Occurrence and Abundance of Hemiptera Auchenorrhyncha Associated with Traditional and Super-High-Density Olive Groves in Tuscany (Central Italy), with a Particular Focus on Xylella fastidiosa Vectors
Abstract
1. Introduction
- Identify and characterize the Auchenorrhyncha insect community occurring in three olive farms located in Tuscany, a key region for Italian olive cultivation.
- Assess how different farming systems (traditional vs. super-high-density) and vegetation strata (herbaceous ground cover vs. olive tree canopy) influence the occurrence and abundance of these insects, with particular attention to the confirmed X. fastidiosa vectors, P. spumarius and N. campestris.
2. Materials and Methods
2.1. Study Sites and Experimental Design
2.2. Insect Collection
2.3. Insect Identification
2.4. Data Analysis
3. Results
3.1. Identification and Abundance of Insects
3.2. Site, Management (TRA vs. SHD) and Vegetation Stratum Effects on Auchenorrhyncha Community
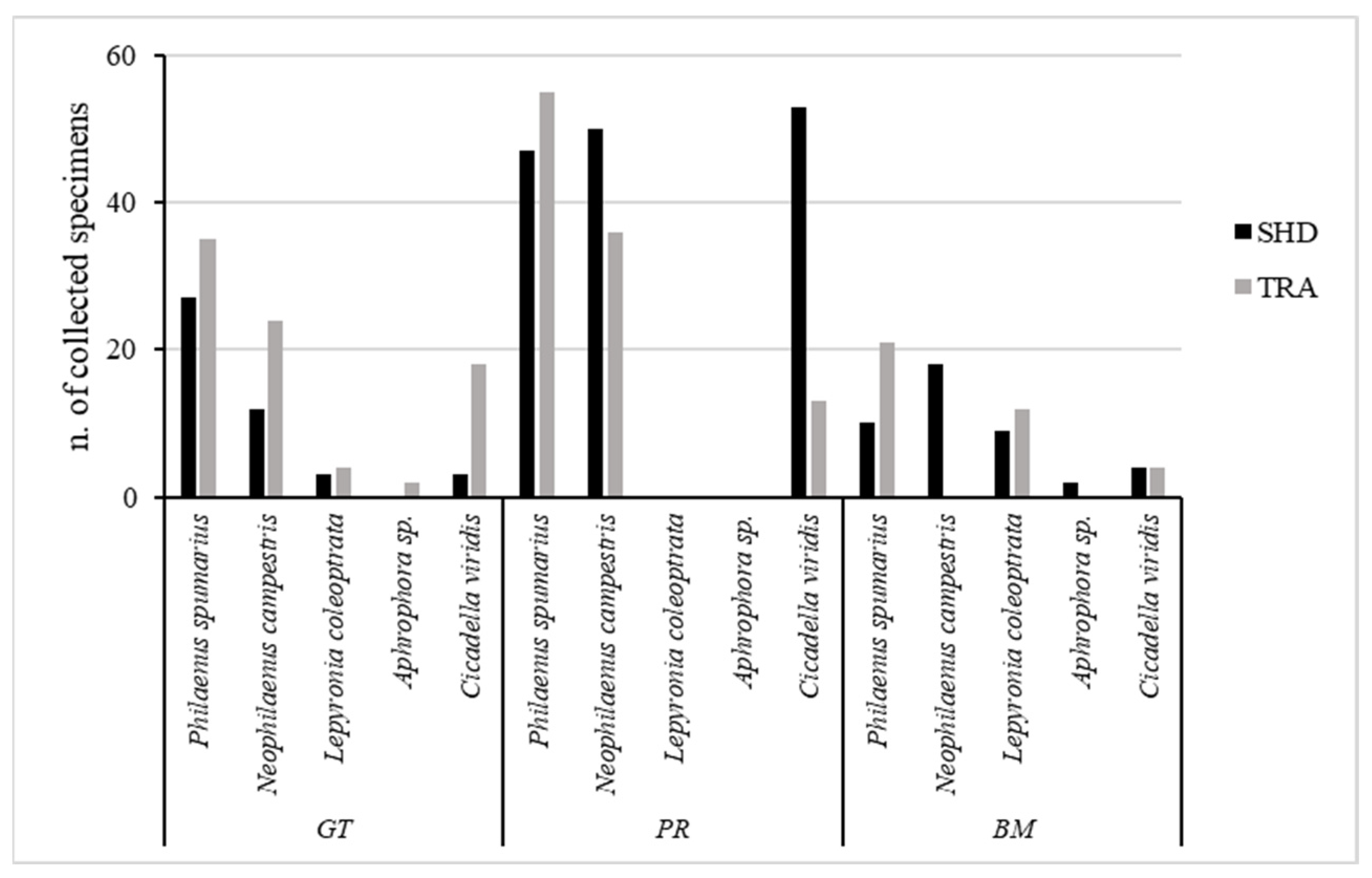
3.3. Focus on Potential and Ascertained Vectors
4. Discussion and Conclusions
- Vector competence under Tuscan conditions: While the presence of vectors is confirmed, their transmission efficiency in local agroecosystems remains unquantified.
- Landscape-level drivers: The role of surrounding vegetation and landscape connectivity in supporting vector populations should be explored further, especially since plantation density appears unrelated to vector abundance.
- Long-term monitoring under climate change: Shifts in temperature and precipitation patterns may alter spittlebug phenology and abundance, potentially influencing epidemiological risk over time.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Degli Innocenti, D. Olivicoltura Italiana: Quale Futuro? Olivo e Olio. 2025. Available online: https://olivoeolio.edagricole.it/attualita/olivicoltura-italiana-quale-futuro (accessed on 4 September 2025).
- Infante-Amate, J.; Villa, I.; Aguilera, E.; Torremocha, E.; Guzmán, G.; Cid, A.; González de Molina, M. The making of olive landscapes in the south of Spain. A history of continuous expansion and intensification. In Biocultural Diversity in Europe; Springer International Publishing: Cham, Switzerland, 2016; pp. 157–179. [Google Scholar]
- Guerrero-Casado, J.; Carpio, A.J.; Tortosa, F.S.; Villanueva, A.J. Environmental challenges of intensive woody crops: The case of super high-density olive groves. Sci. Total Environ. 2021, 798, 149212. [Google Scholar] [CrossRef]
- Giliberti, G. Per un Vero Rilancio Dell’olivicoltura Italiana: Strategie e Prospettive. Notiziario Accademia Georgofili. 2025. Available online: https://www.georgofili.info/contenuti/per-un-vero-rilancio-dellolivicoltura-italiana-strategie-e-prospettive/31027 (accessed on 5 February 2025).
- Díez, C.M.; Moral, J.; Cabello, D.; Morello, P.; Rallo, L.; Barranco, D. Cultivar and tree density as key factors in the long-term performance of super high-density olive orchards. Front. Plant Sci. 2016, 7, 1226. [Google Scholar] [CrossRef]
- Landi, S.; d’Errico, G.; Papini, R.; Cutino, I.; Simoncini, S.; Rocchini, A.; Brandi, G.; Rizzo, R.; Gugliuzza, G.; Germinara, G.S.; et al. Impact of Super-High Density Olive Orchard Management System on Soil Free-Living and Plant-Parasitic Nematodes in Central and South Italy. Animals 2022, 12, 1551. [Google Scholar] [CrossRef]
- ISMEA. Report Agrimercati. 2024. Available online: https://www.ismeamercati.it/ (accessed on 5 February 2025).
- Ottanelli, A. Allevamento in Parete, Esperienze in Toscana. Olivo e Olio. 2020. Available online: https://olivoeolio.edagricole.it/oliveto-e-frantoio/allevamento-olivo-a-parete-esperienze-in-toscana/ (accessed on 5 February 2025).
- Landi, S.; Cutino, I.; Simoni, S.; Simoncini, S.; Benvenuti, C.; Pennacchio, F.; Binazzi, F.; Guidi, S.; Goggioli, D.; Tarchi, F.; et al. Super high-density olive orchard system affects the main olive crop pests. Ital. J. Agron. 2024. [Google Scholar] [CrossRef]
- Saponari, M.; Boscia, D.; Nigro, F.; Martelli, G.P. Identification of DNA sequences related to Xylella fastidiosa in oleander, almond, and olive trees exhibiting leaf scorch symptoms in Apulia (Southern Italy). J. Plant Pathol. 2013, 95, 668. [Google Scholar]
- Martelli, G.P.; Boscia, D.; Porcelli, F.; Saponari, M. The olive quick decline syndrome in south-east Italy: A threatening phytosanitary emergency. Eur. J. Plant Pathol. 2015, 144, 235–243. [Google Scholar] [CrossRef]
- Trkulja, V.; Tomić, A.; Iličić, R.; Nožinić, M.; Milovanović, T.P. Xylella fastidiosa in Europe: From the introduction to the current status. Plant Pathol. J. 2022, 38, 551. [Google Scholar] [CrossRef]
- Redak, R.A.; Purcell, A.H.; Lopes, J.R.S.; Blua, M.J.; Mizell, R.F., 3rd; Andersen, P.C. The biology of xylem fluid feeding insect vectors of Xylella fastidiosa and their relation to disease epidemiology. Annu. Rev. Entomol. 2004, 49, 243–270. [Google Scholar] [CrossRef]
- Saponari, M.; Loconsole, G.; Cornara, D.; Yokomi, R.K.; De Stradis, A.; Boscia, D.; Bosco, D.; Martelli, G.P.; Krugner, R.; Porcelli, F. Infectivity and transmission of Xylella fastidiosa by Philaenus spumarius (Hemiptera: Aphrophoridae) in Apulia, Italy. J. Econ. Entomol. 2014, 107, 1316–1319. [Google Scholar] [CrossRef]
- Cavalieri, V.; Altamura, G.; Fumarola, G.; di Carolo, M.; Saponari, M.; Cornara, D.; Bosco, D.; Dongiovanni, C. Transmission of Xylella fastidiosa subspecies pauca sequence type 53 by different insect species. Insects 2019, 10, 324. [Google Scholar] [CrossRef]
- Sanna, F.; Mori, N.; Santoiemma, G.; D’Ascenzo, D.; Scotillo, M.A.; Marini, L. Ground cover management in olive groves reduces populations of Philaenus spumarius (Hemiptera: Aphrophoridae), vector of Xylella fastidiosa. J. Econ. Entomol. 2021, 114, 1716–1721. [Google Scholar] [CrossRef]
- Elbeaino, T.; Yaseen, T.; Valentini, F.; Moussa, I.E.B.; Mazzoni, V.; D’Onghia, A.M. Identification of three potential insect vectors of Xylella fastidiosa in southern Italy. Phytopath. Mediter. 2014, 53, 328–332. [Google Scholar]
- Antonatos, S.; Papachristos, D.P.; Kapantaidaki, D.E.; Lytra, I.C.; Varikou, K.; Evangelou, V.I.; Milonas, P. Presence of Cicadomorpha in olive orchards of Greece with special reference to Xylella fastidiosa vectors. J. Appl. Entomol. 2020, 144, 1–11. [Google Scholar] [CrossRef]
- Bodino, N.; Cavalieri, V.; Dongiovanni, C.; Saladini, M.A.; Simonetto, A.; Volani, S.; Bosco, D. Spittlebugs of Mediterranean olive groves: Host-plant exploitation throughout the year. Insects 2020, 11, 130. [Google Scholar] [CrossRef]
- Trotta, V.; Forlano, P.; Caccavo, V.; Fanti, P.; Battaglia, D. A survey of potential vectors of the plant pathogenic bacterium Xylella fastidiosa in the Basilicata Region, Italy. Bull. Insect 2021, 74, 273–283. [Google Scholar]
- Bodino, N.; Cavalieri, V.; Dongiovanni, C.; Plazio, E.; Saladini, M.A.; Volani, S.; Bosco, D. Phenology, seasonal abundance and stage-structure of spittlebug (Hemiptera: Aphrophoridae) populations in olive groves in Italy. Sci. Rep. 2019, 9, 17725. [Google Scholar] [CrossRef]
- Santoiemma, G.; Tamburini, G.; Sanna, F.; Mori, N.; Marini, L. Landscape composition predicts the distribution of Philaenus spumarius, vector of Xylella fastidiosa, in olive groves. J. Pest Sci. 2019, 92, 1101–1109. [Google Scholar] [CrossRef]
- Avosani, S.; Tattoni, C.; Mazzoni, V.; Ciolli, M. Occupancy and detection of agricultural threats: The case of Philaenus spumarius, European vector of Xylella fastidiosa. Agric. Ecosyst. Environ. 2022, 324, 107707. [Google Scholar] [CrossRef]
- Godefroid, M.; Duràn, J.M. Composition of landscape impacts the distribution of the main vectors of Xylella fastidiosa in southern Spain. J. Appl. Entomol. 2022, 146, 666–675. [Google Scholar] [CrossRef]
- Lago, C.; Garzo, E.; Moreno, A.; Barrios, L.; Campoy, A.M.; Rodriguez-Ballester, F.; Fereres, A. Flight performance and the factors affecting the flight behaviour of Philaenus spumarius the main vector of Xylella fastidiosa in Europe. Sci. Rep. 2021, 11, 17608. [Google Scholar] [CrossRef]
- Bodino, N.; Cavalieri, V.; Saponari, M.; Dongiovanni, C.; Altamura, G.; Bosco, D. Transmission of Xylella fastidiosa subsp. pauca ST53 by the Sharpshooter Cicadella viridis From Different Source Plants and Artificial Diets. J. Econ. Entomol. 2022, 115, 1852–1858. [Google Scholar]
- Gargani, E.; Benvenuti, C.; Marianelli, L.; Roversi, P.F.; Ricciolini, M.; Scarpelli, I.; Sacchetti, P.; Nencioni, A.; Rizzo, D.; Strangi, A.; et al. A five-year survey in Tuscany (Italy) and detection of Xylella fastidiosa subspecies multiplex in potential insect vectors, collected in Monte Argentario. Redia 2021, 104, 75–88. [Google Scholar] [CrossRef]
- Pignatti, S. Flora d’Italia; Edagricole: Bologna, Italy, 2017; p. 1120. [Google Scholar]
- Drosopoulos, S.; Remane, R. Biogeographic studies on the spittlebug Philaenus signatus Melichar, 1986 species group (Hemiptera: Aphrophoridae) with the description of two new allopatric species. Ann. Soc. Entomol. Fr. 2000, 36, 269–277. [Google Scholar]
- Biedermann, R.; Niedringhaus, R. The Plant-and Leafhoppers of Germany: Identification Key to All Species; WABV Fründ: Scheeßel, Germany, 2009; p. 410. [Google Scholar]
- Holzinger, W.; Kammerlander, I.; Nickel, H. The Auchenorrhyncha of Central Europe-Die Zikaden Mitteleuropas Fulgoromorpha, Cicadomorpha exc. Cicadellidae; Brill Academic Publishers: Leiden, The Netherlands; Boston, MA, USA, 2003; 673p. [Google Scholar]
- Kunz, G.; Nickel, H.; Niedringhaus, R. Fotoatlas der Zikaden Deutschlands: Photographic Atlas of the Planthoppers and Leafhoppers of Germany; WABV Fründ: Scheeßel, Germany, 2011; 293p. [Google Scholar]
- Wilson, M.; Stewart, A.; Biedermann, R.; Nickel, H.; Niedringhaus, R. The Planthoppers and Leafhoppers of Britain and Ireland: Identification Keys to All Families and Genera and All British and Irish Species not Recorded from Germany; WABV Fründ: Scheeßel, Germany, 2015; 138p. [Google Scholar]
- Theodorou, D.; Koufakis, I.; Thanou, Z.; Kalaitzaki, A.; Chaldeou, E.; Afentoulis, D.; Tsagkarakis, A. Management system affects the occurrence, diversity and seasonal fluctuation of Auchenorrhyncha, potential vectors of Xylella fastidiosa, in the olive agroecosystem. Bull. Insectol. 2021, 74, 27–40. [Google Scholar]
- Dellapé, G.; Bouvet, J.P.; Paradell, S.L. Diversity of cicadomorpha (Hemiptera: Auchenorrhyncha) in citrus orchards in Northeastern Argentina. Fla. Entomol. 2013, 96, 1125–1134. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. Past: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Morente, M.; Cornara, D.; Plaza, M.; Durán, J.M.; Capiscol, C.; Trillo, R.; Ruiz, M.; Ruz, C.; Sanjuan, S.; Pereira, J.A. Distribution and relative abundance of insect vectors of Xylella fastidiosa in olive groves of the Iberian Peninsula. Insects 2018, 9, 175. [Google Scholar] [CrossRef]
- Koufakis, I.E.; Kalaitzaki, A.P.; Pappas, M.L.; Tsagkarakis, A.E.; Tzobanoglou, D.K.; Broufas, G.D. Population Dynamics of Potential Insect Vectors of Xylella fastidiosa (Xanthomanadales: Xanthomonadaceae) and Other Auchenorrhyncha in Olive and Citrus Groves of Crete, Greece. Agronomy 2024, 14, 2243. [Google Scholar] [CrossRef]
- Ben Moussa, I.E.; Mazzoni, V.; Valentini, F.; Yasseen, T.; Lorusso, D.; Speranza, S.; Digiaro, M.; Varvaro, L.; Krugner, R.; D’Onghia, A.M. Seasonal fluctuations of sap-feeding insect species infected by Xylella fastidiosa in Apulian olive groves of southern Italy. J. Econ. Entomol. 2016, 109, 1512–1518. [Google Scholar] [CrossRef]
- Thanou, Z.; Stamouli, M.; Magklara, A.; Theodorou, D.; Stamatakou, G.; Konidis, G.; Koufopoulou, P.; Lyberopoulos, C.; Tribonia, S.; Vetsos, P.; et al. Faunistic Study of Auchenorrhyncha in Olive Orchards in Greece, Including First Records of Species. Agronomy 2024, 14, 2792. [Google Scholar] [CrossRef]
- Cornara, D.; Cavalieri, V.; Dongiovanni, C.; Altamura, G.; Palmisano, F.; Bosco, D.; Porcelli, F.; Almeida, R.P.P.; Saponari, M. Transmission of Xylella fastidiosa by naturally infected Philaenus spumarius (Hemiptera, Aphrophoridae) to different host plants. J. Appl. Entomol. 2017, 141, 80–87. [Google Scholar] [CrossRef]


| Sites | Geographical Position | Climate Parameters | Soil texture USDA | Olive Orchard Features | ||||
|---|---|---|---|---|---|---|---|---|
| Coordinates | Altitude a.s.l. (m) | Koppen Climate Types | Mean Air Temperature (°C) | Mean Annual Precipitation (mm) | Olive Cultivar in Super- High Density (SHD) and Traditional (TRA) Plantations | Soil Management | ||
| Poggio a Remole (PR), Florence | 43.800183 N; 11.403579 E | 260 | Cfa | 14.7 | 940 | Clay | SHD: Leccio del Corno, Tosca, Diana TRA: Frantoio, Leccino, Moraiolo | Conventional tillage, mineral, fertilization |
| Giganti (GT), Siena | 43.275739 N; 11.603974 E | 280 | Csa | 14.5 | 880 | Silty clay, Loam | SHD: Frantoio, Leccino, Moraiolo, Pendolino, Leccio del Corno, Correggiolo, Maurino, Vittoria selection TRA: Frantoio, Leccino, Moraiolo, Pendolino | Green Cover, organic fertilization |
| Barban (BM), Siena | 43.0940 N; 11.2316 E | 147 | Csa | 14.7 | 890 | Clay Loam | SHD: Arbequina TRA: Correggiolo, Pendolino, Leccino | Conventional tillage, mineral, fertilization |
| Genus | Number | RI | ||
|---|---|---|---|---|
| Agallia | 38 | ** | ||
| Agalmatium | 777 | *** | ||
| Allygus | 3 | * | ||
| Anoplotettix | 90 | ** | ||
| Aphrodes | 1 | * | ||
| Aphrophora | 4 | * | ||
| Athysanus | 71 | ** | ||
| Cicadella | 95 | ** | ||
| Dictyophara | 28 | ** | ||
| Euscelis | 45 | ** | ||
| Evacanthus | 3 | * | ||
| Hyalesthes | 9 | * | ||
| Issus | 67 | ** | ||
| Ledra | 2 | * | ||
| Lepyronia | 28 | ** | ||
| Macropsis | 70 | ** | ||
| Macrosteles | 18 | ** | ||
| Neophilaenus | 140 | ** | ||
| Oncopsis | 1 | * | ||
| Pediopsis | 2 | * | ||
| Philaenus | 195 | *** | ||
| Platymetopius | 24 | ** | ||
| Reptalus | 2 | * | ||
| Synophropsis | 87 | ** | ||
| Thamnotettix | 44 | ** | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elisabetta, G.; Valeria, F.; Ilaria, C.; Sauro, S.; Anita, N.; Gaia, B.; Silvia, L. Occurrence and Abundance of Hemiptera Auchenorrhyncha Associated with Traditional and Super-High-Density Olive Groves in Tuscany (Central Italy), with a Particular Focus on Xylella fastidiosa Vectors. Crops 2025, 5, 73. https://doi.org/10.3390/crops5050073
Elisabetta G, Valeria F, Ilaria C, Sauro S, Anita N, Gaia B, Silvia L. Occurrence and Abundance of Hemiptera Auchenorrhyncha Associated with Traditional and Super-High-Density Olive Groves in Tuscany (Central Italy), with a Particular Focus on Xylella fastidiosa Vectors. Crops. 2025; 5(5):73. https://doi.org/10.3390/crops5050073
Chicago/Turabian StyleElisabetta, Gargani, Francardi Valeria, Cutino Ilaria, Simoni Sauro, Nencioni Anita, Bigiotti Gaia, and Landi Silvia. 2025. "Occurrence and Abundance of Hemiptera Auchenorrhyncha Associated with Traditional and Super-High-Density Olive Groves in Tuscany (Central Italy), with a Particular Focus on Xylella fastidiosa Vectors" Crops 5, no. 5: 73. https://doi.org/10.3390/crops5050073
APA StyleElisabetta, G., Valeria, F., Ilaria, C., Sauro, S., Anita, N., Gaia, B., & Silvia, L. (2025). Occurrence and Abundance of Hemiptera Auchenorrhyncha Associated with Traditional and Super-High-Density Olive Groves in Tuscany (Central Italy), with a Particular Focus on Xylella fastidiosa Vectors. Crops, 5(5), 73. https://doi.org/10.3390/crops5050073








