Integration of Radical Intent Treatment in Colorectal Liver Metastases
Simple Summary
Abstract
1. Introduction
2. Preoperative Imaging Assessment
3. Definition of Resectability
4. Patient Selection
4.1. Patient-Related Factors
4.2. Cancer-Related Factors
5. Surgery
Liver Transplant
6. Non-Surgical Locoregional Treatments
7. Systemic Treatment
7.1. Perioperative Treatment in Resectable Disease
7.2. Potentially Resectable Disease
- -
- Impossibility of R0 resection, although some studies allow R1 resection [109].
- -
- Residual liver function after surgery less than 30% (less than 20% in healthy liver in some studies [110]), or with insufficient vascular supply and/or biliary drainage.
- -
- -
- Complex hepatectomy that does not allow the preservation of at least two contiguous hepatic segments.
7.3. Unresectable Disease
8. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Jamison, R.L.; Donohue, J.H.; Nagorney, D.M.; Rosen, C.B.; Harmsen, W.S.; Ilstrup, D.M. Hepatic Resection for Metastatic Colorectal Cancer Results in Cure for Some Patients. Arch. Surg. 1997, 132, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Hughes, K.; Scheele, J.; Sugarbaker, P.H. Surgery for Colorectal Cancer Metastatic to the Liver: Optimizing the Results of Treatment. Surg. Clin. N. Am. 1989, 69, 339–359. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, E.K.; Vauthey, J.-N.; Ellis, L.M.; Ellis, V.; Pollock, R.; Broglio, K.R.; Hess, K.; Curley, S.A. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann. Surg. 2004, 239, 818–827. [Google Scholar] [CrossRef] [PubMed]
- Cervantes, A.; Adam, R.; Roselló, S.; Arnold, D.; Normanno, N.; Taïeb, J.; Seligmann, J.; De Baere, T.; Osterlund, P.; Yoshino, T.; et al. Metastatic colorectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2023, 34, 10–32. [Google Scholar] [CrossRef]
- Fong, Y.; Fortner, J.; Sun, R.L.; Brennan, M.F.; Blumgart, L.H. Clinical Score for Predicting Recurrence After Hepatic Resection for Metastatic Colorectal Cancer: Analysis of 1001 Consecutive Cases. Ann. Surg. 1999, 230, 309. [Google Scholar] [CrossRef]
- Nagashima, I.; Takada, T.; Adachi, M.; Nagawa, H.; Muto, T.; Okinaga, K. Proposal of criteria to select candidates with colorectal liver metastases for hepatic resection: Comparison of our scoring system to the positive number of risk factors. World J. Gastroenterol. 2006, 12, 6305–6309. [Google Scholar] [CrossRef]
- Nordlinger, B.; Guiguet, M.; Vaillant, J.-C.; Balladur, P.; Boudjema, K.; Bachellier, P.; Jaeck, D.; Association Française de Chirurgie. Surgical resection of colorectal carcinoma metastases to the liver: A prognostic scoring system to improve case selection, based on 1568 patients. Cancer 1996, 77, 1254–1262. [Google Scholar] [CrossRef]
- Konopke, R.; Kersting, S.; Distler, M.; Dietrich, J.; Gastmeier, J.; Heller, A.; Kulisch, E.; Saeger, H. Prognostic factors and evaluation of a clinical score for predicting survival after resection of colorectal liver metastases. Liver Int. 2009, 29, 89–102. [Google Scholar] [CrossRef]
- Margonis, G.A.; Sasaki, K.; Gholami, S.; Kim, Y.; Andreatos, N.; Rezaee, N.; Deshwar, A.; Buettner, S.; Allen, P.J.; Kingham, T.P.; et al. Genetic and Morphological Evaluation (GAME) score for patients with colorectal liver metastases. Br. J. Surg. 2018, 105, 1210–1220. [Google Scholar] [CrossRef]
- Adam, R.; Piedvache, C.; Chiche, L.; Adam, J.P.; Salamé, E.; Bucur, P.; Cherqui, D.; Scatton, O.; Granger, V.; Ducreux, M.; et al. Liver transplantation plus chemotherapy versus chemot-herapy alone in patients with permanently unresectable colorectal liver metastases (TransMet): Results from a multicentre, open-label, prospective, randomised controlled trial. Lancet 2024, 404, 1107–1118. [Google Scholar] [CrossRef]
- Bolhuis, K.; Wensink, G.E.; Elferink, M.A.G.; Bond, M.J.G.; Dijksterhuis, W.P.M.; Fijneman, R.J.A.; Kranenburg, O.W.; Rinkes, I.H.M.B.; Koopman, M.; Swijnenburg, R.-J.; et al. External Validation of Two Established Clinical Risk Scores Predicting Outcome after Local Treatment of Colorectal Liver Metastases in a Nationwide Cohort. Cancers 2022, 14, 2356. [Google Scholar] [CrossRef]
- Kamel, I.R.; Choti, M.A.; Horton, K.M.; Braga, H.J.V.; Birnbaum, B.A.; Fishman, E.K.; Thompson, R.E.; Bluemke, D.A. Surgically Staged Focal Liver Lesions: Accuracy and Reproducibility of Dual-Phase Helical CT for Detection and Characterization. Radiology 2003, 227, 752–757. [Google Scholar] [CrossRef]
- Bipat, S.; van Leeuwen, M.S.; Comans, E.F.I.; Pijl, M.E.J.; Bossuyt, P.M.M.; Zwinderman, A.H.; Stoker, J. Colorectal Liver Metastases: CT, MR Imaging, and PET for Diagnosis—Meta-analysis. Radiology 2005, 237, 123–131. [Google Scholar] [CrossRef]
- Floriani, I.; Torri, V.; Rulli, E.; Garavaglia, D.; Compagnoni, A.; Salvolini, L.; Giovagnoni, A. Performance of imaging modalities in diagnosis of liver metastases from colorectal cancer: A systematic review and meta-analysis. J. Magn. Reson. Imaging 2010, 31, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Charnsangavej, C.; Clary, B.; Fong, Y.; Grothey, A.; Pawlik, T.M.; Choti, M.A. Selection of Patients for Resection of Hepatic Colorectal Metastases: Expert Consensus Statement. Ann. Surg. Oncol. 2006, 13, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Kim, S.Y.; Park, S.H.; Kim, K.W.; Lee, J.Y.; Lee, S.S.; Lee, M. Diagnostic performance of CT, gadoxetate disodium-enhanced MRI, and PET/CT for the diagnosis of colorectal liver metastasis: Systematic review and meta-analysis. J. Magn. Reson. Imaging 2018, 47, 1237–1250. [Google Scholar] [CrossRef] [PubMed]
- Angliviel, B.; Benoist, S.; Penna, C.; El Hajjam, M.; Chagnon, S.; Julié, C.; Beauchet, A.; Rougier, P.; Nordlinger, B. Impact of Chemotherapy on the Accuracy of Computed Tomography Scan for the Evaluation of Colorectal Liver Metastases. Ann. Surg. Oncol. 2009, 16, 1247–1253. [Google Scholar] [CrossRef]
- Van Kessel, C.S.; Buckens, C.F.; van den Bosch, M.A.; van Leeuwen, M.S.; van Hillegersberg, R.; Verkooijen, H.M. Preoperative Imaging of Colorectal Liver Metastases After Neoadjuvant Chemotherapy: A Meta-Analysis. Ann. Surg. Oncol. 2012, 19, 2805–2813. [Google Scholar] [CrossRef]
- Patel, S.; McCall, M.; Ohinmaa, A.; Bigam, D.; Dryden, D.M. Positron Emission Tomography/Computed Tomographic Scans Com-pared to Computed Tomographic Scans for Detecting Colorectal Liver Metastases: A Systematic Review. Ann. Surg. 2011, 253, 666–671. [Google Scholar] [CrossRef]
- Kulemann, V.; Schima, W.; Tamandl, D.; Kaczirek, K.; Gruenberger, T.; Wrba, F.; Weber, M.; Ba-Ssalamah, A. Preoperative detection of colorectal liver metastases in fatty liver: MDCT or MRI? Eur. J. Radiol. 2011, 79, e1–e6. [Google Scholar] [CrossRef]
- Scharitzer, M.; Ba-Ssalamah, A.; Ringl, H.; Kölblinger, C.; Grünberger, T.; Weber, M.; Schima, W. Preoperative evaluation of colorectal liver metastases: Comparison between gadoxetic acid-enhanced 3.0-T MRI and contrast-enhanced MDCT with histopathological correlation. Eur. Radiol. 2013, 23, 2187–2196. [Google Scholar] [CrossRef]
- Wiering, B.; Krabbe, P.F.M.; Jager, G.J.; Oyen, W.J.G.; Ruers, T.J.M. The impact of fluor-18-deoxyglucose-positron emission tomography in the management of colorectal liver metastases: A systematic review and metaanalysis. Cancer 2005, 104, 2658–2670. [Google Scholar] [CrossRef] [PubMed]
- Glazer, E.S.; Beaty, K.; Abdalla, E.K.; Vauthey, J.N.; Curley, S.A. Effectiveness of Positron Emission Tomography for Predicting Chemotherapy Response in Colorectal Cancer Liver Metastases. Arch. Surg. 2010, 145, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Akhurst, T.; Kates, T.J.; Mazumdar, M.; Yeung, H.; Riedel, E.R.; Burt, B.M.; Blumgart, L.; Jarnagin, W.; Larson, S.M.; Fong, Y. Recent chemotherapy reduces the sensitivity of [18F]fluorodeoxyglucose positron emission tomography in the detection of colorectal metastases. J. Clin. Oncol. 2005, 23, 8713–8716. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Hindman, N.M.; Al-Refaie, W.B.; Arif-Tiwari, H.; Cash, B.D.; Chernyak, V.; Farrell, J.; Grajo, J.R.; Horowitz, J.M.; McNamara, M.M.; et al. ACR Appropriateness Criteria® Suspected Liver Metastases. J. Am. Coll. Radiol. 2017, 14, S314–S325. [Google Scholar] [CrossRef]
- Bitterer, F.; Bauer, A.; Glehr, G.; Brunner, S.; Schmidt, K.; Schlitt, H.J.; Jung, E.M.; Hackl, C. Intraoperative contrast-enhanced ultrasound has an outcome-relevant impact on surgery of primary and metastatic liver lesions. Ultraschall Med. Eur. J. Ultrasound 2024, 46, 49–56. [Google Scholar] [CrossRef]
- Ferrero, A.; Langella, S.; Russolillo, N.; Vigano’, L.; Tesoriere, R.L.; Capussotti, L. Intraoperative Detection of Disappearing Colorectal Liver Metastases as a Predictor of Residual Disease. J. Gastrointest. Surg. 2012, 16, 806–814. [Google Scholar] [CrossRef]
- Arita, J.; Ono, Y.; Takahashi, M.; Inoue, Y.; Takahashi, Y.; Matsueda, K.; Saiura, A. Routine Preoperative Liver-specific Magnetic Resonance Imaging Does Not Exclude the Necessity of Contrast-enhanced Intraoperative Ultrasound in Hepatic Resection for Colorectal Liver Metastasis. Ann. Surg. 2015, 262, 1086–1091. [Google Scholar] [CrossRef]
- Muaddi, H.; Silva, S.; Choi, W.J.; Coburn, N.; Hallet, J.; Law, C.; Cheung, H.; Karanicolas, P.J. When is a Ghost Really Gone? A Systematic Review and Meta-analysis of the Accuracy of Imaging Modalities to Predict Complete Pathological Response of Colorectal Cancer Liver Metastases After Chemotherapy. Ann. Surg. Oncol. 2021, 28, 6805–6813. [Google Scholar] [CrossRef]
- Khan, A.S.; Garcia-Aroz, S.; Ansari, M.A.; Atiq, S.M.; Senter-Zapata, M.; Fowler, K.; Doyle, M.; Chapman, W. Assessment and optimization of liver volume before major hepatic resection: Current guidelines and a narrative review. Int. J. Surg. 2018, 52, 74–81. [Google Scholar] [CrossRef]
- Fromer, M.W.; Aloia, T.A.; Gaughan, J.P.; Atabek, U.M.; Spitz, F.R. The utility of the MELD score in predicting mortality following liver resection for metastasis. Eur. J. Surg. Oncol. (EJSO) 2016, 42, 1568–1575. [Google Scholar] [CrossRef]
- Nardo, B.; Serafini, S.; Ruggiero, M.; Grande, R.; Fugetto, F.; Zullo, A.; Novello, M.; Rizzuto, A.; Bonaiuto, E.; Vaccarisi, S.; et al. Liver resection for metastases from colorectal cancer in very elderly patients: New surgical horizons. Int. J. Surg. 2016, 33, S135–S141. [Google Scholar] [CrossRef]
- Primrose, J.; Falk, S.; Finch-Jones, M.; Valle, J.; O’Reilly, D.; Siriwardena, A.; Hornbuckle, J.; Peterson, M.; Rees, M.; Iveson, T.; et al. Systemic chemotherapy with or without cetuximab in patients with resectable colorectal liver metastasis: The New EPOC randomised controlled trial. Lancet Oncol. 2014, 15, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Bridgewater, J.A.; Pugh, S.A.; Maishman, T.; Eminton, Z.; Mellor, J.; Whitehead, A.; Stanton, L.; Radford, M.; Corkhill, A.; Griffiths, G.O.; et al. Systemic chemotherapy with or without cetuximab in patients with resectable colorectal liver metastasis (New EPOC): Long-term results of a multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2020, 21, 398–411. [Google Scholar] [CrossRef] [PubMed]
- Brudvik, K.W.; Jones, R.P.; Giuliante, F.; Shindoh, J.; Passot, G.; Chung, M.H.; Song, J.; Li, L.; Dagenborg, V.J.; Fretland, Å.A.; et al. RAS Mutation Clinical Risk Score to Predict Survival After Resection of Colorectal Liver Metastases. Ann. Surg. 2019, 269, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Brudvik, K.W.; Shindoh, J. Limitations of molecular biomarkers in patients with resectable colorectal liver metastases. Chin. Clin. Oncol. 2019, 8, 48. [Google Scholar] [CrossRef]
- Martín-Cullell, B.; Virgili, A.C.; Riera, P.; Fumagalli, C.; Mirallas, O.; Pelegrín, F.J.; Sánchez-Cabús, S.; Molina, V.; Szafranska, J.; Páez, D. Histopathological, Clinical, And Molecular (HICAM) score for patients with colorectal liver metastases. Br. J. Surg. 2024, 111, znae016. [Google Scholar] [CrossRef]
- Tai, K.; Komatsu, S.; Sofue, K.; Kido, M.; Tanaka, M.; Kuramitsu, K.; Awazu, M.; Gon, H.; Tsugawa, D.; Yanagimoto, H.; et al. Total tumour volume as a prognostic factor in patients with resectable colorectal cancer liver metastases. BJS Open 2020, 4, 456–466. [Google Scholar] [CrossRef]
- Wesdorp, N.J.; Bolhuis, K.; Roor, J.; van Waesberghe, J.H.T.; van Dieren, S.; van Amerongen, M.J.; Chapelle, T.; Dejong, C.H.; Engelbrecht, M.R.; Gerhards, M.F.; et al. The Prognostic Value of Total Tumor Volume Response Compared with RECIST1.1 in Patients with Initially Unresectable Colorectal Liver Metastases Un-dergoing Systemic Treatment. Ann. Surg. Open 2021, 2, e103. [Google Scholar] [CrossRef]
- Bond, M.J.G.; Bolhuis, K.; Loosveld, O.J.L.; de Groot, J.W.B.; Droogendijk, H.; Helgason, H.H.; Hendriks, M.P.; Klaase, J.M.; Kazemier, G.; Liem, M.S.L.; et al. First-line systemic treatment strategies in patients with initially unresectable colorectal cancer liver metastases (CAIRO5): An open-label, multicentre, randomised, controlled, phase 3 study from the Dutch Colorectal Cancer Group. Lancet Oncol. 2023, 24, 757–771. [Google Scholar] [CrossRef]
- Michiel Zeeuw, J.; Wesdorp, N.J.; Ali, M.; Bakker, A.J.J.; Voigt, K.R.; Starmans, M.P.A.; Roor, J.; Kemna, R.; van Waesberghe, J.; van den Bergh, J.E.; et al. Prognostic value of total tumor volume in patients with colorectal liver metastases: A secondary analysis of the randomized CAIRO5 trial with external cohort validation. Eur. J. Cancer 2024, 207, 114185. [Google Scholar] [CrossRef]
- Yamashita, S.; Chun, Y.S.; Kopetz, S.E.; Vauthey, J.N. Biomarkers in colorectal liver metastases. Br. J. Surg. 2018, 105, 618–627. [Google Scholar] [CrossRef]
- Pulitanò, C.; Bodingbauer, M.; Aldrighetti, L.; Choti, M.A.; Castillo, F.; Schulick, R.D.; Gruenberger, T.; Pawlik, T.M. Colorectal Liver metastasis in the setting of lymph node metastasis: Defining the benefit of surgical resection. Ann. Surg. Oncol. 2012, 19, 435–442. [Google Scholar] [CrossRef]
- Adam, R.; de Haas, R.J.; Wicherts, D.A.; Aloia, T.A.; Delvart, V.; Azoulay, D.; Bismuth, H.; Castaing, D. Is hepatic resection justified after chemotherapy in patients with colorectal liver metastases and lymph node involvement? J. Clin. Oncol. 2008, 26, 3672–3680. [Google Scholar] [CrossRef] [PubMed]
- Jaeck, D.; Nakano, H.; Bachellier, P.; Inoue, K.; Weber, J.C.; Oussoultzoglou, E.; Wolf, P.; Chenard-Neu, M.P. Significance of Hepatic Pedicle Lymph Node Involvement in Patients with Colorectal Liver Metastases: A Prospective Study. Ann. Surg. Oncol. 2002, 9, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Khatri, V.P.; Petrelli, N.J.; Belghiti, J. Extending the frontiers of surgical therapy for hepatic colorectal metastases: Is there a limit? J. Clin. Oncol. 2005, 23, 8490–8499. [Google Scholar] [CrossRef] [PubMed]
- PulMiCC Trial Group; Treasure, T.; Farewell, V.; Macbeth, F.; Monson, K.; Williams, N.R.; Brew-Graves, C.; Lees, B.; Grigg, O.; Fallowfield, L. Pulmonary Metastasectomy versus Continued Active Monitoring in Colorectal Cancer (PulMiCC): A multicentre randomised clinical trial. Trials 2019, 20, 718. [Google Scholar] [CrossRef]
- Ratnayake, C.B.B.; Wells, C.I.; Atherton, P.; Hammond, J.S.; White, S.; French, J.J.; Manas, D.; Pandanaboyana, S. Meta-analysis of survival outcomes following surgical and non surgical treatments for colorectal cancer metastasis to the lung. ANZ J. Surg. 2021, 91, 255–263. [Google Scholar] [CrossRef]
- Cipriani, F.; Shelat, V.G.; Rawashdeh, M.; Francone, E.; Aldrighetti, L.; Takhar, A.; Armstrong, T.; Pearce, N.W.; Abu Hilal, M. Laparoscopic Parenchymal-Sparing Resections for Nonperipheral Liver Lesions, the Diamond Technique: Technical Aspects, Clinical Outcomes, and Oncologic Efficiency. J. Am. Coll. Surg. 2015, 221, 265–272. [Google Scholar] [CrossRef]
- Akyuz, M.; Yazici, P.; Yigitbas, H.; Dural, C.; Okoh, A.; Aliyev, S.; Aucejo, F.; Quintini, C.; Fung, J.; Berber, E. Oncologic results of laparoscopic liver resection for malignant liver tumors. J. Surg. Oncol. 2016, 113, 127–129. [Google Scholar] [CrossRef]
- Ciria, R.; Cherqui, D.; Geller, D.A.; Briceno, J.; Wakabayashi, G. Comparative Short-term Benefits of Laparoscopic Liver Resection: 9000 Cases and Climbing. Ann. Surg. 2016, 263, 761–777. [Google Scholar] [CrossRef]
- Aghayan, D.L.; Kazaryan, A.M.; Dagenborg, V.J.; Røsok, B.I.; Fagerland, M.W.; Bjørnelv, G.M.W.; Kristiansen, R.; Flatmark, K.; Fretland, Å.A.; Edwin, B. Long-Term Oncologic Outcomes After Laparoscopic Versus Open Resection for Colorectal Liver Metastases. Ann. Intern. Med. 2020, 174, 175–182. [Google Scholar] [CrossRef]
- Wong, P.; Vien, P.; Kessler, J.; Lafaro, K.; Wei, A.; Melstrom, L.G. Augmenting the Future Liver Remnant Prior to Major Hepatectomy: A Review of Options on the Menu. Ann. Surg. Oncol. 2025, 32, 5694–5709. [Google Scholar] [CrossRef] [PubMed]
- Werey, F.; Dembinski, J.; Michaud, A.; Sabbagh, C.; Mauvais, F.; Yzet, T.; Regimbeau, J.-M. Right portal vein ligation is still relevant for left hemi-liver hypertrophy: Results of a comparative study using a propensity score between right portal vein ligation and embolization. Langenbeck’s Arch. Surg. 2023, 409, 25. [Google Scholar] [CrossRef] [PubMed]
- Bargellini, I.; Bozzi, E.; Lorenzoni, G.; Boni, G.; Bianchi, F.; Traino, C.A.; Masi, G.; Cioni, R.; Crocetti, L. Role of Transhepatic Arterial Radioembolization in Metastatic Colorectal Cancer. Cardiovasc. Interv. Radiol. 2022, 45, 1579–1589. [Google Scholar] [CrossRef] [PubMed]
- Korenblik, R.; James, S.; Smits, J.; Díaz-Nieto, R.; Davis, R.; Chan, B.K.; Erdmann, J.I.; Zijlstra, I.A.; Arntz, P.J.; Kollmar, O.; et al. Safety and efficacy of combined portal and hepatic vein embolisation in patients with colorectal liver metastases (DRAGON1): A multicentre, single-arm clinical trial. Lancet Reg. Health Eur. 2025, 53, 101284. [Google Scholar] [CrossRef]
- Sandström, P.; Røsok, B.I.; Sparrelid, E.; Larsen, P.N.; Larsson, A.L.; Lindell, G.; Schultz, N.A.; Bjørnbeth, B.A.; Isaksson, B.; Rizell, M.; et al. ALPPS Improves Resectability Compared with Conventional Two-stage Hepatectomy in Patients with Advanced Colorectal Liver Metastasis. Ann. Surg. 2018, 267, 833–840. [Google Scholar] [CrossRef]
- Serenari, M.; Lanari, J.; Dueland, S.; Ettorre, G.M.; Aldrighetti, L.; Vivarelli, M.; Di Benedetto, F.; Pinelli, D.; Mazzaferro, V.; Massani, M.; et al. Liver Transplantation Versus Alpps for Colorectal Liver Metastases: An Entropy Balanced Retrospective Analysis. Ann. Surg. 2025. [Google Scholar] [CrossRef]
- Vico, T.D.; Castro, P.G.; Navarro, L.A.; Sánchez, A.S.; Góngora, L.M.; Orón, E.M.M.; Ibáñez, J.M.; Alonso, N.T.; Arrillaga, I.G.-P.; Trancón, J.E.G. Two stage hepatectomy (TSH) versus ALPPS for initially unresectable colorectal liver metastases: A systematic review and meta-analysis. Eur. J. Surg. Oncol. (EJSO) 2023, 49, 550–559. [Google Scholar] [CrossRef]
- Milazzo, M.; Todeschini, L.; Caimano, M.; Mattia, A.; Cristin, L.; Martinino, A.; Bianco, G.; Spoletini, G.; Giovinazzo, F. Surgical Resection in Colorectal Liver Metastasis: An Umbrella Review. Cancers 2024, 16, 1849. [Google Scholar] [CrossRef]
- Andres, A.; Toso, C.; Adam, R.; Barroso, E.; Hubert, C.; Capussotti, L.; Gerstel, E.; Roth, A.; Majno, P.E.; Mentha, G. A Survival Analysis of the Liver-First Reversed Management of Advanced Simultaneous Colorectal Liver Metastases: A LiverMetSurvey-Based Study. Ann. Surg. 2012, 256, 772–778. [Google Scholar] [CrossRef]
- Mentha, G.; Roth, A.D.; Terraz, S.; Giostra, E.; Gervaz, P.; Andres, A.; Morel, P.; Rubbia-Brandt, L.; Majno, P.E. ‘Liver First’ Approach in the Treatment of Colorectal Cancer with Synchronous Liver Metastases. Dig. Surg. 2009, 25, 430–435. [Google Scholar] [CrossRef]
- Tsilimigras, D.I.; Sahara, K.; Hyer, J.M.; Diaz, A.; Moris, D.; Bagante, F.; Guglielmi, A.; Ruzzenente, A.; Alexandrescu, S.; Poultsides, G.; et al. Trends and outcomes of simultaneous versus staged resection of synchronous colorectal cancer and colorectal liver metastases. Surgery 2021, 170, 160–166. [Google Scholar] [CrossRef]
- Endo, Y.; Alaimo, L.; Moazzam, Z.; Woldesenbet, S.; Lima, H.A.; Munir, M.M.; Shaikh, C.F.; Yang, J.; Azap, L.; Katayama, E.; et al. Postoperative morbidity after simultaneous versus staged resection of synchronous colorectal liver metastases: Impact of hepatic tumor burden. Surgery 2024, 175, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Andreou, A.; Gloor, S.; Inglin, J.; Martinelli, C.D.P.; Banz, V.; Lachenmayer, A.; Kim-Fuchs, C.; Candinas, D.; Beldi, G. Parenchymal-sparing hepatectomy for colorectal liver metastases reduces postoperative morbidity while maintaining equivalent oncologic outcomes compared to non-parenchymal-sparing resection. Surg. Oncol. 2021, 38, 101631. [Google Scholar] [CrossRef] [PubMed]
- Mise, Y.; Aloia, T.A.; Brudvik, K.W.; Schwarz, L.; Vauthey, J.-N.; Conrad, C. Parenchymal-sparing Hepatectomy in Colorectal Liver Metastasis Improves Salvageability and Survival. Ann. Surg. 2016, 263, 146–152. [Google Scholar] [CrossRef]
- Adam, R.; Laurent, A.; Azoulay, D.; Castaing, D.; Bismuth, H. Two-Stage Hepatectomy: A Planned Strategy to Treat Irresectable Liver Tumors. Ann. Surg. 2000, 232, 777–785. [Google Scholar] [CrossRef]
- Chavez, M.I.; Gholami, S.; Kim, B.J.; Margonis, G.A.; Ethun, C.G.; Tsai, S.; Christians, K.K.; Clarke, C.; Mogal, H.; Maithel, S.K.; et al. Two-Stage Hepatectomy for Bilateral Colorectal Liver Metastases: A Multi-institutional Analysis. Ann. Surg. Oncol. 2021, 28, 1457–1465. [Google Scholar] [CrossRef]
- Adam, R.; de Gramont, A.; Figueras, J.; Kokudo, N.; Kunstlinger, F.; Loyer, E.; Poston, G.; Rougier, P.; Rubbia-Brandt, L.; Sobrero, A.; et al. Managing synchronous liver metastases from colorectal cancer: A multidisciplinary international consensus. Cancer Treat. Rev. 2015, 41, 729–741. [Google Scholar] [CrossRef]
- Sadot, E.; Koerkamp, B.G.; Leal, J.N.; Shia, J.; Gonen, M.; Allen, P.J.; DeMatteo, R.P.; Kingham, T.P.; Kemeny, N.; Blumgart, L.H.; et al. Resection Margin and Survival in 2368 Patients Undergoing Hepatic Resection for Metastatic Colorectal Cancer: Surgical Technique or Biologic Surrogate? Ann. Surg. 2015, 262, 476–485. [Google Scholar] [CrossRef]
- Wang, J.; Margonis, G.A.; Amini, N.; Andreatos, N.; Yuan, C.; Damaskos, C.; Antoniou, E.; Garmpis, N.; Buettner, S.; Barbon, C.; et al. The Prognostic Value of Varying Definitions of Positive Resection Margin in Patients with Colorectal Cancer Liver Metastases. J. Gastrointest. Surg. 2018, 22, 1350–1357. [Google Scholar] [CrossRef] [PubMed]
- Viganò, L.; Procopio, F.; Cimino, M.M.; Donadon, M.; Gatti, A.; Costa, G.; Del Fabbro, D.; Torzilli, G. Is Tumor Detachment from Vascular Structures Equivalent to R0 Resection in Surgery for Colorectal Liver Metastases? An Observational Cohort. Ann. Surg. Oncol. 2016, 23, 1352–1360. [Google Scholar] [CrossRef] [PubMed]
- Dueland, S.; Syversveen, T.; Solheim, J.M.; Solberg, S.; Grut, H.; Bjørnbeth, B.A.; Hagness, M.; Line, P.-D. Survival Following Liver Transplantation for Patients with Nonresectable Liver-only Colorectal Metastases. Ann. Surg. 2020, 271, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Dueland, S.; Grut, H.; Syversveen, T.; Hagness, M.; Line, P.-D. Selection criteria related to long-term survival following liver transplantation for colorectal liver metastasis. Am. J. Transplant. 2020, 20, 530–537. [Google Scholar] [CrossRef]
- Bonney, G.K.; Chew, C.A.; Lodge, P.; Hubbard, J.; Halazun, K.J.; Trunecka, P.; Muiesan, P.; Mirza, D.F.; Isaac, J.; Laing, R.W.; et al. Liver transplantation for non-resectable colorectal liver metastases: The International Hepato-Pancreato-Biliary Association consensus guidelines. Lancet Gastroenterol. Hepatol. 2021, 6, 933–946. [Google Scholar] [CrossRef]
- Gorji, L.; Brown, Z.J.; Limkemann, A.; Schenk, A.D.; Pawlik, T.M. Liver Transplant as a Treatment of Primary and Secondary Liver Neoplasms. JAMA Surg. 2024, 159, 211–218. [Google Scholar] [CrossRef]
- Pathak, S.; Jones, R.; Tang, J.M.F.; Parmar, C.; Fenwick, S.; Malik, H.; Poston, G. Ablative therapies for colorectal liver metastases: A systematic review. Color. Dis. 2011, 13, e252–e265. [Google Scholar] [CrossRef]
- Ruers, T.; Van Coevorden, F.; Punt, C.J.A.; Pierie, J.-P.E.N.; Borel-Rinkes, I.; Ledermann, J.A.; Poston, G.; Bechstein, W.; Lentz, M.-A.; Mauer, M.; et al. Local Treatment of Unresectable Colorectal Liver Metastases: Results of a Randomized Phase II Trial. JNCI J. Natl. Cancer Inst. 2017, 109, djx015. [Google Scholar] [CrossRef]
- Meijerink, M.R.; van der Lei, S.; Dijkstra, M.; Versteeg, K.S.; Buffart, T.E.; Lissenberg-Witte, B.I.; Swijnenburg, R.-J.; Tol, M.P.v.D.; Puijk, R.S.; COLLISION Trial Collaborator Group. Surgery versus thermal ablation for small-size colorectal liver metastases (COLLISION): An international, multicenter, phase III randomized controlled trial. J. Clin. Oncol. 2024, 42, LBA3501. [Google Scholar] [CrossRef]
- Palma, D.A.; Olson, R.; Harrow, S.; Gaede, S.; Louie, A.V.; Haasbeek, C.; Mulroy, L.; Lock, M.; Rodrigues, G.B.; Yaremko, B.P.; et al. Stereotactic Ablative Radiotherapy for the Comprehensive Treatment of Oligometastatic Cancers: Long-Term Results of the SABR-COMET Phase II Randomized Trial. J. Clin. Oncol. 2020, 38, 2830–2838. [Google Scholar] [CrossRef]
- Lee, M.T.; Kim, J.J.; Dinniwell, R.; Brierley, J.; Lockwood, G.; Wong, R.; Cummings, B.; Ringash, J.; Tse, R.V.; Knox, J.J.; et al. Phase I Study of Individualized Stereotactic Body Radiotherapy of Liver Metastases. J. Clin. Oncol. 2009, 27, 1585–1591. [Google Scholar] [CrossRef]
- Rusthoven, K.E.; Kavanagh, B.D.; Cardenes, H.; Stieber, V.W.; Burri, S.H.; Feigenberg, S.J.; Chidel, M.A.; Pugh, T.J.; Franklin, W.; Kane, M.; et al. Multi-Institutional Phase I/II Trial of Stereotactic Body Radiation Therapy for Liver Metastases. J. Clin. Oncol. 2009, 27, 1572–1578. [Google Scholar] [CrossRef]
- Hoyer, M.; Roed, H.; Traberg Hansen, A.; Ohlhuis, L.; Petersen, J.; Nellemann, H.; Kill Berthelsen, A.; Grau, C.; Aage Engelholm, S.; Von Der Maase, H. Phase II study on stereotactic body radiotherapy of colorectal metastases. Acta Oncol. 2006, 45, 823–830. [Google Scholar] [CrossRef]
- Chang, D.T.; Swaminath, A.; Kozak, M.; Weintraub, J.; Koong, A.C.; Kim, J.; Dinniwell, R.; Brierley, J.; Kavanagh, B.D.; Dawson, L.A.; et al. Stereotactic body radiotherapy for colorectal liver metastases. Cancer 2011, 117, 4060–4069. [Google Scholar] [CrossRef] [PubMed]
- McPartlin, A.; Swaminath, A.; Wang, R.; Pintilie, M.; Brierley, J.; Kim, J.; Ringash, J.; Wong, R.; Dinniwell, R.; Craig, T.; et al. Long-Term Outcomes of Phase 1 and 2 Studies of SBRT for Hepatic Colorectal Metastases. Int. J. Radiat. Oncol. 2017, 99, 388–395. [Google Scholar] [CrossRef]
- O’Cathail, S.M.; Smith, T.; Owens, R.; Zeniou, A.; Tsang, Y.; Holyoake, D.L.; Murray, L.; Harrison, M.; Hawkins, M.A. Superior outcomes of nodal metastases compared to visceral sites in oligometastatic colorectal cancer treated with stereotactic ablative radiotherapy. Radiother. Oncol. 2020, 151, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Hong, T.S.; Wo, J.Y.; Borger, D.R.; Yeap, B.Y.; McDonnell, E.I.; Willers, H.; Blaszkowsky, L.S.; Kwak, E.L.; Allen, J.N.; Clark, J.W.; et al. Phase II Study of Proton-Based Stereotactic Body Radiation Therapy for Liver Metastases: Importance of Tumor Genotype. JNCI J. Natl. Cancer Inst. 2017, 109, djx031. [Google Scholar] [CrossRef] [PubMed]
- Mulcahy, M.F.; Mahvash, A.; Pracht, M.; Montazeri, A.H.; Bandula, S.; Martin, R.C.G.; Herrmann, K.; Brown, E.; Zuckerman, D.; Wilson, G.; et al. Radioembolization With Chemotherapy for Colorectal Liver Metastases: A Randomized, Open-Label, International, Multicenter, Phase III Trial. J. Clin. Oncol. 2021, 39, 3897–3907. [Google Scholar] [CrossRef]
- Van Hazel, G.A.; Heinemann, V.; Sharma, N.K.; Findlay, M.P.; Ricke, J.; Peeters, M.; Perez, D.; Robinson, B.A.; Strickland, A.H.; Ferguson, T.; et al. SIRFLOX: Randomized Phase III Trial Comparing First-Line mFOLFOX6 (Plus or Minus Bevacizumab) Versus mFOLFOX6 (Plus or Minus Bevacizumab) Plus Selective Internal Radiation Therapy in Patients with Metastatic Colorectal Cancer. J. Clin. Oncol. 2016, 34, 1723–1731. [Google Scholar] [CrossRef]
- Wasan, H.S.; Gibbs, P.; Sharma, N.K.; Taieb, J.; Heinemann, V.; Ricke, J.; Peeters, M.; Findlay, M.; Weaver, A.; Mills, J.; et al. First-line selective internal radiotherapy plus chemotherapy versus chemotherapy alone in patients with liver metastases from colorectal cancer (FOXFIRE, SIRFLOX, and FOXFIRE-Global): A combined analysis of three multicentre, randomised, phase 3 trials. Lancet Oncol. 2017, 18, 1159–1171. [Google Scholar] [CrossRef]
- House, M.G.; Kemeny, N.E.; Gönen, M.; Fong, Y.; Allen, P.J.; Paty, P.B.; DeMatteo, R.P.; Blumgart, L.H.; Jarnagin, W.R.; D’ANgelica, M.I. Comparison of Adjuvant Systemic Chemotherapy with or Without Hepatic Arterial Infusional Chemotherapy After Hepatic Resection for Metastatic Colorectal Cancer. Ann. Surg. 2011, 254, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Kemeny, N.E.; Niedzwiecki, D.; Hollis, D.R.; Lenz, H.-J.; Warren, R.S.; Naughton, M.J.; Weeks, J.C.; Sigurdson, E.R.; Herndon, J.E.; Zhang, C.; et al. Hepatic arterial infusion versus systemic therapy for hepatic metastases from colorectal cancer: A randomized trial of efficacy, quality of life, and molecular markers (CALGB 9481). J. Clin. Oncol. 2006, 24, 1395–1403. [Google Scholar] [CrossRef] [PubMed]
- Groot Koerkamp, B.; Sadot, E.; Kemeny, N.E.; Gönen, M.; Leal, J.N.; Allen, P.J.; Cercek, A.; DeMatteo, R.P.; Kingham, T.P.; Jarnagin, W.R.; et al. Perioperative Hepatic Arterial Infusion Pump Chemotherapy Is Associated with Longer Survival After Resection of Colorectal Liver Me-tastases: A Propensity Score Analysis. J. Clin. Oncol. 2017, 35, 1938–1944. [Google Scholar] [CrossRef] [PubMed]
- Creasy, J.M.; Sadot, E.; Koerkamp, B.G.; Chou, J.F.; Gonen, M.; Kemeny, N.E.; Balachandran, V.P.; Kingham, T.P.; DeMatteo, R.P.; Allen, P.J.; et al. Actual 10-year survival after hepatic resection of colorectal liver metastases: What factors preclude cure? Surgery 2018, 163, 1238–1244. [Google Scholar] [CrossRef]
- Pak, L.M.; Kemeny, N.E.; Capanu, M.; Chou, J.F.; Boucher, T.; Cercek, A.; Balachandran, V.P.; Kingham, T.P.; Allen, P.J.; DeMatteo, R.P.; et al. Prospective phase II trial of combination hepatic artery infusion and systemic chemotherapy for unresectable colorectal liver metastases: Long term results and curative potential. J. Surg. Oncol. 2018, 117, 634–643. [Google Scholar] [CrossRef]
- Fiorentini, G.; Aliberti, C.; Tilli, M.; Mulazzani, L.; Graziano, F.; Giordani, P.; Mambrini, A.; Montagnani, F.; Alessandroni, P.; Catalano, V.; et al. Intra-arterial Infusion of Irinotecan-loaded Drug-eluting Beads (DEBIRI) versus Intravenous Therapy (FOLFIRI) for Hepatic Metastases from Colorectal Cancer: Final Results of a Phase III Study. Anticancer Res. 2012, 32, 1387. [Google Scholar]
- Martin, R.C.G.; Scoggins, C.R.; Schreeder, M.; Rilling, W.S.; Laing, C.J.; Tatum, C.M.; Kelly, L.R.; Garcia-Monaco, R.D.; Sharma, V.R.; Crocenzi, T.S.; et al. Randomized controlled trial of irinotecan drug-eluting beads with simultaneous FOLFOX and bevacizumab for patients with unresectable colorectal liver-limited metastasis. Cancer 2015, 121, 3649–3658. [Google Scholar] [CrossRef]
- Akinwande, O.; Philips, P.; Scoggins, C.R.; Kelly, L.; Tatum, C.; Hahl, M.; McMasters, K.M.; Martin, R.C. Comparison of tumor response assessment methods in patients with metastatic colorectal cancer after locoregional therapy. J. Surg. Oncol. 2016, 113, 443–448. [Google Scholar] [CrossRef]
- Shady, W.; Sotirchos, V.S.; Do, R.K.; Pandit-Taskar, N.; Carrasquillo, J.A.; Gonen, M.; Sofocleous, C.T. Surrogate Imaging Biomarkers of Response of Colorectal Liver Metastases After Salvage Radioembolization Using 90Y-Loaded Resin Microspheres. Am. J. Roentgenol. 2016, 207, 661–670. [Google Scholar] [CrossRef]
- Nigri, G.; Petrucciani, N.; Ferla, F.; La Torre, M.; Aurello, P.; Ramacciato, G. Neoadjuvant chemotherapy for resectable colorectal liver metastases: What is the evidence? Results of a systematic review of comparative studies. Surgeon 2015, 13, 83–90. [Google Scholar] [CrossRef]
- Hasselgren, K.; Malagò, M.; Vyas, S.; Campos, R.R.; Brusadin, R.; Linecker, M.; Petrowsky, H.; Clavien, P.A.; Machado, M.A.; Hernandez-Alejandro, R.; et al. Neoadjuvant chemotherapy does not affect future liver remnant growth and outcomes of associating liver partition and portal vein ligation for staged hepatectomy. Surgery 2017, 161, 1255–1265. [Google Scholar] [CrossRef] [PubMed]
- Sorbye, H.; Mauer, M.; Gruenberger, T.; Glimelius, B.; Poston, G.J.; Schlag, P.M.; Rougier, P.; Primrose, J.N.; Walpole, E.T.; Nordlinger, B. Predictive factors for the effect of perioperative FOLFOX for resectable liver metastasis in colorectal cancer patients (EORTC phase III study 40983). J. Clin. Oncol. 2010, 28, 3544. [Google Scholar] [CrossRef]
- Loupakis, F.; Schirripa, M.; Caparello, C.; Funel, N.; Pollina, L.; Vasile, E.; Cremolini, C.; Salvatore, L.; Morvillo, M.; Antoniotti, C.; et al. Histopathologic evaluation of liver metastases from colorectal cancer in patients treated with FOLFOXIRI plus bevacizumab. Br. J. Cancer 2013, 108, 2549–2556. [Google Scholar] [CrossRef] [PubMed]
- Mitry, E.; Fields, A.L.; Bleiberg, H.; Labianca, R.; Portier, G.; Tu, D.; Nitti, D.; Torri, V.; Elias, D.; O’CAllaghan, C.; et al. Adjuvant chemotherapy after potentially curative resection of metastases from colorectal cancer: A pooled analysis of two randomized trials. J. Clin. Oncol. 2008, 26, 4906–4911. [Google Scholar] [CrossRef]
- Portier, G.; Elias, D.; Bouche, O.; Rougier, P.; Bosset, J.-F.; Saric, J.; Belghiti, J.; Piedbois, P.; Guimbaud, R.; Nordlinger, B.; et al. Multicenter Randomized Trial of Adjuvant Fluorouracil and Folinic Acid Compared with Surgery Alone After Resection of Colorectal Liver Metastases: FFCD ACHBTH AURC 9002 Trial. J. Clin. Oncol. 2006, 24, 4976–4982. [Google Scholar] [CrossRef]
- Kanemitsu, Y.; Shimizu, Y.; Mizusawa, J.; Inaba, Y.; Hamaguchi, T.; Shida, D.; Ohue, M.; Komori, K.; Shiomi, A.; Shiozawa, M.; et al. Hepatectomy Followed by mFOLFOX6 Versus Hepatectomy Alone for Liver-Only Metastatic Colorectal Cancer (JCOG0603): A Phase II or III Randomized Controlled Trial. J. Clin. Oncol. 2021, 39, 3789–3799. [Google Scholar] [CrossRef]
- André, T.; Shiu, K.-K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; et al. Pembrolizumab in Microsatellite-Instability–High Advanced Colorectal Cancer. N. Engl. J. Med. 2020, 383, 2207–2218. [Google Scholar] [CrossRef]
- André, T.; Elez, E.; Van Cutsem, E.; Jensen, L.H.; Bennouna, J.; Mendez, G.; Schenker, M.; de la Fouchardiere, C.; Limon, M.L.; Yoshino, T.; et al. Nivolumab plus Ipilimumab in Microsatellite-Instability–High Metastatic Colorectal Cancer. N. Engl. J. Med. 2024, 391, 2014–2026. [Google Scholar] [CrossRef]
- Folprecht, G.; Grothey, A.; Alberts, S.; Raab, H.-R.; Köhne, C.-H. Neoadjuvant treatment of unresectable colorectal liver metastases: Correlation between tumour response and resection rates. Ann. Oncol. 2005, 16, 1311–1319. [Google Scholar] [CrossRef]
- Gruenberger, T.; Bridgewater, J.; Chau, I.; Alfonso, P.G.; Rivoire, M.; Mudan, S.; Lasserre, S.; Hermann, F.; Waterkamp, D.; Adam, R. Bevacizumab plus mFOLFOX-6 or FOLFOXIRI in patients with initially unresectable liver metastases from colorectal cancer: The OLIVIA multinational randomised phase II trial. Ann. Oncol. 2015, 26, 702–708. [Google Scholar] [CrossRef]
- Ye, L.C.; Liu, T.S.; Ren, L.; Wei, Y.; Zhu, D.X.; Zai, S.Y.; Ye, Q.H.; Yu, Y.; Xu, B.; Qin, X.Y.; et al. Randomized controlled trial of cetuximab plus chemotherapy for patients with KRAS wild-type unresectable colorectal liver-limited metastases. J. Clin. Oncol. 2013, 31, 1931–1938. [Google Scholar] [CrossRef]
- Folprecht, G.; Gruenberger, T.; Bechstein, W.O.; Raab, H.-R.; Lordick, F.; Hartmann, J.T.; Lang, H.; Frilling, A.; Stoehlmacher, J.; Weitz, J.; et al. Tumour response and secondary resectability of colorectal liver metastases following neoadjuvant chemotherapy with cetuximab: The CELIM randomised phase 2 trial. Lancet Oncol. 2010, 11, 38–47. [Google Scholar] [CrossRef]
- Gholami, S.; Grothey, A.; Lenz, H.-J. Microsatellite Stable Colorectal Liver Metastases—Understanding the Mechanisms of Immune Resistance. JAMA Netw. Open 2021, 4, e2119025. [Google Scholar] [CrossRef]
- Oki, E.; Emi, Y.; Yamanaka, T.; Uetake, H.; Muro, K.; Takahashi, T.; Nagasaka, T.; Hatano, E.; Ojima, H.; Manaka, D.; et al. Randomised phase II trial of mFOLFOX6 plus bevacizumab versus mFOLFOX6 plus cetuximab as first-line treatment for colorectal liver metastasis (ATOM trial). Br. J. Cancer 2019, 121, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Ren, L.; Liu, T.; Ye, Q.; Wei, Y.; He, G.; Lin, Q.; Wang, X.; Wang, M.; Liang, F.; et al. Bevacizumab Plus mFOLFOX6 Versus mFOLFOX6 Alone as First-Line Treatment for RAS Mutant Unresectable Colorectal Liver-Limited Metastases: The BECOME Randomized Controlled Trial. J Clin. Oncol. 2020, 38, 3175–3184. [Google Scholar] [CrossRef] [PubMed]
- Chrabaszcz, S.; Rajeev, R.M.; Witmer, H.D.M.; Dhiman, A.M.; Klooster, B.; Gamblin, T.C.M.; Banerjee, A.; Johnston, F.M.M.; Turaga, K.K. A Systematic Review of Conversion to Resectability in Unresectable Metastatic Colorectal Cancer Chemotherapy Trials. Am. J. Clin. Oncol. 2022, 45, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, J.; Muro, K.; Shitara, K.; Yamazaki, K.; Shiozawa, M.; Ohori, H.; Takashima, A.; Yokota, M.; Makiyama, A.; Akazawa, N.; et al. Panitumumab vs Bevacizumab Added to Standard First-line Chemotherapy and Overall Survival Among Patients with RAS Wild-type, Left-Sided Metastatic Colorectal Cancer. JAMA 2023, 329, 1271–1282. [Google Scholar] [CrossRef]
- Ychou, M.; Rivoire, M.; Thezenas, S.; Quenet, F.; Delpero, J.-R.; Rebischung, C.; Letoublon, C.; Guimbaud, R.; Francois, E.; Ducreux, M.; et al. A randomized phase ii trial of three intensified chemotherapy regimens in first-line treatment of colorectal cancer patients with initially unresectable or not optimally resectable liver metastases. The METHEP trial. Ann. Surg. Oncol. 2013, 20, 4289–4297. [Google Scholar] [CrossRef]
- Ychou, M.; Rivoire, M.; Thezenas, S.; Guimbaud, R.; Ghiringhelli, F.; Mercier-Blas, A.; Mineur, L.; Francois, E.; Khemissa, F.; Moussata, D.; et al. FOLFIRINOX combined to targeted therapy according RAS status for colorectal cancer patients with liver metastases initially non-resectable: A phase II randomized Study—Prodige 14—ACCORD 21 (METHEP-2), a unicancer GI trial. J. Clin. Oncol. 2016, 34, 3512. [Google Scholar] [CrossRef]
- Gagnière, J.; Dupré, A.; Gholami, S.S.; Pezet, D.; Boerner, T.; Gönen, M.; Kingham, T.P.; Allen, P.J.; Balachandran, V.P.; De Matteo, R.P.; et al. Is Hepatectomy Justified for BRAF Mutant Colorectal Liver Metastases?: A Multi-institutional Analysis of 1497 Patients. Ann. Surg. 2020, 271, 147–154. [Google Scholar] [CrossRef]
- Margonis, G.A.; Buettner, S.; Andreatos, N.; Kim, Y.; Wagner, D.; Sasaki, K.; Beer, A.; Schwarz, C.; Løes, I.M.; Smolle, M.; et al. Association of BRAF Mutations with Survival and Recurrence in Surgically Treated Patients with Metastatic Colorectal Liver Cancer. JAMA Surg. 2018, 153, e180996. [Google Scholar] [CrossRef] [PubMed]
- Booth, C.M.; Berry, S.R. Perioperative Chemotherapy for Resectable Liver Metastases in Colorectal Cancer: Do We Have a Blind Spot? J. Clin. Oncol. 2021, 39, 3767–3769. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Green, M.D.; Li, S.; Sun, Y.; Journey, S.N.; Choi, J.E.; Rizvi, S.M.; Qin, A.; Waninger, J.J.; Lang, X.; et al. Liver metastasis restrains immunotherapy efficacy via macrophage-mediated T cell elimination. Nat. Med. 2021, 27, 152–164. [Google Scholar] [CrossRef]
- Shiri, A.M.; Zhang, T.; Bedke, T.; Zazara, D.E.; Zhao, L.; Lücke, J.; Sabihi, M.; Fazio, A.; Zhang, S.; Tauriello, D.V.; et al. IL-10 dampens antitumor immunity and promotes liver metastasis via PD-L1 induction. J. Hepatol. 2024, 80, 634–644. [Google Scholar] [CrossRef]
- Beiter, E.R.; Patel, S.R.; Chen, C.T. Immunotherapy Efficacy in Mismatch Repair–Proficient Colorectal Cancer Patients with and Without Liver Metastases. J. Clin. Oncol. 2025, JCO2501044. [Google Scholar] [CrossRef]
- Kopetz, S.; Yoshino, T.; Kim, T.W.; Wasan, H.S.; Van Cutsem, E.; Ciardiello, F.; Maughan, T.S.; Eng, C.; Yaeger, R.; Desai, J.; et al. BREAKWATER: An open-label, multicenter, randomized, phase 3 study, with a safety lead-in (SLI), of first-line (1L) encorafenib (E) + cetuximab (C) ± chemotherapy (CT) vs standard-of-care (SOC) CT for BRAF V600E-mutant metastatic colorectal cancer (mCRC). J. Clin. Oncol. 2023, 41, TPS3627. [Google Scholar] [CrossRef]
- Vera, R.; González-Flores, E.; Rubio, C.; Urbano, J.; Camps, M.V.; Ciampi-Dopazo, J.J.; Rincón, J.O.; Macías, V.M.; Braco, M.A.G.; Suarez-Artacho, G. Multidisciplinary management of liver metastases in patients with colorectal cancer: A consensus of SEOM, AEC, SEOR, SERVEI, and SEMNIM. Clin. Transl. Oncol. 2020, 22, 647–662. [Google Scholar] [CrossRef]
- Vogel, J.D.; Felder, S.I.; Bhama, A.R.; Hawkins, A.T.; Langenfeld, S.J.; Shaffer, V.O.; Thorsen, A.J.; Weiser, M.R.; Chang, G.J.; Lightner, A.L.; et al. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the Management of Colon Cancer. Dis. Colon Rectum 2022, 65, 148–177. [Google Scholar] [CrossRef]
- Zuo, D.; Wang, L.; Jin, K.; Zhang, Y.; Wang, Y.; Sun, Y.; Tang, J. Genomic and Clinical Predictors of Conversion in Initially Unresectable Colorectal Cancer Liver Metastases. Ann. Surg. Oncol. 2025, 32, 7173–7182. [Google Scholar] [CrossRef]
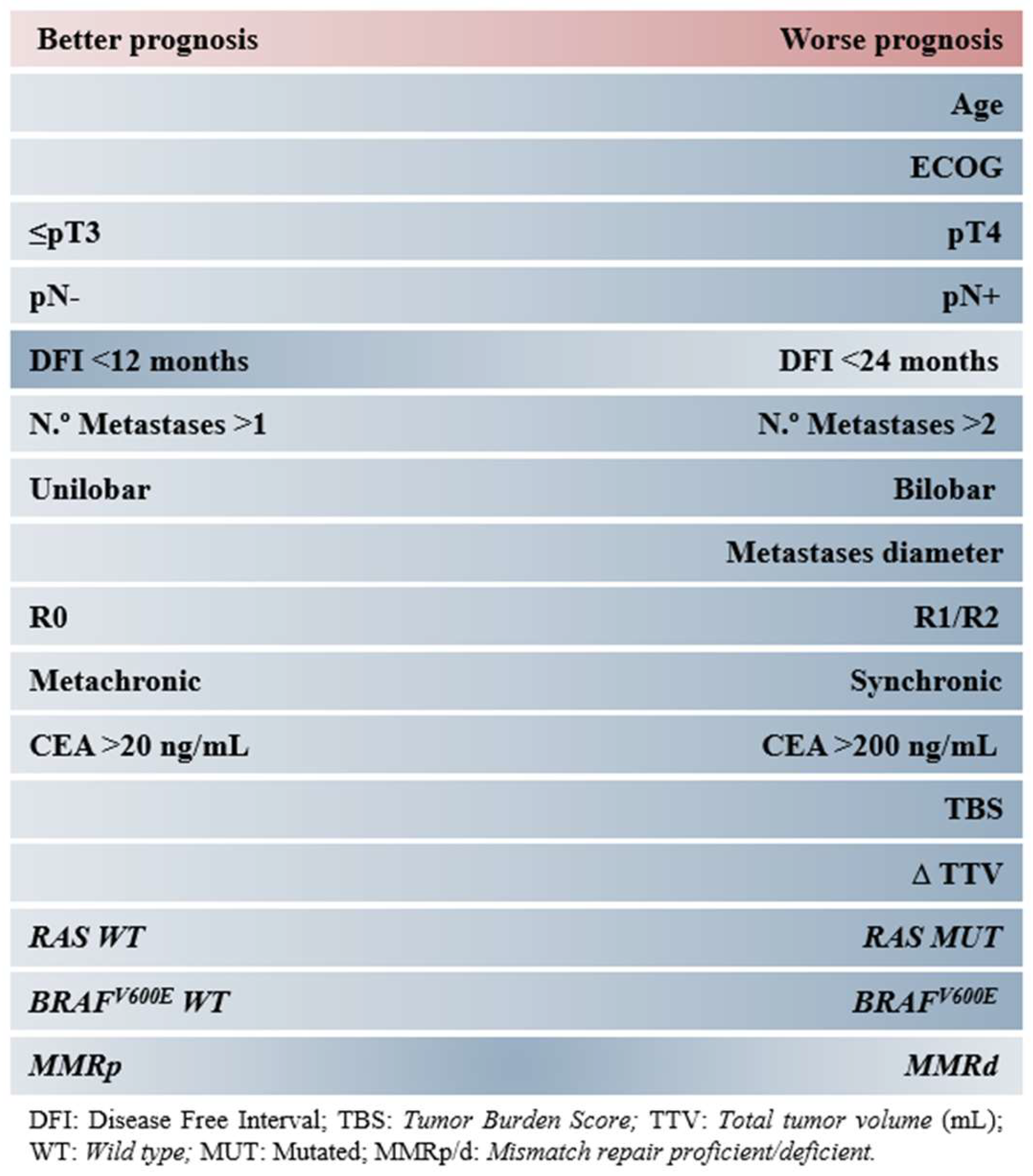
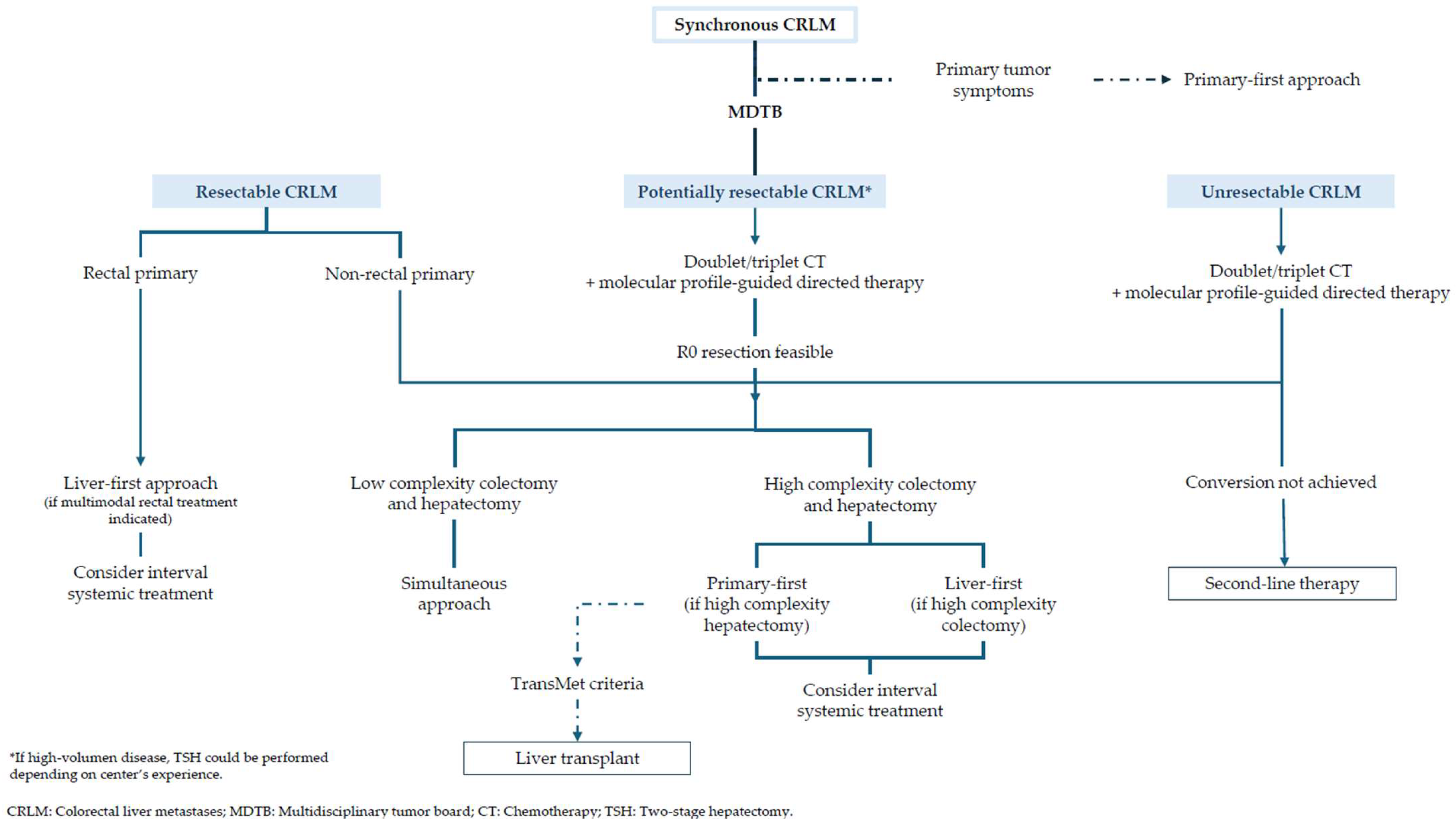
| Score | Criteria | Risk |
|---|---|---|
| Nordlinger [7] | Age > 60 years Serous invasion of primary tumor (>pT3) Primary locoregional lymph node involvement Disease-free interval < 24 months Number of liver metastases > 3 Maximum diameter of liver metastases > 5 cm | Low: 0–2 Intermediate: 3–4 High: 5–6 |
| Fong [5] | Primary locoregional lymph node involvement Disease-free interval < 12 months Number of liver metastases > 1 Maximum diameter of liver metastases > 5 cm Preoperative CEA > 200 ng/mL | Low: 0–2 High: 3–5 |
| Nagashima [6] | Serous invasion of primary tumor (>pT3) Primary locoregional lymph node involvement Number of liver metastases ≥ 2 Maximum diameter of liver metastases > 5 cm Resectable extrahepatic metastases | Low: 0–1 Intermediate: 2–3 High: ≥4 |
| Konopke [8] | Number of liver metastases ≥ 4 Preoperative CEA ≥ 200 ng/mL Synchronous liver metastases |
| Criteria | Score | Risk |
|---|---|---|
| Primary locoregional lymph node involvement | 1 | Low: 0–1 Intermediate: 2–3 High: ≥4 |
| Preoperative CEA ≥ 20 ng/mL | 1 | |
| Extrahepatic liver disease | 2 | |
| KRAS mutation ¥ | 1 | |
| TBS2 * 3–8 | 1 | |
| TBS2 ≥ 9 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pelegrín-Mateo, F.J.; Gallego Plazas, J. Integration of Radical Intent Treatment in Colorectal Liver Metastases. Onco 2025, 5, 45. https://doi.org/10.3390/onco5040045
Pelegrín-Mateo FJ, Gallego Plazas J. Integration of Radical Intent Treatment in Colorectal Liver Metastases. Onco. 2025; 5(4):45. https://doi.org/10.3390/onco5040045
Chicago/Turabian StylePelegrín-Mateo, Francisco J., and Javier Gallego Plazas. 2025. "Integration of Radical Intent Treatment in Colorectal Liver Metastases" Onco 5, no. 4: 45. https://doi.org/10.3390/onco5040045
APA StylePelegrín-Mateo, F. J., & Gallego Plazas, J. (2025). Integration of Radical Intent Treatment in Colorectal Liver Metastases. Onco, 5(4), 45. https://doi.org/10.3390/onco5040045






