabetic Retinopathy in Mice. Mol. Methods Clin. Dev. 2019, 14, 161–170. [Google Scholar] [CrossRef]
- Volcy, K.; Fraser, N.W. DNA damage promotes herpes simplex virus-1 protein expression in a neuroblastoma cell line. J. Neurovirol. 2013, 19, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Hau, P.M.; Tsao, S.W. Epstein-Barr Virus Hijacks DNA Damage Response Transducers to Orchestrate Its Life Cycle. Viruses 2017, 9, 341. [Google Scholar] [CrossRef] [PubMed]
- Dickerson, F.; Jones-Brando, L.; Ford, G.; Genovese, G.; Stallings, C.; Origoni, A.; O’Dushlaine, C.; Katsafanas, E.; Sweeney, K.; Khushalani, S.; et al. Schizophrenia is Associated with an Aberrant Immune Response to Epstein-Barr Virus. Schizophr. Bull. 2019, 45, 1112–1119. [Google Scholar] [CrossRef]
- Yolken, R. Viruses and schizophrenia: A focus on herpes simplex virus. Herpes 2004, 11 (Suppl. 2), 83A–88A. [Google Scholar]
- Bedri, S.; Sultan, A.A.; Alkhalaf, M.; Al Moustafa, A.E.; Vranic, S. Epstein-Barr virus (EBV) status in colorectal cancer: A mini review. Hum. Vaccin Immunother. 2019, 15, 603–610. [Google Scholar] [CrossRef]
- Wang, Y.; Ren, Y.; Huang, Y.; Yu, X.; Yang, Y.; Wang, D.; Shi, L.; Tao, K.; Wang, G.; Wu, K. Fungal dysbiosis of the gut microbiota is associated with colorectal cancer in Chinese patients. Am J. Transl. Res. 2021, 13, 11287–11301. [Google Scholar]
- Handley, S.A.; Devkota, S. Going Viral: A Novel Role for Bacteriophage in Colorectal Cancer. mBio 2019, 10, e02626-18. [Google Scholar] [CrossRef] [PubMed]
- Sfera, A.; Osorio, C.; Zapata Martín Del Campo, C.M.; Pereida, S.; Maurer, S.; Maldonado, J.C.; Kozlakidis, Z. Endothelial Senescence and Chronic Fatigue Syndrome, a COVID-19 Based Hypothesis. Front. Cell Neurosci. 2021, 15, 673217. [Google Scholar] [CrossRef] [PubMed]
- Sfera, A.; Osorio, C.; Hazan, S.; Kozlakidis, Z.; Maldonado, J.C.; Zapata-Martín del Campo, C.M.; Anton, J.J.; Rahman, L. Long COVID and the Neuroendocrinology of Microbial Translocation Outside the GI Tract: Some Treatment Strategies. Endocrines 2022, 3, 703–725. [Google Scholar] [CrossRef]
- Nicolson, G.L.; Breeding, P.C. Membrane Lipid Replacement with Glycerolphospholipids Slowly Reduces Self-Reported Symptom Severities in Chemically Exposed Gulf War Veterans. Int. J. Transl. Med. 2022, 2, 164–173. [Google Scholar] [CrossRef]
- Jin, L.; Kim, E.-Y.; Chung, T.-W.; Han, C.W.; Park, S.Y.; Han, J.H.; Bae, S.-J.; Lee, J.R.; Kim, Y.W.; Jang, S.B.; et al. Hemistepsin A suppresses colorectal cancer growth through inhibiting pyruvate dehydrogenase kinase activity. Sci. Rep. 2020, 10, 21940. [Google Scholar] [CrossRef] [PubMed]
- Kirkland, J.L.; Tchkonia, T. Senolytic drugs: From discovery to translation. J. Intern. Med. 2020, 288, 518–536. [Google Scholar] [CrossRef]
- Sgrignani, J.; Cecchinato, V.; Fassi, E.M.A.; D’Agostino, G.; Garofalo, M.; Danelon, G.; Pedotti, M.; Simonelli, L.; Varani, L.; Grazioso, G.; et al. Systematic Development of Peptide Inhibitors Targeting the CXCL12/HMGB1 Interaction. J. Med. Chem. 2021, 64, 13439–13450. [Google Scholar] [CrossRef]
- Musumeci, D.; Roviello, G.N.; Montesarchio, D. An overview on HMGB1 inhibitors as potential therapeutic agents in HMGB1-related pathologies. Pharm. Ther. 2014, 141, 347–357. [Google Scholar] [CrossRef]
- Folkes, A.S.; Feng, M.; Zain, J.M.; Abdulla, F.; Rosen, S.T.; Querfeld, C. Targeting CD47 as a cancer therapeutic strategy: The cutaneous T-cell lymphoma experience. Curr. Opin. Oncol. 2018, 30, 332–337. [Google Scholar] [CrossRef]
- Coutinho de Sousa, B.; Reis Machado, J.; da Silva, M.V.; da Costa, T.A.; Lazo-Chica, J.E.; Degasperi, T.D.; Rodrigues Junior, V.; Sales-Campos, H.; Uber Bucek, E.; Freire Oliveira, C.J. Morinda citrifolia (Noni) Fruit Juice Reduces Inflammatory Cytokines Expression and Contributes to the Maintenance of Intestinal Mucosal Integrity in DSS Experimental Colitis. Mediat. Inflamm. 2017, 2017, 6567432. [Google Scholar] [CrossRef]
- De Leo, F.; Quilici, G.; Tirone, M.; De Marchis, F.; Mannella, V.; Zucchelli, C.; Preti, A.; Gori, A.; Casalgrandi, M.; Mezzapelle, R.; et al. Diflunisal targets the HMGB1/CXCL12 heterocomplex and blocks immune cell recruitment. EMBO Rep. 2019, 20, e47788. [Google Scholar] [CrossRef] [PubMed]
- Lohmann, K.L.; Vandenplas, M.L.; Barton, M.H.; Bryant, C.E.; Moore, J.N. The equine TLR4/MD-2 complex mediates recognition of lipopolysaccharide from Rhodobacter sphaeroides as an agonist. J. Endotoxin Res. 2007, 13, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Nicolson, G.L.; Ferreira de Mattos, G.; Ash, M.; Settineri, R.; Escribá, P.V. Fundamentals of Membrane Lipid Replacement, a natural medicine approach to reducing fatigue, pain, and other symptoms while restoring function in chronic illnesses and aging. Membranes 2021, 11, 944. [Google Scholar] [CrossRef]
- Szeligowski, T.; Yun, A.L.; Lennox, B.R.; Burnet, P.W.J. The Gut Microbiome and Schizophrenia: The Current State of the Field and Clinical Applications. Front. Psychiatry 2020, 11, 156. [Google Scholar] [CrossRef]
- Valatas, V.; Kolios, G.; Bamias, G. TL1A (TNFSF15) and DR3 (TNFRSF25): A Co-stimulatory System of Cytokines With Diverse Functions in Gut Mucosal Immunity. Front. Immunol. 2019, 10, 583. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.J.; Choi, I.A.; Meylan, F.; Demoruelle, M.K.; Farley, T.; Richard, A.C.; Hawley, E.; Botson, J.; Hong, Y.J.; Lee, E.Y.; et al. Circulating TNF-like protein 1A (TL1A) is elevated early in rheumatoid arthritis and depends on TNF. Arthritis Res. Ther. 2020, 22, 106. [Google Scholar] [CrossRef]
- Wang, E.C.; Kitson, J.; Thern, A.; Williamson, J.; Farrow, S.N.; Owen, M.J. Genomic structure, expression, and chromosome mapping of the mouse homologue for the WSL-1 (DR3, Apo3, TRAMP, LARD, TR3, TNFRSF12) gene. Immunogenetics 2001, 53, 59–63. [Google Scholar] [CrossRef]
- Newman, S.J.; Bond, B.; Crook, B.; Darker, J.; Edge, C.; Maycox, P.R. Neuron-specific localisation of the TR3 death receptor in Alzheimer’s disease. Brain Res. 2000, 857, 131–140. [Google Scholar] [CrossRef]
- Twohig, J.P.; Roberts, M.I.; Gavalda, N.; Rees-Taylor, E.L.; Giralt, A.; Adams, D.; Brooks, S.; Bull, M.J.; Calder, C.J.; Cuff, S.; et al. Age-dependent maintenance of motor control and corticostriatal innervation by death receptor 3. J. Neurosci. 2010, 30, 3782–3792. [Google Scholar] [CrossRef] [PubMed]
- Mück, C.; Herndler-Brandstetter, D.; Micutkova, L.; Grubeck-Loebenstein, B.; Jansen-Dürr, P. Two functionally distinct isoforms of TL1A (TNFSF15) generated by differential ectodomain shedding. J. Gerontol. A Biol. Sci. Med. Sci. 2010, 65, 1165–1180. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, Y.; Baker, D.J.; Tachibana, M.; Liu, C.C.; van Deursen, J.M.; Brott, T.G.; Bu, G.; Kanekiyo, T. Vascular Cell Senescence Contributes to Blood-Brain Barrier Breakdown. Stroke 2016, 47, 1068–1077. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Dev, S.I.; Chen, G.; Liou, S.C.; Martin, A.S.; Irwin, M.R.; Carroll, J.E.; Tu, X.; Jeste, D.V.; Eyler, L.T. Abnormal levels of vascular endothelial biomarkers in schizophrenia. Eur. Arch. Psychiatry Clin. Neurosci. 2018, 268, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Kellogg, D.L.; Kellogg, D.L., Jr.; Musi, N.; Nambiar, A.M. Cellular Senescence in Idiopathic Pulmonary Fibrosis. Curr. Mol. Biol. Rep. 2021, 7, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Fakhri, S.; Zachariah Moradi, S.; DeLiberto, L.K.; Bishayee, A. Cellular senescence signaling in cancer: A novel therapeutic target to combat human malignancies. Biochem. Pharmacol. 2022, 199, 114989. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Pappu, R.; Ramirez-Carrozzi, V.; Ota, N.; Caplazi, P.; Zhang, J.; Yan, D.; Xu, M.; Lee, W.P.; Grogan, J.L. TNF superfamily member TL1A elicits type 2 innate lymphoid cells at mucosal barriers. Mucosal. Immunol. 2014, 7, 730–740. [Google Scholar] [CrossRef]
- Zhu, M.; Min, S.; Mao, X.; Zhou, Y.; Zhang, Y.; Li, W.; Li, L.; Wu, L.; Cong, X.; Yu, G. Interleukin-13 promotes cellular senescence through inducing mitochondrial dysfunction in IgG4-related sialadenitis. Int. J. Oral Sci. 2022, 14, 29. [Google Scholar] [CrossRef] [PubMed]
- Coppé, J.P.; Desprez, P.Y.; Krtolica, A.; Campisi, J. The senescence-associated secretory phenotype: The dark side of tumor suppression. Annu. Rev. Pathol. 2010, 5, 99–118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, M.; Liu, W.; Hu, C.; Li, H.; Deng, J.; Cao, Q.; Wang, Y.; Hu, W.; Li, Q. Th17/IL-17 induces endothelial cell senescence via activation of NF-κB/p53/Rb signaling pathway. Lab. Investig. 2021, 101, 1418–1426. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Feng, D.; Wang, H.; Hong, F.; Bertola, A.; Wang, F.S.; Gao, B. Interleukin-22 induces hepatic stellate cell senescence and restricts liver fibrosis in mice. Hepatology 2012, 56, 1150–1159. [Google Scholar] [CrossRef] [PubMed]
- Hayflick, L. The Limited in Vitro Lifetime of Human Diploid Cell Strains. Exp. Cell Res. 1965, 37, 614–636. [Google Scholar] [CrossRef] [PubMed]
- Schosserer, M.; Grillari, J.; Breitenbach, M. The Dual Role of Cellular Senescence in Developing Tumors and Their Response to Cancer Therapy. Front. Oncol. 2017, 7, 278. [Google Scholar] [CrossRef]
- Sfera, A.; Bullock, K.; Price, A.; Inderias, L.; Osorio, C. Ferrosenescence: The iron age of neurodegeneration? Mech. Ageing Dev. 2018, 174, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Hamsanathan, S.; Gurkar, A.U. Lipids as Regulators of Cellular Senescence. Front. Physiol. 2022, 13, 796850. [Google Scholar] [CrossRef] [PubMed]
- An, S.; Cho, S.-Y.; Kang, J.; Lee, S.; Kim, H.-S.; Min, D.-J.; Son, E.; Cho, K.-H. Inhibition of 3-phosphoinositide–dependent protein kinase 1 (PDK1) can revert cellular senescence in human dermal fibroblasts. Proc. Natl. Acad. Sci. USA 2020, 117, 31535–31546. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Viloca, M.; Bayascas, J.R.; Lluch, J.M.; González-Lafont, À. Molecular Insights into the Regulation of 3-Phosphoinositide-Dependent Protein Kinase 1: Modeling the Interaction between the Kinase and the Pleckstrin Homology Domains. ACS Omega 2022, 7, 25186–25199. [Google Scholar] [CrossRef] [PubMed]
- Heras-Martínez, G.D.L.; Calleja, V.; Bailly, R.; Dessolin, J.; Larijani, B.; Requejo-Isidro, J. A Complex Interplay of Anionic Phospholipid Binding Regulates 3′-Phosphoinositide-Dependent-Kinase-1 Homodimer Activation. Sci. Rep. 2019, 9, 14527. [Google Scholar] [CrossRef]
- Wei, Y.; Han, X.; Zhao, C. PDK1 regulates the survival of the developing cortical interneurons. Mol. Brain 2020, 13, 65. [Google Scholar] [CrossRef]
- Yang, K.; Li, B.; Chen, J. Knockdown of phosphoinositide-dependent kinase 1 (PDK1) inhibits fibrosis and inflammation in lipopolysaccharide-induced acute lung injury rat model by attenuating NF-κB/p65 pathway activation. Ann. Transl. Med. 2021, 9, 1671. [Google Scholar] [CrossRef]
- Du, J.; Yang, M.; Chen, S.; Li, D.; Chang, Z.; Dong, Z. PDK1 promotes tumor growth and metastasis in a spontaneous breast cancer model. Oncogene 2016, 35, 3314–3323. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).