Precision Medicine for Older AML Patients
Simple Summary
Abstract
1. Introduction
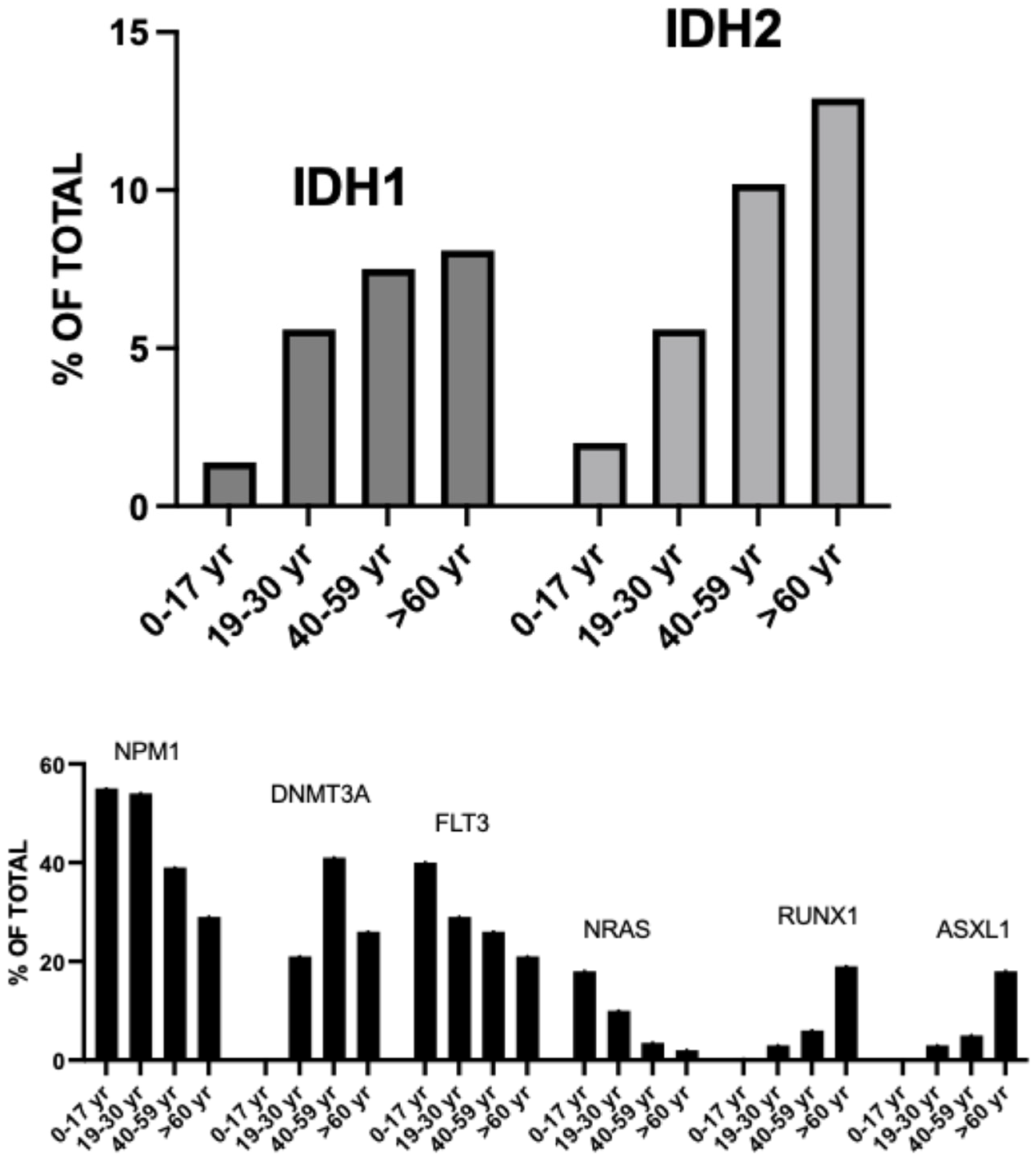
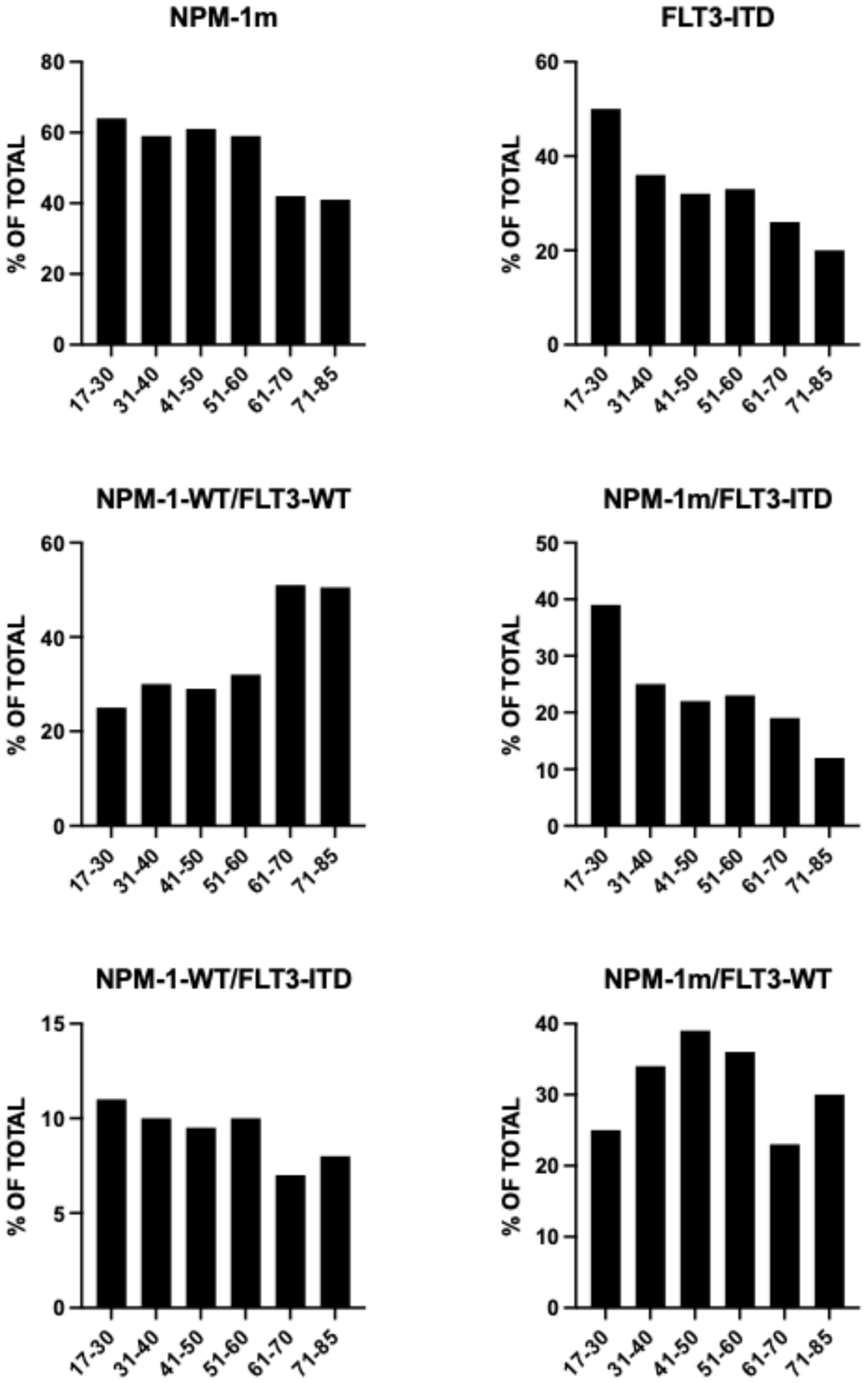
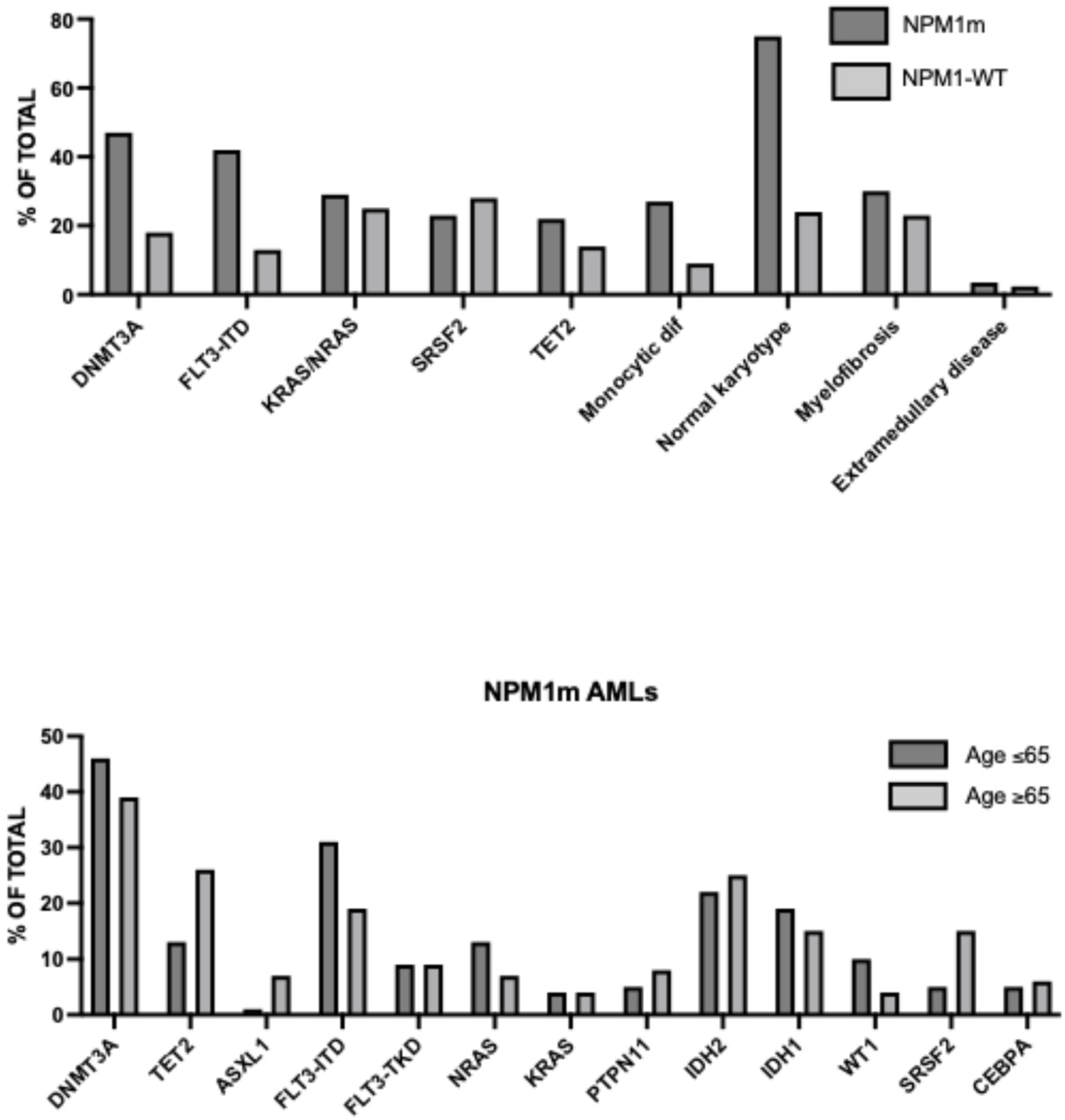
Landscape of Genetic Alterations in Older AML
2. Development of Precision Medicine for AML Patients
2.1. Genomic Profiling
2.2. Diagnostic Tools for AML
2.3. Artificial Intelligence and Machine Learning in AML Diagnosis, Prognosis and Treatment
2.4. Drug Sensitivity Assays for AML
2.5. Development of Precision Medicine for Older AML Patients
3. FLT3-Mutant AMLs
| Drug Name | Molecular Target | Clinical Trial (Phase) | Patient Number and Disease Status | Therapeutic Regimen | Trial Outcomes | Toxicity and Adverse Events |
|---|---|---|---|---|---|---|
| GILTERITINIB | FLT3 | LACEWING (III) | 123 (median age 77 yrs) ND FLT3m AML GIL + AZA 74 pts AZA 49 pts | AZA (75 mg/m2) Gilteritinib (80 or 120 mg/QD) | GIL + AZA mOS 9.8 mp AZA mOS 8.9 mo | Common AE Pyrexia 47% Diarrhea 38% Febrile Neutropenia 35% Constipation 34% Nausea 33% |
| GILTERITINIB | FLT3 | NCT 041440487 (I/II) | 52 (median age 71 yrs) 22, R/R FLT3m AML 30, ND FLT3m AML 73% FLT3-ITD (ND) 45% FLT3-ITD (R/R) | AZA (75 mg/m2) VEN (200–400 mg/QD) | ND AMLL CRR 96% 18-mo RFS 71% 18-mo OS 72% mRFS ans mOS no reached R/R AML CRR 27% mRFS 4.3 mo mOS 5.8 mo | AE, grade 3 or 4 Febrile Neutropenia 33% (ND). 45% (R/R) Infection 50% (ND) 59% (R/R) |
| QUIZARTINIB | FLT3 | Yilmaz et al. 2025 [64] | 73 (median age 70 yrs) 26, ND FLT3-ITD AML 47, R/R FLT3-ITD AML | DECITABINR (20 mng/m2) VEN (400 mg/QD) Quizartinib (30 mg or 40 mg/QD) | ND AML CR + CRi 92% MRD-neg (PCR) 71% mOS not reached R/R AML CR + CRi 60% MRD-neg (PCR) 28% mOS 6.3 mo | ND AML Pneumonia 38% Neutropenic fever 57% Infections 22% R/R AML Pneumonia 72% Neutropenic fever 62% Infections 47% |
Mechanisms of Resistance of FLT3 Mutations to Venetoclax
4. IDH-Mutant AMLs
5. NPM1-Mutant and KMT2A-Rearranged AMLs
6. The Development of Triplet Regimens for an Efficacious Targeting of AML Disease in Elderly Patients
7. Ongoing Barriers to the Development of Precision Pargeted Therapies in Older AML Patients
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Prassek, V.V.; Rothenberg-Thurley, M.; Sauerland, M.C.; Herold, T.; Janke, H.; Kslenzyk, B.; Konstandnin, N.P.; Goerlich, D.; Krug, U.; Faldum, A.; et al. Genetics of acute myeloid leukemia in the elderly: Mutation spectrum and clinical impact in intensively treated patients aged 75 years or older. Haematologica 2018, 103, 1853–1861. [Google Scholar] [CrossRef]
- Bataller, A.; Di Nardo, C.D.; Bazinet, A.; Daver, N.; Maiti, A.; Borthakur, G.; Short, N.; Sasaki, K.; Jabbour, E.J.; Issa, G.C.; et al. Targetable genetic abnormalities in patients with acute myeloid leukemia across age groups. Am. J. Hematol. 2024, 99, 792–796. [Google Scholar] [CrossRef]
- Li, J.F.; Cheng, W.Y.; Lin, X.J.; Wen, L.J.; Wang, K.; Zhu, Y.M.; Zhu, H.M.; Chen, X.J.; Zhang, Y.L.; Yin, W.; et al. Aging and comprehensive molecular profiling in acute myeloid leukemia. Proc. Natl. Acad. Sci. USA 2024, 121, e2319366121. [Google Scholar] [CrossRef]
- Hoff, F.W.; Huang, Y.; Welkie, R.l.; Swords, R.T.; Traer, E.; Stein, E.M.; Lin, T.L.; Patel, P.A.; Collins, R.H., Jr.; Baer, M.R.; et al. Genomic characterization of newly diagnosed acute myeloid leukemia patients aged 60 years or older: A report from the Beat AML master trial. Blood 2023, 142 (Suppl. 1), 4296. [Google Scholar] [CrossRef]
- Dohner, H.; Wei, A.H.; Appelbaum, F.R.; Creddock, C.; DiNardo, C.D.; Dombret, H. Daignosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood 2022, 140, 1345–1377. [Google Scholar]
- Tsai, X.C.H.; Sun, K.J.; Lo, M.Y.; Tien, F.M.; Kuo, Y.Y.; Tseng, M.H.; Peng, Y.L.; Chuang, Y.K.; Ko, B.S.; Tang, J.L.; et al. Poor prognostic implications of myelodysplasia-related mutations in both older and younger patients with de novo AML. Blood Cancer J. 2023, 13, 4. [Google Scholar] [CrossRef] [PubMed]
- Meclenbrauck, R.; Bochert, N.; Gabdoulline, R.; Poll, P.; Funke, C.; Brandes, M.; Dallman, L.K.; Fiedler, W.; Krauter, J.; Trummer, A.; et al. Prognostic impact of clonal representation of myelodysplasia-related gene mutations in acute myeloid leukemia. Leukemia 2025, 39, 1773–1777. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; You, X.; Droin, N.; Banasrak, L.G.; Churpak, J.; Padron, E.; Geissler, K.; Solary, E.; Patnaik, M.M.; Zhang, J. Role of ASXL1 in hematopoiesis and myeloid disaeses. Exp. Hematol. 2022, 115, 14–19. [Google Scholar] [CrossRef]
- Dawoud, A.A.Z.; Tapper, W.J.; Cros, N.C.P. Clonal myelopoiesis in the UK Biobank cohort: ASXL1 mutations are strongly associated with smoking. Leukemia 2020, 34, 2660–2672. [Google Scholar] [CrossRef] [PubMed]
- Lattorre-Crespo, E.; Robertson, N.A.; Kobent, E.G.; MacGillivroy, L.; Murphy, L.; Uddin, M.; Whitsel, E.; Honigber, M.; Bick, A.; Reiner, A.P.; et al. Clinical progression of clonal hematopoiesisis detrmined by a combination of mutation timing, fitness and clonal structure. bioRxiv 2025, in press. [Google Scholar] [CrossRef]
- Mill, C.P.; Fiskus, W.C.; Birdwell, C.; Davis, J.A.; Das, K.; Hou, H.; Sahrma, S.; Sasaki, K.; Loghavi, S.; Kadia, T.M.; et al. ASXL1 mutations in AML are associated with a distinct epigenetic state whigh highlights vulnerabilities to specific epigenetic-targeted agents. Blood 2024, 144 (Suppl. 1), 1349–1350. [Google Scholar] [CrossRef]
- Jahn, E.; Saadati, M.; Fenaux, P.; Gobbi, M.; Roboz, G.; Bullinger, L.; Lutsik, P.; Riedel, A.; Plass, C.; Jahn, N.; et al. Clinical impact of the genomic landscape and leukemogenic trajectories in non-intensively treated elderly acute myeloid leukemia patients. Leukemia 2023, 37, 2187–2196. [Google Scholar] [CrossRef]
- Khan, M.; Cortes, J.; Kadia, T.; Naqvi, V.; Brandt, M.; Pierce, S.; Patel, K.P.; Borthekur, G.; Ravandi, F.; Konopleva, M.; et al. Clonal outcomes and co-occurring mutations in patients with RUNX1-mutated acute myeloid leukemia. Int. J. Mol. Sci. 2017, 18, 1618. [Google Scholar]
- Kim, H.; Lee, J.Y.; Yu, S.; Yoo, E.; Kim, H.R.; Lee, S.M.; Lee, W.S. Acute myeloid leukemia and myelodysplastic neoplasms: Clinical implications of myelodysplasia-related genes mutations and TP53 aberrations. Blood Res. 2024, 59, 41. [Google Scholar] [PubMed]
- Gao, Y.; Jia, M.; Mao, Y.; Cai, H.; Zhou, D.; Li, J. Distinct mutation landscapes between acurte myelodysplasia-related changes and de novo acute myeloid leukemia. Am. J. Clin. Pathol. 2022, 157, 691–700. [Google Scholar] [CrossRef]
- Li, Y.; Xu, M.; Deng, X.; Dong, L.; Nguyen, L.X.T.; Ren, L.; Han, L.; LI, C.; Xue, J.; Zhao, Z.; et al. TET2-mediated mRNA demethylation regulates leukemia stem cell homing and self-renewal. Cell Stem Cell 2023, 30, 1072–1090. [Google Scholar] [CrossRef] [PubMed]
- Iyama, S.; Chi, S.G.; Idogawa, M.; Ikazoe, T.; Fukushima, K.; Utsu, Y.; Kanda, T.; Yoshimoto, G.; Haono, N. Prognostic impact of TET2 mutations in patients with acute myeloid leukemia: HM-SCREEN-Japan 01 and 02 study. Ann. Hematol. 2025, 104, 275–284. [Google Scholar]
- Chen, Y.; Wu, Z.; Chen, Y.; Wang, Z.; Cai, R.; Wu, Y.; Zhang, J. Prognostic impact of methylation-related gene mutations in elderly acute myeloid leukemia: A real-world retrospective analysis. Front. Med. 2025, 12, 1594784. [Google Scholar]
- Shahzad, M.; Amin, M.K.; Daver, N.G.; Shah, M.V.; Hiwase, D.; Arber, D.A.; Karfan-Dabaja, M.A.; Bador, T. What have we learned about TP53-mutated acute myeloid leukemia? Blood Cancer J. 2024, 14, 202. [Google Scholar] [CrossRef]
- Bador, T.; Kettani, M.; Shah, K.; Hassan, O.; Shallis, R.; Diebold, K.; Coltoff, A.; Goldberg, A.D.; Patel, A.A.; Bewersdorf, J.P.; et al. Should we treat TP53-mutated high-risk myeloid neoplasms in older patients? J. Clin. Oncol. 2025, 43, 6538. [Google Scholar] [CrossRef]
- DiNardo, C.D.; Jonas, B.A.; Pullarkat, V.; Thirman, M.J.; Garcia, J.S.; Wei, A.H.; Konopleva, M.; Pratz, K.W. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N. Engl. J. Med. 2020, 383, 617–629. [Google Scholar] [CrossRef]
- DiNardo, C.D.; Pratz, K.; Pullarkat, V.; Arellano, M.; Becker, P.S.; Frankfurt, O.; Wei, A.H.; Konopleva, M.; Xu, T. Venetoclax combined with decitabine or azacitidine in treatment-naïve, elderly patients with acute myeloid leukemia. Blood 2019, 133, 7–17. [Google Scholar] [CrossRef]
- Pratz, K.W.; Jonas, B.A.; Pullarkat, V.A.; Thirman, M.J.; Garcia, J.S.; Fiedler, W.; Yamamoto, K.; Wang, J.; Yoos, S.; Wolach, O.; et al. Long-term follow-up of the phase 3 Viale A clinical trial of venetoclax plus azacitidine for patients with untreated acute myeloid leukemia ineligible for intensive chemotherapy. Blood 2022, 140 (Suppl. 1), 529–531. [Google Scholar] [CrossRef]
- Madarong, E.; Likon, J.; Zhao, W.; Sekeres, M.A.; Bradley, T.; Chandhok, N.S.; Taylor, J.; Venigopal, S.; Koru-Sengul, T.; Iyer, S.G.; et al. Venetoclax and hypomethylating agents in octogenarians and nonagenarians with acute myeloid leukemia. Blood Neoplasia 2024, 1, 100016. [Google Scholar] [CrossRef]
- Shimony, S.; Garcia, J.S.; Keating, J.; Chen, E.C.; Luskin, M.R.; Stahl, M.; Neuberg, D.S.; De Angelo, D.J.; Stone, R.M.; Lindsley, R.C. Molecular ontogeny underlies the benefit of adding venetoclax to hypomethylating agents in newly diagnosed AML patients. Leukemia 2024, 38, 1494–1500. [Google Scholar] [CrossRef] [PubMed]
- Shimony, S.; Bewersdorf, J.P.; Shallis, R.M.; Liu, Y.; Schaefer, E.J.; Zeidan, A.M.; Goldberg, A.D.; Stein, E.M.; Marcucci, G.; Lindsley, R.C.; et al. Hypomethylating agents plus venetoclax compared with intensive induction chemotherapy regimens in molecularly defined secondary AML. Leukemia 2024, 38, 762–768. [Google Scholar] [CrossRef]
- Salom, H.; Joshi, R.; Mongrolia, U.; Waghoo, S.; Zaidi, G.; Rawool, S.; Thakare, R.; Banday, S.; Mishra, A.; Das, G.; et al. Next-generation sequencing technology: Current trends and advancements. Biology 2023, 12, 997. [Google Scholar] [CrossRef] [PubMed]
- Guijarro, F.; Garrata, M.; Villamer, N.; Colomer, D.; Eshave, J.; Lopez-Guerra, H. Novel tools for diagnosis and monitoring of AML. Curr. Oncol. 2024, 30, 395. [Google Scholar] [CrossRef] [PubMed]
- Papaemmanuil, E.; Gerstung, M.; Bullinger, L.; Gaidzik, V.I.; Paschka, P.; Roberts, N.D.; Potter, N.E.; Heuser, M.; Thol, F.; Bolli, N.; et al. Genomic classification and prognosis in acute myeloid leukemia. N. Engl. J. Med. 2016, 374, 2209–2221. [Google Scholar] [CrossRef]
- Testa, U.; Pelosi, E.; Castelli, G. Acute myeloid leukemia in the elderly: Molecular abnormalities and molecular classification. Hemato 2025, 6, 22. [Google Scholar] [CrossRef]
- Hunter, B.; Hindocha, S.; Lee, R.W. The role of artificial intelligence in early cancer diagnosis. Cancers 2022, 14, 1524. [Google Scholar] [CrossRef]
- Ghete, T.; Keek, F.; Pontones, M.; Pfang, D.; Westphal, M.; Hofener, H.; Metzeler, M. Models for the marrow, a comprehensive review of AI-based cell classification methods and malignancy detection in bone marrow aspirate smears. Hemasphere 2024, 8, e70048. [Google Scholar] [CrossRef]
- Didi, I.; Alleot, J.M.; Dimas, P.Y.; Vergez, F.; Tavition, S.; Lageaud, L.; Bidet, A.; Rieu, J.B.; Luquet, I.; Lechavalier, N.; et al. Artificial intelligence-based prediction models for acute myeloid leukemia using real-life data: A DATAML registry study. Leuk. Res. 2024, 136, 107437. [Google Scholar] [CrossRef]
- Cheng, F.M.; Lo, S.C.; Lin, C.C.; Lo, W.J.; Chien, S.Y.; Sun, T.H.; Hsu, K.C. Deep learning assist in acute leukemia detection and cell classification via flow cytometry using the acute leukemia orientation tube. Sci. Rep. 2024, 14, 8350. [Google Scholar] [CrossRef]
- Awada, H.; Durmaz, A.; Gurnari, C.; Kishtagari, A.; Meggendorfer, M.; Kerr, C.; Kuzmanovic, T.; Durrani, J.; Shrave, J.; Nagata, Y.; et al. Machine learning integrates genomic signatures for subclassification beyond primary and secondary acute myeloid leukemia. Blood 2021, 138, 1885–1895. [Google Scholar] [CrossRef] [PubMed]
- Marchi, F.; Shastrio, V.; Marrero, R.; Nguyen, N.; Ottl, A.; Schade, A.K.; Landwehr, M.; Krali, O.; Nordlund, J.; Ghevami, M.; et al. Epigenomic diagnosis and prognosis of acute myeloid leukemia. Nat. Commun. 2025, 16, 6961. [Google Scholar] [CrossRef]
- Moeking, T.; van de Loossdrecht, A.; Cloos, J.; Bachas, C. Applications of medicine learning for immunophenotypic measurable residual disease attachement in acute myeloid leukemia. HemaSphere 2025, 9, e70138. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; He, L.; Iunevski, A.; Nader, K.; Ruokoranta, T.; Linnavirta, N.; Miettinen, J.; Vahe-Kakela, M.; Vanttinen, I.; Kuusamaki, H.; et al. A machine learning-based strategy predicts selective and synergistic drug combinations for relapsed acute myeloid leukemia. Cancer Res. 2025, 85, 2753–2768. [Google Scholar]
- Eckardt, J.N.; Hahn, W.; Ries, R.E.; Chrost, S.D.; Winter, S.; Stasik, S.; Rollig, C.; Platzbecker, U.; Muller-Tidow, C.; Serve, H.; et al. Age-stratified machine learning identifies divergent prognostic significance of molecular alterations in AML. HemaSphere 2025, 9, e71032. [Google Scholar] [CrossRef]
- Qin, G.; Dai, J.; Chien, S.; Martins, T.J.; Loera, B.; Nguyen, Q.H.; Oakes, M.L.; Tercan, B.; Aguilar, B.; Hagen, L.; et al. Mutation patterns predict drug sensitivity in acute myeloid leukemia. Clin. Cancer Res. 2024, 30, 2659–2671. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, M.; Mandal, B.; Mandal, V.; Karki, S.; Thapa, R. Drug sensitivity patterns across FAB subtypes and molecular mutations in AML: A comprehensive analysis for precision medicine. Clin. Transl. Discov. 2025, 5, e70046. [Google Scholar] [CrossRef]
- Andersen, A.N.; Brodersen, A.M.; Ayuda-Duron, P.; Piechaczyk, L.; Tadele, D.S.; Bakan, L.; Fredriksen, J.; Stoksfold, M.; Lenartova, A.; Floisand, Y.; et al. Clinical forecasting of acute myeloid leukemia using ex vivo drug-sensitivity profiling. Cell Rep. Methods 2023, 3, 100654. [Google Scholar]
- Liebers, N.; Bruch, P.M.; Terzer, T.; Hernandez-Hernandez, M.; Paramavisam, N.; Fitzgerald, D.; Altmann, H.; Reider, T.; Kolb, C.; Knoll, M.; et al. Ex vivo drug response profiling for response and outcome prediction in hematologic malignancies: The prospective non-interventional SMART trial. Nat. Cancer 2023, 4, 1648–1659. [Google Scholar]
- Tyner, J.W.; Tognon, C.E.; Bottomly, D.; Wilmot, B.; Kurtz, S.E.; Savage, S.L.; Long, N.; Schultz, A.R.; Traer, E.; Agarwal, A.; et al. Functional genomic landscape of acute myeloid leukemia. Nature 2018, 562, 526–531. [Google Scholar] [CrossRef]
- Bottomly, D.; Long, N.; Schultz, A.R.; Kurtz, S.E.; Tognon, C.E.; Johnson, K.; Abel, M.; Agarwal, A.; Avaylon, S.; Beuton, E.; et al. Integrative analysis of drug response and clinical outcome in acute myeloid leukemia. Cancer Cell 2022, 40, 850–864. [Google Scholar] [CrossRef]
- Malani, D.; Kumar, A.; Bruck, O.; Kontro, M.; Yadav, B.; Hellesay, M.; Kuusanmaki, H.; Dufva, O.; Kantainen, M.; Eldfus, K.; et al. Implementing a functional precision medicine tumor board for acute myeloid leukemia. Cancer Discov. 2022, 12, 388–401. [Google Scholar]
- Kuusanmaki, H.; Kruytola, S.; Vantinen, I.; Ruokoranta, T.; Ranta, A.; Huuhtanen, J. Ex vivo venetoclax sensitivity testing predicts treatment response in acute myeloid leukemia. Haematologica 2023, 108, 1768–1781. [Google Scholar]
- Kytola, S.; Vanttinen, I.; Ruokoranta, T.; Partanen, A.; Holopainen, A.; Saad, J.; Kuusisto, M.; Koskela, S.; Itala-Remes, M.; Vatstrik, I.; et al. Ex vivo venetoclax sensitivity predicts clinical response in acute myeloid leukemia in the prospective VenEx trial. Blood 2025, 145, 409–421. [Google Scholar] [CrossRef] [PubMed]
- Eide, C.A.; Kutz, S.E.; Kaempf, A.; Long, N.; Joshi, S.K.; Nechiporuk, T.; Huang, A.; Dibb, C.A.; Taylor, A.; Bottomly, D.; et al. Clinical correaltes of venetoclax-based combination sensitivities to augment acute myeloid therapy. Blood Cancer Discov. 2023, 4, 452–467. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.L.; Gibbs, J.; Bottomly, D.; Kaempf, A.; Kurtz, S.E.; Eide, C.A.; Huang, A.; Sax, L.; Long, N.; McWeeney, S.K.; et al. Combination with palbociclib overcomes venetoclax resistance mechanisms and outperforms single agent efficacy in acute myeloid leukemia. Blood 2024, 144 (Suppl. 1), 1566–1567. [Google Scholar] [CrossRef]
- Burd, A.; Levine, R.L.; Ruppert, A.S.; Minus, A.S.; Borate, U.; Stein, E.M.; Patel, P.; Baer, M.R.; Stock, W.; Deininger, M.; et al. Precision medicine treatment in acute myeloid leukemia using prospective genomic profiling: Feasibility and preliminary efficacy of the Beat AML master trial. Nat. Med. 2020, 26, 1852–1858. [Google Scholar] [CrossRef]
- Hoff, F.W.; Huang, Y.; Welkie, R.L.; Swords, R.T.; Traer, E.; Stein, E.M.; Lin, T.L.; Patel, P.A.; Collins, R.H.; Baer, M.R.; et al. Molecular characterization of newly diagnosed acute myeloid leukemia patients aged 60 years or older: A report from the Beat AML clinical trial. Blood Cancer J. 2025, 15, 55. [Google Scholar] [CrossRef] [PubMed]
- Duong, V.H.; Ruppert, A.S.; Mims, A.S.; Borate, U.; Stein, E.M.; Baer, M.R.; Stock, W.; Kovacsovics, T.; Blum, W.; Arellano, M.L. Entospletinib with decitabine in acute myeloid leukemia with mutant TP53 or complex karyotype: A phase 2 substudy of the Beat AML master trial. Cancer 2023, 126, 2308–2320. [Google Scholar] [CrossRef]
- Little, R.F.; Othus, M.; Assouline, S.; Ansher, S.; Atallah, E.L.; Lindsley, R.C.; Freidlin, B.; Gore, S.D.; Harris, L.; Hourigan, C.S.; et al. Umbrella Trial in Myeloid Malignancies: The Myelomatch National Clinical Trials Network Precision Medicine Initiative. Blood 2022, 140, 9057–9060. [Google Scholar] [CrossRef]
- Yeung, C.; Narava, S.; Chang, T.C.; Saeed, M.; Aicher, L.; Beppu, L.W.; Majana, M.S.; Taylor, E.M.; Camaller, C.E.; Sandhuria, P.; et al. Analytical performance of the NCI-myeloMATCH assay: A parid turnaround genomic profiling assay for myeloid disorders. J. Mol. Diagn. 2025, 27, 783–795. [Google Scholar] [CrossRef] [PubMed]
- Short, N.J.; Nguyen, D.; Ravandi, F. Treatment of older adults with FLT3-mutated AML ongoing paradigms and the role of frontline FLT3 inhibitors. Blood Cancer J. 2023, 13, 142. [Google Scholar] [CrossRef] [PubMed]
- Konopleva, M.; Thirman, M.J.; Pratz, K.W.; Garcia, J.S.; Recher, C.; Pullarkat, V.; Kantarjian, H.M.; DiNardo, C.D.; Dail, M.; Duan, Y.; et al. Impact of FLT3 mutation on outcomes after venetoclax and azacitidine for patients with treatment-naïve acute myeloid leukemia. Clin. Cancer Res. 2022, 28, 2744–2752. [Google Scholar] [CrossRef]
- DiNardo, C.D.; Tiong, I.S.; Quasglieri, A.; MacRaild, S.; Loghavi, S.; Brown, F.C.; Thijssen, R.; Pomilio, G.; Ivey, A.; Salmon, J.M.; et al. Molecular patterns of response and treatment failure after frontline venetoclax combinations in older patients with AML. Blood 2020, 135, 791–803. [Google Scholar] [CrossRef]
- Perl, A.E.; Martinelli, G.; Cortes, J.E.; Neubauer, A.; Berman, E.; Paolini, S.; Montesinos, P.; Levis, M. Gilteritinib or chemotherapy for relapsed or refractory FLT3-mutated AML. N. Engl. J. Med. 2019, 381, 1728–1740. [Google Scholar] [CrossRef]
- Ma, J.; Zhao, S.; Qiao, X.; Knight, T.; Edwards, H.; Polin, L.; Kushner, J.; Dzinic, S.; Whitek, K.; Wabg, G.; et al. Inhibition of Bcl-2 synergistically enhances the antileukemic activity of midostaurin and gilteritinib in preclinical models of FLT3-mutated acute myeloid leukemia. Clin. Cancer Res. 2019, 25, 6815–6826. [Google Scholar] [CrossRef]
- Daver, N.; Perl, A.E.; Maly, J.; Ritchie, E.; Litzow, M.; McCloskey, J.; Smith, C.C.; Schiller, G.; Bradley, T.; Tiu, R.V.; et al. Venetoclax plus gilteritinib for FLT3-mutated relapsed/refractory acute myeloid leukemia. J. Clin. Oncol. 2022, 40, 4048–4059. [Google Scholar] [CrossRef]
- Wang, E.S.; Montesinos, P.; Minden, M.D.; Lee, J.H.; Heuser, M.; Naoe, T.; Chou, W.C.; Laribi, K.; Esteve, J.; Altman, J.K.; et al. Phase 3 trial of gilteritinib plus azacitidine vs azacitidine for newly diagnosed FLT3mut+ AML ineligible for intensive chemotherapy. Blood 2022, 140, 1845–1857. [Google Scholar] [CrossRef] [PubMed]
- Short, N.J.; Daver, N.; DiNardo, C.D.; Kadia, T.; Nasr, L.F.; Macoron, W.; Yilmaz, M.; Borthakur, G.; Montalban-Bravo, G.; Garcia-Manero, G.; et al. Azacitidine, venetoclax, and gilteritinib in newly diagnosed and relapsed or refractory FLT3-mutated AML. J. Clin. Oncol. 2024, 42, 1499–1508. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, M.; Mufluoglu, M.; Short, N.; Loghavi, S.; Kadia, T.; DiNardo, C.; Borthakur, G.; Pemmaraju, N.; Alvardo, Y.; Maiti, A.; et al. Phase I/II study of decitabine, venetoclax, and quizartinib triplet combination in FLT3-ITD mutated AML. In Proceedings of the 30th Annual Congress of the European Hematology Association (EHA), Milan, Italy, 12–15 June 2025. Abstract S142. [Google Scholar]
- Altman, J.K.; Sun, Z.; Perl, A.E.; Little, R.; Gore, S.D.; Atallah, E.L.; Luger, S.; Litzow, M.R. A randomized phase II study of Venetoclax and HMA-based therapies for the treatment of older and unfit adults with newly diagnosed FLT3-mutatted acute myeloid leukemia (AML): A Myelomatch treatment trial: ECOG-ACRIN MM 20A-EA02. Blood 2024, 144 (Suppl. 1), 6027–6028. [Google Scholar]
- Liu, Q.; Welkie, R.L.; Huang, Y.; Swords, R.T.; Lin, T.L.; Koenig, K.L.; Madanat, Y.F.; Patel, P.A.; Collins, R.H.; Blum, W.; et al. Beat AML S8 group 2: Gilteritinib (GILT) in combination with decitabine (DEC) and venetoclax (VEN) in untreated FLT3 mutated acute myeloid leukemia (AML) patients age >60 with high and low variant allele frequency. Blood 2023, 142 (Suppl. 1), 5933–5937. [Google Scholar] [CrossRef]
- Dohner, H.; Weber, D.; Krkykalla, J.; Fiedler, W.; Wulf, G.; Salih, H.; Lubbert, M.; Kuhn, M.; Schoeder, T.; Salvender, H.; et al. Midostaurin plus intensive chemotherapy for younger and older patients with AML and FLT3 internal tandem duplications. Blood Adv. 2022, 6, 5345–5355. [Google Scholar] [CrossRef]
- Erba, H.P.; Montesinos, P.; Kim, H.J.; Patkowska, E.; Vrhovac, R.; Zak, P.; Wang, P.N.; Mitov, T.; Hanyok, J.; Kamel, Y.M.; et al. Quizartinib plus chemotherapy in newly diagnosed patients with FLT3-internal-tandem-duplication-positive acute myeloid leukemia (QUANTUM-First): A randomized, double-blind, placebo-controlled, phase 3 trial. Lancet 2023, 401, 1571–1583. [Google Scholar]
- Levis, M.J.; Erba, H.P.; Montesinos, P.; Patkowska, E.; Cortes, J.E.; Dombret, H.; Perl, A.E.; Amadori, S.; Wang, J.; Schlenk, R.F.; et al. Quantum-first: Effects of Quizartinib (Q) on RFAS, OS, CIR, amd MRD in newly diagnosed (nd) patients (pts) with FMS-like tyrosine kinase 3-internal tandem duplication-posiitve (FLT3-ITD) acute myeloid leukemia (AML) who received continuum (CONT) therapy (tx). Blood 2024, 144 (Suppl. 1), 2890. [Google Scholar]
- Chua, C.C.; Hsu, B.; Enjeti, A.; Bajel, A.; Marlton, P.; Fleming, S.; Hiwase, D.; Kris Ma, C.K.; Browett, P.J.; Perera, T.; et al. A phase II randomized trial comparing low-dose cytarabine and venetoclax +/− midostaurin in non-adverse cytogenetic risk acute myeloid leukemia: The ALLG AMLM25 Intervene trial. Blood 2024, 144 (Suppl. 1), 217–219. [Google Scholar]
- Wang, E.S.; Goldberg, A.D.; Tallman, M.; Walter, R.B.; Karanes, C.; Sandhu, K.; Vigil, C.E.; Collins, R.; Jain, V.; Stone, R.M. Crenolanib and intensive chemotherapy in adults with newly diagnosed FLT3-mutated AML. J. Clin. Oncol. 2024, 42, 1776–1788. [Google Scholar] [CrossRef]
- Bazinet, A.; Bataller, A.; Kadia, T.; Daver, N.; Short, N.J.; Yilmaz, M.; Sasaki, K.; DiNardo, C.D.; Borthakur, G.M.; Issa, G.; et al. A retrospective study of outcomes across time and treatment regimens in newly diagnosed, FMS-like tyrosine kinase 3 (FLT3)-mutated acute myeloid leukemia. Cancers 2025, 131, e35813. [Google Scholar] [CrossRef]
- Dohner, H.; Pratz, K.W.; DiNardo, C.D.; Wei, A.H.; Jones, B.A.; Pullarkat, V.A.; Thirman, M.J.; Recher, C.; Schuh, A.C.; Babus, S.; et al. Genetic risk stratification and outcomes among treatment-naïve patients with AML treated with venetoclax and azacytidine. Blood 2024, 144, 2211–2222. [Google Scholar] [CrossRef] [PubMed]
- McMahon, C.M.; Ferng, T.; Canaani, J.; Wang, E.S.; Morrissette, J.; Eastburn, D.; Pellegrino, M.; Durruthy-Durruthy, R.; Watt, C.D.; Asthana, S.; et al. Clonal selection with RAS pathway activation mediates secondary clinical resistance to selective FLT3 inhibition in acute myeloid leukemia. Cancer Discov. 2019, 9, 1050–1063. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Riley-Gillis, B.; Han, L.; Jia, Y.; Lodi, A.; Zhang, H.; Ganesan, S.; Pan, R.; Konoplev, S.; Sweeney, S.; et al. Activation of RAS/MAPK confers MCL-1-mediated acquired resistance to BCL-2 inhibitor venetoclax in acute myeloid leukemia. Signal Transduct. Target. Ther. 2022, 7, 51. [Google Scholar] [CrossRef] [PubMed]
- Nwosa, G.O.; Ross, D.M.; Powell, J.A.; Pitson, S.M. Venetoclax therapy and emerging resistance mechanisms in acute myeloid leukemia. Cell Death Dis. 2024, 15, 413. [Google Scholar] [CrossRef]
- Kennedy, V.E.; Pereztz, G.A.; Whalia, A.; Chyla, B.; Sun, Y.; Hill, J.; Tran, E.; Koh, A.; Ferng, T.; Pintor, S.; et al. RAS pathway activation drives clonal selection and monocytic differentiation in FLT3 and BCL2 inhibitor resistance. bioRxiv 2025, in press. [Google Scholar] [CrossRef]
- Zarnegar-Lumley, S.; Alonzo, T.A.; Gerbing, R.B.; Othus, M.; Sun, Z.; Ries, R.E.; Wang, J.; Leonti, A.; Kutny, M.A.; Oatronoiff, F.; et al. Characteristics and prognostic impact of IDH mutations in AML: A COG, SWOG, and ECOG analysis. Blood Adv. 2023, 7, 5941–5950. [Google Scholar] [CrossRef]
- Hoff, F.W.; Huang, Y.; Welkie, P.L.; Swortds, R.T.; Traer, E.; Stein, E.M.; Lin, T.L.; Patel, P.A.; Collins, R.H. IDH2 mutation is associated with favorable outcome among older adults with newly diagnosed acute myeloid leukemia treated with lower-intensity therapy. Blood 2024, 144, 4325–4327. [Google Scholar] [CrossRef]
- Sakamoto, T.; Leca, J.; Zhang, X.; Meydan, C.; Foox, J.; Ramachandran, P.; Hendrikse, L.D.; Zhou, W.; Berger, T.; Fortin, J.; et al. Mutant IDH1 cooperates with NPM1c or FLT3ITD to drive distinct myeloid diseases and molecular outcomes. Proc. Natl. Acad. Sci. USA 2025, 122, e2415779122. [Google Scholar] [PubMed]
- Sirenko, M.; Lee, S.; Sun, Z.; Chaligne, R.; Loghavi, S.; Asimomitis, G.; Brierley, C.K.; Bernard, E.; Cai, S.F.; Myers, R.M.; et al. Deconvoluting clonal and cellular architecture in IDH-mutant acute myeloid leukemia. Cell Stem Cell 2025, 32, 1102–1121.e5. [Google Scholar] [CrossRef]
- Montesinos, P.; Recher, C.; Vives, S.; Zarzycka, E.; Wang, J.; Bertani, G.; Heuser, M.; Calado, R.T.; Schuh, A.; Yeh, S.P.; et al. Ivosidenib and azacitidine in IDH1-mutated acute myeloid leukemia. N. Engl. J. Med. 2022, 386, 1519–1531. [Google Scholar] [CrossRef]
- Montesinos, P.; Marchione, D.M.; Recher, C.; Heuser, M.; Vives, S.; Zarzycka, E.; Wang, J.; Riva, M.; Calado, R.T.; Schuh, A.C.; et al. Long-term results from the AGILE study of azacitidine plus ivosidenibv vs placebo in newly diagnosed IDH1-mutated AML. Blood Adv. 2025, in press. [Google Scholar]
- Cortes, J.E.; Roboz, G.J.; Watts, J.; Baer, M.R.; Jonas, B.A.; Schiller, G.J.; Yee, K.; Ferrell, B.; Yang, J.; Wang, E.S.; et al. Olutasidenib in combination with azacitidine induces durable complete remissions in patients with relapsed or refractory mIDH1 acute myeloid leukemia: A multicohort open-label phase 1–2 trial. J. Hematol. Oncol. 2025, 18, 7. [Google Scholar] [CrossRef] [PubMed]
- Marvin-Peek, J.; Garcia, J.S.; Borthakur, G.; Garcia-Manero, G.; Short, N.J.; Kadia, T.M.; Loghavi, S.; Masarova, L.; Daver, N.; Maiti, A.; et al. A phase Ib/II study of ivosidenib plus venetoclax ± azacitidine in IDH1-mutated hematologic malignancies: A 2024 update. Blood 2024, 144 (Suppl. 1), 219–221. [Google Scholar]
- DiNardo, C.D.; Marvin-Peek, J.; Loghavi, S.; Takahashi, K.; Issa, G.C.; Jen, W.Y.; Daver, N.G.; Reville, P.K.; Short, N.J.; Sasaki, K.; et al. Outcomes of frontline triplet regimens with a hypomethylating agent, venetoclax, and isocitrate dehydrogenase inhibitor for intensive chemotherapy-ineligible patients with isocitrate dehydrogenase-mutated AML. J. Clin. Oncol. 2025, 43, 2692–2699. [Google Scholar] [PubMed]
- DiNardo, C.D.; Schuh, A.; Stein, E.M.; Montesinos, P.; Wei, A.H.; de Botton, S.; Zeidan, A.M.; Fathi, A.T.; Kantarjian, H.M.; Beneet, J.M.; et al. Enasidenib plus azacitidine versus azacitidine alone in patients with newly diagnoses, mutant-IDH2 acute myeloid leukemia (AG221-AML-005): A single-arm, phase 1b and randomized, phase 2 trial. Lancet Oncol. 2021, 22, 1597–1608. [Google Scholar] [PubMed]
- De Botton, S.; Montesinos, P.; Schuh, A.C.; Papayannidis, C.; Vyes, P.; Wei, A.H.; Ommen, H.; Semochkin, S.; Kim, H.J.; Larsom, R.A.; et al. Enasidenib versus conventional care in older patients with late-stage mutant IDH2-relapsed/refractory AML: A randomized phase 3 trial. Blood 2023, 141, 156–164. [Google Scholar]
- Richard-Carpentier, G.; Gupta, G.; Cameron, C.; Chatelin, S.; Bankar, A.; Davidson, M.B.; Gupta, V.; Maze, D.C.; Minden, M.D.; Murphy, T.; et al. Final results of the phase Ib/II study evaluating enasidenib in combination with venetoclax in patients with IDH2-mutated relapsed/refractory myeloid malignancies. Blood 2023, 142 (Suppl. S1), 159–161. [Google Scholar]
- Cai, S.F.; Huang, Y.; Lance, J.R.; Mao, H.C.; Dunbar, A.J.; McNulti, S.; Druley, T.; Li, Y.; Baer, M.R.; Stock, W.; et al. A study to assess the efficacy of enasidenib and risk-adapted addition of azacitidine in newly diagnosed IDH2-mutant AML. Blood Adv. 2024, 8, 429–440. [Google Scholar] [CrossRef]
- Ozga, M.P.; Dvorek-Kornaus, K.; Zhao, Q.; Langanson, A.; Hamp, E.; Madanat, Y.; Pollyea, D.A.; Stein, E.M.; Zeidner, J.F.; Mardis, E.R.; et al. I-DATA study: Randomized, sequential, open-label study to evaluate the efficacy of IDH targeted/non-targeted versus non-targeted/IDH-targeted approaches in the treatment of newly diagnosed IDH mutated adult AML patients not candidates for intensive induction therapy. Blood 2023, 142 (Suppl. 1), 1534–1536. [Google Scholar]
- Stein, E.M.; DiNardo, C.D.; Fathi, A.T.; Mims, A.S.; Pratz, K.W.; Savona, M.R.; Stein, A.S.; Stone, R.M.; Winer, E.S.; Seet, C.S.; et al. Ivosidenib or enasidenib with intensive chemotherapy in patients with newly diagnosed AML: A phase I study. Blood 2021, 137, 1792–1803. [Google Scholar] [CrossRef]
- Pratz, K.W.; Jonas, B.A.; Pullarkat, V.A.; Thirman, M.J.; Garcia, J.S.; Dohner, H.; Récher, C.; Fiedler, W.; Yamamoto, K.; Wang, J.; et al. Long-term follow-up of VIALE-A: Venetoclax and azacitidine in chemotherapy-ineligible untreated acute myeloid leukemia. Am. J. Hematol. 2024, 99, 615–624. [Google Scholar]
- Pollyea, D.A.; DiNardo, C.D.; Arellano, M.L.; Pigneux, A.; Fiedler, W.; Konopleva, M.; Rizzieri, D.A.; Smith, B.D.; Shinagawa, A.; Lemoli, R.M.; et al. Impact of venetoclax and azacitidine in treatment-naïve patients with acute myeloid leukemia and IDH1/2 mutations. Clin. Cancer Res. 2022, 28, 2753–2761. [Google Scholar] [CrossRef] [PubMed]
- Lachowiez, C.A.; Barcello, A.; Zettler, C.M.; Belli, A.J.; Fernandes, L.L.; Hansen, E.; Wang, C.K.; Owusu, H.F.; Zeidan, A.M.; Stein, E.M.; et al. Treatment patterns and real-world outcomes of molecular subgroups in patients with AML receiving frontline venetoclax-based therapy. JCO Oncol. Pract. 2025, in press. [Google Scholar]
- Lachowiez, C.A.; Smith, D.; Ambinder, A.J.; Binder, G.; Angiolillo, A.; Potiuri, R.; Papademetriou, E.; Leblanc, T.W. Ivosidenib or venetoclax combined with hypomethylating agents in IDH1-mutated acute myeloid leukemia: A real world study. Blood Neoplasia 2025, in press. [Google Scholar] [CrossRef]
- Hammond, D.; Loghavi, S.; Wang, S.A.; Konopleva, M.; Kadia, T.M.; Daver, N.G.; Ohanian, M.; Issa, G.C.; Alvarado, Y.; Short, N.J.; et al. Response patterns and impact of MRD in patients with IDH1/2-mutated AML treated with venetoclax and hypomethylating agents. Blood Cancer J. 2023, 13, 148. [Google Scholar] [CrossRef]
- Lachowiez, C.A.; Loghavi, S.; Zeng, Z.; Tanaka, T.; Kim, Y.J.; Uryu, H.; Turkalj, S.; Jakobsen, N.A.; Luskin, M.R.; Duose, Y.D.; et al. A phase Ib/II study of ivosidenib with venetoclax ± azacitidine in IDH1-mutated myeloid malignancies. Blood Cancer Discov. 2023, 4, 276–293. [Google Scholar]
- Wang, L.; Song, J.; Xiao, X.; Li, D.; Liu, T.; He, X. Comparison of venetoclax and ivosidenib/enasidenib for unfit newly diagnosed patients with acute myeloid leukemia and IDH1/2 mutation: A network meta-analysis. J. Chemother. 2024, 36, 202–207. [Google Scholar]
- Othman, J.; Potter, N.; Ivey, A.; Tazi, Y.; Papaemmanuil, E.; Jovanovic, J.; Freeman, S.D.; Gilkes, A.; Gale, R.; Rapoz-D’Silva, T.; et al. Molecular, clinical, and therapeutic determinants of outcome in NPM1-mutated AML. Blood 2024, 144, 714–728. [Google Scholar] [CrossRef] [PubMed]
- Farhat, A.; Kantarjian, H.M.; Sasaki, K.; Short, N.J.; Cuglievan, B.; Loghavi, S.; Patel, K.P.; Bataller, A.; Yilmaz, M.; Montalban-Bravo, G.; et al. Clinical outcomes associated with NPM1 mutations in newly diagnosed AML. Blood 2024, 144 (Suppl. 1), 2853–2855. [Google Scholar] [CrossRef]
- Schneider, F.; Hoster, E.; Unterhalt, M.; Dufour, A.; Benthaus, T.; Mellert, G.; Zellmeier, E.; Bohlander, S.K.; Feuring-Buske, M.; Buske, C.; et al. Age-dependent frequencies of NPM1/FLT3-ITD mutations in patients with normal karyotype AML. Blood 2008, 112, 2531. [Google Scholar] [CrossRef]
- Dhillon, V.; Khan, A.M.; Aguilar, J.J.; Reddy, S.N.; Aly, M.M.; Kewan, T.; Bahaj, W.; Gurnari, C.; Visconte, V.; Carr, D.; et al. Comprehensive age-stratified impact of NPM1 mutation in acute myeloid leukemia: A real-world experience. Cancers 2025, 17, 1020. [Google Scholar] [CrossRef]
- Falini, B.; Mecucci, C.; Tiacci, E.; Alcalay, M.; Rosati, R.; Pasqualucci, L.; La Starza, R.; Diverio, D.; Colombo, E.; Santucci, A. Cytoplasmic nucleophosmin in acute myelogenous leukemia with a normal karyotype. N. Engl. J. Med. 2005, 352, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Ostronoff, F.; Othus, M.; Lazenby, M.; Estey, E.; Appelbaum, F.R.; Evans, A.; Godwin, J.; Gilkes, A.; Kopecky, K.J.; Burnett, A. Prognostic significance of NPM1 mutations in the absence of fLT3-internal tandem duplication in older patients with acute myeloid leukemia: A SWOG and UK National Cancer Research Institute/Medical Research Council Report. J. Clin. Oncol. 2015, 33, 1157–1164. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Zhou, Y.; Zhuo, N.; Xie, W.; Meng, H.; Lou, Y.; Muo, L.; Tong, H.; Qian, J.; Yiang, M.; et al. Co-mutation landscape and its prognostic impact on newly diagnosed adult patients with NPM1-mutated de novo acute myeloid leukemia. Blood Cancer J. 2024, 14, 118. [Google Scholar] [CrossRef] [PubMed]
- Borate, U.; Welkie, R.L.; Huang, Y.; Swords, R.T.; Traer, E.; Stein, E.M.; Lin, T.L.; Madanat, Y.F.; Patel, P.A.; Collins, R.H.; et al. Demographics, characteristics, survival and outcomes in older, untreated, acute myeloid leukemia patients with NPM1 mutations or KMT2A rearrangements from the BEAT AML master clinical trial. Blood 2024, 144 (Suppl. 1), 1564–1566. [Google Scholar] [CrossRef]
- Abdelhakim, H.; Elkhanany, A.; Telfah, M.; Lin, T.L.; Godwin, A.K. Older patients with NPM1 mutated AML have distinctive genomic mutation landscape associated with enrichment in immunosuppressive gene signature. Blood 2019, 134, 1402. [Google Scholar] [CrossRef]
- Chua, C.C.; Loo, S.; Fong, C.Y.; Ting, S.B.; Tions, I.S.; Fleming, S.; Anstee, N.S.; Ivey, A.; Ashby, M.; Teh, T.C.; et al. Final analysis of the phase 1b chemotherapy and venetoclax in elderly acute myeloid leukemic trial (CAVEAT). Blood 2025, 9, 1827–1836. [Google Scholar]
- Bewensdorf, J.P.; Shimony, S.; Shallis, R.M.; Liu, Y.; Berton, G.; Schaefer, E.J.; Zeidan, A.M.; Goldberg, A.D.; Stein, E.M.; Marcucci, G.; et al. Intensive induction chemotherapy vs hypomethylating agents in combination with venetoclax in NPM1-mutant AML. Blood Adv. 2024, 8, 4845–4854. [Google Scholar] [CrossRef]
- Zale, A.; Ambinder, A.J.; Sandeep Kaduluri, V.P. A retrospective analysis of intensive chemotherapy vs venetoclax/hypomethylating agents for patients aged 60–75 with favorable risk, NPM1-mutated AML. Blood 2024, 146 (Suppl. 1), 450. [Google Scholar] [CrossRef]
- Dali, S.A.; Al-Mashdali, A.F.; Kalfah, A.; Mohamed, S.F. Menin inhibitors in KMT2A-rearranged and NPM1-mutated acute myeloid leukemia: A scoping review of safety and efficacy. Crit. Rev. Oncol. Hematol. 2025, 13, 104783. [Google Scholar]
- Falini, B.; Sorcini, D.; Perriello, V.M.; Sportoletti, P. Functions of the native NPM1 protein and its leukemic mutant. Leukemia 2025, 39, 276–290. [Google Scholar] [CrossRef] [PubMed]
- Arellano, M.L.; Thirman, M.J.; DiPersio, J.F.; Heiblig, M.; Stein, E.M.; Schuh, A.C.; Zucenka, A.; DeBotton, S.; Grove, C.S.; Mannis, G.N.; et al. Menin inhibition with revumenib for NPM1-mutated relapsed or refractory acute myeloid leukemia: The AUGMENT-101 study. Blood 2025, 146, 1065–1077. [Google Scholar] [CrossRef]
- Zeidner, J.F.; Lin, T.L.; Welkie, R.L.; Curran, T.; Koenig, K.; Stock, W.; Madanat, Y.F.; Swords, R.; Baer, M.R.; Blum, W.; et al. Azacitidine, Venetoclax, and revanumenib for newly diagnosed NPM1-mutated or KMT2A-rearranged AML. J. Clin. Oncol. 2025, 43, 2606–2615. [Google Scholar] [CrossRef]
- Wei, A.H.; Reyner, J.E.; Garciaz, S.; Aldoss, I.; Piérola, A.A.; Alfred, A.; Dominguez, J.M.A.; Berreyro, L.; Beries, P.; Dehalkis, N.; et al. RP2D determination of blexomenib in combination with venetoclax/azacytidine: Phase 1b study in ND & R/R AML with KMT2A/NPM1 alterations. In Proceedings of the 30th Annual Congress of the European Hematology Association (EHA), Milan, Italy, 12–15 June 2025. Abstract 5137. [Google Scholar]
- Recher, C.; O’Nions, J.; Aldoss, I.; Pierola, A.A.; Allred, A.; Alonso-Dominguez, J.M.; Barreyro, L.; Bories, P.; Curtis, M.; Daskalakis, N.; et al. Phase 1b study of Menin-KMT2A inhibitor bleximenib in combination with intensive chemotherapy in newly diagnosed acute myeloid leukemia with KMT2Ar or NPM1 alterations. Blood 2024, 144 (Suppl. 1), 215–218. [Google Scholar]
- Zeidan, A.M.; Wang, E.S.; Issa, G.C.; Erba, H.; Kaplan Altman, J.; Balasubramanian, S.K.; Strickland, S.A.; Roboz, G.J.; Schiller, G.J.; McMahon, C.M.; et al. Ziftomenib combined with intensive induction (7+3) in newly diagnosed NMP1-m or KMT2A-r acute myeloid leukemia: Interim phase 1a results from KOMET-007. Blood 2024, 144 (Suppl. 1), 214–216. [Google Scholar]
- Erba, H.; Wang, E.S.; Fathi, A.T.; Roboz, G.; Madanat, Y.; Strickland, S.; Balasubramanian, S.; Mangan, J.; Pratz, K.; Advani, A.; et al. Ziftomenib combined with intensive induction chemotherapy (7+3) in newly diagnosed NPM1-m or KMT2A-r acute myeloid leukemia: Updated phase 1a/b results from KOMET-007. In Proceedings of the 30th Annual Congress of the European Hematology Association (EHA), Milan, Italy, 12–15 June 2025. Abstract S136. [Google Scholar]
- Testa, U.; Castelli, G.; Pelosi, E. Recent developments in differentiation therapy of acute myeloid leukemia. Cancers 2025, 17, 1141. [Google Scholar] [CrossRef]
- Candoni, A.; Coppola, G. A 2024 update on menin inhibitors. A new class of target agents against KMT2A-rearranged and NPM1-mutated acute myeloid leukemia. Hematol. Rep. 2024, 16, 244–254. [Google Scholar] [PubMed]
- Farina, M.; Malagola, M.; Bernardi, S.; Re, F.; Russo, D.; Avenoso, D. Intensive chemotherapy versus venetoclax-based regimens in elderly patients with acute myeloid leukemia: Is the chemotherapy era ending? J. Clin. Med. 2025, 14, 2759. [Google Scholar] [CrossRef]
- Goulart, H.; Kantarjian, H.; Pemmaraju, N.; Dover, N.; DiNardo, C.D.; Rausch, C.R.; Ravandi, F.; Kadia, T.M. Venetoclax-based combination regimens in acute myeloid leukemia. Blood Cancer Discov. 2025, 6, 23–37. [Google Scholar] [CrossRef] [PubMed]
- DiNardo, C.D.; Maiti, A.; Rausch, C.R.; Pemmaraju, N.; Naqvi, K.; Daver, N.G.; Kadia, T.M.; Borthakur, P.G.; Ohanian, M.; Alvarado, Y.; et al. 10-day decitabine with venetoclax for newly diagnosed intensive chemotherapy ineligible and relapsed or refractory acute myeloid leukemia: A single-centre phase 2 trial. Lancet Hematol. 2020, 7, e724–e736. [Google Scholar] [CrossRef]
- Hochman, M.T.; Munir, J.P.; Papadantovakis, N. Precision medicine in myeloid neoplasia: Challenges and opportunities. J. Pers. Med. 2025, 15, 49. [Google Scholar] [CrossRef]
- Marvin-Peck, J.; Maiti, A.; Alvarado, Y.; Daver, N.; Sasaki, K.; Borthakur, G.; Short, N.; Chien, K.; Haddad, F.; Hammond, D.; et al. A phase IB/II trial of all-oral “triplet” regimen for IDH-mutated myeloid malignancies: Decitabine/cedazuridine and venetoclax in combination with ivosidenib/enasidenib. In Proceedings of the 30th Annual Congress of the European Hematology Association (EHA), Milan, Italy, 12–15 June 2025. Abstract PS1471. [Google Scholar]
- Sonewal, H.; Rice, W.G.; Bajar, R.; Byyun, J.Y.; Jung, S.H.; Sinba, R.; Howell, S.B. Preclinical development of tuspetinib for the treatment of acute myeloid leukemia. Cancer Res. Commun. 2025, 5, 74–83. [Google Scholar] [CrossRef]
- Daver, N.; Mannis, G.; Watts, J.M.; Podolster, N.; Jonas, B.A.; Boratye, U.; Jeyakumar, D.; Tam, E.; Vachani, P.; Erba, H.; et al. Tuscany study of safetry and efficacy of tuspetinib plus standard of. Care ven and aza in study participants with newly di-agnosed AML ineligible for induction chemotherapy. In Proceedings of the 30th Annual Congress of the European Hematology Association (EHA), Milan, Italy, 12–15 June 2025. Abstract S139. [Google Scholar]
- Vijayanarayanan, A.; Shaw, B.M.; Gibbons, K.; Inamdor, K.V.; Kuriakose, P.; Menon, M. The need for rappid cytogenetics in the era of unique therapies for acute myeloid leukemia. Blood Adv. 2022, 6, 6210–6212. [Google Scholar] [CrossRef] [PubMed]
- Norsworthy, K.J.; Mulkey, F.; Scott, E.C.; Ward, A.F.; Przepiorka, D.; Charlab, R.; Dorff, S.E.; Deisseroth, A.; Kazandjian, D.; Sridhara, R.; et al. Differentiation Syndrome with Ivosidenib and Enasidenib Treatment in Patients with Relapsed or Refractory IDH-Mutated AML: A U.S. Food and Drug Administration Systematic Analysis. Clin. Cancer Res. 2020, 26, 4280–4288. [Google Scholar] [CrossRef] [PubMed]
- Zeidner, J.F. Differentiating the Differentiation Syndrome associated with IDH inhibitors in AML. Clin. Cancer Res. 2020, 26, 4174–4176. [Google Scholar] [CrossRef] [PubMed]
- Perner, F.; Stein, E.M.; Wenge, D.V.; Singh, S.; Kim, J.; Apazidis, A.; Rahnamoun, H.; Anand, D.; Marinaccio, C.; Hatton, C.; et al. MEN1 mutations mediate clinical resistance to menin inhibition. Nature 2023, 615, 913–919. [Google Scholar] [CrossRef]
- Alotaibi, A.S.; Yilmaz, M.; KanagalShamanna, R.; Loghavi, S.; Kadia, T.M.; DiNardo, C.D.; Borthakur, G.; Konopleva, M.; Pierce, S.A.; Wang, S.A.; et al. Patterns of Resistance differ in Patients with Acute Myeloid Leukemia treated with Type I versus Type II FLT3 Inhibitors. Blood Cancer Discov. 2021, 2, 125–134. [Google Scholar] [CrossRef]
- Smith, C.C.; Levis, M.J.; Perl, A.E.; Hill, J.E.; Rosales, M.; Bahceci, E. MolecularProfileofFLT3-Mutated relapsed or refractory patients with AML in the Phase 3 ADMIRAL Study of Gilteritinib. Blood Adv. 2022, 6, 2144–2155. [Google Scholar] [CrossRef]
- Baden, D.; Zukunft, S.; Hernandez, G.; Wolgast, N.; Steinhauser, S.; Pehlnann, A.; Schliemann, C.; Mikesch, J.H.; Steffen, B.; Sauer, T.; et al. Time from diagnosis to treatment has no impact on survival in newly diagnosed acute myeloid leukemia treated with venetoclax-based regimens. Haematologica 2024, 109, 1269–2477. [Google Scholar] [CrossRef]
- Venditti, A.; Palmieri, R.; Laurillo, L.; Rollig, C.; Wierzbowska, A.; de Leeuw, D.; Efficace, F.; Curti, A.; Negai, L.L.; Tettero, J.; et al. Fitness assessment in acute myeloid leukemia: Recommendations from an expert panel on behalf of the European LeukemiaNet. Blood Adv. 2025, 9, 2207–2220. [Google Scholar] [CrossRef] [PubMed]
| Drug Name | Molecular Target | Clinical Trial (Phase) | Patient Number and Disease Status | Therapeutic Regimen | Trial Outcomes | Toxicity and Adverse Events |
|---|---|---|---|---|---|---|
| Ivosidenib (IVO) | IDH1 | AGILE (III) | 146, ND IDH1m-AML | Azacitidine (75 mg/m2) + Ivosidenib (500 mg/QD) vs. Azacitidine + placebo | ORR 62% vs. 19% CR + CRi 58% vs. 19% mEFS at 12 mo 22.9 vs. 4.1 mo mOS 24 vs. 7.9 mo | Differentiation syndrome 4% vs. 4% Febrile neutropenia 20% vs. 34% Thrombocytopenia 20% vs. 15% Infection 21% vs. 31% |
| Ivosidenib (IVO) | IDH1 | NCT03471260 | 37, ND IDH1m-AML | Azacitidine (75 mg/m2) + Ivosidenib (500 mg/QD) Venetoclax (400 or 800 mg) | CR + CRi 86% MRD-neg 81% mOS not reached 2-yr OS 73% 2 yr CIR 24% | Grade 3 or 4 events Hyperbiliribinemia 5% Differentiation syn 3% Transmaninitis 2% |
| Olutasidenib | IDH1 | NCT02577406 (phase I/II) | 67 (mean age 66 yr) R/R IDH1m AML | Olutasidenib (150 mg BID) AZA (75 mg/m2) | ORR 51% CR + CRi 31% mOS 12.5 mo mOS in patients achieving CR 36 mo | AE Grade 3–4 Anemia 25% Thromobocytopenia 37% Neutropenia 19% Leukocytosis 6% |
| Enasidenib (ENA) | IDH2 | AG221-AML-005 (phase I/II) | 101, ND IDH2m AML | Phase Ib: AZA (75 mg/m2) + ENA (100 mg or 200 mg/day) Phase II: AZA + ENA vs. AZA | AZA + ENA vs. AZA ORR: 76% vs. 33% CRR: 54% vs. 12% mOS: 22 vs. 22.3 months | Grade 3 or 4 events AZA + ENA vs. AZA Thrombocytopenia 37% vs. 19% Neutropenis 37% vs. 25% Anemia 19% vs. 22% |
| Enasidenib (ENA) | IDH2 | NCT092719524 (phase I) | 319 (mean age 72 yr) R/R IDH2m AML | ENA (100 mg/QD) vs. Conventional therapy | ORR 40.5% vs. 9.9% CR + CRi 29.7% vs. 6.6% OS at 12 mo 38% vs. 26% mEFS 4.1 mo vs. 2.6 mo | AE grade 3–4 Diff Synd 5% vs. 0% Hyperbilirubinemia 10.8% vs. 0% Anemia 5% vs. 19% Leukopenia 3% vs. 33% |
| Enasidenib (ENA) | IDH2 | NCT04092179 (phase I/II) | 27 (median age 70 yr) R/R IDH2m AML | VEN (400 mg) AZA (100 mg/QD) | ORR 70% CR 57% mOS 9.4 mo IDH2R172 ORR 83% CR 67% IDH2R140 ORR 55% CR 45% | AE grade 3–4 Neutropenia 41% Lung infection 22% Thrombocytopenia 26% |
| Drug Name | Molecular Target | Clinical Trial (Phase) | Patient Number and Disease Status | Therapeutic Regimen | Trial Outcomes | Toxicity and Adverse Events |
|---|---|---|---|---|---|---|
| VENETOCLAX | BCL-2 | CAVEAT (Ib) | 85, mean age 71 yr De novo AML 61% sAML 39% | IC (Cytarabine, Idarubicin) VEN (50–600 mg) | All AMLs CRR 75% mOS 19.3 mo De novo AML CRR 89% mOS 33.1 mo | AE grade 3 or 4 Febrile Neutropenia 55% Spesis 35% Localized infection 10% |
| VENETOCLAX | BCL-2 | Retrospective study Bewersdorf et al. [84] | 221, median age 68.6 yr ND NPM1m AML IC 147 HMA + VEN 74 | IC HMA + VEN | IC vs. HMA + VEN CRR 85% vs. 74% 24 mo OS (all)b39% vs. 38% 24 mo OS (60–75 yr) 60% vs. 44% | Not Reported |
| REVUMENIB | MENIN | AUGMENT-101 (I/II) | 84, median age 63 yr R/R NPM1m AML | Revumenib (160 mg/m2) Q 12 h in 28-day continuous cycles | CR + CRi 26% ORR 48% MRD-neg 63& in CR Duration of CR 4.7 mo | AE grade 3 or greater QTc prolongation 23.4% Anemia 14.3% Neutropenia 13.1% Diff syndrome 13.1% Thrombocytopenia 10.7% |
| REVUMENIB | MENIN | NCT 03013998 (I, in the context of BEAT AML Master Trial) | 43, >60 yr ND NPM1m AML ND KMT2Ar AML DL1: mean age 75 yr DL2: mean age 69.5 yr | AZA (75 mg/m2) VEN Revumenib at two doses: DL1 (113 mg); DL2 (163 mg) | DL1 ORR 90.5% DL2 ORR 86.4% KMT2Ar ORR 100%, CRR 88.9% NPM1m ORR 85.3%, CRR 79.4% KMT2Ar mOS 18 mo NPM1m mOS 15.5 mo | AE grade 3 or greater Neutroppenia 26% Acute kidney injury 14% QTc prolongation 12% Hypokalemia 12% Diff syndrome 4% |
| BLEXIMENIB | MENIN | NCT 05453903 | 120, median age 66.5 yr 68 NPM1m AML 52 KMT2Ar AML R/R 86 patients ND 52 patients | AZA VEN Bleximenib (BL, 50 mg, 100 mg, 150 mg) | R/R AML BL 50 mg ORR 76% CRR 32% R/R AML BL 100 mg ORR 79% CRR 54% ND AML BL 50 mg ORR 77%. CRR 62% ND AML BL 100 mg ORR 92% CRR 85% | AE, grade 3 or greater Thrombocytopenia 53% Anemia 48% Neutropenia 46% Diff syndrome 4% |
| ZIFTOMENIB | MENIN | KOMET-007 (NCT 05735184) (I-II) | 82 49 ND NPM1m AML 33 ND KMT2Ar AML | IC (7 + 3) Ziftomenib 600 m QD | NPM1m ORR 93% MRD-neg 68% KMT2Ar ORR 89% MRD-neg 83% At 25 wk, NPM1m OS 96% At 16 wk KMT2Ar OS 88% | AE, grade 3 or greater Neutropenia 47% Thrombocytopenia 35% Anemia 22% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Testa, U.; Castelli, G.; Pelosi, E. Precision Medicine for Older AML Patients. Onco 2025, 5, 42. https://doi.org/10.3390/onco5030042
Testa U, Castelli G, Pelosi E. Precision Medicine for Older AML Patients. Onco. 2025; 5(3):42. https://doi.org/10.3390/onco5030042
Chicago/Turabian StyleTesta, Ugo, Germana Castelli, and Elvira Pelosi. 2025. "Precision Medicine for Older AML Patients" Onco 5, no. 3: 42. https://doi.org/10.3390/onco5030042
APA StyleTesta, U., Castelli, G., & Pelosi, E. (2025). Precision Medicine for Older AML Patients. Onco, 5(3), 42. https://doi.org/10.3390/onco5030042







