Biodiversity of the Genus Trichoderma in the Rhizosphere of Coffee (Coffea arabica) Plants in Ethiopia and Their Potential Use in Biocontrol of Coffee Wilt Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection of Soil Samples and Isolation of Trichoderma Species
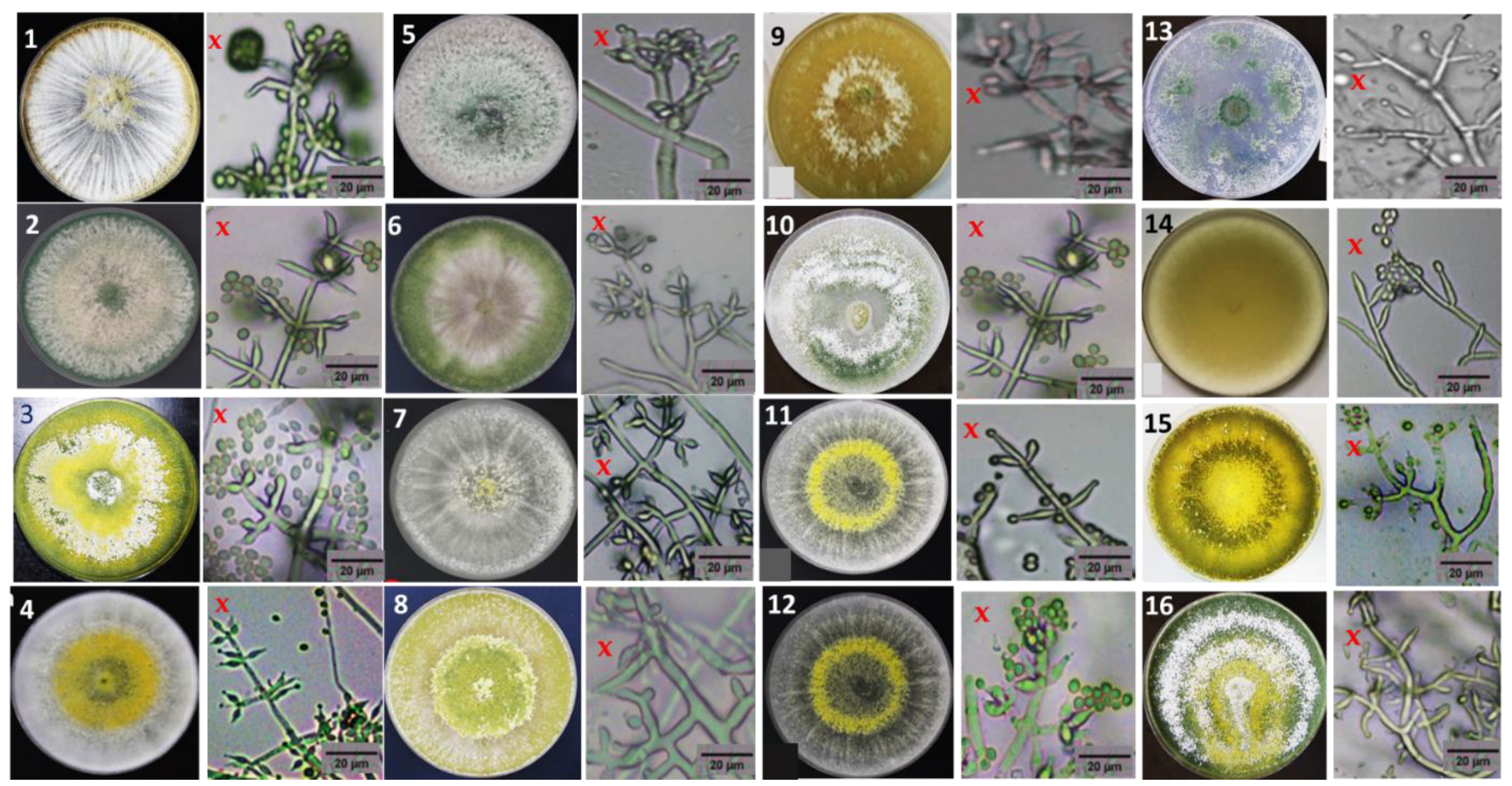
2.2. Morphological Characteristics
2.3. DNA Extraction, PCR Amplification and Sequencing
2.4. Phylogenetic Analysis
2.5. Diversity Analysis of Trichoderma Species
2.6. In Vitro Bioassay
2.7. Statistical Data Analysis
3. Results
3.1. Isolation and Morphological Characterization of Trichoderma Isolates
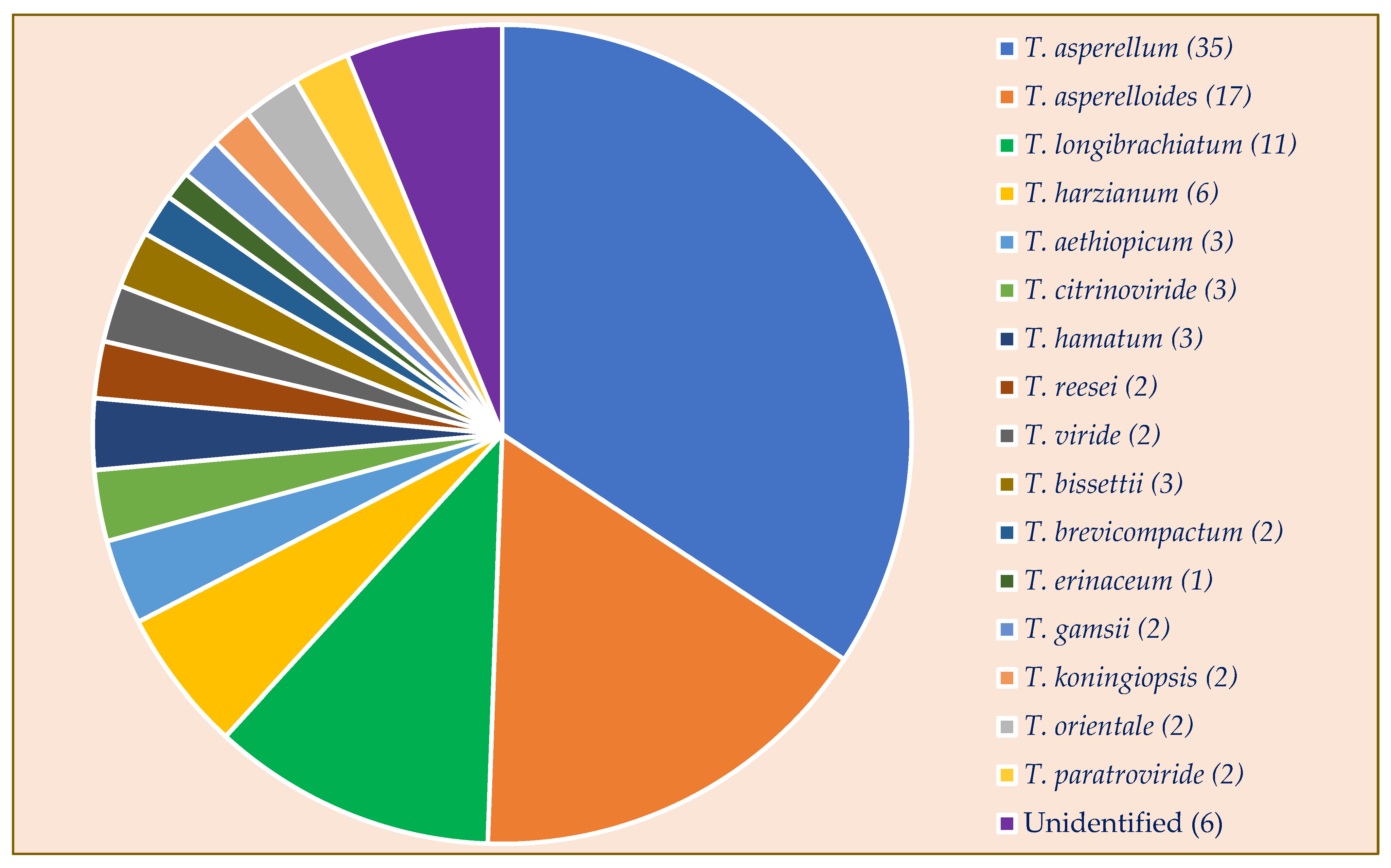
3.2. Molecular Identification of Trichoderma Isolates
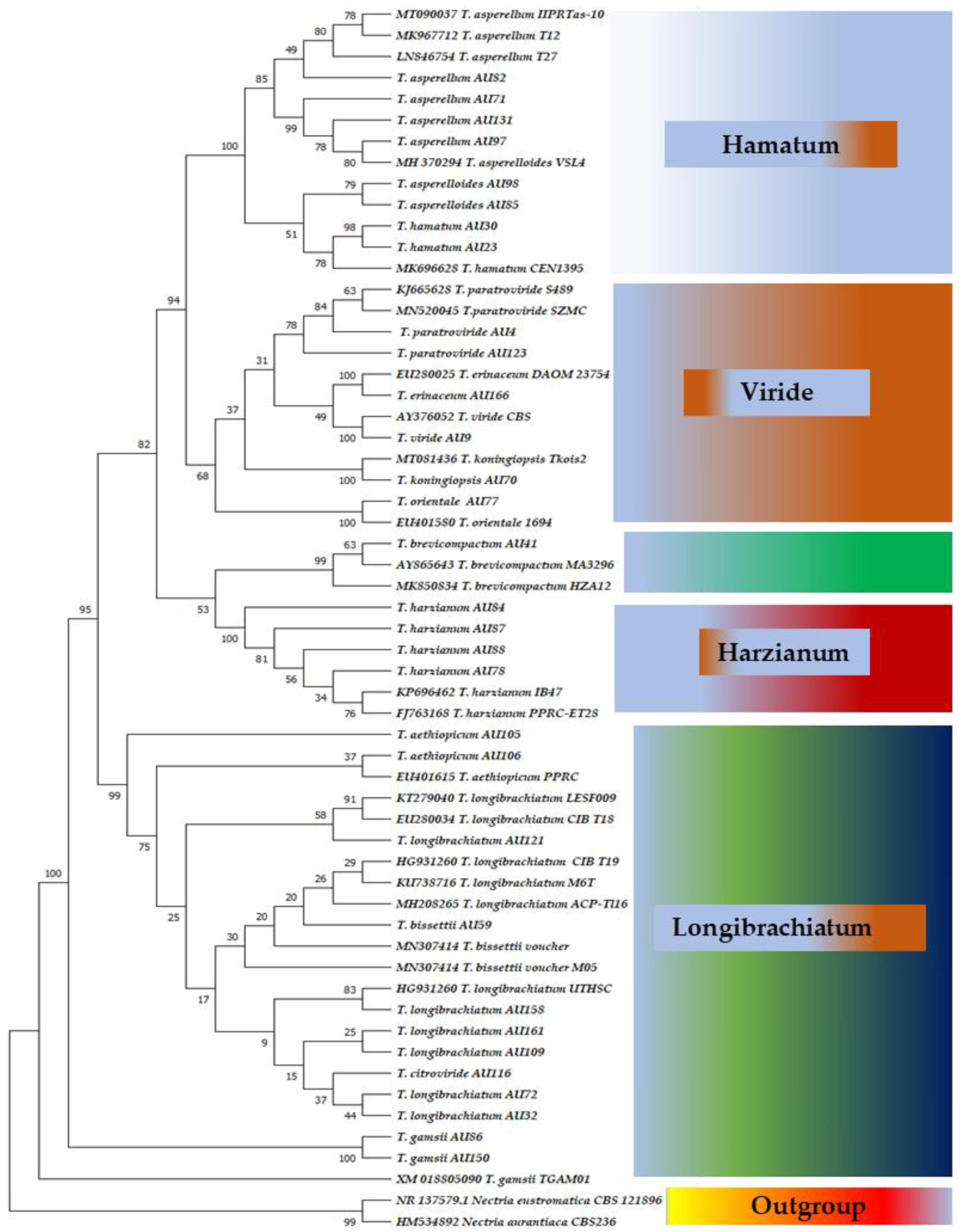
3.3. Phylogenetic Analysis
3.4. Biodiversity and Distribution of Trichoderma Isolates
3.4.1. Diversity Analysis of Trichoderma Species
3.4.2. Distribution of Trichoderma Species in Different Coffee-Growing Zones
3.4.3. Distribution of Trichoderma Species in a Coffee Ecosystem
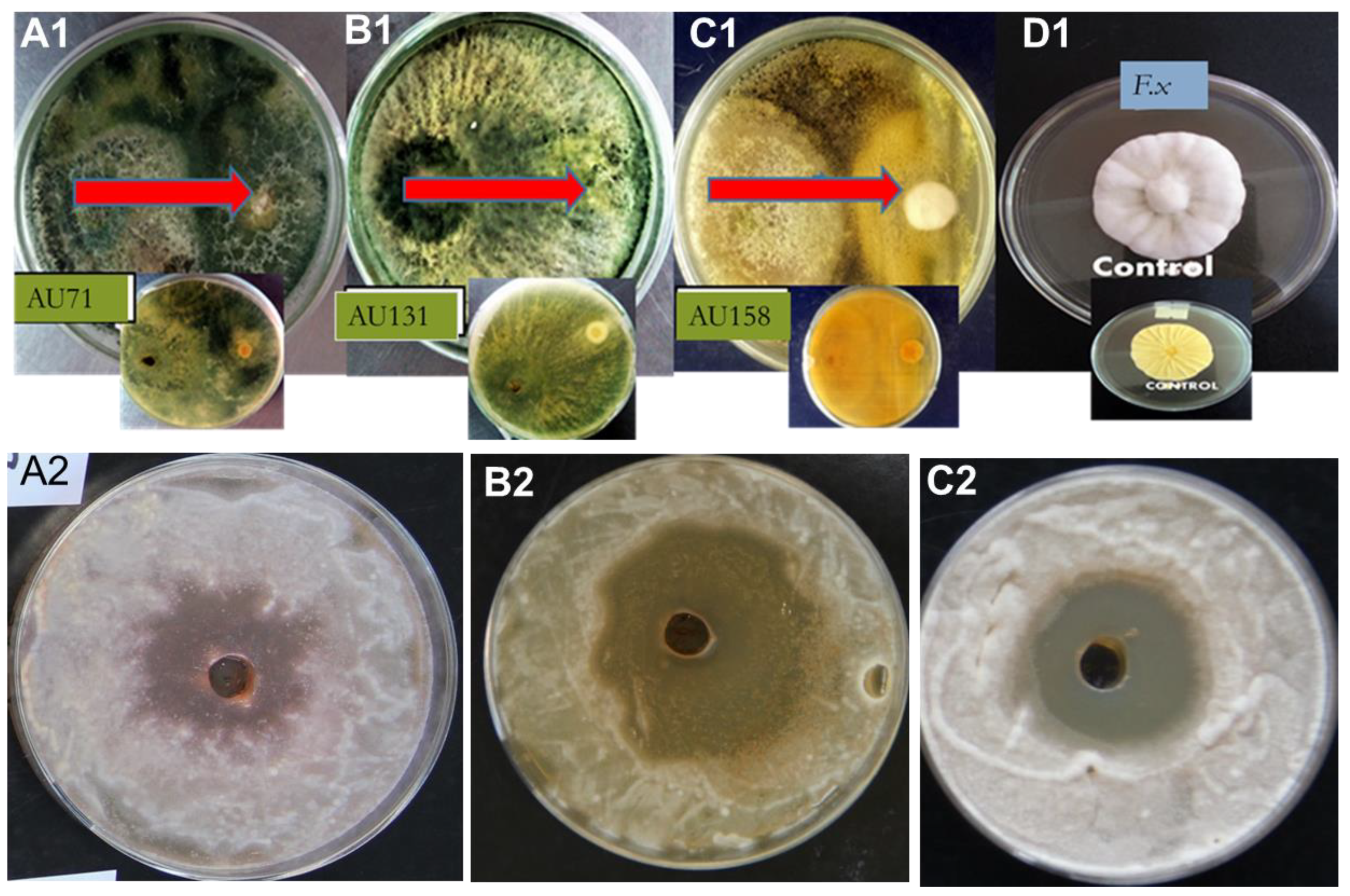
3.5. Screening of Biocontrol Trichoderma
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Srinivasa, N.; Devi, T.P.; Sudhirkumar, S.; Kamil, D.; Borah, J.L.; Prabhakaran, N. Bioefficacy of Trichoderma isolates against soil-borne pathogens. Afr. J. Microbiol. Res. 2014, 8, 2710–2723. [Google Scholar] [CrossRef] [Green Version]
- Parulekar-Berde, C.V.; Joshi, S.A.; Berde, V.B. Fungal Communities as Biological Control Agents for Different Phytopathogenic Organisms. In Recent Trends in Mycological Research; Springer: Berlin/Heidelberg, Germany, 2021; pp. 189–201. [Google Scholar]
- Zamanizadeh, H.R.; Hatami, N.; Aminaee, M.M.; Rakhshandehroo, F. Application of biofungicides in control of damping disease off in greenhouse crops as a possible substitute to synthetic fungicides. Int. J. Environ. Sci. Technol. 2011, 8, 129–136. [Google Scholar] [CrossRef] [Green Version]
- Woo, S.L.; Ruocco, M.; Vinale, F.; Nigro, M.; Marra, R.; Lombardi, N.; Pascale, A.; Lanzuise, S.; Manganiello, G.; Lorito, M. Trichoderma-based products and their widespread use in agriculture. Open Mycol. J. 2014, 8, 71–126. [Google Scholar] [CrossRef] [Green Version]
- Sood, M.; Kapoor, D.; Kumar, V.; Sheteiwy, M.S.; Ramakrishnan, M.; Landi, M.; Araniti, F.; Sharma, A. Trichoderma: The “Secrets” of a Multitalented Biocontrol Agent. Plants 2020, 9, 762. [Google Scholar] [CrossRef]
- López-Bucio, J.; Pelagio-Flores, R.; Herrera-Estrella, A. Trichoderma as biostimulant: Exploiting the multilevel properties of a plant beneficial fungus. Sci. Hortic. 2015, 196, 109–123. [Google Scholar] [CrossRef]
- Altomare, C.; Norvell, W.; Bjorkman, T.; Harman, G. Solubilization of phosphates and micronutrients by the plant-growth-promoting and biocontrol fungus Trichoderma harzianum Rifai 1295-22. Appl. Environ. Microbiol. 1999, 65, 2926–2933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shoresh, M.; Harman, G.E.; Mastouri, F. Induced systemic resistance and plant responses to fungal biocontrol agents. Annu. Rev. Phytopathol. 2010, 48, 21–43. [Google Scholar] [CrossRef] [Green Version]
- Kubicek, C.P.; Herrera-Estrella, A.; Seidl-Seiboth, V.; Martinez, D.A.; Druzhinina, I.S.; Thon, M.; Zeilinger, S.; Casas-Flores, S.; Horwitz, B.A.; Mukherjee, P.K. Comparative genome sequence analysis underscores mycoparasitism as the ancestral life style of Trichoderma. Genome Biol. 2011, 12, R40. [Google Scholar] [CrossRef] [Green Version]
- Jaklitsch, W.; Voglmayr, H. Biodiversity of Trichoderma (Hypocreaceae) in Southern Europe and Macaronesia. Stud. Mycol. 2015, 80, 1–87. [Google Scholar] [CrossRef] [Green Version]
- du Plessis, I.L.; Druzhinina, I.S.; Atanasova, L.; Yarden, O.; Jacobs, K. The diversity of Trichoderma species from soil in South Africa, with five new additions. Mycologia 2018, 110, 559–583. [Google Scholar] [CrossRef]
- Haouhach, S.; Karkachi, N.; Oguiba, B.; Sidaoui, A.; Chamorro, I.; Kihal, M.; Monte, E. Three New Reports of Trichoderma in Algeria: T. atrobrunneum (South), T. longibrachiatum (South), and T. afroharzianum (Northwest). Microorganisms 2020, 8, 1455. [Google Scholar] [CrossRef] [PubMed]
- Belayneh, T.; Kubicek, C.P.; Druzhinina, I.S. The rhizosphere of Coffea arabica in its native highland forests of Ethiopia provides a niche for a distinguished diversity of Trichoderma. Diversity 2010, 2, 527–549. [Google Scholar] [CrossRef]
- Rifai, M.A. Revision of the genus Trichoderma. Mycol. Pap. 1969, 116, 1–56. [Google Scholar]
- Bissett, J. A revision of the genus Trichoderma. III. Section Pachybasium. Can. J. Bot. 1991, 69, 2373–2417. [Google Scholar] [CrossRef]
- Bissett, J. A revision of the genus Trichoderma. I. Section Longibrachiatum sect. nov. Can. J. Bot. 1984, 62, 924–931. [Google Scholar] [CrossRef]
- Bissett, J. A revision of the genus Trichoderma. II. Infrageneric classification. Can. J. Bot. 1991, 69, 2357–2372. [Google Scholar] [CrossRef]
- Bissett, J. A revision of the genus Trichoderma. IV. Additional notes on section Longibrachiatum. Can. J. Bot. 1991, 69, 2418–2420. [Google Scholar] [CrossRef]
- Spring, O.; Gomez-Zeledon, J.; Hadziabdic, D.; Trigiano, R.N.; Thines, M.; Lebeda, A. Biological characteristics and assessment of virulence diversity in pathosystems of economically important biotrophic oomycetes. Crit. Rev. Plant Sci. 2018, 37, 439–495. [Google Scholar] [CrossRef]
- Cai, F.; Druzhinina, I.S. In honor of John Bissett: Authoritative guidelines on molecular identification of Trichoderma. Fungal Divers. 2021, 107, 1–69. [Google Scholar] [CrossRef]
- Samuels, G.J.; Dodd, S.L.; Gams, W.; Castlebury, L.A.; Petrini, O. Trichoderma species associated with the green mold epidemic of commercially grown Agaricus bisporus. Mycologia 2002, 94, 146–170. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protoc. Guide Meth. Appl. 1990, 18, 315–322. [Google Scholar]
- Schoch, C.L.; Seifert, K.A.; Huhndorf, S.; Robert, V.; Spouge, J.L.; Levesque, C.A.; Chen, W. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proc. Natl. Acad. Sci. USA 2012, 109, 6241–6246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaverri, P.; Branco-Rocha, F.; Jaklitsch, W.; Gazis, R.; Degenkolb, T.; Samuels, G.J. Systematics of the Trichoderma harzianum species complex and the re-identification of commercial biocontrol strains. Mycologia 2015, 107, 558–590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, J.E.; Vannette, R.L.; Igwe, A.; Blundell, R.; Casteel, C.L.; Gaudin, A.C. Effects of agricultural management on rhizosphere microbial structure and function in processing tomato plants. Appl. Environ. Microbiol. 2019, 85, e01064-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leelavathi, M.; Vani, L.; Reena, P. Antimicrobial activity of Trichoderma harzianum against bacteria and fungi. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 96–103. [Google Scholar]
- Anwar, J.; Iqbal, Z. Effect of growth conditions on antibacterial activity of Trichoderma harzianum against selected pathogenic bacteria. Sarhad J. Agric. 2017, 33, 501–510. [Google Scholar] [CrossRef]
- Motlagh, M.R.S.; Samimi, Z. Evaluation of Trichoderma spp., as biological agents in some of plant pathogens. Ann. Biol. Res. 2013, 4, 173–179. [Google Scholar]
- Nandini, B.; Hariprasad, P.; Shankara, H.N.; Prakash, H.S.; Geetha, N. Total crude protein extract of Trichoderma spp. induces systemic resistance in pearl millet against the downy mildew pathogen. 3 Biotec. 2017, 7, 183. [Google Scholar] [CrossRef]
- Lamondia, J.A.; Cowles, R.S. Effect of Entomopathogenic Nematodes and Trichoderma harzianum on the Strawberry Black Root Rot Pathogens Pratylenchus penetrans and Rhizoctonia fragariae. J. Nematol. 2002, 34, 351–357. [Google Scholar]
- Poveda, J.; Abril-Urias, P.; Escobar, C. Biological control of plant-parasitic nematodes by filamentous fungi inducers of resistance: Trichoderma, mycorrhizal and endophytic fungi. Front. Microbiol. 2020, 11, 992. [Google Scholar] [CrossRef]
- Tefera, A.; Tefera, T. Ethiopia Coffee Annual Report ET2020-0004; USDA Foreign Agricultural Service: Addis Ababa, Ethiopia, 2021; pp. 1–6.
- Moat, J.; Williams, J.; Baena, S.; Wilkinson, T.; Gole, T.W.; Challa, Z.K.; Demissew, S.; Davis, A.P. Resilience potential of the Ethiopian coffee sector under climate change. Nat. Plants 2017, 3, 17081. [Google Scholar] [CrossRef] [PubMed]
- ICO. Total Production by All Exporting Countries; ICO: London, UK, 2016. [Google Scholar]
- Panggabean, Y.; Arsyad, M. Coffee farming business development: E-commerce technology utilization. IOP Conf. Ser. Earth Environ. Sci. 2021, 807, 032011. [Google Scholar] [CrossRef]
- Tefera, A.; Tefera, T.; Gray, Q. Coffee Annual Report; USDA Foreign Agricultural Service: Addis Ababa, Ethiopia, 2012.
- Ethiopian Economic Association. Report on the Ethiopian Economy; Ethiopian Economic Association: Addis Ababa, Ethiopia, 2005; Volume 5. [Google Scholar]
- International Coffee Organization. Total Production by All Exporting Countries. 2016. Available online: http://www.ico.org (accessed on 18 June 2021).
- Teferi, D.; Belachew, K. Evaluation of released arabica coffee varieties (Coffea arabica L.) for major coffee diseases with especial emphasis to coffee wilt disease (Gibberella xylarioides) at jimma, Ethiopia. Evaluation 2015, 5, 81–87. [Google Scholar]
- Adugna, G.; Million, A.; Hindorf, H.; Zeru, A.; Teferi, D.; Jefuka, C. (Eds.) Coffee Wilt Disease in Ethiopia; CAB International: Oxfordshire, UK, 2010; p. 50. [Google Scholar]
- Zeru, A.; Assefa, F.; Adugna, G.; Hindorf, H. Occurrence of fungal diseases of Coffea arabica L. in montane rainforests of Ethiopia. J. Appl. Bot. Food Qual. 2012, 82, 148–151. [Google Scholar]
- Getachew, S.; Adugna, G.; Lemessa, F.; Hindorf, H. Coffee wilt disease (Gibberella xylarioides Heim and Saccas) in forest coffee systems of southwest and southeast Ethiopia. Plant Pathol. J. 2012, 11, 10–17. [Google Scholar] [CrossRef] [Green Version]
- Tiru, M.; Muleta, D.; Bercha, G.; Adugna, G. Antagonistic effect of rhizobacteria against coffee wilt disease caused by Gibberella xylarioides. Asian J. Plant Pathol. 2013, 7, 109–122. [Google Scholar] [CrossRef] [Green Version]
- Adugna, G. Diversity in Pathogenicity and Genetics of Gibberella xylarioides (Fusarium xylarioides) Populations and Resistance of Coffea spp. Ph.D. Thesis, University of Bonn, Bonn, Germany, 2004. [Google Scholar]
- Gil, S.V.; Pastor, S.; March, G. Quantitative isolation of biocontrol agents Trichoderma spp., Gliocladium spp. and Actinomycetes from soil with culture media. Microbiol. Res. 2009, 164, 196–205. [Google Scholar]
- Saravanakumar, K.; Yu, C.; Dou, K.; Wang, M.; Li, Y.; Chen, J. Biodiversity of Trichoderma community in the tidal flats and wetland of southeastern China. PLoS ONE 2016, 11, e0168020. [Google Scholar] [CrossRef]
- Adugna, G.; Hindorf, H.; Steiner, U.; Nirenberg, H.; Dehne, H.-W.; Schellander, K. Genetic diversity in the coffee wilt pathogen (Gibberella xylarioides) populations: Differentiation by host specialization and RAPD analysis/Genetische Diversität in der Population des Erregers der Kaffeewelke (Gibberella xylarioides): Differenzierung durch Wirtsspezifität und RAPD Analyse. J. Plant Dis. Protec. 2005, 112, 134–145. [Google Scholar]
- Adugna, G.; Hindorf, H. Tracheomycosis (Gibberella xylarioides) on coffee (Coffea arabica). In Proceedings of the Conference on International Agricultural Research for Development, Berlin, Germany, 5–7 October 2004; Deutscher Tropentag: Berlin, Germany; pp. 9–11. [Google Scholar]
- Samuels, G.J.; Hebbar, P.K. Trichoderma: Identification and Agricultural Applications; APS Press: Eagan, MN, USA, 2015. [Google Scholar]
- Leahy, J.G.; Colwell, R.R. Microbial degradation of hydrocarbons in the environment. Microbiol. Rev. 1990, 54, 305–315. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. Mycologia 2013, 64, 315–322. [Google Scholar]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Tsegaye, E.; Li, M.; Wu, B.; Jiang, X. Biodiversity of Trichoderma from grassland and forest ecosystems in Northern Xinjiang, China. 3 Biotech 2020, 10, 362. [Google Scholar] [CrossRef] [PubMed]
- Simpson, E.H. Measurement of Diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Shannon, C.E. A Mathematical theory of communication. Bell Syst. Technol. J. 1948, 27, 379–423. [Google Scholar] [CrossRef] [Green Version]
- Pielou, E.C. The measurement of diversity in different types of biological collections. J. Theor. Biol. 1966, 13, 131–144. [Google Scholar] [CrossRef]
- Margalef, R. Information theory in biology. Gen. Syst. Yearb. 1958, 3, 36–71. [Google Scholar]
- Konopiński, M.K. Shannon diversity index: A call to replace the original Shannon’s formula with unbiased estimator in the population genetics studies. PeerJ 2020, 8, e9391. [Google Scholar] [CrossRef]
- Gregorius, H.-R.; Gillet, E.M. Generalized Simpson-diversity. Ecol. Model. 2008, 211, 90–96. [Google Scholar] [CrossRef]
- Dennis, C.; Webster, J. Antagonistic properties of species-groups of Trichoderma: II. Production of volatile antibiotics. Trans. Br. Mycol. Soc. 1971, 57, 41–48. [Google Scholar] [CrossRef]
- Szekeres, A.; Leitgeb, B.; Kredics, L.; Antal, Z.; Hatvani, L.; Manczinger, L.; Vágvölgyi, C. Peptaibols and related peptaibiotics of Trichoderma. Acta Microbiol. Immunol. Hung. 2005, 52, 137–168. [Google Scholar] [CrossRef] [PubMed]
- Samuels, G.J.; Ismaiel, A.; Bon, M.-C.; De Respinis, S.; Petrini, O. Trichoderma asperellum sensu lato consists of two cryptic species. Mycologia 2010, 102, 944–966. [Google Scholar] [CrossRef]
- Mazrou, Y.S.; Makhlouf, A.H.; Elseehy, M.M.; Awad, M.F.; Hassan, M.M. Antagonistic activity and molecular characterization of biological control agent Trichoderma harzianum from Saudi Arabia. Egypt J. Biol. Pest Contr. 2020, 30, 4. [Google Scholar] [CrossRef] [Green Version]
- Hermosa, R.; Cardoza, R.E.; Rubio, M.B.; Gutiérrez, S.; Monte, E. Secondary metabolism and antimicrobial metabolites of Trichoderma. In Biotechnology and Biology of Trichoderma; Elsevier: Amsterdam, The Netherlands, 2014; pp. 125–137. [Google Scholar]
- Bridge, P.; Spooner, B.; Roberts, P. The impact of molecular data in fungal systematics. Adv. Bot. Res. 2005, 42, 33–67. [Google Scholar]
- Atanasova, L.; Le Crom, S.; Gruber, S.; Coulpier, F.; Seidl-Seiboth, V.; Kubicek, C.P.; Druzhinina, I.S. Comparative transcriptomics reveals different strategies of Trichoderma mycoparasitism. BMC Gen. 2013, 14, 121. [Google Scholar] [CrossRef] [Green Version]
- Druzhinina, I.; Kubicek, C.P. Species concepts and biodiversity in Trichoderma and Hypocrea: From aggregate species to species clusters? J. Zhejiang Univ. Sci. B 2005, 6, 100. [Google Scholar] [CrossRef] [Green Version]
- Druzhinina, I.S.; Kubicek, C.P. Genetic engineering of Trichoderma reesei cellulases and their production. Microb. Biotechnol. 2017, 10, 1485–1499. [Google Scholar] [CrossRef]
- Liu, B.; Ji, S.; Zhang, H.; Wang, Y.; Liu, Z. Isolation of Trichoderma in the rhizosphere soil of Syringa oblata from Harbin and their biocontrol and growth promotion function. Microbiol. Res. 2020, 235, 126445. [Google Scholar] [CrossRef]
- Sumida, C.H.; Daniel, J.F.; Araujod, A.P.C.; Peitl, D.C.; Abreu, L.M.; Dekker, R.F.; Canteri, M.G. Trichoderma asperelloides antagonism to nine Sclerotinia sclerotiorum strains and biological control of white mold disease in soybean plants. Biocontr. Sci. Technol. 2018, 28, 142–156. [Google Scholar] [CrossRef]
- Mutawila, C.; Vinale, F.; Halleen, F.; Lorito, M.; Mostert, L. Isolation, production and in vitro effects of the major secondary metabolite produced by Trichoderma species used for the control of grapevine trunk diseases. Plant Pathol. 2016, 65, 104–113. [Google Scholar] [CrossRef] [Green Version]
- Belayneh, T.; Druzhinina, I.S.; Kubicek, C.P.; Atanasova, L. Novel endophytic Trichoderma spp. isolated from healthy Coffea arabica roots are capable of controlling coffee Tracheomycosis. Diversity 2013, 5, 750–766. [Google Scholar]
- Holmes, K.A.; Schroers, H.-J.; Thomas, S.E.; Evans, H.C.; Samuels, G.J. Taxonomy and biocontrol potential of a new species of Trichoderma from the Amazon basin of South America. Mycol. Progr. 2004, 3, 199–210. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, J.-L.; Chen, J.; Mao, L.-J.; Feng, X.-X.; Zhang, C.-L.; Lin, F.-C. Trichoderma biodiversity of agricultural fields in east china reveals a gradient distribution of species. PLoS ONE 2016, 11, e0160613. [Google Scholar] [CrossRef] [PubMed]
- Błaszczyk, L.; Strakowska, J.; Chełkowski, J.; Gąbka-Buszek, A.; Kaczmarek, J. Trichoderma species occurring on wood with decay symptoms in mountain forests in Central Europe: Genetic and enzymatic characterization. J. Appl. Gen. 2016, 57, 397–407. [Google Scholar] [CrossRef] [Green Version]
- Hoyos-Carvajal, L.; Bissett, J. Biodiversity of Trichoderma in neotropics. In The Dynamical Processes of Biodiversity-Case Studies of Evolution and Spatial Distribution; InTech: London, UK, 2011. [Google Scholar]
- Morris, E.K.; Caruso, T.; Buscot, F.; Fischer, M.; Hancock, C.; Maier, T.S.; Meiners, T.; Müller, C.; Obermaier, E.; Prati, D. Choosing and using diversity indices: Insights for ecological applications from the German Biodiversity Exploratories. Ecol. Evol. 2014, 4, 3514–3524. [Google Scholar] [CrossRef] [Green Version]
- Migheli, Q.; Balmas, V.; Komoñ-Zelazowska, M.; Scherm, B.; Fiori, S.; Kopchinskiy, A.G.; Kubicek, C.P.; Druzhinina, I.S. Soils of a Mediterranean hot spot of biodiversity and endemism (Sardinia, Tyrrhenian Islands) are inhabited by pan-European, invasive species of Hypocrea/Trichoderma. Environ. Microbiol. 2009, 11, 35–46. [Google Scholar] [CrossRef]
- Błaszczyk, L.; Popiel, D.; Chełkowski, J.; Koczyk, G.; Samuels, G.J.; Sobieralski, K.; Siwulski, M. Species diversity of Trichoderma in Poland. J. Appl. Genet. 2011, 52, 233–243. [Google Scholar] [CrossRef] [Green Version]
- Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; Del-Val, E.; Larsen, J. Ecological functions of Trichoderma spp. and their secondary metabolites in the rhizosphere: Interactions with plants. FEMS Microbiol. Ecol. 2016, 92, fiw036. [Google Scholar] [CrossRef] [Green Version]
- Kumar, G.; Maharshi, A.; Patel, J.; Mukherjee, A.; Singh, H. Trichoderma: A potential fungal antagonist to control plant diseases. ATSA Mukhapatra-Ann. Technol. Issue 2017, 21, 206–218. [Google Scholar]
- Kredics, L.; Hatvani, L.; Naeimi, S.; Körmöczi, P.; Manczinger, L.; Vágvölgyi, C.; Druzhinina, I. Biodiversity of the genus Hypocrea/Trichoderma in different habitats. In Biotechnology and Biology of Trichoderma; Elsevier: Amsterdam, The Netherlands, 2014; pp. 3–24. [Google Scholar]
- Cummings, N.; Ambrose, A.; Braithwaite, M.; Bissett, J.; Roslan, H.; Abdullah, J.; Stewart, A.; Agbayani, F.; Steyaert, J.; Hill, R.A. Diversity of root-endophytic Trichoderma from Malaysian Borneo. Mycol. Progr. 2016, 15, 50. [Google Scholar] [CrossRef]
- Flood, J. Coffee Wilt Disease; CABI: London, UK, 2010. [Google Scholar]
- Mulatu, A.; Megersa, N.; Alemu, T. Characterization of fungal extracts from Trichoderma isolates: Their effects against coffee wilt pathogen (Gibberella xylarioides). SINET Ethiop. J. Sci. 2013, 36, 81–92. [Google Scholar]
- Mulatu, A.; Alemu, T.; Megersa, N.; Vetukuri, R.R. Optimization of Culture Conditions and Production of Bio-Fungicides from Trichoderma Species under Solid-State Fermentation using Mathematical Modeling. Microorganisms 2021, 9, 1675. [Google Scholar] [CrossRef] [PubMed]
- Filizola, P.R.B.; Luna, M.A.C.; de Souza, A.F.; Coelho, I.L.; Laranjeira, D.; Campos-Takaki, G.M. Biodiversity and phylogeny of novel Trichoderma isolates from mangrove sediments and potential of biocontrol against Fusarium strains. J. Microb. Cell Factor. 2019, 18, 89. [Google Scholar] [CrossRef]
- Waghunde, R.R.; Shelake, R.M.; Sabalpara, A.N. Trichoderma: A significant fungus for agriculture and environment. Afr. J. Agri. Res. 2016, 11, 1952–1965. [Google Scholar]
- Zhang, S.; Xu, B.; Zhang, J.; Gan, Y. Identification of the antifungal activity of Trichoderma longibrachiatum T6 and assessment of bioactive substances in controlling phytopathgens. Pestic. Biochem. Phys. 2018, 147, 59–66. [Google Scholar] [CrossRef]
- Degani, O.; Khatib, S.; Becher, P.; Gordani, A.; Harris, R. Trichoderma asperellum Secreted 6-Pentyl-α-Pyrone to Control Magnaporthiopsis maydis, the Maize Late Wilt Disease Agent. Biology 2021, 10, 897. [Google Scholar] [CrossRef]
- Carrero-Carrón, I.; Trapero-Casas, J.L.; Olivares-García, C.; Monte, E.; Hermosa, R.; Jiménez-Díaz, R.M. Trichoderma asperellum is effective for biocontrol of Verticillium wilt in olive caused by the defoliating pathotype of Verticillium dahliae. Crop Prot. 2016, 88, 45–52. [Google Scholar] [CrossRef]
- Wu, Q.; Sun, R.; Ni, M.; Yu, J.; Li, Y.; Yu, C.; Dou, K.; Ren, J.; Chen, J. Identification of a novel fungus, Trichoderma asperellum and comprehensive evaluation of its biocontrol efficacy. PLoS ONE 2017, 12, e0179957. [Google Scholar] [CrossRef]
- Elamawi, R.M.; Al-Harbi, R.E.; Hendi, A.A. Biosynthesis and characterization of silver nanoparticles using Trichoderma longibrachiatum and their effect on phytopathogenic fungi. Egy. J. Biol. Pest Contr. 2018, 28, 28. [Google Scholar] [CrossRef] [Green Version]
- Moretto, K.C.; Gimenes-Fernandes, N.; dos Santos, J.M. Influence of Trichoderma spp. on Colletotrichum acutatum mycelial growth and morphology and on infection of “Tahiti” lime detached flowers. Summa Phytopathol. 2001, 27, 357–364. [Google Scholar]
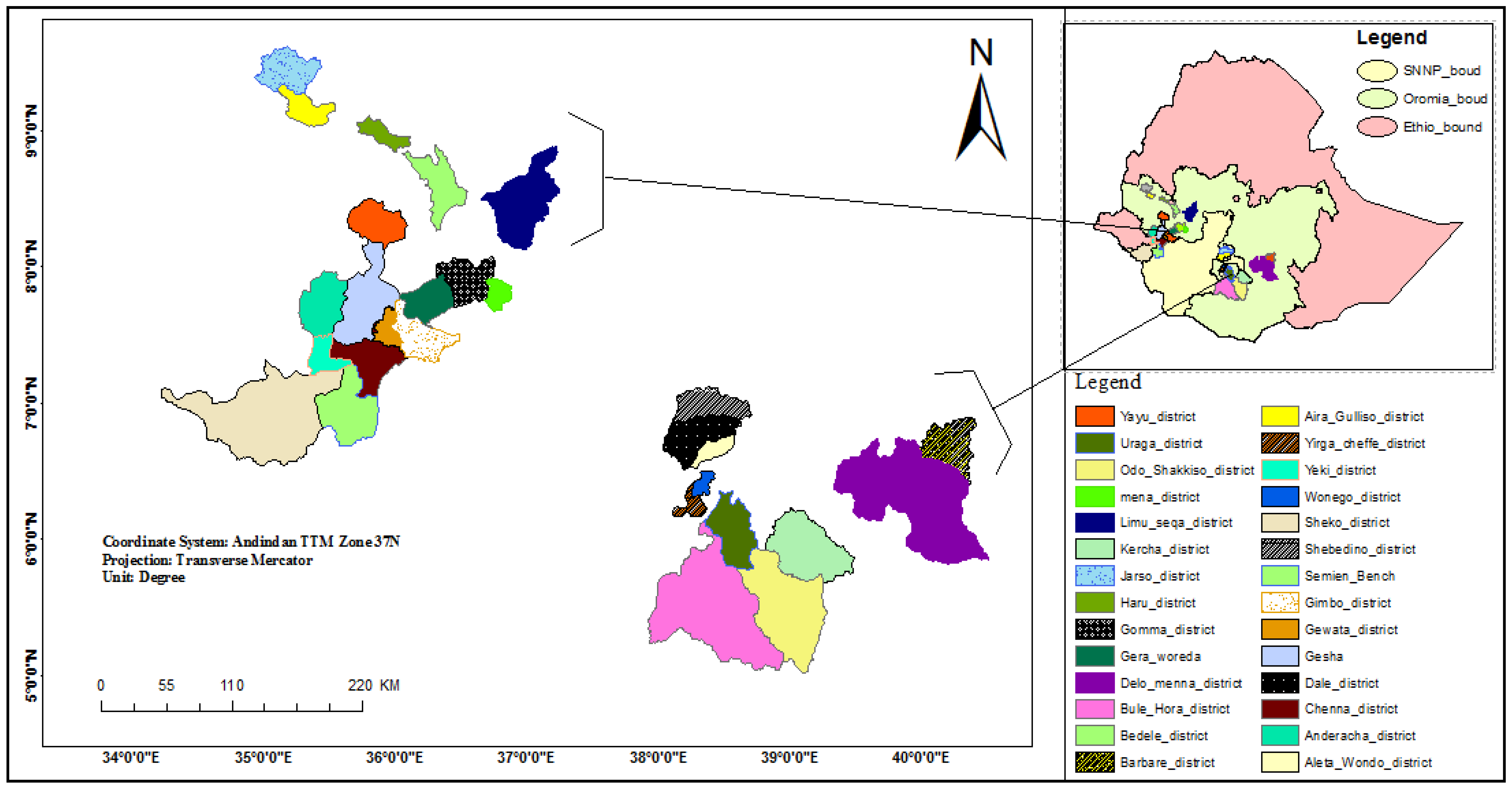
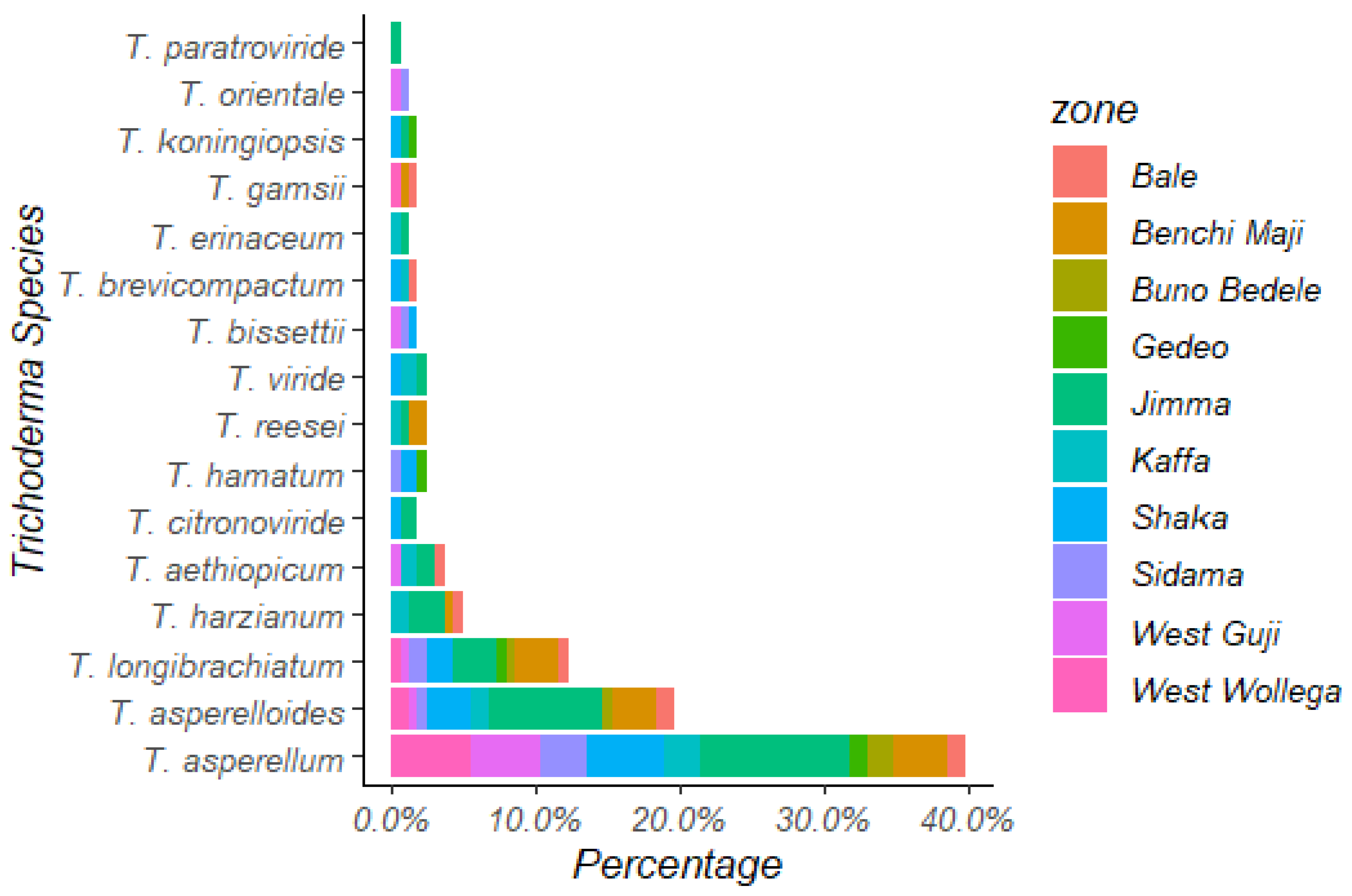
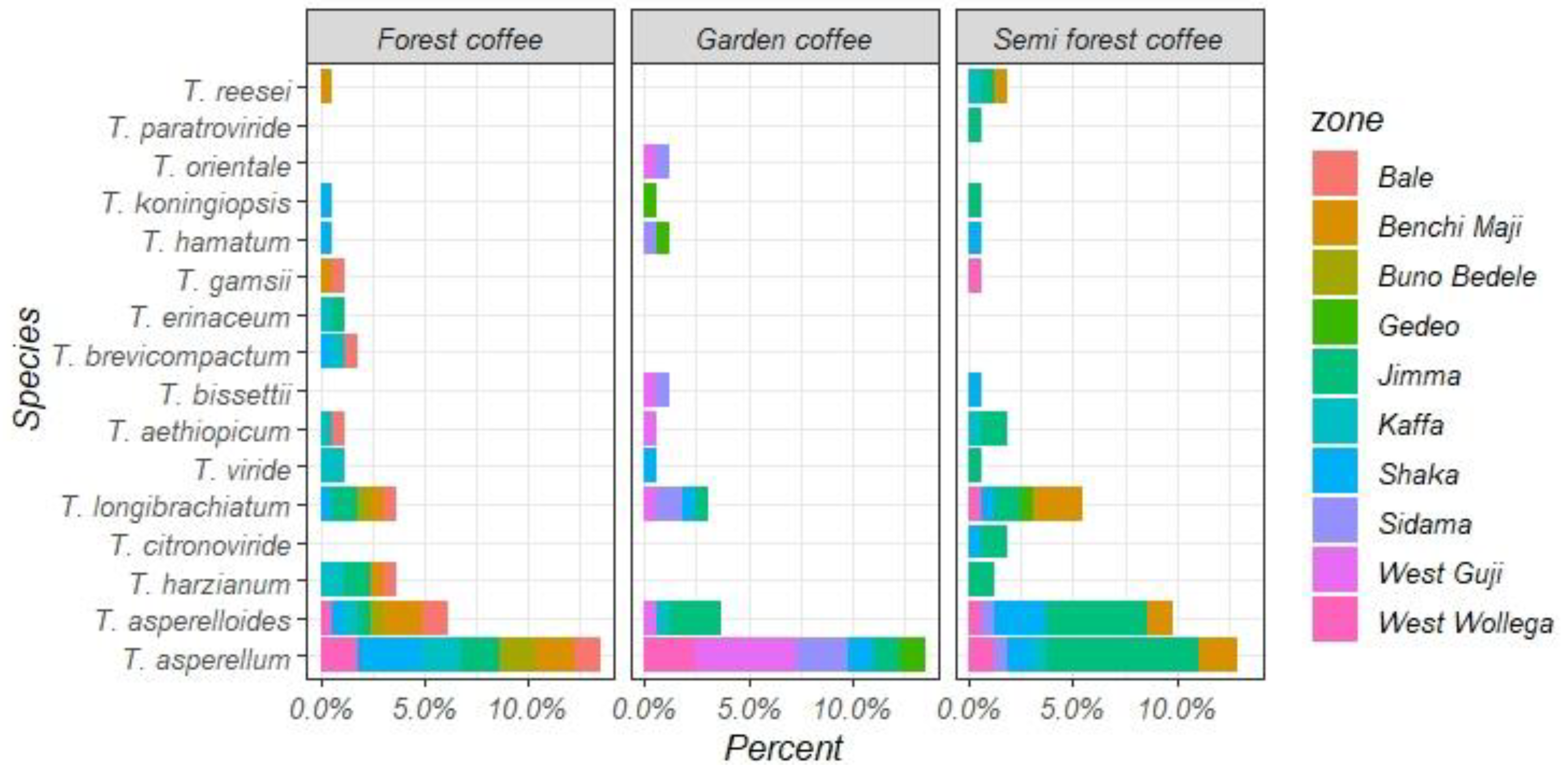
| Trichoderma Species | Isolate ID | Accession Number (TEF1-α) | District/Location | Zone | Coffee Ecosystem |
|---|---|---|---|---|---|
| Trichoderma hamatum | AU2 | MZ361591 | Gera | Jimma | Semi-forest |
| Trichoderma asperellum | AU3 | MZ361592 | Gera | Jimma | Semi-forest |
| Trichoderma asperellum | AU6 | MZ361593 | Melko | Jimma | Semi-forest |
| Trichoderma asperellum | AU8 | MZ361594 | Gera | Jimma | Semi-forest |
| Trichoderma viride | AU9 | MZ361595 | Yeki | Jimma | Semi-forest |
| Trichoderma asperelloides | AU10 | MZ361596 | Gera | Jimma | Semi-forest |
| Trichoderma asperelloides | AU11 | MZ361597 | Gera | Jimma | Semi-forest |
| Trichoderma asperellum | AU13 | MZ361598 | Gera | Jimma | Semi-forest |
| Trichoderma longibrachiatum | AU14 | MZ361599 | Gera | Jimma | Semi-forest |
| Trichoderma asperellum | AU15 | MZ361600 | Yeki | Sheka | Semi-forest |
| Trichoderma hamatum | AU19 | MZ361601 | Gera | Jimma | Semi-forest |
| Trichoderma asperellum | AU21 | MZ361602 | Gera | Jimma | Semi-forest |
| Trichoderma asperellum | AU22 | MZ361603 | Yeki | Sheka | Semi-forest |
| Trichoderma hamatum | AU23 | MZ361604 | Gera | Jimma | Semi-forest |
| Trichoderma asperellum | AU24 | MZ361605 | Odo Shakiso | West Guji | Garden Coffee |
| Trichoderma asperellum | AU26 | MZ361606 | Odo Shakiso | West Guji | Garden Coffee |
| Trichoderma asperelloides | AU28 | MZ361607 | Gera | Jimma | Garden Coffee |
| Trichoderma asperelloides | AU29 | MZ361608 | Gera | Jimma | Garden Coffee |
| Trichoderma hamatum | AU30 | MZ361609 | Shebedino | Sidama | Garden Coffee |
| Trichoderma longibrachiatum | AU32 | MZ361610 | Gera | Jimma | Garden Coffee |
| Trichoderma asperelloides | AU34 | MZ361611 | Yeki | Sheka | Forest |
| Trichoderma asperellum | AU37 | MZ361612 | Gera | Jimma | Forest |
| Trichoderma asperellum | AU38 | MZ361613 | Yeki | Sheka | Forest |
| Trichoderma asperellum | AU39 | MZ361614 | Gimbo | Kaffa | Forest |
| Trichoderma longibrachiatum | AU40 | MZ361615 | Gomma | Jimma | Forest |
| Trichoderma brevicompactum | AU41 | MZ361615 | Yeki | Sheka | Forest |
| Trichoderma asperellum | AU42 | MZ361615 | Gomma | Jimma | Forest |
| Trichoderma asperellum | AU44 | MZ361618 | Gomma | Jimma | Forest |
| Trichoderma asperellum | AU46 | MZ361619 | Gomma | Jimma | Forest |
| Trichoderma asperelloides | AU47 | MZ361620 | Chena | Kaffa | Forest |
| Trichoderma longibrachiatum | AU49 | MZ361621 | Andaracha | Sheka | Forest |
| Trichoderma hamatum | AU50 | MZ361622 | Andaracha | Sheka | Forest |
| Trichoderma asperellum | AU51 | MZ361623 | Andaracha | Sheka | Forest |
| Trichoderma asperellum | AU53 | MZ361624 | Andaracha | Sheka | Forest |
| Trichoderma asperelloides | AU55 | MZ361624 | Mena | Jimma | Semi-forest |
| Trichoderma asperellum | AU58 | MZ361626 | Gewata | Kaffa | Forest |
| Trichoderma bissettii | AU59 | MZ361627 | Aleta Wondo | Sidama | Garden Coffee |
| Trichoderma asperelloides | AU61 | MZ361628 | Gomma | Kaffa | Garden Coffee |
| Trichoderma asperellum | AU69 | MZ361629 | Wonago | Gedeo | Garden Coffee |
| Trichoderma koningiopsis | AU70 | MZ361630 | Wonago | Gedeo | Garden Coffee |
| Trichoderma asperellum | AU71 | MZ361631 | Yirga cheffe | Sidama | Semi-forest |
| Trichoderma longibrachiatum | AU72 | MZ361632 | Yirga cheffe | Gedeo | Semi-forest |
| Trichoderma asperellum | AU73 | MZ361633 | Gewata | Kaffa | Semi-forest |
| Trichoderma asperellum | AU74 | MZ361634 | Aleta Wondo | Sidama | Semi-forest |
| Trichoderma asperellum | AU75 | MZ361635 | Dale | Sidama | Garden |
| Trichoderma orientale | AU77 | MZ361636 | Dale | Sidama | Garden |
| Trichoderma harzianum | AU78 | MZ361637 | Gimbo | Kaffa | Forest |
| Trichoderma asperellum | AU81 | MZ361638 | Haru | West Wollega | Forest |
| Trichoderma asperellum | AU82 | MZ361639 | Sheko | Benchi Maji | Forest |
| Trichoderma harzianum | AU84 | MZ361640 | Sheko | Benchi Maji | Forest |
| Trichoderma asperelloides | AU85 | MZ361641 | Sheko | Benchi Maji | Forest |
| Trichoderma gamsii | AU86 | MZ361642 | Sheko | Benchi Maji | Forest |
| Trichoderma harzianum | AU87 | MZ361643 | Gera | Jimma | Forest |
| Trichoderma harzianum | AU88 | MZ361644 | Gera | Jimma | Forest |
| Trichoderma asperellum | AU91 | MZ361645 | Chena | Kaffa | Forest |
| Trichoderma asperelloides | AU93 | MZ361646 | Chena | Kaffa | Semi-forest |
| Trichoderma asperelloides | AU94 | MZ361647 | Chena | Kaffa | Semi-forest |
| Trichoderma asperellum | AU95 | MZ361648 | Limmu Saka | Jimma | Semi-forest |
| Trichoderma asperellum | AU97 | MZ361649 | Limmu Saka | Jimma | Garden Coffee |
| Trichoderma asperelloides | AU98 | MZ361650 | Limmu Saka | Jimma | Garden Coffee |
| Trichoderma asperelloides | AU99 | MZ361651 | Limmu Saka | Jimma | Garden Coffee |
| Trichoderma asperellum | AU100 | MZ361652 | Limmu Saka | Jimma | Garden Coffee |
| Trichoderma asperelloides | AU103 | MZ361653 | Limmu Saka | Jimma | Semi-forest |
| Trichoderma asperellum | AU104 | MZ361654 | Geisha | Kaffa | Forest |
| Trichoderma aethiopicum | AU106 | MZ361655 | Geisha | Kaffa | Forest |
| Trichoderma asperellum | AU108 | MZ361656 | Yeki | Sheka | Semi-forest |
| Trichoderma longibrachiatum | AU109 | MZ361657 | Yeki | Sheka | Semi-forest |
| Trichoderma asperellum | AU110 | MZ361658 | Yeki | Sheka | Semi-forest |
| Trichoderma viride | AU112 | MZ361659 | Yeki | Sheka | Garden Coffee |
| Trichoderma longibrachiatum | AU114 | MZ361660 | Yeki | Sheka | Garden Coffee |
| Trichoderma asperellum | AU115 | MZ361661 | Yeki | Sheka | Garden Coffee |
| Trichoderma citroviride | AU116 | MZ361662 | Yeki | Sheka | Semi-forest |
| Trichoderma asperelloides | AU118 | MZ361663 | Limmu Saka | Jimma | Semi-forest |
| Trichoderma asperellum | AU122 | MZ361664 | Yeki | Sheka | Semi-forest |
| Trichoderma paratroviride | AU123 | MZ361665 | Limmu Saka | Jimma | Semi-forest |
| Trichoderma longibrachiatum | AU125 | MZ361666 | Yayu | Buno Bedele | Forest |
| Trichoderma asperellum | AU126 | MZ361667 | Yayu | Buno Bedele | Forest |
| Trichoderma asperellum | AU129 | MZ361668 | Yayu | Buno Bedele | Forest |
| Trichoderma asperellum | AU131 | MZ361669 | Gera | Jimma | Forest Coffee |
| Trichoderma asperellum | AU133 | MZ361670 | Sheko | Benchi Maji | Semi-forest |
| Trichoderma asperellum | AU134 | MZ361671 | Sheko | Benchi Maji | Semi-forest |
| Trichoderma asperellum | AU135 | MZ361672 | Sheko | Benchi Maji | Semi-forest |
| Trichoderma longibrachiatum | AU136 | MZ361673 | Sheko | Benchi Maji | Forest |
| Trichoderma longibrachiatum | AU138 | MZ361674 | Semien Benchi | Benchi Maji | Semi-forest |
| Trichoderma asperelloides | AU139 | MZ361675 | Semein Benchi | Benchi Maji | Semi-forest |
| Trichoderma longibrachiatum | AU141 | MZ361676 | Semein Benchi | Benchi Maji | Semi-forest |
| Trichoderma longibrachiatum | AU143 | MZ361677 | Sheko | Benchi Maji | Semi-forest |
| Trichoderma asperelloides | AU144 | MZ361678 | Sheko | Benchi Maji | Forest |
| Trichoderma reesei | AU145 | MZ361679 | Sheko | Benchi Maji | Forest |
| Trichoderma harzianum | AU148 | MZ361680 | Haru | West Wollega | Forest |
| Trichoderma asperelloides | AU149 | MZ361681 | Haru | West Wollega | Semi-forest |
| Trichoderma gamsii | AU150 | MZ361682 | Haru | West Wollega | Semi-forest |
| Trichoderma asperelloides | AU155 | MZ361683 | Delo Mena | Bale | Forest |
| Trichoderma asperelloides | AU158 | MZ361684 | Yeki | Sheka | Semi-forest |
| Trichoderma longibrachiatum | AU161 | MZ361685 | Berbere | Bale | Forest |
| Trichoderma asperelloides | AU162 | MZ361686 | Kercha | West Guji | Garden Coffee |
| Trichoderma longibrachiatum | AU164 | MZ361687 | Jarso | West Wollega | Semi-forest |
| Trichoderma asperellum | AU165 | MZ361688 | Jarso | West Wollega | Semi-forest |
| Trichoderma erinaceum | AU166 | MZ361689 | Aira Guliso | West Wollega | Semi-forest |
| Trichoderma asperellum | AU167 | MZ361690 | Semein Benchi | Benchi Maji | Semi-forest |
| Trichoderma asperellum | AU169 | MZ361691 | Kercha | West Guji | Garden Coffee |
| Trichoderma asperellum | AU171 | MZ361692 | Aira Guliso | West Wollega | Garden Coffee |
| Trichoderma longibrachiatum | AU173 | MZ361693 | Bule Hora | West Guji | Garden Coffee |
| Trichoderma asperellum | AU174 | MZ361694 | Bule Hora | West Guji | Garden Coffee |
| Trichoderma asperellum | AU175 | MZ361695 | Bedele | Buno Bedele | Forest |
| Ecological Indices | Coffee Ecosystem | Major Coffee-Growing Zones | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Native Forest | Semi-Forest | Garden Coffee | Average | Jimma | Kaffa | Bench Maji | Sheka | Bunno Bedale | West Wollega | West Guji | Gedio | Sidama | Bale | |
| Simpson index (D) | 0.81 | 0.81 | 0.7 | 0.76 | 0.91 | 0.94 | 0.83 | 0.82 | 0.7 | 0.64 | 0.53 | 0.9 | 0.8 | 0.87 |
| Shannon’s index (H) | 1.97 | 1.96 | 1.57 | 1.77 | 1.97 | 1.82 | 1.7 | 1.83 | 0.75 | 0.94 | 1.29 | 1.33 | 1.54 | 1.89 |
| Pielou evenness index (E) | 0.79 | 0.79 | 0.71 | 0.75 | 0.76 | 0.95 | 0.87 | 0.83 | 0.86 | 0.72 | 0.68 | 0.96 | 0.86 | 0.97 |
| Abundance index (J) | 2.71 | 2.64 | 2.14 | 2.4 | 2.73 | 2.58 | 1.97 | 2.49 | 1.24 | 1.95 | 1.17 | 1.86 | 2.09 | 2.6 |
| Trichoderma Species | Mycelia Inhibition over Control (%) | Scale of Antagonistic Activity | |
|---|---|---|---|
| Dual Culture | Agar Diffusion Assay | ||
| T. hamatum AU23 | 76.9 ab ± 1.07 | 67.32 ab ± 4.06 | +++ |
| T. longibrachiatum AU32 | 75.2 b ± 0.7 | 69.82 ab ± 4.20 | +++ |
| T. asperellum AU53 | 72.6 b ± 0.3 | 66.3 c ± 2.3 | +++ |
| T. koningiopsis AU70 | 62.59 d ± 0.9 | 70.71 ab ± 4.82 | ++ |
| T. asperellum AU71 | 81.8 a ± 3.03 | 83.5 a ± 4.83 | ++++ |
| T. asperellum AU97 | 79.3 b ± 1.0 | 76.42 b ± 3.68 | ++++ |
| T. harzianum AU105 | 78.7 c ± 1.2 | 75.82 b ± 4.81 | +++ |
| T. aethiopicum AU106 | 79.3 d ± 5.1 | 68.50 c ± 5.12 | ++ |
| T. longibrachiatum AU121 | 79.2 b ± 0.9 | 63.4 c ± 3.4 | +++ |
| T. asperellum AU131 | 84.8 a ± 0.9 | 86.7 a ± 1.6 | ++++ |
| T. longibrachiatum AU158 | 82.4 a ± 0.5 | 88.2 a ± 3.5 | ++++ |
| T. asperellum AU171 | 77.7 ab ± 0.3 | 66.4 c ± 2.5 | +++ |
| Mean ± standard deviation | 77.54 ± 1.3 | 74.25 ± 3.74 | +++ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mulatu, A.; Megersa, N.; Abena, T.; Kanagarajan, S.; Liu, Q.; Tenkegna, T.A.; Vetukuri, R.R. Biodiversity of the Genus Trichoderma in the Rhizosphere of Coffee (Coffea arabica) Plants in Ethiopia and Their Potential Use in Biocontrol of Coffee Wilt Disease. Crops 2022, 2, 120-141. https://doi.org/10.3390/crops2020010
Mulatu A, Megersa N, Abena T, Kanagarajan S, Liu Q, Tenkegna TA, Vetukuri RR. Biodiversity of the Genus Trichoderma in the Rhizosphere of Coffee (Coffea arabica) Plants in Ethiopia and Their Potential Use in Biocontrol of Coffee Wilt Disease. Crops. 2022; 2(2):120-141. https://doi.org/10.3390/crops2020010
Chicago/Turabian StyleMulatu, Afrasa, Negussie Megersa, Tariku Abena, Selvaraju Kanagarajan, Qinsong Liu, Tesfaye Alemu Tenkegna, and Ramesh R. Vetukuri. 2022. "Biodiversity of the Genus Trichoderma in the Rhizosphere of Coffee (Coffea arabica) Plants in Ethiopia and Their Potential Use in Biocontrol of Coffee Wilt Disease" Crops 2, no. 2: 120-141. https://doi.org/10.3390/crops2020010
APA StyleMulatu, A., Megersa, N., Abena, T., Kanagarajan, S., Liu, Q., Tenkegna, T. A., & Vetukuri, R. R. (2022). Biodiversity of the Genus Trichoderma in the Rhizosphere of Coffee (Coffea arabica) Plants in Ethiopia and Their Potential Use in Biocontrol of Coffee Wilt Disease. Crops, 2(2), 120-141. https://doi.org/10.3390/crops2020010







