Preoperative Assessment of Surgical Resectability in Ovarian Cancer Using Ultrasound: A Narrative Review Based on the ISAAC Trial
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Conventional Imaging Techniques for Staging and Predicting Resectability in Ovarian Cancer
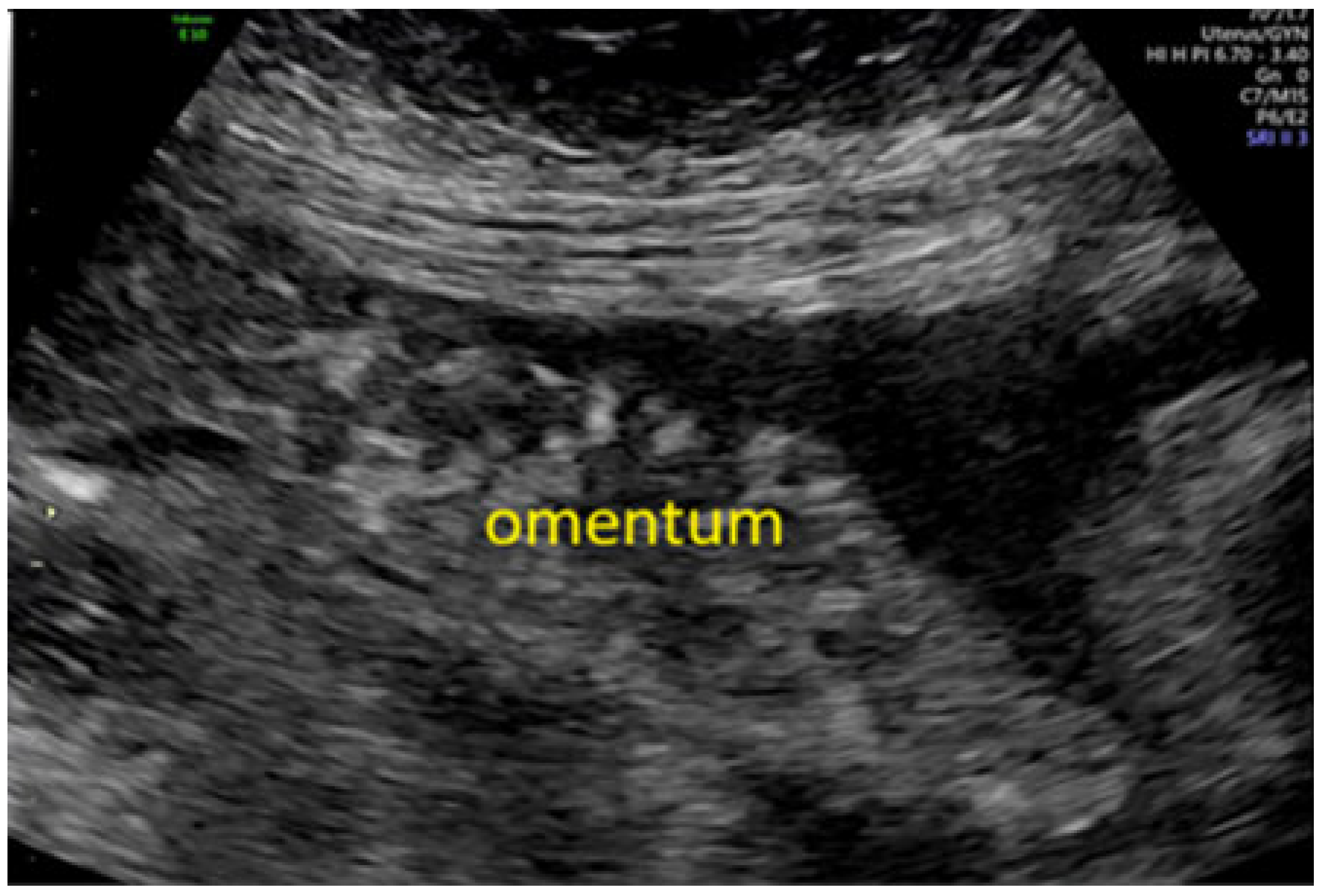
3.2. The Role of Ultrasound for Staging Ovarian Cancer and for Predicting Resectability: Pre-ISAAC Study Evidence
3.3. The ISAAC Study
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CT | Computed tomography |
| MRI | Magnetic resonance imaging |
| WB | Whole Body |
| DWI | Diffusion weighted imaging |
| PET | Positron Emission tomography |
| PCI | Peritoneal carcinomatosis index |
| PIV | Predictive Index Value |
| PCS | Primary cytoreductive surgery |
| NACT | NeoAdjuvant Chemotherapy |
References
- Webb, P.M.; Jordan, S.J. Epidemiology of epithelial ovarian cancer. Best Pract. Res. Clin. Obstet. Gynaecol. 2017, 41, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Autier, P.; Boniol, M.; Heanue, M.; Colombet, M.; Boyle, P. Estimates of the cancer incidence and mortality in Europe in 2006. Ann. Oncol. 2007, 18, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Kuroki, L.; Guntupalli, S.R. Treatment of epithelial ovarian cancer. BMJ 2020, 371, m3773. [Google Scholar] [CrossRef]
- Mazidimoradi, A.; Momenimovahed, Z.; Allahqoli, L.; Tiznobaik, A.; Hajinasab, N.; Salehiniya, H.; Alkatout, I. The global, regional and national epidemiology, incidence, mortality, and burden of ovarian cancer. Health Sci. Rep. 2022, 5, e936. [Google Scholar] [CrossRef]
- Berek, J.S.; Kehoe, S.T.; Kumar, L.; Friedlander, M. Cancer of the ovary, fallopian tube, and peritoneum. Int. J. Gynaecol. Obstet. 2018, 143 (Suppl. S2), 59–78. [Google Scholar] [CrossRef]
- Lee, K.R.; Tavassoli, F.A.; Prat, J.; Dietel, M.; Gersell, D.J.; Karseladze, A.I.; Hauptmann, S.; Rutgers, J.; Russell, P.; Buckley, C.H.; et al. Tumors of the ovary and peritoneum. In World Health Organization Classification of Tumors; Tavassoli, F.A., Devilee, P., Eds.; Pathology and Genetics of Tumors of the Breast and Female Genital Organs; IARC Press: Paris, France, 2023; pp. 113–202. [Google Scholar]
- Kurman, R.J.; Shih, I.M. Molecular pathogenesis and extraovarian origin of epithelial ovarian cancer—Shifting the paradigm. Hum. Pathol. 2011, 42, 918–931. [Google Scholar] [CrossRef]
- Burges, A.; Schmalfeldt, B. Ovarian cancer: Diagnosis and treatment. Deut Ärzt Internat 2011, 108, 635–641. [Google Scholar]
- Ledermann, J.A.; Matias-Guiu, X.; Amant, F.; Concin, N.; Davidson, B.; Fotopoulou, C.; González-Martin, A.; Gourley, C.; Leary, A.; Lorusso, D.; et al. ESGO-ESMO-ESP consensus conference recommendations on ovarian cancer: Pathology and molecular biology and early, advanced and recurrent disease. Ann. Oncol. 2024, 35, 248–266. [Google Scholar] [CrossRef]
- du Bois, A.; Reuss, A.; Pujade-Lauraine, E.; Harter, P.; Ray-Coquard, I.; Pfisterer, J. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: A combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials. Cancer 2009, 115, 1234–1244. [Google Scholar] [CrossRef]
- Zapardiel, I.; Morrow, C.W. New terminology for cytoreduction in advanced ovarian cancer. Lancet Oncol. 2011, 12, 214. [Google Scholar] [CrossRef]
- Polterauer, S.; Vergote, I.; Concin, N.; Braicu, I.; Chekerov, R.; Mahner, S.; Woelber, L.; Cadron, I.; Van Gorp, T.; Zeillinger, R.; et al. Prognostic value of residual tumor size in patients with epithelial ovarian cancer FIGO stages IIA–IV: Analysis of the OVCAD data. Int. J. Gynecol. Cancer 2012, 22, 380–385. [Google Scholar] [CrossRef]
- Vernooij, F.; Heintz, P.; Witteveen, E.; van der Graaf, Y. The outcomes of ovarian cancer treatment are better when provided by gynecologic oncologists and in specialized hospitals: A systematic review. Gynecol. Oncol. 2007, 105, 801–812. [Google Scholar] [CrossRef] [PubMed]
- du Bois, A.; Baert, T.; Vergote, I. Role of neoadjuvant chemotherapy in advanced epithelial ovarian cancer. J. Clin. Oncol. 2019, 37, 2398–2405. [Google Scholar] [CrossRef] [PubMed]
- Vergote, I.; du Bois, A.; Amant, F.; Heitz, F.; Leunen, K.; Harter, P. Neoadjuvant chemotherapy in advanced ovarian cancer: On what do we agree and disagree? Gynecol. Oncol. 2013, 128, 6–11. [Google Scholar] [CrossRef]
- Salani, R.; Axtell, A.; Gerardi, M.; Holschneider, C.; Bristow, R.E. Limited utility of conventional criteria for predicting unresectable disease in patients with advanced stage epithelial ovarian cancer. Gynecol. Oncol. 2008, 108, 271–275. [Google Scholar] [CrossRef]
- Gueli Alletti, S.; Capozzi, V.A.; Rosati, A.; De Blasis, I.; Cianci, S.; Vizzielli, G.; Uccella, S.; Gallotta, V.; Fanfani, F.; Fagotti, A.; et al. Laparoscopy vs. laparotomy for advanced ovarian cancer: A systematic review of the literature. Minerva Med. 2019, 110, 341–357. [Google Scholar] [CrossRef] [PubMed]
- Pinto, P.; Burgetova, A.; Cibula, D.; Haldorsen, I.S.; Indrielle-Kelly, T.; Fischerova, D. Prediction of Surgical Outcome in Advanced Ovarian Cancer by Imaging and Laparoscopy: A Narrative Review. Cancers 2023, 15, 1904. [Google Scholar] [CrossRef]
- Alcázar, J.L.; Vidal, J.R.; Tameish, S.; Chacón, E.; Manzour, N.; Mínguez, J.A. Ultrasound for assessing tumor spread in ovarian cancer. A systematic review of the literature and meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2024, 292, 194–200. [Google Scholar] [CrossRef]
- Green, B.N.; Johnson, C.D.; Adams, A. Writing narrative literature reviews for peer-reviewed journals: Secrets of the trade. J. Chiropr. Med. 2006, 5, 101–117. [Google Scholar] [CrossRef]
- Kang, S.K.; Reinhold, C.; Atri, M.; Benson, C.B.; Bhosale, P.R.; Jhingran, A.; Lakhman, Y.; Maturen, K.E.; Nicola, R.; Pandharipande, P.V.; et al. ACR Appropriateness Criteria®: Staging and follow-up of ovarian cancer. J. Am. Col. Radiol. 2018, 15, S198–S207. [Google Scholar] [CrossRef]
- Suidan, R.S.; Ramirez, P.T.; Sarasohn, D.M.; Teitcher, J.B.; Iyer, R.B.; Zhou, Q.; Iasonos, A.; Denesopolis, J.; Zivanovic, O.; Long Roche, K.C.; et al. A multicenter assessment of the ability of preoperative computed tomography scan and CA-125 to predict gross residual disease at primary debulking for advanced epithelial ovarian cancer. Gynecol. Oncol. 2017, 145, 27–31. [Google Scholar] [CrossRef]
- Dowdy, S.C.; Ullany, S.A.; Brandt, K.R.; Huppert, B.J.; Cliby, W.A. The utility of computed tomography scans in predicting suboptimal cytoreductive surgery in women with advanced ovarian carcinoma. Cancer 2004, 101, 346–352. [Google Scholar] [CrossRef]
- Suidan, R.S.; Ramirez, P.T.; Sarasohn, D.M.; Teitcher, J.B.; Mironov, S.; Iyer, R.B.; Zhou, Q.; Iasonos, A.; Paul, H.; Hosaka, M.; et al. A multicenter prospective trial evaluating the ability of preoperative computed tomography scan and serum CA-125 to predict suboptimal cytoreduction at primary debulking surgery for advanced ovarian, fallopian tube, and peritoneal cancer. Gynecol. Oncol. 2014, 134, 455–461. [Google Scholar] [CrossRef]
- Roze, J.F.; Hoogendam, J.P.; van de Wetering, F.T.; Spijker, R.; Verleye, L.; Vlayen, J.; Veldhuis, W.B.; Scholten, R.J.P.M.; Zweemer, R.P. Positron emission tomography (PET) and magnetic resonance imaging (MRI) for assessing tumour resectability in advanced epithelial ovarian/fallopian tube/primary peritoneal cancer. Cochrane Database Syst. Rev. 2018, 10, CD012567. [Google Scholar] [CrossRef]
- Chong, G.O.; Jeong, S.Y.; Lee, Y.H.; Lee, H.J.; Lee, S.W.; Han, H.S.; Hong, D.G.; Lee, Y.S. The ability of whole-body SUVmax in F-18 FDG PET/CT to predict suboptimal cytoreduction during primary debulking surgery for advanced ovarian cancer. J. Ovar. Res. 2019, 12, 12. [Google Scholar] [CrossRef]
- Han, S.; Woo, S.; Suh, C.H.; Lee, J.J. Performance of pre-treatment 18F-fluorodeoxyglucose positron emission tomography/computed tomography for detecting metastasis in ovarian cancer: A systematic review and meta-analysis. J. Gynecol. Oncol. 2018, 29, e98. [Google Scholar] [CrossRef] [PubMed]
- Sadowski, E.A.; Thomassin-Naggara, I.; Rockall, A.; Maturen, K.E.; Forstner, R.; Jha, P.; Nougaret, S.; Siegelman, E.S.; Reinhold, C. O-RADS MRI risk stratification system: Guide for assessing adnexal lesions from the ACR O-RADS Committee. Radiology 2022, 303, 35–47. [Google Scholar] [CrossRef]
- Rizzo, S.; De Piano, F.; Buscarino, V.; Pagan, E.; Bagnardi, V.; Zanagnolo, V.; Colombo, N.; Maggioni, A.; Del Grande, M.; Del Grande, F. Pre-operative evaluation of epithelial ovarian cancer patients: Role of whole body diffusion weighted imaging MR and CT scans in the selection of patients suitable for primary debulking surgery. A single-centre study. Eur. J. Radiol. 2020, 123, 108786. [Google Scholar] [CrossRef]
- Michielsen, K.; Vergote, I.; Op de Beeck, K.; Amant, F.; Leunen, K.; Moerman, P.; Deroose, C.; Souverijns, G.; Dymarkowski, S.; De Keyzer, F. Whole-body MRI with diffusion-weighted sequence for staging of patients with suspected ovarian cancer: A clinical feasibility study in comparison to CT and FDG-PET/CT. Eur. Radiol. 2014, 24, 889–901. [Google Scholar] [CrossRef]
- Gareeballah, A.; Gameraddin, M.; Alshoabi, S.A.; Alsaedi, A.; Elzaki, M.; Alsharif, W.; Daoud, I.M.; Aldahery, S.; Alelyani, M.; AbdElrahim, E.; et al. The diagnostic performance of International Ovarian Tumor Analysis: Simple Rules for diagnosing ovarian tumors—A systematic review and meta-analysis. Front. Oncol. 2025, 14, 1474930. [Google Scholar] [CrossRef]
- Vara, J.; Manzour, N.; Chacón, E.; López-Picazo, A.; Linares, M.; Pascual, M.Á.; Guerriero, S.; Alcázar, J.L. Ovarian Adnexal Reporting Data System (O-RADS) for Classifying Adnexal Masses: A Systematic Review and Meta-Analysis. Cancers 2022, 14, 3151. [Google Scholar] [CrossRef]
- Perez, M.; Meseguer, A.; Vara, J.; Vilches, J.C.; Brunel, I.; Lozano, M.; Orozco, R.; Alcazar, J.L. GI-RADS versus O-RADS in the differential diagnosis of adnexal masses: A systematic review and head-to-head meta-analysis. Ultrasonography 2024, 43, 438–447. [Google Scholar] [CrossRef]
- Almeida, G.; Bort, M.; Alcázar, J.L. Comparison of the Diagnostic Performance of Ovarian Adnexal Reporting Data System (O-RADS) with IOTA Simple Rules and ADNEX Model for Classifying Adnexal Masses: A Head-To-Head Meta-Analysis. J. Clin. Ultrasound, 2025; online ahead of print. [Google Scholar] [CrossRef]
- Van Calster, B.; Van Hoorde, K.; Valentin, L.; Testa, A.C.; Fischerova, D.; Van Holsbeke, C.; Savelli, L.; Franchi, D.; Epstein, E.; Kaijser, J.; et al. Evaluating the risk of ovarian cancer before surgery using the ADNEX model to differentiate between benign, borderline, early and advanced stage invasive, and secondary metastatic tumours: Prospective multicentre diagnostic study. BMJ 2014, 349, g5920. [Google Scholar] [CrossRef]
- Van Calster, B.; Van Hoorde, K.; Froyman, W.; Kaijser, J.; Wynants, L.; Landolfo, C.; Anthoulakis, C.; Vergote, I.; Bourne, T.; Timmerman, D. Practical guidance for applying the ADNEX model from the IOTA group to discriminate between different subtypes of adnexal tumors. Facts Views Vis. ObGyn 2015, 7, 32–41. [Google Scholar]
- Barreñada, L.; Ledger, A.; Dhiman, P.; Collins, G.; Wynants, L.; Verbakel, J.Y.; Timmerman, D.; Valentin, L.; Van Calster, B. ADNEX risk prediction model for diagnosis of ovarian cancer: Systematic review and meta-analysis of external validation studies. BMJ Med. 2024, 3, e000817. [Google Scholar] [CrossRef]
- Han, J.; Wen, J.; Hu, W. Comparison of O-RADS with the ADNEX model and IOTA SR for risk stratification of adnexal lesions: A systematic review and meta-analysis. Front. Oncol. 2024, 14, 1354837. [Google Scholar] [CrossRef]
- Tempany, C.M.; Zou, K.H.; Silverman, S.G.; Brown, D.L.; Kurtz, A.B.; McNeil, B.J. Staging of advanced ovarian cancer: Comparison of imaging modalities—Report from the Radiological Diagnostic Oncology Group. Radiology 2000, 215, 761–767. [Google Scholar] [CrossRef]
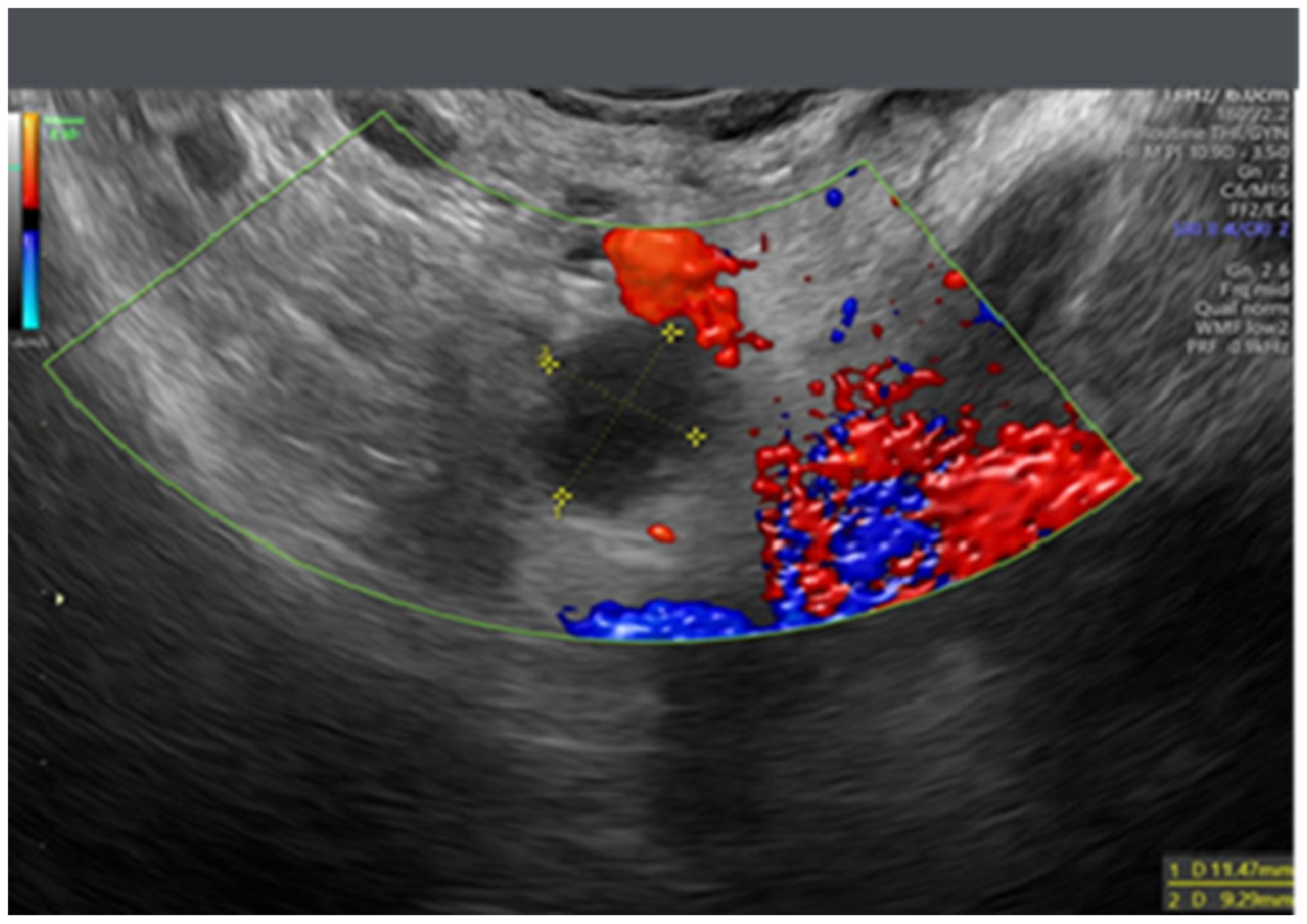
- Testa, A.C.; Ludovisi, M.; Savelli, L.; Fruscella, E.; Ghi, T.; Fagotti, A.; Scambia, G.; Ferrandina, G. Ultrasound and color power Doppler in the detection of metastatic omentum: A prospective study. Ultrasound Obstet. Gynecol. 2005, 27, 65–70. [Google Scholar] [CrossRef]
- Savelli, L.; De Iaco, P.; Ceccaroni, M.; Ghi, T.; Ceccarini, M.; Seracchioli, R.; Cacciatore, B. Transvaginal sonographic features of peritoneal carcinomatosis. Ultrasound Obstet. Gynecol. 2005, 26, 552–557. [Google Scholar] [CrossRef]
- Weinberger, V.; Fischerova, D.; Semeradova, I.; Slama, J.; Dundr, P.; Dusek, L.; Cibula, D.; Zikan, M. Prospective Evaluation of Ultrasound Accuracy in the Detection of Pelvic Carcinomatosis in Patients with Ovarian Cancer. Ultrasound Med. Biol. 2016, 42, 2196–2202. [Google Scholar] [CrossRef]
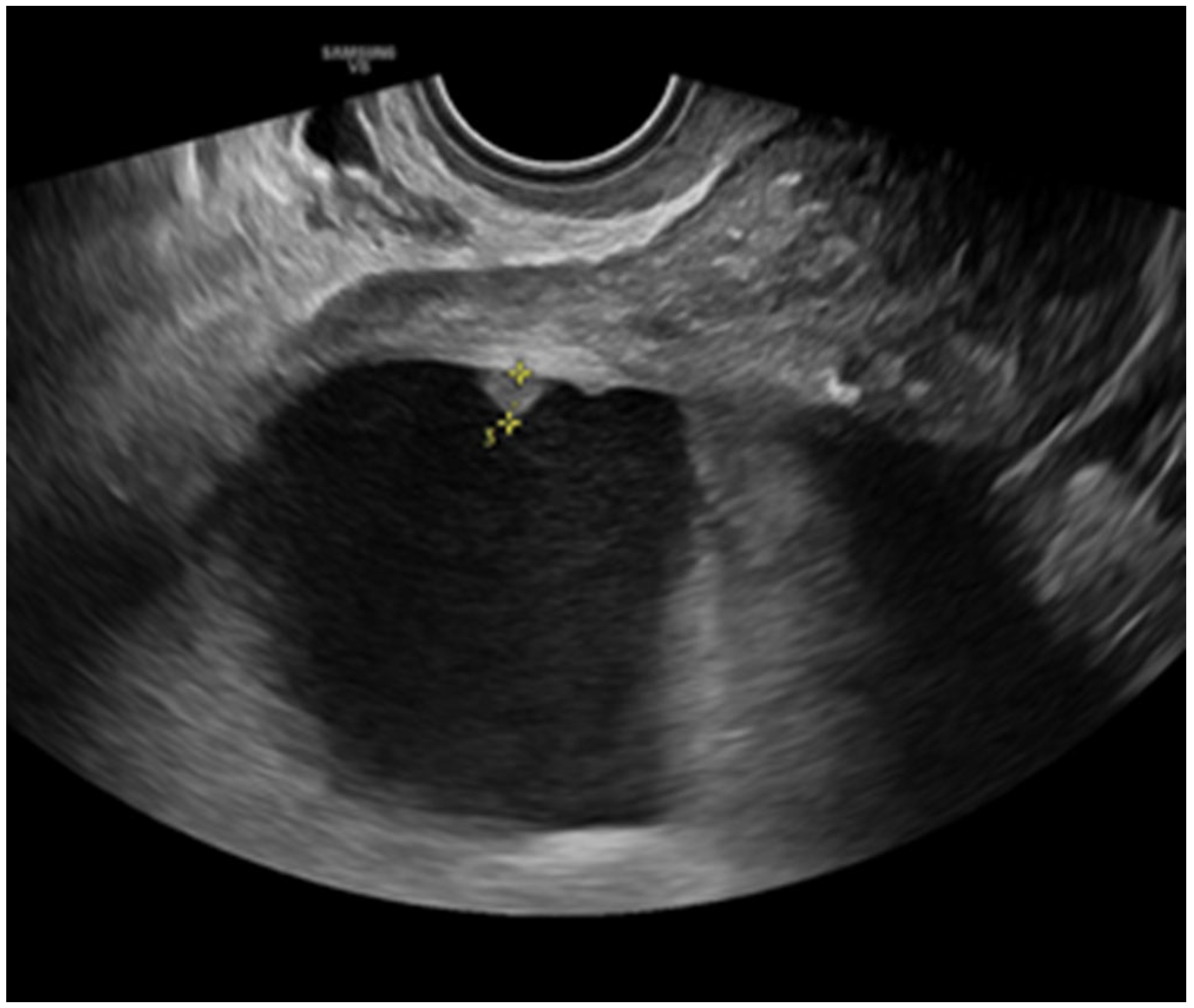
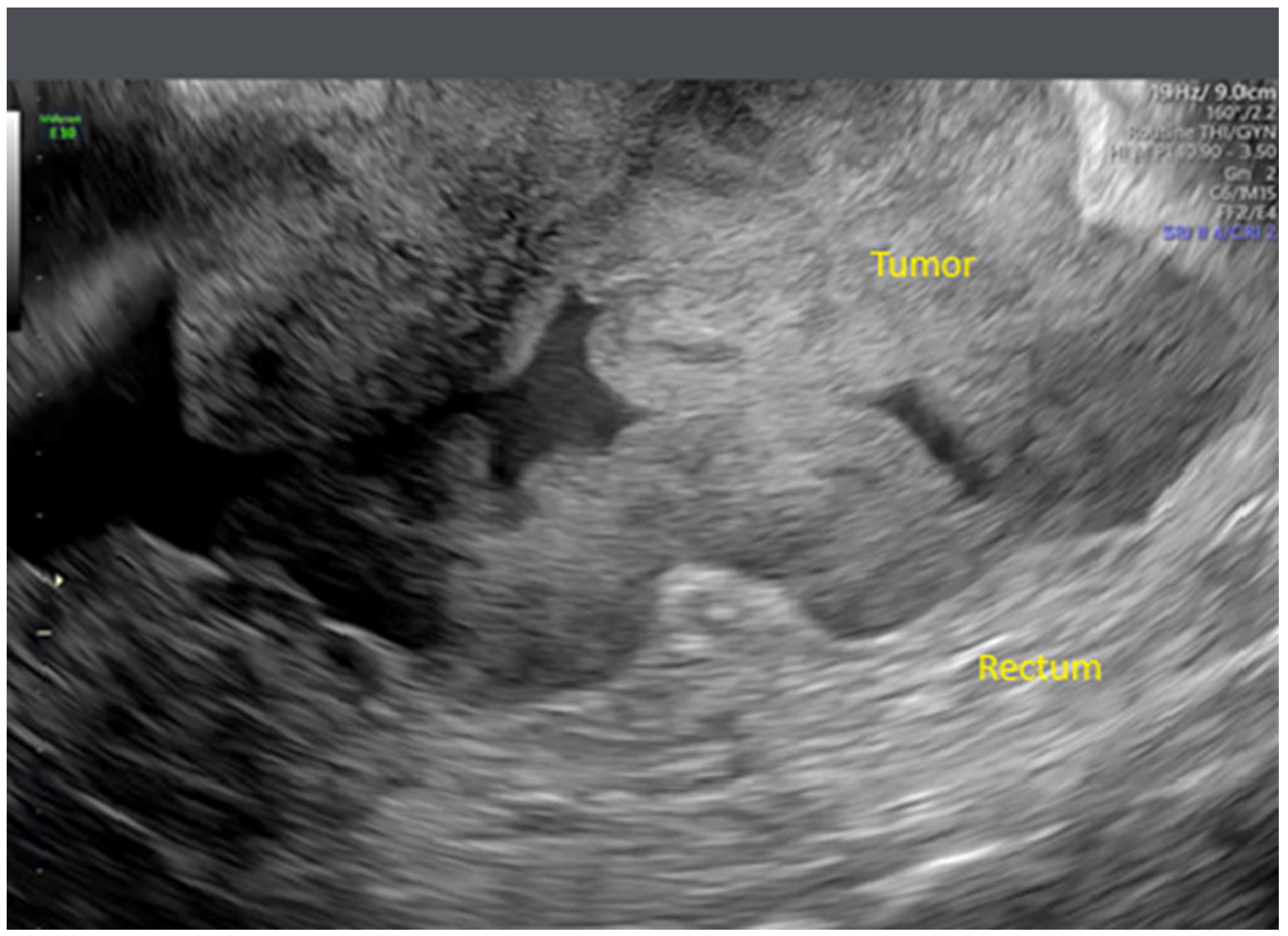
- Zikan, M.; Fischerova, D.; Semeradova, I.; Slama, J.; Dundr, P.; Weinberger, V.; Dusek, L.; Cibula, D. Accuracy of ultrasound in prediction of rectosigmoid infiltration in epithelial ovarian cancer. Ultrasound Obstet. Gynecol. 2017, 50, 533–538. [Google Scholar] [CrossRef]
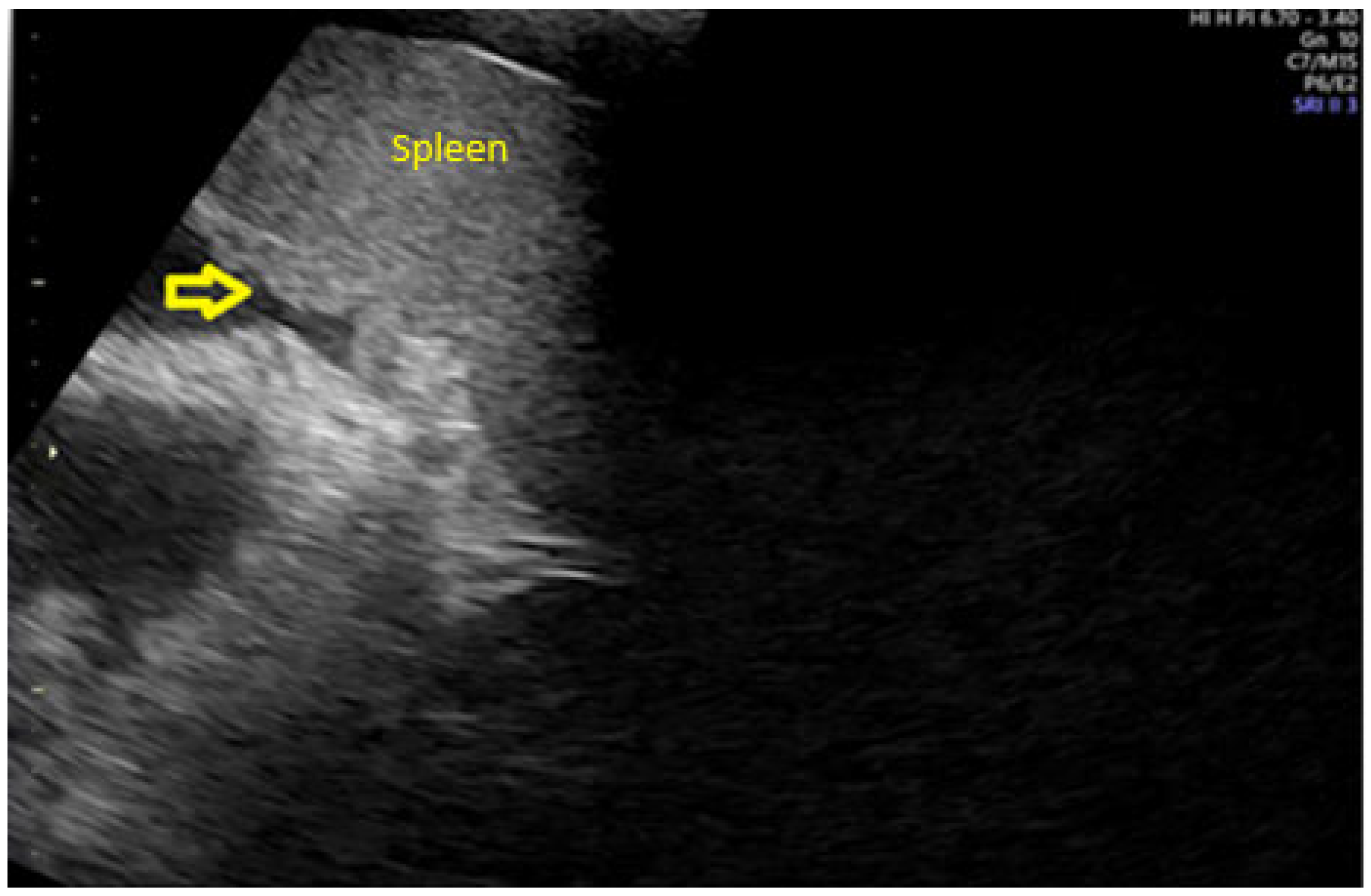
- Fischerova, D. Ultrasound scanning of the pelvis and abdomen for staging of gynecological tumors: A review. Ultrasound Obstet. Gynecol. 2011, 38, 246–266. [Google Scholar] [CrossRef]
- Testa, A.C.; Ludovisi, M.; Mascilini, F.; Di Legge, A.; Malaggese, M.; Fagotti, A.; Fanfani, F.; Salerno, M.G.; Ercoli, A.; Scambia, G.; et al. Ultrasound evaluation of intra-abdominal sites of disease to predict likelihood of suboptimal cytoreduction in advanced ovarian cancer: A prospective study. Ultrasound Obstet. Gynecol. 2012, 39, 99–105. [Google Scholar] [CrossRef]
- Fischerova, D.; Zikan, M.; Semeradova, I.; Slama, J.; Kocian, R.; Dundr, P.; Nemejcova, K.; Burgetova, A.; Dusek, L.; Cibula, D. Ultrasound in preoperative assessment of pelvic and abdominal spread in patients with ovarian cancer: A prospective study. Ultrasound Obstet. Gynecol. 2017, 49, 263–274. [Google Scholar] [CrossRef]
- Tomasinska, A.; Stukan, M.; Badocha, M.; Myszewska, A. Accuracy of Pretreatment Ultrasonography Assessment of Intra-Abdominal Spread in Epithelial Ovarian Cancer: A Prospective Study. Diagnostics 2021, 11, 1600. [Google Scholar] [CrossRef]
- Alcázar, J.L.; Caparrós, M.; Arraiza, M.; Mínguez, J.A.; Guerriero, S.; Chiva, L.; Jurado, M. Pre-operative assessment of intraabdominal disease spread in epithelial ovarian cancer: A comparative study between ultrasound and computed tomography. Int. J. Gynecol. Cancer 2019, 29, 227–233. [Google Scholar] [CrossRef]
- Timmerman, D.; Planchamp, F.; Bourne, T.; Landolfo, C.; du Bois, A.; Chiva, L.; Cibula, D.; Concin, N.; Fischerova, D.; Froyman, W.; et al. ESGO/ISUOG/IOTA/ESGE consensus statement on preoperative diagnosis of ovarian tumors. Ultrasound Obstet. Gynecol. 2021, 58, 148–168. [Google Scholar] [CrossRef]
- Fischerova, D.; Smet, C.; Scovazzi, U.; Sousa, D.N.; Hundarova, K.; Haldorsen, I.S. Staging by imaging in gynecologic cancer and the role of ultrasound: An update of European joint consensus statements. Int. J. Gynecol. Cancer 2024, 34, 363–378. [Google Scholar] [CrossRef]
- Fischerová, D.; Pinto, P.; Burgetová, A.; Masek, M.; Sláma, J.; Kocián, R.; Frühauf, F.; Zikán, M.; Dušek, L.; Dundr, P.; et al. Preoperative staging of ovarian cancer: Comparison between ultrasound, CT and whole-body diffusion-weighted MRI (ISAAC study). Ultrasound Obstet. Gynecol. 2022, 59, 248–262. [Google Scholar] [CrossRef]
- Education and Practical Standards Committee, European Federation of Societies for Ultrasound in Medicine and Biology (EFSUMB). Minimum training recommendations for the practice of medical ultrasound. Ultraschall Med. 2006, 27, 79–105. [Google Scholar]
- Jacquet, P.; Sugarbaker, P.H. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat. Res. 1996, 82, 359–374. [Google Scholar] [PubMed]
- Fagotti, A.; Ferrandina, G.; Fanfani, F.; Fagotti, A.; Ferrandina, G.; Fanfani, F.; Scambia, G. A laparoscopy-based score to predict surgical outcome in patients with advanced ovarian carcinoma: A pilot study. Ann. Surg. Oncol. 2006, 13, 1156–1161. [Google Scholar] [CrossRef]
- Petrillo, M.; Vizzielli, G.; Fanfani, F.; Gallotta, V.; Costantini, B.; Alletti, S.G.; Scambia, G. Definition of a dynamic laparoscopic model for the prediction of incomplete cytoreduction in advanced epithelial ovarian cancer: Proof of a concept. Gynecol. Oncol. 2015, 139, 5–9. [Google Scholar] [CrossRef]
- Pinto, P.; Moro, F.; Alcázar, J.L.; Alessi, S.; Avesani, G.; Benesova, K.; Burgetova, A.; Calareso, G.; Chiappa, V.; Cibula, D.; et al. Prediction of non-resectability in tubo-ovarian cancer patients using Peritoneal Cancer Index—A prospective multicentric study using imaging (ISAAC study). Gynecol. Oncol. 2024, 191, 132–142. [Google Scholar] [CrossRef]
- Moro, F.; Pinto, P.; Chiappa, V.; Testa, A.C.; Alcázar, J.L.; Franchi, D.; Benesova, K.; Jarkovsky, J.; Frühauf, F.; Borčinová, M.; et al. Prediction of nonresectability using the updated Predictive Index value model assessed by imaging and surgery in tubo-ovarian cancer: A prospective multicenter ISAAC study. Am. J. Obstet. Gynecol. 2024, 231, 632.e1–632.e14. [Google Scholar] [CrossRef] [PubMed]
- Fischerova, D.; Pinto, P.; Pesta, M.; Blasko, M.; Moruzzi, M.C.; Testa, A.C.; Franchi, D.; Chiappa, V.; Alcázar, J.L.; Wiesnerova, M.; et al. Ultrasound examiners’ ability to describe ovarian cancer spread using preacquired ultrasound videoclips from a selected patient sample with high prevalence of cancer spread. Ultrasound Obstet. Gynecol. 2025, 65, 641–652. [Google Scholar] [CrossRef]
- Pinto, P.; Valentin, L.; Borčinová, M.; Wiesnerová, M.; Filip, F.; Burgetova, A.; Masek, M.; Lambert, L.; Chiappa, V.; Franchi, D.; et al. Patient satisfaction with ultrasound, whole-body CT and whole-body diffusion-weighted MRI for pre-operative ovarian cancer staging: A multicenter prospective cross-sectional survey. Int. J. Gynecol. Cancer 2024, 34, 871–878. [Google Scholar] [CrossRef]
- Fischerova, D.; Planchamp, F.; Alcázar, J.L.; Dundr, P.; Epstein, E.; Felix, A.; Frühauf, F.; Garganese, G.; Salvesen Haldorsen, I.; Jurkovic, D.; et al. ISUOG/ESGO Consensus Statement on ultrasound-guided biopsy in gynecological oncology. Int. J. Gynecol. Cancer 2025, 35, 101732. [Google Scholar] [CrossRef]
- Eddama, O.; Coast, J. A systematic review of the use of economic evaluation in local decision-making. Health Policy 2008, 86, 129–141. [Google Scholar] [CrossRef]
- Michelly Gonçalves Brandão, S.; Brunner-La Rocca, H.P.; Pedroso de Lima, A.C.; Alcides Bocchi, E. A review of cost-effectiveness analysis: From theory to clinical practice. Medicine 2023, 102, e35614. [Google Scholar] [CrossRef] [PubMed]
| Sensitivity | Specificity | PPV | NPV | |
|---|---|---|---|---|
| Ultrasound | 71.6% | 94.2% | 90.9% | 80.4% |
| CT scan | 71.2% | 79.6% | 73.8% | 77.4% |
| MRI | 71.2% | 84.7% | 81.8% | 75.2% |
| Sensitivity | Specificity | PPV | NPV | |
|---|---|---|---|---|
| Ultrasound | 83.3% | 78.2% | 60.6% | 92.1% |
| CT scan | 79.2% | 68.1% | 50.5% | 89.0% |
| MRI | 81.3% | 79.8% | 61.9% | 91.3% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alcázar, J.L.; Morales, C.; Venturo, C.; de la Maza, F.; Lucio, L.; Lozano, M.; Vilches, J.C.; Orozco, R.; Ludovisi, M. Preoperative Assessment of Surgical Resectability in Ovarian Cancer Using Ultrasound: A Narrative Review Based on the ISAAC Trial. Onco 2025, 5, 46. https://doi.org/10.3390/onco5040046
Alcázar JL, Morales C, Venturo C, de la Maza F, Lucio L, Lozano M, Vilches JC, Orozco R, Ludovisi M. Preoperative Assessment of Surgical Resectability in Ovarian Cancer Using Ultrasound: A Narrative Review Based on the ISAAC Trial. Onco. 2025; 5(4):46. https://doi.org/10.3390/onco5040046
Chicago/Turabian StyleAlcázar, Juan Luis, Cristian Morales, Carolina Venturo, Florencia de la Maza, Laura Lucio, Manuel Lozano, José Carlos Vilches, Rodrigo Orozco, and Manuela Ludovisi. 2025. "Preoperative Assessment of Surgical Resectability in Ovarian Cancer Using Ultrasound: A Narrative Review Based on the ISAAC Trial" Onco 5, no. 4: 46. https://doi.org/10.3390/onco5040046
APA StyleAlcázar, J. L., Morales, C., Venturo, C., de la Maza, F., Lucio, L., Lozano, M., Vilches, J. C., Orozco, R., & Ludovisi, M. (2025). Preoperative Assessment of Surgical Resectability in Ovarian Cancer Using Ultrasound: A Narrative Review Based on the ISAAC Trial. Onco, 5(4), 46. https://doi.org/10.3390/onco5040046







