Biocontrol of Grapevine Crown Gall Performed Using Allorhizobium vitis Strain ARK-1
Abstract
1. Introduction
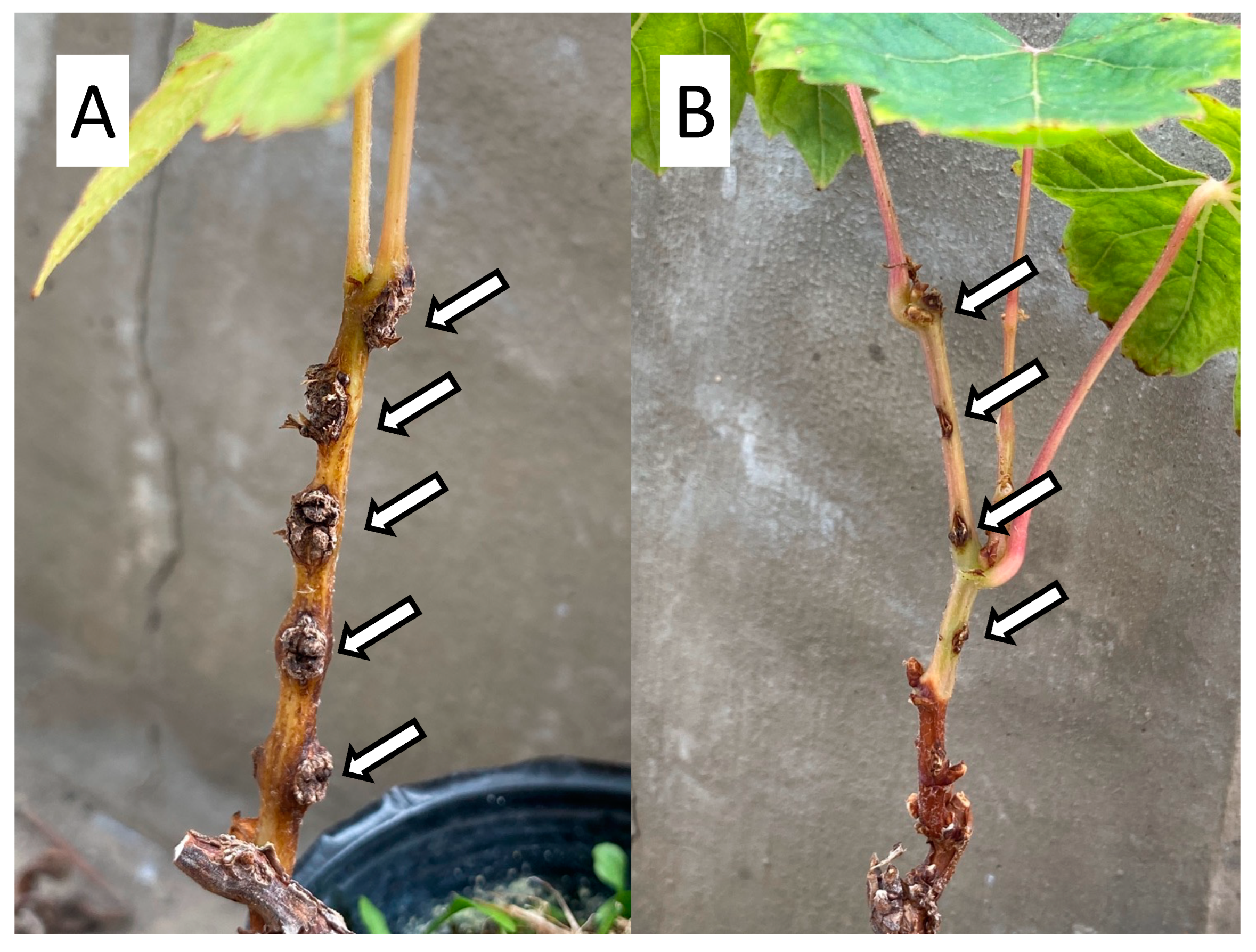
1.1. What Is Crown Gall?
1.2. Mechanism of GCG Development
1.3. Necessity of GCG Management
2. Screening Tests for Biocontrol Agents
3. Root-Dipping Inoculation for Practical Use
3.1. Field Trials
3.2. Population Dynamics of ARK-1 in Roots of Grapevine
4. Unique Mechanism of ARK-1 Strain
4.1. Live Strain of ARK-1 Is Needed to Control GCG
4.2. Reducing Pathogen Population in Plants
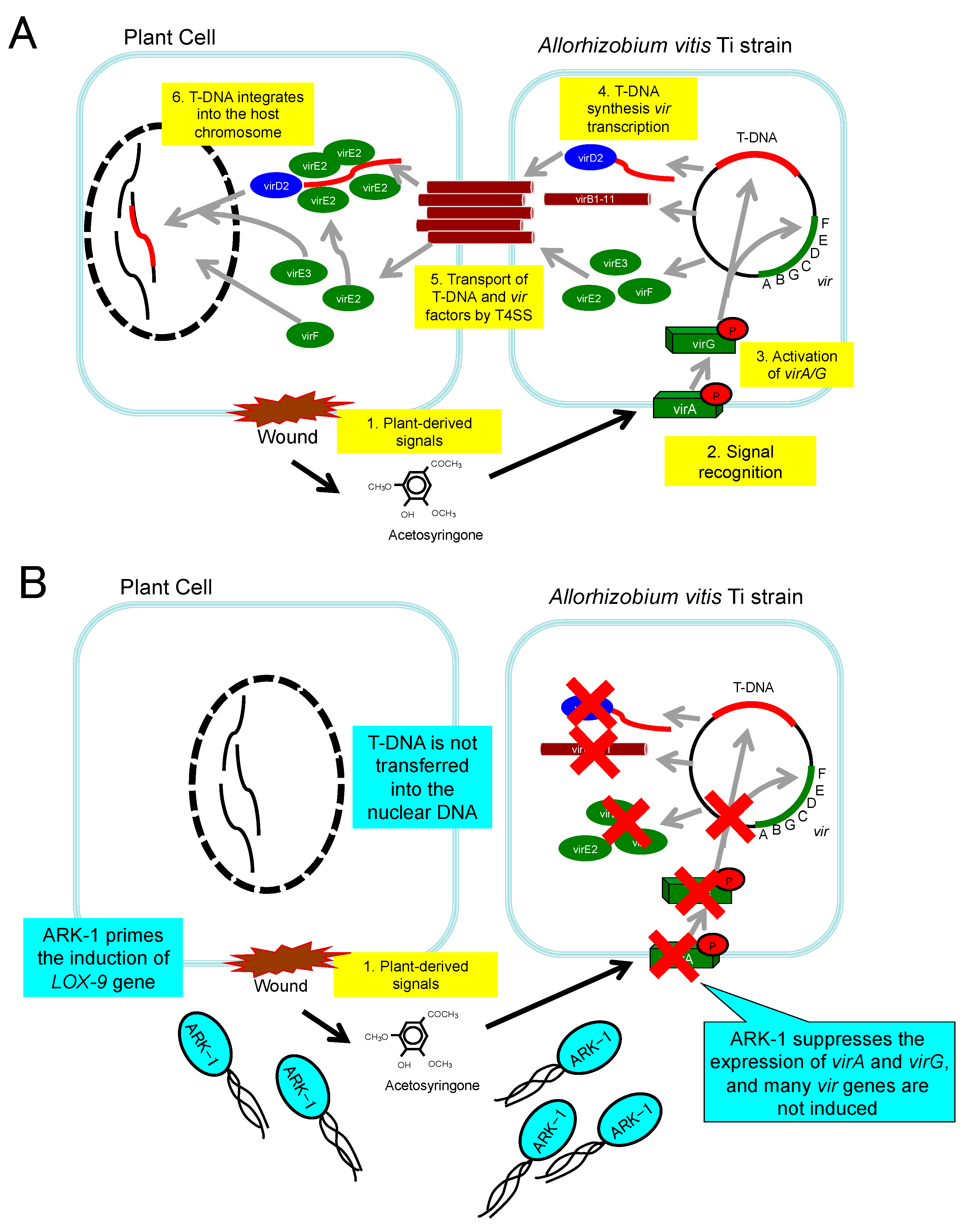
4.3. Suppressing Expression of vir Genes in Ti Strains
4.4. Inducing Disease Resistance with ARK-1
5. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kado, C.I. Crown gall. The Plant Health Instructor 2002. [Google Scholar] [CrossRef]
- Kawaguchi, A. Biological control for grapevine crown gall. In Grapevines: Varieties, Cultivation and Management; Szabo, P.V., Shojania, J., Eds.; Nova Science Publishers: New York, NY, USA, 2012; pp. 153–167. [Google Scholar]
- Burr, T.J.; Bazzi, C.; Süle, S.; Otten, L. Crown gall of grape: Biology of Agrobacterium vitis and the development of disease control strategies. Plant Dis. 1998, 82, 1288–1297. [Google Scholar] [CrossRef]
- Kawaguchi, A.; Inoue, K.; Tanina, K.; Nita, M. Biological control for grapevine crown gall using nonpathogenic Rhizobium vitis strain ARK-1. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2017, 93, 547–560. [Google Scholar] [CrossRef]
- Kawaguchi, A.; Inoue, K. Grapevine crown gall caused by Rhizobium radiobacter (Ti) in Japan. J. Gen. Plant Pathol. 2009, 75, 205–212. [Google Scholar] [CrossRef]
- Mousavi, S.A.; Willems, A.; Nesme, X.; de Lajudie, P.; Lindstrom, K. Revised phylogeny of Rhizobiaceae: Proposal of the delineation of Pararhizobium gen. nov., and 13 new species combinations. Syst. Appl. Microbiol. 2015, 38, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, A. Risk assessment of inferior growth and death of grapevines due to crown gall. Euro. J. Plant Pathol. 2022, 162. in press. [Google Scholar] [CrossRef]
- Chilton, M.D.; Drummond, M.H.; Merlo, D.J.; Sciaky, D.; Montoya, A.L.; Gordon, M.P.; Nester, E.W. Stable incorporation of plasmid DNA into higher plant cells: The moleculer basis of crown gall tumorigenesis. Cell 1977, 11, 263–271. [Google Scholar] [CrossRef]
- Zupan, J.R.; Zambryski, P. The Agrobacterium DNA transfer complex. Crit. Rev. Plant Sci. 1997, 16, 279–295. [Google Scholar] [CrossRef]
- Gelvin, S.B. Traversing the cell: Agrobacterium T-DNA’s journey to the host genome. Front. Plant Sci. 2012, 3, 52. [Google Scholar] [CrossRef] [PubMed]
- Nester, E.W. Agrobacterium: Nature’s genetic engineer. Front. Plant Sci. 2015, 5, 730. [Google Scholar] [CrossRef]
- New, P.B.; Kerr, A. Biological control of grown gall: Field measurements and glasshouse experiments. J. Appl. Bacteriol. 1972, 35, 279–287. [Google Scholar] [CrossRef]
- Kerr, A.; Htay, K. Biological control of crown gall through bacteriocin production. Physiol. Plant Pathol. 1974, 4, 37–44. [Google Scholar] [CrossRef]
- Moore, L.W.; Warren, G. Agrobacterium radiobacter strain 84 and biological control of crown gall. Ann. Rev. Phytopathol. 1979, 17, 163–179. [Google Scholar] [CrossRef]
- Kerr, A. Biological control of crown gall through production of agrocin 84. Plant Dis. 1980, 64, 24–30. [Google Scholar]
- Kerr, A.; Bullard, G. Biocontrol of crown gall by Rhizobium rhizogenes: Challenges in biopesticide commercialization. Agronomy 2020, 20, 1126. [Google Scholar] [CrossRef]
- Reader, J.S.; Ordoukhanian, P.T.; Kim, J.G.; de Crécy-Lagard, V.; Hwang, I.; Farrand, S.; Schimmel, P. Major biocontrol of plant tumors targets tRNA synthetase. Science 2005, 309, 1533. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.A.; Kerr, A. Agrobacterium radiobacter strain K1026, a genetically engineered derivative of strain K84, for biological control of crown gall. Plant Dis. 1989, 73, 15–18. [Google Scholar] [CrossRef]
- Penyalver, R.; Vicedo, B.; López, M.M. Use of the genetically engineered Agrobacterium strain K1026 for biological control of crown gall. Euro. J. Plant Pathol. 2000, 106, 801–810. [Google Scholar] [CrossRef]
- Staphorst, J.L.; van Zyl, F.G.H.; Strijdom, B.W.; Groenewold, Z.E. Agrocin-producing pathogenic and nonpathogenic biotype-3 strains of Agrobacterium tumefaciens active against biotype-3 pathogens. Curr. Microbiol. 1985, 12, 45–52. [Google Scholar] [CrossRef]
- Burr, T.J.; Reid, C.L. Biological control of grape crown gall with nontumorigenic Agrobacterium vitis F2 ⁄ 5. Am. J. Enol. Viticul. 1994, 45, 213–219. [Google Scholar]
- Burr, T.J.; Reid, C.L.; Taglicti, E.; Bazzi, C.; Süle, S. Biological control of grape crown gall by strain F2/5 is not associated with agrocin production or competition for attachment site on grape cells. Phytopathology 1997, 87, 706–711. [Google Scholar] [CrossRef]
- Burr, T.J.; Otten, L. Crown gall of grape: Biology and disease management. Annu. Rev. Phytopathol. 1999, 37, 53–80. [Google Scholar] [CrossRef]
- Kaewnum, S.; Zheng, D.; Reid, C.L.; Johnson, K.L.; Gee, J.C.; Burr, T.J. A host-specific biological control of grape crown gall by Agrobacterium vitis strain F2/5; its regulation and population dynamics. Phytopathology 2013, 103, 427–435. [Google Scholar] [CrossRef]
- Webster, J.; Thomson, J.A. Agrocin-producing Agrobacterium tumefaciens strain active against grapevine isolates. Appl. Environ. Microbiol. 1986, 52, 217–219. [Google Scholar] [CrossRef]
- Chen, X.Y.; Xiang, W.N. A strain of Agrobacterium radiobacter inhibits growth and gall formation by biotype III strains of Agrobacterium tumefaciens from grapevine. Acta. Microbiol. Sin. 1986, 26, 193–199. [Google Scholar] [CrossRef]
- Wang, H.M.; Wang, H.X.; Ng, T.B.; Li, J.Y. Purification and characterization of an antibacterial compound produced by Agrobacterium vitis strain E26 with activity against A. tumefaciens. Plant Pathol. 2003, 52, 134–139. [Google Scholar] [CrossRef]
- Liang, Y.; Di, Y.; Zhao, J.; Ma, D. A biotype 3 strain of Agrobacterium radiobacter inhibits crown gall formation on grapevine. Acta Microbiol. Sin. 1990, 30, 165–171. [Google Scholar]
- Chen, F.; Guo, Y.B.; Wang, J.H.; Li, J.Y.; Wang, H.M. Biological control of grape crown gall by Rahnella aquatilis HX2. Plant Dis. 2007, 91, 957–963. [Google Scholar] [CrossRef]
- Xie, X.M.; You, J.F.; Chen, P.M.; Guo, J.M. On a strain MI15 of Agrobacterium radiobacter for biological control of grapevine crown gall. Acta Phytopathol. Sin. 1993, 23, 137–141. [Google Scholar]
- Ferrigo, D.; Causin, R.; Raiola, A. Effect of potential biocontrol agents selected among grapevine endophytes on crown gall disease. Bio Control. 2017, 62, 821–833. [Google Scholar] [CrossRef]
- Kawaguchi, A.; Sawada, H.; Inoue, K.; Nasu, H. Multiplex PCR for the identification of Agrobacterium biover 3 strains. J. Gen. Plant Pathol. 2005, 71, 54–59. [Google Scholar] [CrossRef]
- Kawaguchi, A.; Inoue, K.; Nasu, H. Inhibition of crown gall formation by Agrobacterium radiobacter biovar 3 strains isolated from grapevine. J. Gen. Plant Pathol. 2005, 71, 422–430. [Google Scholar] [CrossRef]
- Kawaguchi, A.; Inoue, K. New antagonistic strains of non-pathogenic Agrobacterium vitis to control grapevine crown gall. J. Phytopathol. 2012, 160, 509–518. [Google Scholar] [CrossRef]
- Kawaguchi, A. Studies on the diagnosis and biological control of grapevine crown gall and phylogenetic analysis of tumorigenic Rhizobium vitis. J. Gen. Plant Pathol. 2009, 75, 462–463. [Google Scholar] [CrossRef]
- Kawaguchi, A.; Sawada, H.; Ichinose, Y. Phylogenetic and serological analyses reveal genetic diversity of Agrobacterium vitis strains in Japan. Plant Pathol. 2008, 57, 747–753. [Google Scholar] [CrossRef]
- Kawaguchi, A. Genetic diversity of Rhizobium vitis strains in Japan based on multilocus sequence analysis of pyrG, recA and rpoD. J. Gen. Plant Pathol. 2011, 77, 299–303. [Google Scholar] [CrossRef]
- Kawaguchi, A.; Sone, T.; Ochi, S.; Matsushita, Y.; Noutoshi, Y.; Nita, M. Origin of pathogens of grapevine crown gall disease in Hokkaido in Japan as characterized by molecular epidemiology of Allorhizobium vitis strains. Life 2021, 11, 1265. [Google Scholar] [CrossRef]
- Wong, A.T.; Kawaguchi, A.; Nita, M. Efficacy of a biological control agent Rhizobium vitis ARK-1 against Virginia R. vitis isolates, and relative relationship among Japanese and Virginia R. vitis isolates. Crop Prot. 2021, 146, 105685. [Google Scholar] [CrossRef]
- Kawaguchi, A.; Noutoshi, Y. Migration of biological control agent Rhizobium vitis strain ARK-1 in grapevine stems and inhibition of galls caused by tumorigenic strain of R. vitis. J. Gen. Plant Pathol. 2022, 88, 63–68. [Google Scholar] [CrossRef]
- Kawaguchi, A.; Inoue, K.; Nasu, H. Biological control of grapevine crown gall by nonpathogenic Agrobacterium vitis strain VAR03-1. J. Gen. Plant Pathol. 2007, 73, 133–138. [Google Scholar] [CrossRef]
- Kawaguchi, A.; Inoue, K.; Ichinose, Y. Biological control of crown gall of grapevine, rose, and tomato by nonpathogenic Agrobacterium vitis strain VAR03-1. Phytopathology 2008, 98, 1218–1225. [Google Scholar] [CrossRef]
- Kawaguchi, A.; Kondo, K.; Inoue, K. Biological control of apple crown gall by nonpathogenic Rhizobium vitis strain VAR03-1. J. Gen. Plant Pathol. 2012, 78, 287–293. [Google Scholar] [CrossRef]
- Kawaguchi, A. Biological control of crown gall on grapevine and root colonization by nonpathogenic Rhizobium vitis strain ARK-1. Microbes Environ. 2013, 28, 306–311. [Google Scholar] [CrossRef]
- Kawaguchi, A.; Inoue, K.; Tanina, K. Evaluation of the nonpathogenic Agrobacterium vitis strain ARK-1 for crown gall control in diverse plant species. Plant Dis. 2015, 99, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Gan, H.M.; Szegedi, E.; Fersi, R.; Chebil, S.; Kovács, L.; Kawaguchi, A.; Hudson, A.O.; Burr, T.J.; Michael, A.; Savka, M.A. Insight into the microbial co-occurrence and diversity of 73 grapevine (Vitis vinifera) crown galls collected across the northern hemisphere. Front. Microbiol. 2019, 10, 1896. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Watanabe, M.; Matsui, H.; Yamamoto, M.; Ichinose, Y.; Toyoda, K.; Kawaguchi, A.; Noutoshi, Y. Characterization of the suppressive effects of the biological control strain VAR03-1 of Rhizobium vitis on the virulence of tumorigenic R. vitis. J. Gen. Plant Pathol. 2018, 84, 58–64. [Google Scholar] [CrossRef]
- Kawaguchi, A. Reduction in pathogen populations at grapevine wound sites is associated with the mechanism underlying the biological control of crown gall by Rhizobium vitis strain ARK-1. Microbes Environ. 2014, 29, 296–302. [Google Scholar] [CrossRef]
- Kawaguchi, A. Biological control agent Agrobacterium vitis strain ARK-1 suppresses expression of the virD2 and virE2 genes in tumorigenic A. vitis. Eur. J. Plant Pathol. 2015, 143, 789–799. [Google Scholar] [CrossRef]
- Jones, H.D.; Doherty, A.; Wu, H. Review of methodologies and a protocol for the Agrobacterium-mediated transformation of wheat. Plant Methods 2005, 1, 5–11. [Google Scholar] [CrossRef]
- Kawaguchi, A.; Nita, M.; Ishii, T.; Watanabe, M.; Noutoshi, Y. Biological control agent Rhizobium (=Agrobacterium) vitis strain ARK-1 suppresses expression of the essential and non-essential vir genes of tumorigenic R. vitis. BMC Res. Notes 2019, 12, 1–6. [Google Scholar] [CrossRef]
- Thomma, B.P.; Eggermont, K.; Penninckx, I.A.; Mauch-Mani, B.; Vogelsang, R.; Cammue, B.; Broekaert, W.F. Separate jasmonate-dependent and salicylate- dependent defense response pathways in Arabidopsis are essential for resistance to distinct microbial pathogens. Proc. Natl. Acad. Sci. USA 1998, 95, 15107–15111. [Google Scholar] [CrossRef] [PubMed]
- Goellner, K.; Conrath, U. Priming: It’s all the world to induced disease resistance. Eur. J. Plant Pathol. 2008, 121, 233–242. [Google Scholar] [CrossRef]
- Pieterse, C.M.J.; Leon-Reyes, A.; Van der Ent, S.; Van Wees, S.C.M. Networking by small-molecule hormones in plant immunity. Nat. Chem. Biol. 2009, 5, 308–316. [Google Scholar] [CrossRef]
- Kawaguchi, A.; Noutoshi, Y. Insight into inducing disease resistance with Allorhizobium vitis strain ARK-1, a biological control agent against grapevine crown gall disease. Eur. J. Plant Pathol. 2022, 162, 981–987. [Google Scholar] [CrossRef]
- Perazzolli, M.; Roatti, B.; Bozza, E.; Pertot, I. Trichoderma harzianum T39 induces resistance against downy mildew by priming for defense without costs for grapevine. Biol. Cont. 2011, 58, 74–82. [Google Scholar] [CrossRef]
- Akram, A.; Ongena, M.; Duby, F.; Dommes, J.; Thonart, P. Systemic resistance and lipoxygenase-related defense response induced in tomato by Pseudomonas putida strain BTP1. BMC Plant Biol. 2008, 8, 113. [Google Scholar] [CrossRef] [PubMed]
- Filo, A.; Sabbatini, P.; Sundin, G.W.; Zabadal, T.J.; Safir, G.R.; Cousins, P.S. Grapevine crown gall suppression using biological control and genetic engineering: A review of recent research. Am. J. Enol. Vitic. 2013, 64, 1–14. [Google Scholar] [CrossRef]
- Stewart, E.L.; Wenner, N.G. Grapevine decline in Pennsylvania and New York. Wine East. 2004, 32, 12–53. [Google Scholar]
- Kuzmanović, N.; Biondi, E.; Overmann, J.; Puławska, J.; Verbarg, S.; Kornelia Smalla, K.; Lassalle, F. Genomic analysis provides novel insights into diversification and taxonomy of Allorhizobium vitis (i.e. Agrobacterium vitis). BMC Genomic. 2022, 23, 462. [Google Scholar] [CrossRef]
- Noutoshi, Y.; Toyoda, A.; Ishii, T.; Saito, K.; Watanabe, M.; Kawaguchi, A. Complete genome sequence data of tumorigenic Rhizobium vitis strain VAT03-9, a causal agent of grapevine crown gall disease. Mol. Plant Microbe Interact. 2020, 33, 1280–1282. [Google Scholar] [CrossRef]
- Noutoshi, Y.; Toyoda, A.; Ishii, T.; Saito, K.; Watanabe, M.; Kawaguchi, A. Complete genome sequence data of nonpathogenic and nonantagonistic strain of Rhizobium vitis VAR06-30 isolated from grapevine rhizosphere. Mol. Plant Microbe Interact. 2020, 33, 1283–1285. [Google Scholar] [CrossRef] [PubMed]
- Noutoshi, Y.; Toyoda, A.; Ishii, T.; Saito, K.; Watanabe, M.; Kawaguchi, A. Complete genome sequence data of nonpathogenic Rhizobium vitis strain VAT03-1, a biological control agent for grapevine crown gall disease. Mol. Plant Microbe Interact. 2020, 33, 1451–1453. [Google Scholar] [CrossRef] [PubMed]

| Bacterium | Strain | Origin | Reference |
|---|---|---|---|
| Allorhizobium vitis (nonpathogenic) | VAR03-1 | Grapevine, Japan | [2,4,33,34,36,37,42,43,44] |
| Allorhizobium vitis (nonpathogenic) | ARK-1 | Grapevine, Japan | [4,35,40,41,45,46] |
| Allorhizobium vitis (nonpathogenic) | ARK-2 | Grapevine, Japan | [4,35] |
| Allorhizobium vitis (nonpathogenic) | ARK-3 | Grapevine, Japan | [4,35] |
| Allorhizobium vitis (nonpathogenic) | F2/5 | Grapevine, South Africa | [20,21,22,23,24] |
| Rhizobium rhizogenes (tumorigenic) | J73 | Plum, South Africa | [25] |
| Allorhizobium vitis (nonpathogenic) | E26 | Grapevine, China | [27,28] |
| Agrobacterium radiobacter (nonpathogenic) | HLB-2 | Hop, China | [26] |
| Rahnella aquatilis | HX2 | Grapevine, China | [29] |
| Agrobacterium radiobacter (nonpathogenic) | MI15 | Grapevine, China | [30] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawaguchi, A. Biocontrol of Grapevine Crown Gall Performed Using Allorhizobium vitis Strain ARK-1. Appl. Microbiol. 2022, 2, 981-991. https://doi.org/10.3390/applmicrobiol2040075
Kawaguchi A. Biocontrol of Grapevine Crown Gall Performed Using Allorhizobium vitis Strain ARK-1. Applied Microbiology. 2022; 2(4):981-991. https://doi.org/10.3390/applmicrobiol2040075
Chicago/Turabian StyleKawaguchi, Akira. 2022. "Biocontrol of Grapevine Crown Gall Performed Using Allorhizobium vitis Strain ARK-1" Applied Microbiology 2, no. 4: 981-991. https://doi.org/10.3390/applmicrobiol2040075
APA StyleKawaguchi, A. (2022). Biocontrol of Grapevine Crown Gall Performed Using Allorhizobium vitis Strain ARK-1. Applied Microbiology, 2(4), 981-991. https://doi.org/10.3390/applmicrobiol2040075






