Exploring the Potential of Biotics in Cancer Prevention and Treatment—Mechanisms, Experimental, and Clinical Insights
Simple Summary
Abstract
1. Introduction
2. Gut Microbiome Homeostasis and Dysbiosis
2.1. Composition of the Gut Microbiome and Gut Homeostasis

2.2. Factors Affecting Gut Dysbiosis
2.3. Dysbiosis and Cancer
3. Prebiotics, Probiotics, Synbiotics, and Postbiotics
3.1. Prebiotics
3.2. Probiotics
3.3. Synbiotics
3.4. Postbiotics
3.5. Therapeutic Potential of the Biotics
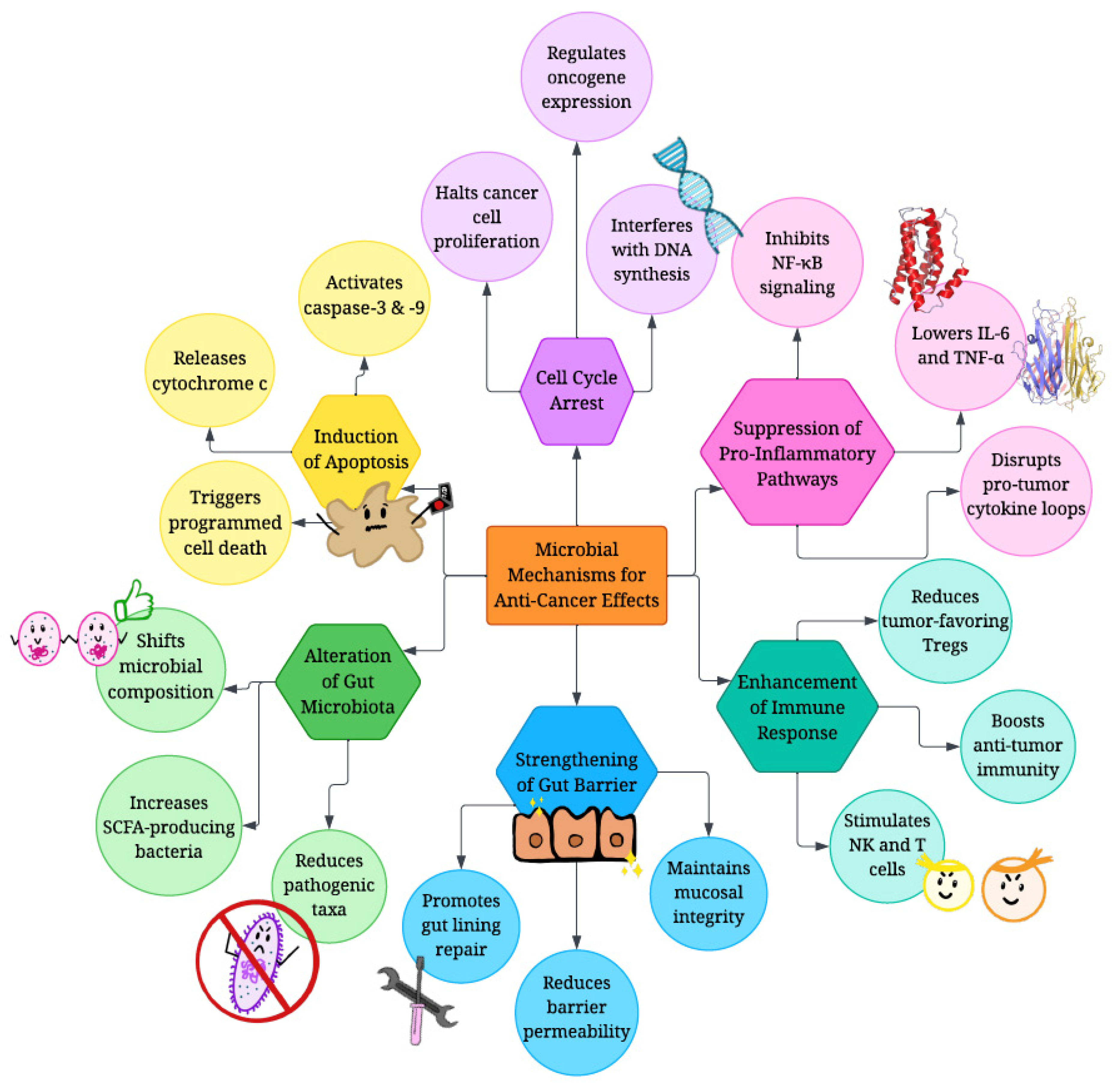
4. Mechanisms of Anti-Cancer Effects Displayed by Different Biotics
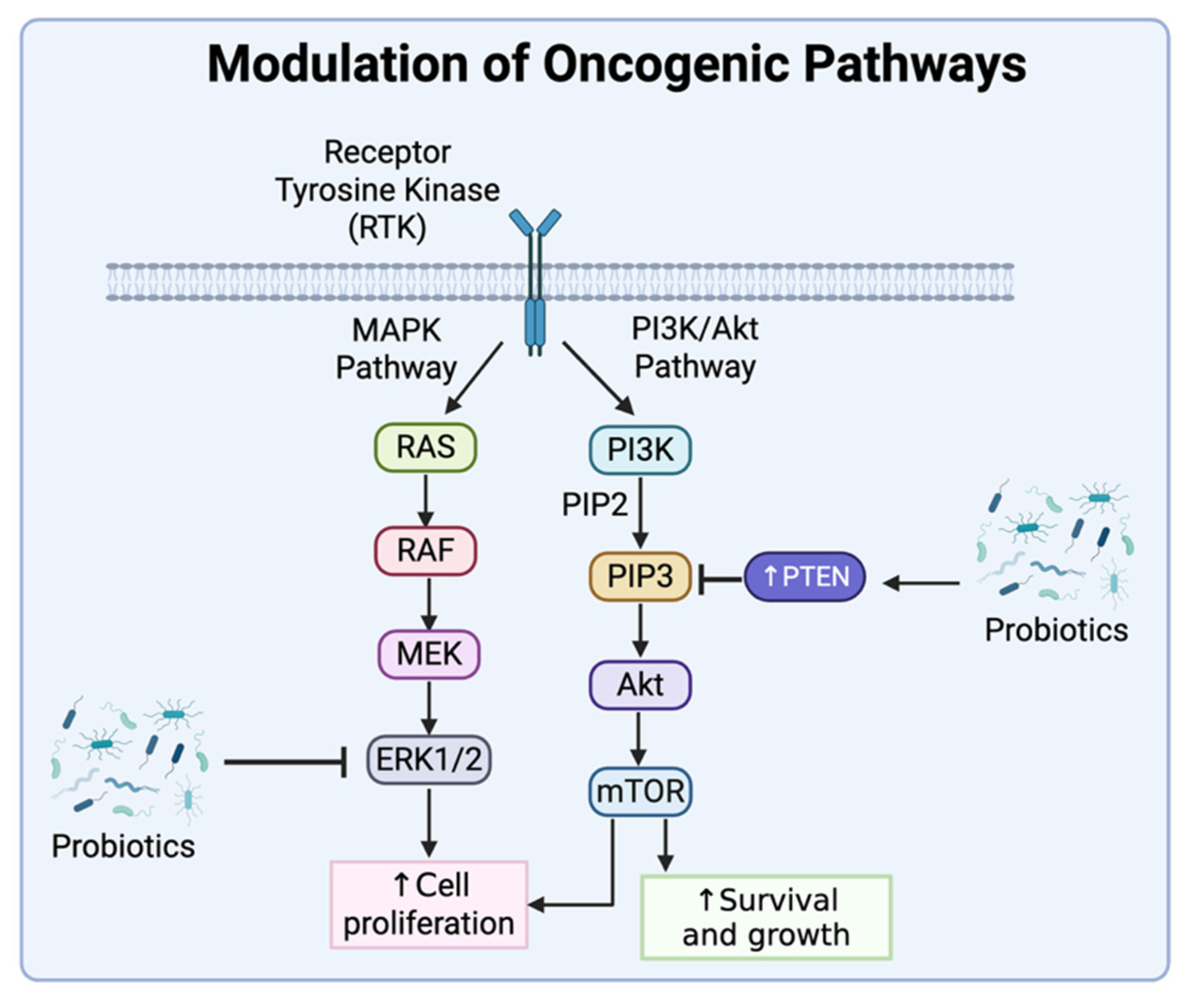
4.1. Modulation of Oncogenic Pathways
4.2. Induction of Apoptosis
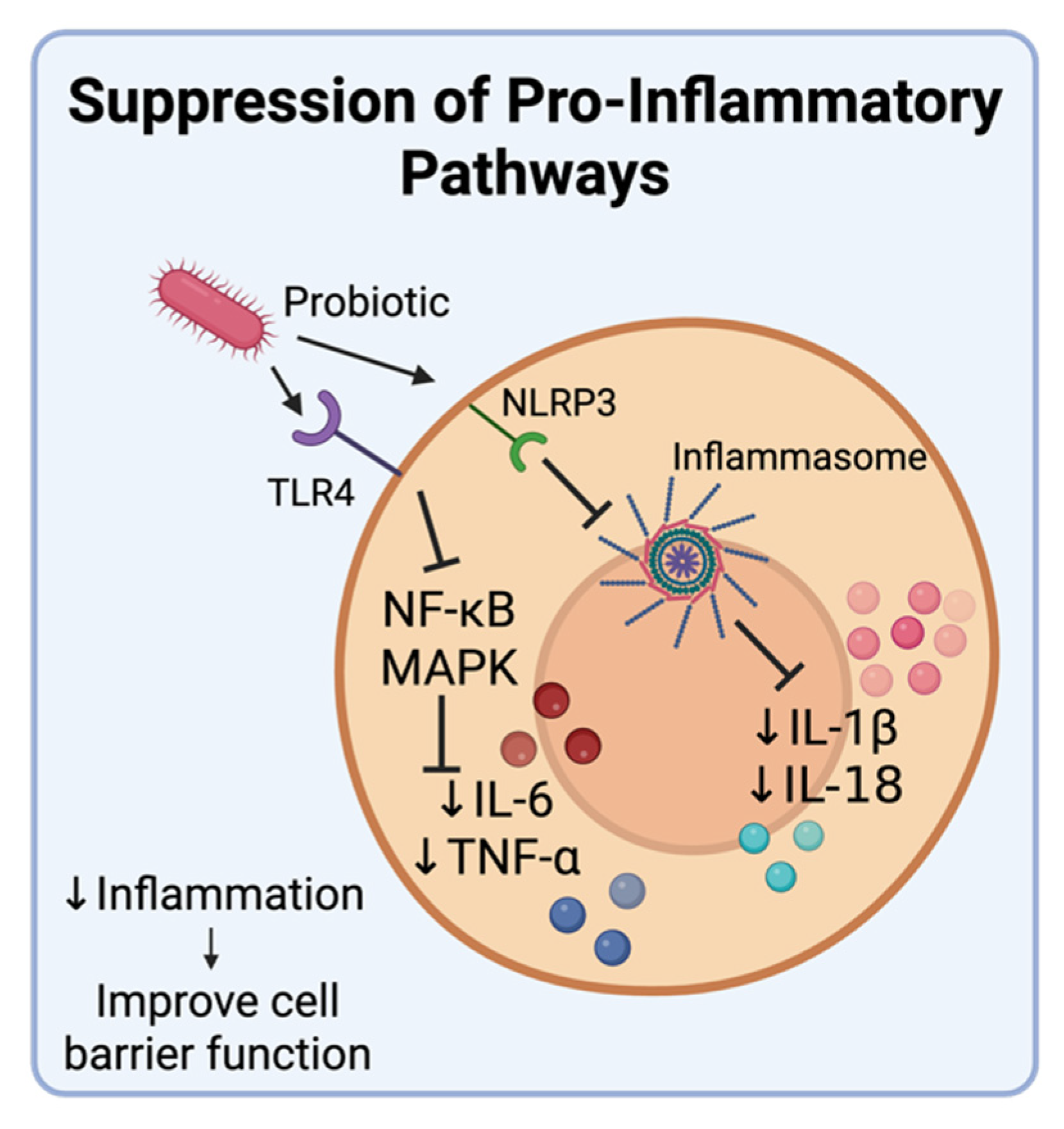
4.3. Modulating Inflammatory Cytokine Production
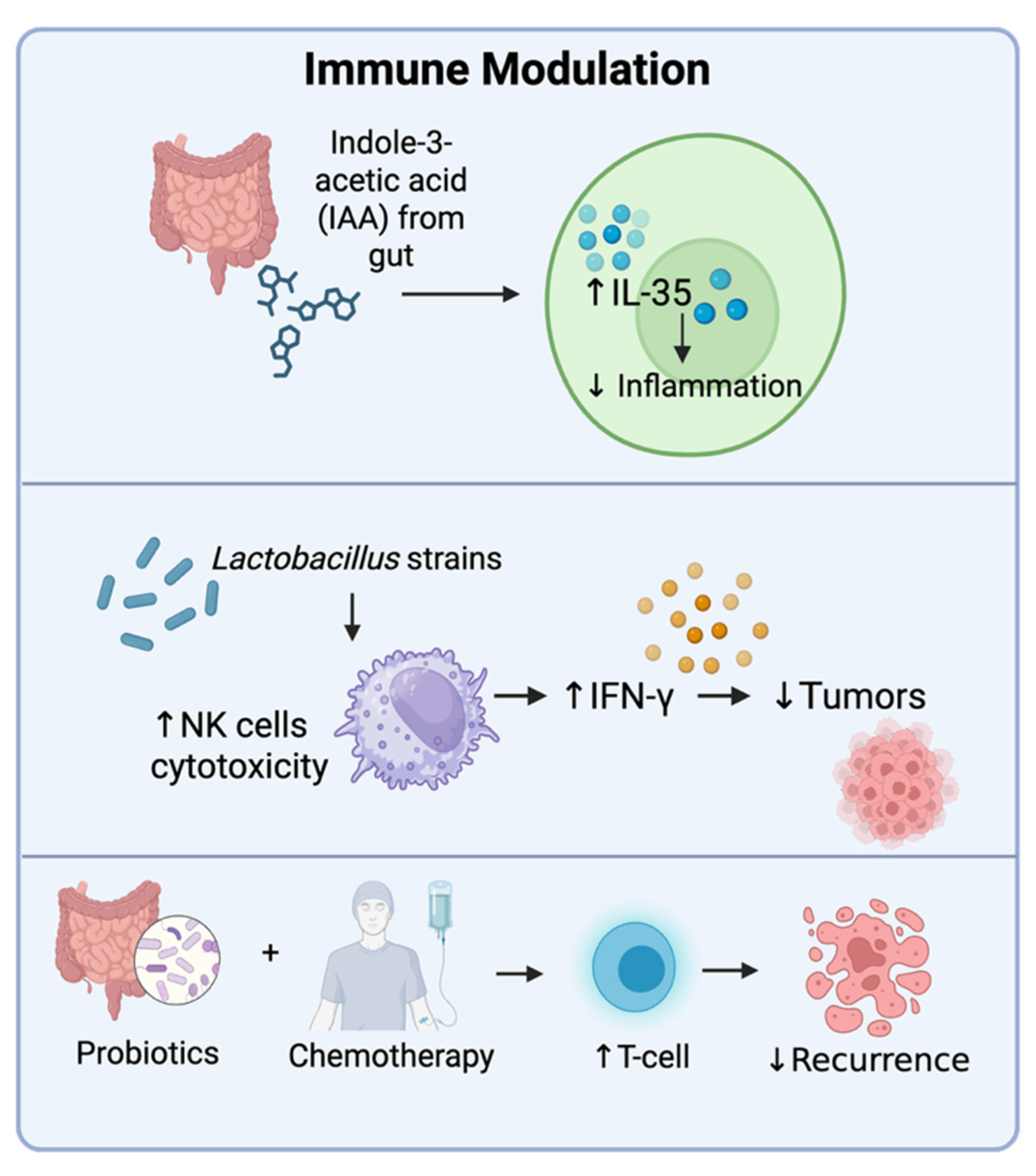
4.4. Immune Modulation
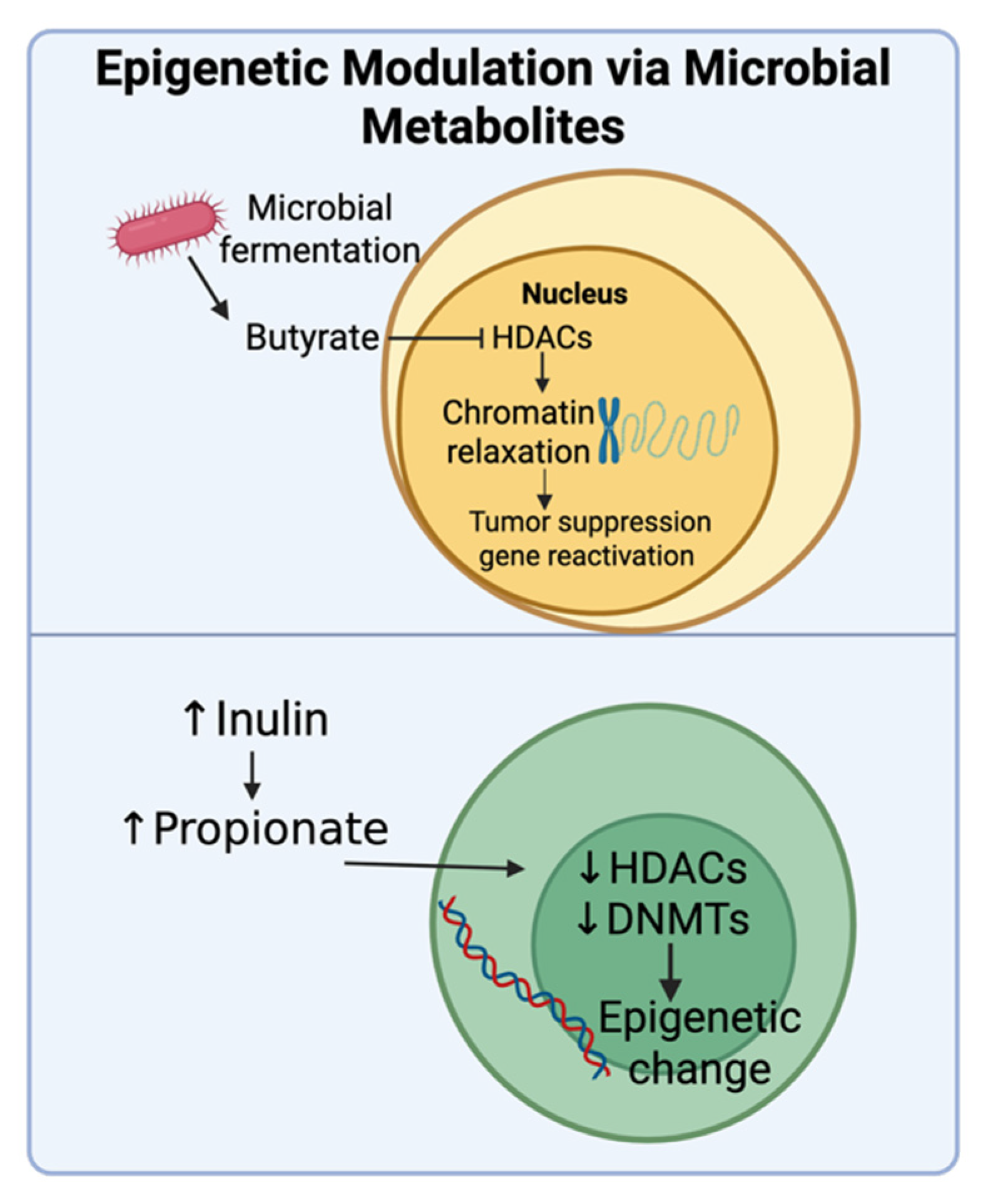
4.5. Epigenetic Modulation via Microbial Metabolites
5. Role of Prebiotics, Probiotics, Synbiotics, and Postbiotics in the Prevention and Treatment of Cancer
5.1. Colorectal Cancer
5.1.1. Prevention and Treatment of CRC Using Different Biotics
5.1.2. Effect of Biotics on Side Effects of CRC Treatment
5.2. Cervical Cancer
5.2.1. Prevention and Treatment of Cervical Cancer Using Biotics
5.2.2. Effect of Biotics on Traditional Cervical Cancer Treatments
5.3. Breast Cancer
5.3.1. Prevention and Treatment of Breast Cancer Using Biotics
5.3.2. Effect of Biotics on Breast Cancer Treatments
5.4. Other Cancers
5.5. Engineered Probiotics and Cancer
| Reference | Synbiotic Combination | Type of Cancer | Type of Trial | Effect |
|---|---|---|---|---|
| Rafter, et al. (2007) [128]. | Oligofructose-enriched inulin (SYN1) + Lactobacillus rhamnosus GG and Bifidobacterium lactis Bb12 | CRC | Double-blind, placebo-controlled trial of a synbiotic food composed of the synbiotic conducted in 37 colon cancer patients and 43 polypectomized patients. | Change in fecal flora, reduced IL-2 production, increased necrosis, reduced genotoxicity. |
| Flesch, et al. (2017) [180]. | Lactobacillus acidophilus, Lactobacillus rhamnosus, Lactobacillus casei, Bifi dobacterium, and fructo-oligosaccharide (FOS) | CRC | Double-blind randomized trial of 91 patients with 42 patients in the placebo group and 49 patients in the synbiotic treated group. | Decreased postoperative wound infection, inhibition of pathogens, and shortened hospital time |
| Krebs, (2016) [181]. | Synbiotic 2000 FORTE (Medipharm). It consists of Pediacoccus pentosaceus, Leuconostoc mesenteroides, Lactobacillus paracasei subsp. Paracasei, and Lactobacillus plantarum 2362 + 2.5 g of each of betaglucan, inulin, pectin, and resistant starch. | CRC | Randomized, controlled, double-blind trial of 54 patients who underwent surgery for colorectal cancer | Enriched colon microbiota, reduction in hospitalization time. Reduction in immune markers was not significantly different from the control group. |
| Farshi Radvar, et al. (2020) [182]. | Synbiotic capsules (Protexin) contained Lactobacillus casei PXN 37, Lactobacillus rhamnosus PXN 54, Streptococcus thermophilus 81 PXN 66, Bifidobacterium breve PXN 25, Lactobacillus acidophilus PXN 35, Bifidobacterium longum PXN 30, 82 Lactobacillus bulgaricus PXN 39, and Fructo-oligosaccharide | Rectal Cancer | Double-blind randomized parallel trial of 38 patients with 19 patients in the placebo group and 19 in the synbiotic treated group. | Increased intake of carbohydrates and proteins, reduction in matrix metalloproteins in patients undergoing chemoradiotherapy |
| Nascimento, et al. (2020) [183]. | Lactobacillus reuteri and soluble fiber (Nestlé) | Prostate cancer | Randomized, double-blind, placebo-controlled pilot trial of 20 patients with 10 patients in the placebo group and 10 in the synbiotic treated group. | Synbiotics prevented rectal inflammation/proctitis caused by radiation therapy for cancer |
| Sugawara, et al. (2006) [184]. | Lactobacillus casei strain Shirota; Bifiel (Yakult Honsha) containing Bifidobacterium breve strain Yakult; and galactooligosaccharide (Oligomate 55, Yakult Honsha) | Biliary cancer | Randomized controlled trial of 81 patients | Decrease in IL-6, CRP, and WBCs. Increase in beneficial bacteria population, decrease in pathogenic bacteria. |
| Tanaka, et al. (2012) [185]. | Yakult BL Seichoyaku (Yakult Honsha, Tokyo) Bifidobacterium breve strain Yakult (B. breve strain Yakult), Lactobacillus casei strain Shirota, and galacto-oligosaccharides (Oligomate S-HP; Yakult Honsha). | Esophageal cancer | Randomized controlled trial of 64 patients undergoing surgery | Reduced inflammation, increase in beneficial bacteria and SCFA production, 20% reduced infection occurrence in comparison to control |
| Sugimoto, et al. (2023) [186]. | Lacticaseibacillus paracasei strain Shirota, Bifidobacterium breve strain Yakult, and galacto-oligosaccharides | Esophageal cancer | Ancillary study to a randomized controlled trial in 73 cancer patients | Reduced febrile neutropenia and diarrhea in patients receiving neoadjuvant chemotherapy, correlated to an increase in beneficial bacteria and SCFA production. |
| Motoori, et al. (2017) [187]. | Yakult BL Seichoyaku containing Bifidobacterium breve strain Yakult and Lactobacillus casei strain and galacto-oligosaccharides | Esophageal cancer | Open-labeled randomized prospective clinical trial of 67 advanced-stage patients | Increased SCFA production reduced lymphopenia and diarrhea and reduced side effects of chemotherapy. |
| Manifar, et al. (2023) [188]. | FamiLact-Bifidobacterium breve, Bifidobacterium longum, Lactobacillus acidophilus, Lactobacillus casei, Lactobacillus bulgaricus, Lactobacillus rhamnosus, streptococcus salivarius +FOS | Oral Cancer | Double-blind randomized clinical trial on 64 oral cancer patients undergoing radiotherapy | Reduced oral mucositis after radiotherapy, changes in microbiome. |
| Koopaie, et al. (2025) [189]. | Synbiotic mouthwash containing Lactobacillus acidophilus, Lactobacillus casei, Lactobacillus rhamnosus, Lactobacillus salivarius, Lactobacillus reuteri, Bifidobacterium lactis, Bifidobacterium longum, Bifidobacterium bifidum and FOS | Head and neck cancer | Triple-blind, placebo-controlled, randomized clinical trial of 44 patients with squamous carcinoma. 22 patients were in the placebo group and 22 in the synbiotic treated group. | Non-significant reduction in salivary TLR 2, significant delay in onset of mucositis and intensity in synbiotic group compared to placebo group. |
| Sommacal, et al. (2015) [190]. | Lactobacillus acidophilus 10, Lactobacillus rhamnosus HS 111, Lactobacillus casei 10, Bifidobacterium bifidum, and fructooligosaccharides (FOS) | Periampullary neoplasm | Randomized double-blind clinical trial of 46 patients with 23 patients in the placebo group and 23 in the synbiotic treated group. | Reduced rate of infection and hospitalization period |
| Monshikarimi, et al. (2020) [191]. | L. rhamnosus Heriz I and soluble1–3,1–6,D-beta glucan | Breast cancer | Randomized double-blind placebo-controlled clinical trial in 30 patients | Significantly improved functional scale scores and reduced symptoms in patients receiving chemotherapy. |
| Tirgar, et al. (2024) [192]. | Lactobacillus casei, L. acidophilus, L. rhamnosus, L. salivarius, L. reuteri, Bifidobacterium lactis, B. longum, and B. bifidum + FOS + Vitamin D | Breast cancer | Double-blind randomized trial of 76 patients | Increased anti-inflammatory index ratio in symbiotic group |
| Khazaei, et al. (2023) [193]. | Lactocare® (Lactobacillus rhamnosus, Lactobacillus casei, Lactobacillus acidophilus, Lactobacillus bulgaricus, Bifidobacterium breve, Bifidobacterium longum, Lactobacillus helveticus, Lactobacillus lactis, Lactobacillus paraplantarum, Bifidobacterium bifidum, Streptococcus thermophilus and Lactobacillus gasseri + Fructo-oligosaccharides) | Breast cancer | Double-blind randomized clinical trial on 67 patients with 33 patients in the placebo group and 34 in the synbiotic treated group. | Reduced side effects of chemotherapy, nausea, fatigue, and anorexia. |
6. Safety Considerations Associated with Biotic Therapies
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BRCA | Breast cancer gene |
| BCR-Abl | Breakpoint cluster region-Abelson |
| CRC | Colorectal cancer |
| DNMT | DNA methyltransferase |
| DSB | Double-strand break |
| FMT | Fecal microbiota transplantation |
| FOS | Fructo-oligosaccharides |
| GOS | Galacto-oligosaccharides |
| HDAC | Histone deacetylase |
| HMOs | Human milk oligosaccharides |
| HPV | Human papillomavirus |
| IAA | Indole acetic acid |
| IgA | Immunoglobulin A |
| ISAPP | International Scientific Association for Probiotics and Prebiotics |
| MAPK | Mitogen-activated protein kinase |
| MLH1 | mutL homolog 1 |
| NK cells | Natural killer cells |
| mTOR | Mechanistic target of rapamycin |
| PI3K | Phosphoinositide 3-kinase |
| PIP3 | Phosphatidylinositol (3,4,5)-trisphosphate |
| SCFAs | Short chain fatty acids |
| Tregs | Regulatory T-cells |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Blackadar, C.B. Historical review of the causes of cancer. World J. Clin. Oncol. 2016, 7, 54–86. [Google Scholar] [CrossRef] [PubMed]
- Kandalai, S.; Li, H.; Zhang, N.; Peng, H.; Zheng, Q. The human microbiome and cancer: A diagnostic and therapeutic perspective. Cancer Biol. Ther. 2023, 24, 2240084. [Google Scholar] [CrossRef]
- Wang, Q. Cancer predisposition genes: Molecular mechanisms and clinical impact on personalized cancer care: Examples of Lynch and HBOC syndromes. Acta Pharmacol. Sin. 2016, 37, 143–149. [Google Scholar] [CrossRef]
- Madia, F.; Worth, A.; Whelan, M.; Corvi, R. Carcinogenicity assessment: Addressing the challenges of cancer and chemicals in the environment. Environ. Int. 2019, 128, 417–429. [Google Scholar] [CrossRef]
- Ofoezie, E.F.; Ogbonna, C.A.; Olisakwe, S.C.; Anunobi, C.J.; George, E.T.; Babarinde, S.; Chuweumeka, C.G.; Ogbonna, U.E.; Amafili, C.C.; Alisigwe, C.V.; et al. Role of infectious agents in cancer pathogenesis and therapy. Microbe 2025, 6, 100284. [Google Scholar] [CrossRef]
- Kennedy, M.S.; Chang, E.B. The microbiome: Composition and locations. Prog. Mol. Biol. Transl. Sci. 2020, 176, 1–42. [Google Scholar] [PubMed]
- Lloyd-Price, J.; Abu-Ali, G.; Huttenhower, C. The healthy human microbiome. Genome Med. 2016, 8, 51. [Google Scholar] [CrossRef] [PubMed]
- Mariotto, A.B.; Enewold, L.; Zhao, J.; Zeruto, C.A.; Yabroff, K.R. Medical Care Costs Associated with Cancer Survivorship in the United States. Cancer Epidemiol. Biomark. Prev. 2020, 29, 1304–1312. [Google Scholar] [CrossRef]
- Liu, B.; Zhou, H.; Tan, L.; Siu, K.T.H.; Guan, X.Y. Exploring treatment options in cancer: Tumor treatment strategies. Signal Transduct. Target. Ther. 2024, 9, 175. [Google Scholar] [CrossRef]
- Leonard, J.M.; Toro, D.D. Defining the Microbiome Components (Bacteria, Viruses, Fungi) and Microbiome Geodiversity. Surg. Infect. 2023, 24, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Matijašić, M.; Meštrović, T.; Paljetak, H.Č.; Perić, M.; Barešić, A.; Verbanac, D. Gut microbiota beyond bacteria-mycobiome, virome, archaeome, and eukaryotic parasites in IBD. Int. J. Mol. Sci. 2020, 21, 2668. [Google Scholar] [CrossRef]
- Rosenberg, E. Diversity of bacteria within the human gut and its contribution to the functional unity of holobionts. NPJ Biofilms Microbiomes 2024, 10, 134. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes ratio: A relevant marker of gut dysbiosis in obese patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef]
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Reddy, D.N. Role of the normal gut microbiota. World J. Gastroenterol. 2015, 21, 8836–8847. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, S.; Li, S.; Jiang, W.; Wang, J.; Xiao, J.; Chen, T.; Ma, J.; Khan, M.Z.; Wang, W.; et al. Unlocking the power of postbiotics: A revolutionary approach to nutrition for humans and animals. Cell Metab. 2024, 36, 725–744. [Google Scholar] [CrossRef] [PubMed]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef]
- Petersen, C.; Round, J.L. Defining dysbiosis and its influence on host immunity and disease. Cell Microbiol. 2014, 16, 1024–1033. [Google Scholar] [CrossRef]
- Lin, D.; Medeiros, D.M. The microbiome as a major function of the gastrointestinal tract and its implication in micronutrient metabolism and chronic diseases. Nutr. Res. 2023, 112, 30–45. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.; Kolodziejczyk, A.A.; Thaiss, C.A.; Elinav, E. Dysbiosis and the immune system. Nat. Rev. Immunol. 2017, 17, 219–232. [Google Scholar] [CrossRef]
- Vimal, J.; Himal, I.; Kannan, S. Role of microbial dysbiosis in carcinogenesis & cancer therapies. Indian. J. Med. Res. 2020, 152, 553–561. [Google Scholar]
- Hou, K.; Wu, Z.X.; Chen, X.Y.; Wang, J.Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef]
- Maciel-Fiuza, M.F.; Muller, G.C.; Campos, D.M.S.; do Socorro Silva Costa, P.; Peruzzo, J.; Bonamigo, R.R.; Veit, T.; Vianna, J.S.L. Role of gut microbiota in infectious and inflammatory diseases. Front. Microbiol. 2023, 14, 1098386. [Google Scholar] [CrossRef] [PubMed]
- Kesavelu, D.; Jog, P. Current understanding of antibiotic-associated dysbiosis and approaches for its management. Ther. Adv. Infect. Dis. 2023, 10, 20499361231154443. [Google Scholar] [CrossRef]
- Winter, S.E.; Bäumler, A.J. Gut dysbiosis: Ecological causes and causative effects on human disease. Proc. Natl. Acad. Sci. USA 2023, 120, e2316579120. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; El-Fahmawi, A.; Christian, D.A.; Fang, Q.; Radaelli, E.; Chen, L.; Sullivan, M.C.; Misic, A.M.; Ellringer, J.A.; Zhu, X.Q.; et al. Infection-induced intestinal dysbiosis is mediated by macrophage activation and nitrate production. mBio 2019, 10, e00935-19. [Google Scholar] [CrossRef] [PubMed]
- Aljahdali, N.H.; Sanad, Y.M.; Han, J.; Foley, S.L. Current knowledge and perspectives of potential impacts of Salmonella enterica on the profile of the gut microbiota. BMC Microbiol. 2020, 20, 353. [Google Scholar] [CrossRef]
- Zeng, M.Y.; Inohara, N.; Nuñez, G. Mechanisms of inflammation-driven bacterial dysbiosis in the gut. Mucosal Immunol. 2017, 10, 18–26. [Google Scholar] [CrossRef]
- Blanton, L.V.; Charbonneau, M.R.; Salih, T.; Barratt, M.J.; Venkatesh, S.; Ilkaveya, O.; Subramanian, S.; Manary, M.J.; Trehan, I.; Jorgensan, J.M.; et al. Gut bacteria that prevent growth impairments transmitted by microbiota from malnourished children. Science 2016, 351, aad3311. [Google Scholar] [CrossRef]
- Castro-Mejía, J.L.; O’Ferrall, S.; Krych, Ł.; O’Mahony, E.; Namusoke, H.; Lanyero, B.; Kot, W.; Nabukeera Barungi, N.; Michaelsen, K.F.; Molgaard, C.; et al. Restitution of gut microbiota in Ugandan children administered with probiotics (Lactobacillus rhamnosus GG and Bifidobacterium animalis subsp. lactis BB-12) during treatment for severe acute malnutrition. Gut Microbes 2020, 11, 855–867. [Google Scholar] [PubMed]
- Yang, J.; Wei, H.; Zhou, Y.; Szeto, C.H.; Li, C.; Lin, Y.; Coker, O.O.; Lau, H.C.H.; Chan, A.W.; Sung, J.J.; et al. High-Fat Diet Promotes Colorectal Tumorigenesis Through Modulating Gut Microbiota and Metabolites. Gastroenterology 2022, 162, 135–149.e2. [Google Scholar] [CrossRef] [PubMed]
- García-Montero, C.; Fraile-Martínez, O.; Gómez-Lahoz, A.M.; Pekarek, L.; Castellanos, A.J.; Noguerales-Fraguas, F.; Coca, S.; Guijjaro, L.G.; García-Honduvilla, N.; Asúnsolo, A.; et al. Nutritional components in Western diet versus Mediterranean diet at the gut microbiota-immune system interplay. Implications for health and disease. Nutrients 2021, 13, 699. [Google Scholar] [CrossRef]
- Rondinella, D.; Raoul, P.C.; Valeriani, E.; Venturini, I.; Cintoni, M.; Severino, A.; Galli, F.S.; Mora, V.; Mele, M.C.; Cammarota, G.; et al. The detrimental impact of ultra-processed foods on the human gut microbiome and gut barrier. Nutrients 2025, 17, 859. [Google Scholar] [CrossRef]
- Fan, X.; Jin, Y.; Chen, G.; Ma, X.; Zhang, L. Gut microbiota dysbiosis drives the development of colorectal cancer. Digestion 2021, 102, 508–515. [Google Scholar] [CrossRef]
- Moreira, M.M.; Carriço, M.; Capelas, M.L.; Pimenta, N.; Santos, T.; Ganhão-Arranhado, S.; Mäkitie, A.; Ravasco, P. The impact of pre-, pro- and synbiotics supplementation in colorectal cancer treatment: A systematic review. Front. Oncol. 2024, 14, 1395966. [Google Scholar] [CrossRef]
- Garrett, W.S.; Punit, S.; Gallini, C.A.; Michaud, M.; Zhang, D.; Sigrist, K.S.; Lord, G.M.; Glickman, J.N.; Glimcher, L.H. Colitis-associated colorectal cancer driven by T-bet deficiency in dendritic cells. Cancer Cell 2009, 16, 208–219. [Google Scholar] [CrossRef]
- Gopalakrishnan, V.; Spencer, C.N.; Nezi, L.; Reuben, A.; Andrews, M.C.; Karpinets, T.V.; Prieto, P.A.; Vicente, D.; Hoffman, K.; Wei, S.C.; et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 2018, 359, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Kaakoush, N.O. Fecal transplants as a microbiome-based therapeutic. Curr. Opin. Microbiol. 2020, 56, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Davar, D.; Dzutsev, A.K.; McCulloch, J.A.; Rodrigues, R.R.; Chauvin, J.M.; Morrison, R.M.; Deblasio, R.N.; Menna, C.; Ding, Q.; Pagliano, O.; et al. Fecal microbiota transplant overcomes resistance to anti-PD-1 therapy in melanoma patients. Science 2021, 371, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Baruch, E.N.; Youngster, I.; Ben-Betzalel, G.; Ortenberg, R.; Lahat, A.; Katz, L.; Adler, K.; Dick-Necula, D.; Raskin, S.; Bloch, N.; et al. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science 2021, 371, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Yadegar, A.; Bar-Yoseph, H.; Monaghan, T.M.; Pakpour, S.; Severino, A.; Kuijper, E.J.; Smits, W.K.; Terveer, E.M.; Neupane, S.; Nabavi-Rad, A.; et al. Fecal microbiota transplantation: Current challenges and future landscapes. Clin. Microbiol. Rev. 2024, 37, e0006022. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Barile, D.; Rastall, R.A. Human milk and related oligosaccharides as prebiotics. Curr. Opin. Biotechnol. 2013, 24, 214–219. [Google Scholar] [CrossRef]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, types, sources, mechanisms, and clinical applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef]
- Fusco, W.; Lorenzo, M.B.; Cintoni, M.; Porcari, S.; Rinninella, E.; Kaitsas, F.; Lener, E.; Mele, M.C.; Gasbarrini, A.; Collado, M.C.; et al. Short-Chain Fatty-Acid-Producing Bacteria: Key Components of the Human Gut Microbiota. Nutrients 2023, 15, 2211. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Farias, C.; Slezak, K.; Fuller, Z.; Duncan, A.; Holtrop, G.; Louis, P. Effect of inulin on the human gut microbiota: Stimulation of Bifidobacterium adolescentis and Faecalibacterium prausnitzii. Br. J. Nutr. 2008, 101, 541–550. [Google Scholar] [CrossRef]
- Hodgkinson, K.; El Abbar, F.; Dobranowski, P.; Manoogian, J.; Butcher, J.; Figeys, D.; Mack, D.; Stintzi, A. Butyrate’s role in human health and the current progress towards its clinical application to treat gastrointestinal disease. Clin. Nutr. 2023, 42, 61–75. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document: The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Fijan, S. Microorganisms with claimed probiotic properties: An overview of recent literature. Int. J. Environ. Res. Public Health 2014, 11, 4745–4767. [Google Scholar] [CrossRef]
- Hemarajata, P.; Versalovic, J. Effects of probiotics on gut microbiota: Mechanisms of intestinal immunomodulation and neuromodulation. Therap Adv. Gastroenterol. 2013, 6, 39–51. [Google Scholar] [CrossRef]
- You, S.; Ma, Y.; Yan, B.; Pei, W.; Wu, Q.; Ding, C.; Huang, C. The promotion mechanism of prebiotics for probiotics: A review. Front. Nutr. 2022, 9, 1000517. [Google Scholar] [CrossRef] [PubMed]
- Kolida, S.; Gibson, G.R. Synbiotics in Health and Disease. Annu. Rev. Food Sci. Technol. 2011, 2, 373–393. [Google Scholar] [CrossRef]
- Markowiak, P.; Śliżewska, K. Effects of Probiotics, Prebiotics, and Synbiotics on Human Health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef] [PubMed]
- Khursheed, R.; Gulati, M.; Wadhwa, S.; Vishwas, S.; Sharma, D.S.; Corrie, L.; Alam, A.; Alnasser, S.M.; Alkhayl, F.F.A.; Parveen, Z.; et al. Multifaceted role of synbiotics as nutraceuticals, therapeutics and carrier for drug delivery. Chem. Biol. Interact. 2022, 368, 110223. [Google Scholar] [CrossRef]
- Mosca, A.; Abreu, Y.; Abreu, A.T.; Gwee, K.A.; Ianiro, G.; Tack, J.; Nguyen, T.V.H.; Hill, C. The clinical evidence for postbiotics as microbial therapeutics. Gut Microbes 2022, 14, 2117508. [Google Scholar] [CrossRef]
- Adams, C.A. The probiotic paradox: Live and dead cells are biological response modifiers. Nutr. Res. Rev. 2010, 23, 37–46. [Google Scholar] [CrossRef]
- Caetano, M.A.F.; Castelucci, P. Role of short chain fatty acids in gut health and possible therapeutic approaches in inflammatory bowel diseases. World J. Clin. Cases 2022, 10, 9985–10003. [Google Scholar] [CrossRef]
- da Silva Vale, A.; de Melo Pereira, G.V.; de Oliveira, A.C.; de Carvalho Neto, D.P.; Herrmann, L.W.; Karp, S.G.; Soccol, V.T.; Soccol, T.R. Production, formulation, and application of postbiotics in the treatment of skin conditions. Fermentation 2023, 9, 264. [Google Scholar] [CrossRef]
- Piqué, N.; Berlanga, M.; Miñana-Galbis, D. Health benefits of heat-killed (tyndallized) probiotics: An overview. Int. J. Mol. Sci. 2019, 20, 2534. [Google Scholar] [CrossRef] [PubMed]
- Lopez, A.; Iyer, J.K. Insights into bacteriotherapy: Harnessing bacteria for the greater good. Biomed. J. Sci. Tech. Res. 2025, 62, 54253–54261. [Google Scholar] [CrossRef]
- Gupta, V.; Mastromarino, P.; Garg, R. Effectiveness of prophylactic oral and/or vaginal probiotic supplementation in the prevention of recurrent urinary tract infections: A Randomized, Double-Blind, Placebo-Controlled Trial. Clin. Infect. Dis. 2024, 78, 1154–1161. [Google Scholar] [CrossRef] [PubMed]
- Kukkonen, K.; Savilahti, E.; Haahtela, T.; Juntunen-Backman, K.; Korpela, R.; Poussa, T.; Tuure, T.; Kuitunen, M. Long-term safety and impact on infection rates of postnatal probiotic and prebiotic (synbiotic) treatment: Randomized, double-blind, placebo-controlled trial. Pediatrics 2008, 122, 8–12. [Google Scholar] [CrossRef]
- Yang, Z.; Zhou, Y.; Han, Z.; He, K.; Zhang, Y.; Wu, D.; Chen, H. The effects of probiotics supplementation on Helicobacter pylori standard treatment: An umbrella review of systematic reviews with meta-analyses. Sci. Rep. 2024, 14, 10069. [Google Scholar] [CrossRef] [PubMed]
- Al Sharaby, A.; Abugoukh, T.M.; Ahmed, W.; Ahmed, S.; Elshaikh, A.O. Do probiotics prevent Clostridium difficile-associated diarrhea? Cureus 2022, 14, e27624. [Google Scholar] [CrossRef]
- Lopez-Santamarina, A.; Lamas, A.; Del Carmen Mondragón, A.; Cardelle-Cobas, A.; Regal, P.; Rodriguez-Avila, J.A.; Miranda, J.M.; Franco, C.M.; Cepeda, A. Probiotic effects against virus infections: New weapons for an old war. Foods 2021, 10, 130. [Google Scholar] [CrossRef]
- Tamaki, H.; Nakase, H.; Inoue, S.; Kawanami, C.; Itani, T.; Ohana, M.; Kusaka, T.; Uose, S.; Hisatsune, H.; Tojo, M.; et al. Efficacy of probiotic treatment with Bifidobacterium longum 536 for induction of remission in active ulcerative colitis: A randomized, double-blinded, placebo-controlled multicenter trial. Dig. Endosc. 2016, 28, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Sood, A.; Midha, V.; Makharia, G.K.; Ahuja, V.; Singal, D.; Goswami, P.; Tandon, R.K. The probiotic preparation, VSL#3 induces remission in patients with mild-to-moderately active ulcerative colitis. Clin. Gastroenterol. Hepatol. 2009, 7, 1202–1209. [Google Scholar]
- Ambalam, P.; Raman, M.; Purama, R.K.; Doble, M. Probiotics, prebiotics and colorectal cancer prevention. Best. Pract. Res. Clin. Gastroenterol. 2016, 30, 119–131. [Google Scholar] [CrossRef]
- Peitsidou, K.; Karantanos, T.; Theodoropoulos, G.E. Probiotics, prebiotics, synbiotics: Is there enough evidence to support their use in colorectal cancer surgery? Dig. Surg. 2012, 29, 426–438. [Google Scholar] [CrossRef]
- Gutiérrez Salmeán, G.; Delgadillo González, M.; Rueda Escalona, A.A.; Leyva Islas, J.A.; Castro-Eguiluz, D. Effects of prebiotics, probiotics, and synbiotics on the prevention and treatment of cervical cancer: Mexican consensus and recommendations. Front. Oncol. 2024, 14, 1383258. [Google Scholar] [CrossRef]
- Lu, K.; Dong, S.; Wu, X.; Jin, R.; Chen, H. Probiotics in cancer. Front. Oncol. 2021, 11, 638148. [Google Scholar] [CrossRef]
- Śliżewska, K.; Markowiak-Kopeć, P.; Śliżewska, W. The role of probiotics in cancer prevention. Cancers 2021, 13, 20. [Google Scholar] [CrossRef] [PubMed]
- Alam, Z.; Shang, X.; Effat, K.; Kanwal, F.; He, X.; Li, Y.; Xu, C.; Niu, W.; War, A.R.; Zhang, Y. The potential role of prebiotics, probiotics, and synbiotics in adjuvant cancer therapy especially colorectal cancer. J. Food Biochem. 2022, 46, e14302. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.M.; Versalovic, J. Probiotics-host communication. Gut Microbes 2010, 1, 148–163. [Google Scholar] [CrossRef] [PubMed]
- Morgos, D.T.; Stefani, C.; Miricescu, D.; Greabu, M.; Stanciu, S.; Nica, S.; Stanescu-Spinu, I.I.; Balan, D.G.; Balcangiu-Stroescu, A.E.; Coculescu, E.C.; et al. Targeting PI3K/AKT/mTOR and MAPK signaling pathways in gastric cancer. Int. J. Mol. Sci. 2024, 25, 1848. [Google Scholar] [CrossRef]
- Guo, Q.; Jin, Y.; Chen, X.; Ye, X.; Shen, X.; Lin, M.; Zeng, C.; Zhou, T.; Zhang, J. NF-κB in biology and targeted therapy: New insights and translational implications. Signal Transduct. Target. Ther. 2024, 9, 53. [Google Scholar] [CrossRef]
- He, Y.; Sun, M.M.; Zhang, G.G.; Yang, J.; Chen, K.S.; Xu, W.W.; Li, B. Targeting PI3K/Akt signal transduction for cancer therapy. Signal Transduct. Target. Ther. 2021, 6, 425. [Google Scholar] [CrossRef]
- Asoudeh-Fard, A.; Barzegari, A.; Dehnad, A.; Bastani, S.; Golchin, A.; Omidi, Y. Lactobacillus plantarum induces apoptosis in oral cancer KB cells through upregulation of PTEN and downregulation of MAPK signaling pathways. BioImpacts 2017, 7, 193–198. [Google Scholar] [CrossRef]
- Zhou, L.; Xie, Y.; Li, Y. Bifidobacterium infantis promotes Foxp3 expression in colon cells via PD-L1-mediated inhibition of the PI3K-Akt-mTOR signaling pathway. Front. Immunol. 2022, 13, 871705. [Google Scholar] [CrossRef]
- Jastrząb, R.; Graczyk, D.; Siedlecki, P. Molecular and cellular mechanisms influenced by postbiotics. Int. J. Mol. Sci. 2021, 22, 13475. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, M.; Ahmad, R.; Tantry, I.Q.; Ahmad, W.; Siddiqui, S.; Alam, M.; Abbas, K.; Moinuddin Hassan, M.I.; Habib, S.; Islam, S. Apoptosis: A comprehensive overview of signaling pathways, morphological changes, and physiological significance and therapeutic implications. Cells 2024, 13, 1838. [Google Scholar] [CrossRef]
- Nami, Y.; Abdullah, N.; Haghshenas, B.; Radiah, D.; Rosli, R.; Khosroushahi, A.Y. Assessment of probiotic potential and anticancer activity of newly isolated vaginal bacterium Lactobacillus plantarum 5BL. Microbiol. Immunol. 2014, 58, 492–502. [Google Scholar] [CrossRef]
- Jam, S.A.M.; Morshedi, M.; Khosroushahi, A.Y.; Eftekharsadat, A.T.; Alipour, M.; Alipour, B. Preventive and tumor-suppressive effects of Lactobacillus paracasei x12 in rat model of colorectal cancer. Iran. J. Pharm. Res. 2020, 19, 330–342. [Google Scholar]
- Kim, S.J.; Kang, C.H.; Kim, G.H.; Cho, H. Anti-tumor effects of heat-killed L. reuteri MG5346 and L. casei MG4584 against human colorectal carcinoma through caspase-9-dependent apoptosis in xenograft model. Microorganisms 2022, 10, 533. [Google Scholar] [CrossRef]
- Cui, L.; Xu, X.; Fan, H.; Wan, X.; Chen, Q.; Zhang, J.; Tao, C.; Du, Z.; Wang, Y.; Zhang, J.; et al. Reuterin promotes pyroptosis in hepatocellular cancer cells through mtDNA-mediated STING activation and caspase 8 expression. Cancer Lett. 2024, 601, 217183. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Dai, M.; Qiu, C.; Sun, Q.; Fan, T.; Guo, Y.; Zhao, L.; Jiang, Y. γ-Linolenic acid derived from Lactobacillus plantarum MM89 induces ferroptosis in colorectal cancer. Food Funct. 2025, 16, 1760–1771. [Google Scholar] [CrossRef]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and tumor progression: Signaling pathways and targeted intervention. Signal Transduct. Target. Ther. 2021, 6, 263. [Google Scholar] [CrossRef] [PubMed]
- Cristofori, F.; Dargenio, V.N.; Dargenio, C.; Miniello, V.L.; Barone, M.; Francavilla, R. Anti-inflammatory and immunomodulatory effects of probiotics in gut inflammation: A door to the body. Front. Immunol. 2021, 12, 578386. [Google Scholar] [CrossRef]
- Yue, Y.; Wang, Y.; Xie, Q.; Lv, X.; Zhou, L.; Smith, E.E.; Cao, T.; Zhang, Y.; Li, B.; Huo, G.; et al. Bifidobacterium bifidum E3 combined with Bifidobacterium longum subsp. infantis E4 improves LPS-induced intestinal injury by inhibiting the TLR4/NF-κB and MAPK signaling pathways in vivo. J. Agric. Food Chem. 2023, 71, 8915–8930. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hao, Y.; Yang, Y.; Zhang, Y.; Xu, C.; Yang, R. Gut microbiota derived indole-3-acetic acid ameliorates precancerous inflammatory intestinal milieu to inhibit tumorigenesis through IL-35. J. Immunother. Cancer 2025, 13, e011155. [Google Scholar] [CrossRef]
- Kasti, A.N.; Synodinou, K.D.; Pyrousis, I.A.; Nikolaki, M.D.; Triantafyllou, K.D. Probiotics regulating inflammation via NLRP3 inflammasome modulation: A potential therapeutic approach for COVID-19. Microorganisms 2021, 9, 2376. [Google Scholar] [CrossRef]
- Galassi, C.; Chan, T.A.; Vitale, I.; Galluzzi, L. The hallmarks of cancer immune evasion. Cancer Cell 2024, 42, 1825–1863. [Google Scholar] [CrossRef]
- Mazziotta, C.; Tognon, M.; Martini, F.; Torreggiani, E.; Rotondo, J.C. Probiotics mechanism of action on immune cells and beneficial effects on human health. Cells 2023, 12, 184. [Google Scholar] [CrossRef]
- Le Noci, V.; Guglielmetti, S.; Arioli, S.; Camisaschi, C.; Bianchi, F.; Sommariva, M.; Storti, C.; Triulzi, T.; Castelli, C.; Balsari, A.; et al. Modulation of pulmonary microbiota by antibiotic or probiotic aerosol therapy: A strategy to promote immunosurveillance against lung metastases. Cell Rep. 2018, 24, 3528–3538. [Google Scholar] [CrossRef]
- Zhao, M.; Liang, X.; Meng, Y.; Lu, H.; Lin, K.; Gong, P.; Liu, T.; Yi, H.; Pan, J.; Zhang, Y.; et al. Probiotics induce intestinal IgA secretion in weanling mice potentially through promoting intestinal APRIL expression and modulating the gut microbiota composition. Food Funct. 2024, 15, 4862–4873. [Google Scholar] [CrossRef]
- Zhang, J.W.; Du, P.; Yang, B.R.; Gao, J.; Fang, W.J.; Ying, C.M. Preoperative probiotics decrease postoperative infectious complications of colorectal cancer. Am. J. Med. Sci. 2012, 343, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Miyake, M.; Oda, Y.; Owari, T.; Iida, K.; Ohnishi, S.; Fujii, T.; Nishimura, N.; Miyamoto, T.; Shimizu, T.; Ohnishi, K.; et al. Probiotics enhances anti-tumor immune response induced by gemcitabine plus cisplatin chemotherapy for urothelial cancer. Cancer Sci. 2023, 114, 1118–1130. [Google Scholar] [CrossRef] [PubMed]
- Legesse Bedada, T.; Feto, T.K.; Awoke, K.S.; Garedew, A.D.; Yifat, F.T.; Birri, D.J. Probiotics for cancer alternative prevention and treatment. Biomed. Pharmacother. 2020, 129, 110409. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Rodrigues e-Lacerda, R.; Barra, N.G.; Kukje Zada, D.; Robin, N.; Mehra, A.; Schertzer, J.D. Postbiotic Impact on Host Metabolism and immunity provides therapeutic potential in metabolic disease. Endocr. Rev. 2025, 46, 60–79. [Google Scholar] [CrossRef]
- Pujari, R.; Banerjee, G. Impact of prebiotics on immune response: From the bench to the clinic. Immunol. Cell Biol. 2021, 99, 255–273. [Google Scholar] [CrossRef]
- Chriett, S.; Dąbek, A.; Wojtala, M.; Vidal, H.; Balcerczyk, A.; Pirola, L. Prominent action of butyrate over β-hydroxybutyrate as histone deacetylase inhibitor, transcriptional modulator and anti-inflammatory molecule. Sci. Rep. 2019, 9, 742. [Google Scholar] [CrossRef]
- Wu, H.; Van Der Pol, W.J.; Dubois, L.G.; Morrow, C.D.; Tollefsbol, T.O. Dietary supplementation of inulin contributes to the prevention of estrogen receptor-negative mammary cancer by alteration of gut microbial communities and epigenetic regulations. Int. J. Mol. Sci. 2023, 24, 9015. [Google Scholar] [CrossRef]
- Mishra, P.; Badiyani, V.M.; Jain, S.; Subramanian, S.; Maharaj, S.V.; Kumar, A.; Singh, B.N. Prebiotics: Ignored player in the fight against cancer. Cancer Rep. 2023, 6, e1870. [Google Scholar] [CrossRef]
- Geier, M.S.; Butler, R.N.; Howarth, G.S. Probiotics, prebiotics and synbiotics: A role in chemoprevention for colorectal cancer? Cancer Biol. Ther. 2006, 5, 1265–1269. [Google Scholar] [CrossRef]
- Mahdavi, M.; Laforest-Lapointe, I.; Massé, E. Preventing colorectal cancer through prebiotics. Microorganisms 2021, 9, 1325. [Google Scholar] [CrossRef] [PubMed]
- Tukenmez, U.; Aktas, B.; Aslim, B.; Yavuz, S. The relationship between the structural characteristics of lactobacilli-EPS and its ability to induce apoptosis in colon cancer cells in vitro. Sci. Rep. 2019, 9, 8268. [Google Scholar] [CrossRef] [PubMed]
- Bauer-Marinovic, M. Dietary resistant starch type 3 prevents tumor induction by 1,2-dimethylhydrazine and alters proliferation, apoptosis and dedifferentiation in rat colon. Carcinogenesis 2006, 27, 1849–1859. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, P.; Xiao, Z. Resistant starch prevents tumorigenesis of dimethylhydrazine-induced colon tumors via regulation of an ER stress-mediated mitochondrial apoptosis pathway. Int. J. Mol. Med. 2018, 41, 1887–1898. [Google Scholar] [CrossRef]
- Chen, L.; Brar, M.S.; Leung, F.C.C.; Hsiao, W.L.W. Triterpenoid herbal saponins enhance beneficial bacteria, decrease sulfate-reducing bacteria, modulate inflammatory intestinal microenvironment and exert cancer preventive effects in ApcMin/+ mice. Oncotarget 2016, 7, 31226–31242. [Google Scholar] [CrossRef]
- Liao, W.; Khan, I.; Huang, G.; Chen, S.; Liu, L.; Leong, W.K.; Li, X.A.; Wu, J.; Wendy Hsiao, W.L. Bifidobacterium animalis: The missing link for the cancer-preventive effect of Gynostemma pentaphyllum. Gut Microbes 2021, 13, 1847629. [Google Scholar] [CrossRef]
- Fernández, J.; Moreno, F.J.; Olano, A.; Clemente, A.; Villar, C.J.; Lombó, F. A galacto-oligosaccharides preparation derived from lactulose protects against colorectal cancer development in an animal model. Front. Microbiol. 2018, 9, 2004. [Google Scholar] [CrossRef] [PubMed]
- Schatzkin, A.; Lanza, E.; Corle, D.; Lance, P.; Iber, F.; Caan, B.; Shike, M.; Weissfeld, J.; Burt, R.; Cooper, M.R.; et al. Lack of effect of a low-fat, high-fiber diet on the recurrence of colorectal adenomas. N. Engl. J. Med. 2000, 342, 1149–1155. [Google Scholar] [CrossRef]
- Peters, U.; Sinha, R.; Chatterjee, N.; Subar, A.F.; Ziegler, R.G.; Kulldorff, M.; Bresalier, R.; Weissfeld, J.L.; Flood, A.; Schatzkin, A.; et al. Dietary fibre and colorectal adenoma in a colorectal cancer early detection programme. Lancet 2003, 361, 1491–1495. [Google Scholar] [CrossRef]
- Tantamango, Y.M.; Knutsen, S.F.; Beeson, L.; Fraser, G.; Sabate, J. Association between dietary fiber and incident cases of colon polyps: The adventist health study. Gastrointest. Cancer Res. 2011, 4, 161–167. [Google Scholar]
- Ben, Q.; Sun, Y.; Chai, R.; Qian, A.; Xu, B.; Yuan, Y. Dietary, fiber intake reduces risk for colorectal adenoma: A meta-analysis. Gastroenterology 2014, 146, 689–699.e6. [Google Scholar] [CrossRef]
- Yue, Y.; Ye, K.; Lu, J.; Wang, X.; Zhang, S.; Liu, L.; Yang, B.; Nassar, K.; Xu, X.; Pang, X.; et al. Probiotic strain Lactobacillus plantarum YYC-3 prevents colon cancer in mice by regulating the tumour microenvironment. Biomed. Pharmacother. 2020, 127, 110159. [Google Scholar] [CrossRef]
- Dubey, V.; Ghosh, A.R.; Bishayee, K.; Khuda-Bukhsh, A.R. Appraisal of the anti-cancer potential of probiotic Pediococcus pentosaceus GS4 against colon cancer: In vitro and in vivo approaches. J. Funct. Foods 2016, 23, 66–79. [Google Scholar] [CrossRef]
- Ohkawara, S.; Furuya, H.; Nagashima, K.; Asanuma, N.; Hino, T. Oral administration of Butyrivibrio fibrisolvens, a butyrate-producing bacterium, decreases the formation of aberrant crypt foci in the colon and rectum of mice. J. Nutr. 2005, 135, 2878–2883. [Google Scholar] [CrossRef] [PubMed]
- Rowland, I.R.; Rumney, C.J.; Coutts, J.T.; Lievense, L.C. Effect of Bifidobacterium longum and inulin on gut bacterial metabolism and carcinogen-induced aberrant crypt foci in rats. Carcinogenesis 1998, 19, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Hinoi, T.; Adachi, T.; Miguchi, M.; Niitsu, H.; Kochi, M.; Sada, H.; Sotomaru, Y.; Sakamoto, N.; Sentani, K.; et al. Synbiotics suppress colitis-induced tumorigenesis in a colon-specific cancer mouse model. PLoS ONE 2019, 14, e0216393. [Google Scholar] [CrossRef]
- Shang, F.; Jiang, X.; Wang, H.; Chen, S.; Wang, X.; Liu, Y.; Guo, S.; Li, D.; Yu, W.; Zhao, Z.; et al. The inhibitory effects of probiotics on colon cancer cells: In vitro and in vivo studies. J. Gastrointest. Oncol. 2020, 11, 1224. [Google Scholar] [CrossRef]
- Rad, A.H.; Aghebati-Maleki, L.; Kafil, H.S.; Abbasi, A. Molecular mechanisms of postbiotics in colorectal cancer prevention and treatment. Crit. Rev. Food Sci. Nutr. 2021, 61, 1787–1803. [Google Scholar] [CrossRef]
- Khoury, N.; El-Hayek, S.; Tarras, O.; El-Sabban, M.; El-Sibai, M.; Rizk, S. Kefir exhibits anti-proliferative and pro-apoptotic effects on colon adenocarcinoma cells with no significant effects on cell migration and invasion. Int. J. Oncol. 2014, 45, 2117–2127. [Google Scholar] [CrossRef]
- Amin, M.; Navidifar, T.; Saeb, S.; Barzegari, E.; Jamalan, M. Tumor-targeted induction of intrinsic apoptosis in colon cancer cells by Lactobacillus plantarum and Lactobacillus rhamnosus strains. Mol. Biol. Rep. 2023, 50, 5345–5354. [Google Scholar] [CrossRef]
- Sun, J.; Shi, Y.H.; Le, G.W.; Ma, X.Y. Distinct immune response induced by peptidoglycan derived from Lactobacillus sp. World J. Gastroenterol. 2005, 11, 6330–6337. [Google Scholar] [CrossRef] [PubMed]
- Guzowska, M.; Dziendzikowska, K.; Kopiasz, Ł.; Gajewska, M.; Wilczak, J.; Harasym, J.; Czerwińska, M.; Gromadzka-Ostrowska, J. Oat beta-glucans modulate the gut microbiome, barrier function, and immune responses in an in vivo model of early-stage colorectal cancer. Int. J. Mol. Sci. 2024, 25, 13586. [Google Scholar] [CrossRef]
- Rafter, J.; Bennett, M.; Caderni, G.; Clune, Y.; Hughes, R.; Karlsson, P.C.; Klinder, A.; O’Riordan, M.; O’Sullivan, G.C.; Pool-Zobel, B.; et al. Dietary synbiotics reduce cancer risk factors in polypectomized and colon cancer patients. Am. J. Clin. Nutr. 2007, 85, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, H.; Akedo, I.; Otani, T.; Suzuki, T.; Nakamura, T.; Takeyama, I.; Ishiguro, S.; Miyaoka, E.; Sobue, T.; Kakizoe, T. Randomized trial of dietary fiber and Lactobacillus casei administration for prevention of colorectal tumors. Int. J. Cancer 2005, 116, 762–767. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.E.; Yoon, L.S.; Michels, K.B.; Tranfield, W.; Jacobs, J.P.; May, F.P. The impact of prebiotic, probiotic, and synbiotic supplements and yogurt consumption on the risk of colorectal neoplasia among adults: A systematic review. Nutrients 2022, 14, 4937. [Google Scholar] [CrossRef]
- Pala, V.; Sieri, S.; Berrino, F.; Vineis, P.; Sacerdote, C.; Palli, D.; Masala, G.; Panico, S.; Mattiello, A.; Tumino, R.; et al. Yogurt consumption and risk of colorectal cancer in the Italian European prospective investigation into cancer and nutrition cohort. Int. J. Cancer 2011, 129, 2712–2719. [Google Scholar] [CrossRef]
- Zaharuddin, L.; Mokhtar, N.M.; Muhammad Nawawi, K.N.; Raja Ali, R.A. A randomized double-blind placebo-controlled trial of probiotics in post-surgical colorectal cancer. BMC Gastroenterol. 2019, 19, 131. [Google Scholar] [CrossRef]
- Österlund, P.; Ruotsalainen, T.; Korpela, R.; Saxelin, M.; Ollus, A.; Valta, P.; Kouri, M.; Elomaa, I.; Joensuu, H. Lactobacillus supplementation for diarrhoea related to chemotherapy of colorectal cancer: A randomised study. Br. J. Cancer 2007, 97, 1028–1034. [Google Scholar] [CrossRef]
- Huang, F.; Li, S.; Chen, W.; Han, Y.; Yao, Y.; Yang, L.; Li, Q.; Xiao, Q.; Wei, J.; Liu, Z.; et al. Postoperative probiotics administration attenuates gastrointestinal complications and gut microbiota dysbiosis caused by chemotherapy in colorectal cancer patients. Nutrients 2023, 15, 356. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Liu, W.; Cai, S.; Feng, X.; Chen, Y.; Cheng, X.; Ma, J.; Ma, W.; Tian, Z.; Yang, W. Evaluation of the efficacy of probiotics in the chemoradiotherapy of colorectal cancer: A meta-analysis of randomized controlled trials. BMC Gastroenterol. 2025, 25, 312. [Google Scholar] [CrossRef] [PubMed]
- Amitay, E.L.; Carr, P.R.; Gies, A.; Laetsch, D.C.; Brenner, H. Probiotic/synbiotic treatment and postoperative complications in colorectal cancer patients: Systematic review and meta-analysis of randomized controlled trials. Clin. Transl. Gastroenterol. 2020, 11, e00268. [Google Scholar] [CrossRef]
- Kandati, K.; Belagal, P.; Nannepaga, J.S.; Viswanath, B. Role of probiotics in the management of cervical cancer: An update. Clin. Nutr. ESPEN 2022, 48, 5–16. [Google Scholar] [CrossRef]
- Xu, P.; Mageswaran, U.M.; Nisaa, A.A.; Balasubramaniam, S.D.; Rajendran, D.; Ismail, E.H.B.E.; Kadir, M.N.; Oon, C.E.; Tan, C.S.; Sany, S.B.; et al. Roles of probiotics against HPV through the gut-vaginal axis. Int. J. Gynecol. Obstet. 2025, 169, 1–8. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, F.; Chen, C.; Chen, S.; Huang, X.; Wang, Y.; Qiu, P.; Deng, G.; Gao, J. Dietary fiber and human papillomavirus infection among US women: The national health and nutrition examination survey, 2003–2016. Nutr. Cancer 2021, 73, 2515–2522. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, X.; Wu, F.; Chen, J.; Luo, J.; Wu, C.; Chen, T. Effectiveness of vaginal probiotics Lactobacillus crispatus chen-01 in women with high-risk HPV infection: A prospective controlled pilot study. Aging 2024, 16, 11446–11459. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Gu, L.; Li, Y.; Chen, S.; You, J.; Fan, L.; Wang, Y.; Zhao, L. Chitooligosaccharides display anti-tumor effects against human cervical cancer cells via the apoptotic and autophagic pathways. Carbohydr. Polym. 2019, 224, 115171. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, H.; Du, X.; Yu, W.; Jiang, J.; Geng, Y.; Guo, X.; Fan, X.; Ma, C. Lactobacilli inhibit cervical cancer cell migration in vitro and reduce tumor burden in vivo through upregulation of E-cadherin. Oncol. Rep. 2017, 38, 1561–1568. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.D.; Xu, D.J.; Wang, B.Y.; Yan, D.H.; Lv, Z.; Su, J.R. Inhibitory effect of vaginal Lactobacillus supernatants on cervical cancer cells. Probiotics Antimicrob. Proteins 2018, 10, 236–242. [Google Scholar] [CrossRef]
- Asoudeh-Fard, A.; Beygi, M.Y.; Parsaei, A.; Mohkam, M.; Asoudeh-Fard, M.; Gholami, A. Postbiotic metabolites derived from Lactobacillus fermentum as potent antiproliferative bioresources on HeLa cells with promising biocompatibility. BMC Complement. Med. Ther. 2024, 24, 420. [Google Scholar] [CrossRef]
- Negi, D.; Singh, A.; Joshi, N.; Mishra, N. Cisplatin and probiotic biomass loaded pessaries for the management of cervical cancer. Anticancer. Agents Med. Chem. 2019, 20, 589–598. [Google Scholar] [CrossRef]
- Okawa, T.; Niibe, H.; Arai, T.; Sekiba, K.; Noda, K.; Takeuchi, S.; Hashimoto, S.; Ogawa, N. Effect of LC9018 combined with radiation therapy on carcinoma of the uterine cervix. A phase III, multicenter, randomized, controlled study. Cancer 1993, 72, 1949–1954. [Google Scholar] [CrossRef]
- Delia, P.; Sansotta, G.; Donato, V.; Frosina, P.; Messina, G.; De Renzis, C.; Famularo, G. Use of probiotics for prevention of radiation-induced diarrhea. World J. Gastroenterol. 2007, 13, 912. [Google Scholar] [CrossRef]
- Linn, Y.H.; Thu, K.K.; Win, N.H.H. Effect of probiotics for the prevention of acute radiation-induced diarrhoea among cervical cancer patients: A randomized double-blind placebo-controlled study. Probiotics Antimicrob. Proteins 2019, 11, 638–647. [Google Scholar] [CrossRef]
- Kim, J.; Harper, A.; McCormack, V.; Sung, H.; Houssami, N.; Morgan, E.; Mutebi, M.; Garvey, G.; Soerjomataram, I.; Fidler-Benaoudia, M.M. Global patterns and trends in breast cancer incidence and mortality across 185 countries. Nat. Med. 2025, 31, 1154–1162. [Google Scholar] [CrossRef]
- van’t Veer, P.; Dekker, J.M.; Lamers, J.W.; Kok, F.J.; Schouten, E.G.; Brants, H.A.; Sturmans, F.; Hermus, R.J. Consumption of fermented milk products and breast cancer: A case-control study in The Netherlands. Cancer Res. 1989, 49, 4020–4023. [Google Scholar] [PubMed]
- Taper, H.S.; Roberfroid, M. Influence of inulin and oligofructose on breast cancer and tumor growth. J. Nutr. 1999, 129, 1488S–1491S. [Google Scholar] [CrossRef]
- Delphi, L.; Sepehri, H.; Khorramizadeh, M.R.; Mansoori, F. Pectic-oligosaccharides from apples induce apoptosis and cell cycle arrest in MDA-MB-231 cells, a model of human breast cancer. Asia Pac. J. Cancer Prev. 2015, 16, 5265–5271. [Google Scholar] [CrossRef]
- Kondegowda, N.G.; Meaney, M.P.; Baker, C.; Ju, Y.H. Effects of non-digestible carbohydrates on the growth of estrogen-dependent human breast cancer (MCF-7) tumors implanted in ovariectomized athymic mice. Nutr. Cancer 2011, 63, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Sampsell, K.; Wang, W.; Ohland, C.; Mager, L.F.; Pett, N.; Lowry, D.E.; Sales, K.M.; McNeely, M.L.; McCoy, K.D.; Culos-Reed, S.N.; et al. Exercise and prebiotic fiber provide gut microbiota-driven benefit in a survivor to germ-free mouse translational model of breast cancer. Cancers 2022, 14, 2722. [Google Scholar] [CrossRef]
- Yazdi, M.H.; Soltan Dallal, M.M.; Hassan, Z.M.; Holakuyee, M.; Agha Amiri, S.; Abolhassani, M.; Mahdavi, M. Oral administration of Lactobacillus acidophilus induces IL-12 production in spleen cell culture of BALB/c mice bearing transplanted breast tumour. Br. J. Nutr. 2010, 104, 227–232. [Google Scholar] [CrossRef]
- Aragón, F.; Carino, S.; Perdigón, G.; De Moreno De LeBlanc, A. Inhibition of growth and metastasis of breast cancer in mice by milk fermented with Lactobacillus casei CRL 431. J. Immunother. 2015, 38, 185–196. [Google Scholar] [CrossRef]
- Zamberi, N.R.; Abu, N.; Mohamed, N.E.; Nordin, N.; Keong, Y.S.; Beh, B.K.; Zakaria, Z.A.B.; Nik Abdul Rahman, N.M.A.; Alitheen, N.B. The antimetastatic and antiangiogenesis effects of Kefir water on murine breast cancer cells. Integr. Cancer Ther. 2016, 15, NP53-66. [Google Scholar] [CrossRef]
- Toi, M.; Hirota, S.; Tomotaki, A.; Sato, N.; Hozumi, Y.; Anan, K.; Nagashima, T.; Tokuda, Y.; Masuda, N.; Ohsumi, S.; et al. Probiotic beverage with soy isoflavone consumption for breast cancer prevention: A case-control study. Curr. Nutr. Food Sci. 2013, 9, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Actis, S.; Cazzaniga, M.; Bounous, V.E.; D’Alonzo, M.; Rosso, R.; Accomasso, F.; Minella, C.; Biglia, N. Emerging evidence on the role of breast microbiota on the development of breast cancer in high-risk patients. Carcinogenesis 2023, 44, 718–725. [Google Scholar] [CrossRef] [PubMed]
- Motevaseli, E.; Dianatpour, A.; Ghafouri-Fard, S. The role of probiotics in cancer treatment: Emphasis on their in vivo and in vitro anti-metastatic effects. Int. J. Mol. Cell Med. 2017, 6, 66. [Google Scholar]
- López, D.M.L. Management of genitourinary syndrome of menopause in breast cancer survivors: An update. World J. Clin. Oncol. 2022, 13, 71–100. [Google Scholar] [CrossRef]
- Marschalek, J.; Farr, A.; Marschalek, M.L.; Domig, K.J.; Kneifel, W.; Singer, C.F.; Kiss, H.; Petricevic, L. Influence of orally administered probiotic Lactobacillus strains on vaginal microbiota in women with breast cancer during chemotherapy: A randomized placebo-controlled double-blinded pilot study. Breast Care 2017, 12, 335–339. [Google Scholar] [CrossRef]
- Juan, Z.; Chen, J.; Ding, B.; Yongping, L.; Liu, K.; Wang, L.; Le, Y.; Liao, Q.; Shi, J.; Huang, J.; et al. Probiotic supplement attenuates chemotherapy-related cognitive impairment in patients with breast cancer: A randomized, double-blind, and placebo-controlled trial. Eur. J. Cancer 2022, 161, 10–22. [Google Scholar] [CrossRef]
- Matsuzaki, T.; Yokokura, T.; Azuma, I. Anti-tumour activity of Lactobacillus casei on Lewis lung carcinoma and line-10 hepatoma in syngeneic mice and guinea pigs. Cancer Immunol. Immunother. 1985, 20, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Yazdi, M.H.; Mahdavi, M.; Setayesh, N.; Esfandyar, M.; Shahverdi, A.R. Selenium nanoparticle-enriched Lactobacillus brevis causes more efficient immune responses in vivo and reduces the liver metastasis in metastatic form of mouse breast cancer. DARU J. Pharm. Sci. 2013, 21, 33. [Google Scholar] [CrossRef] [PubMed]
- Davoodvandi, A.; Fallahi, F.; Tamtaji, O.R.; Tajiknia, V.; Banikazemi, Z.; Fathizadeh, H.; Abbasi-Kolli, M.; Aschner, M.; Ghandali, M.; Sahebkar, A.; et al. An update on the effects of probiotics on gastrointestinal cancers. Front. Pharmacol. 2021, 12, 680400. [Google Scholar] [CrossRef]
- Vesty, A.; Gear, K.; Boutell, S.; Taylor, M.W.; Douglas, R.G.; Biswas, K. Randomized, double-blind, placebo-controlled trial of oral probiotic Streptococcus salivarius M18 on head and neck cancer patients post-radiotherapy: A pilot study. Sci. Rep. 2020, 10, 13201. [Google Scholar] [CrossRef]
- Fu, W.; Kapila, L.Y. Nisin, a probiotic bacteriocin, combined with limited chemoradiation therapy in head and neck cancer. Arch. Clin. Med. Case Rep. 2021, 05, 531–536. [Google Scholar] [CrossRef]
- Torabi, S.; Nahidi, Y.; Ghasemi, S.Z.; Reihani, A.; Samadi, A.; Ramezanghorbani, N.; Nazari, E.; Davoudi, S. Evaluation of skin cancer prevention properties of probiotics. Genes. Nutr. 2025, 20, 12. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Lyu, Y.; Liu, X.; Jia, X.; Cui, F.; Wu, X.; Deng, S.; Yue, C. Engineered probiotics. Microb. Cell Fact. 2022, 21, 72. [Google Scholar] [CrossRef]
- Wang, H.; Chen, T.; Wan, L.; Lu, J.; Wei, H.; Deng, K.Y.; Wei, J.; Xin, H.B. Attenuated Salmonella engineered with an apoptosis-inducing factor (AIF) eukaryotic expressing system enhances its anti-tumor effect in melanoma in vitro and in vivo. Appl. Microbiol. Biotechnol. 2020, 104, 3517–3528. [Google Scholar] [CrossRef]
- He, L.; Yang, H.; Tang, J.; Liu, Z.; Chen, Y.; Lu, B.; He, H.; Tang, S.; Sun, Y.; Liu, F. Intestinal probiotics E. coli Nissle 1917 as a targeted vehicle for delivery of p53 and Tum-5 to solid tumors for cancer therapy. J. Biol. Eng. 2019, 13, 58. [Google Scholar] [CrossRef]
- Buchbinder, E.I.; Desai, A. CTLA-4 and PD-1 pathways similarities, differences, and implications of their inhibition. Am. J. Clin. Oncol. 2016, 39, 98–106. [Google Scholar] [CrossRef]
- Gurbatri, C.R.; Lia, I.; Vincent, R.; Coker, C.; Castro, S.; Treuting, P.M.; Hinchliffe, T.E.; Arpaia, N.; Danino, T. Engineered probiotics for local tumor delivery of checkpoint blockade nanobodies. Sci. Transl. Med. 2020, 12, eaax0876. [Google Scholar] [CrossRef]
- Leventhal, D.S.; Sokolovska, A.; Li, N.; Plescia, C.; Kolodziej, S.A.; Gallant, C.W.; Christmas, R.; Gao, J.R.; James, M.J.; Abin-Fuentes, A.; et al. Immunotherapy with engineered bacteria by targeting the STING pathway for anti-tumor immunity. Nat. Commun. 2020, 11, 2739. [Google Scholar] [CrossRef]
- Luke, J.J.; Piha-Paul, S.A.; Medina, T.; Verschraegen, C.F.; Varterasian, M.; Brennan, A.M.; Riese, R.J.; Sokolovska, A.; Strauss, J.; Hava, D.L.; et al. Phase I study of SYNB1891, an engineered E. coli Nissle strain expressing STING agonist, with and without atezolizumab in advanced malignancies. Clin. Cancer Res. 2023, 29, 2435–2444. [Google Scholar] [CrossRef] [PubMed]
- Hamade, D.F.; Epperly, M.W.; Fisher, R.; Hou, W.; Shields, D.; van Pijkeren, J.P.; Leibowitz, B.J.; Coffman, L.G.; Wang, H.; Huq, M.S.; et al. Genetically engineered probiotic Limosilactobacillus reuteri releasing IL-22 (LR-IL-22) modifies the tumor microenvironment, enabling irradiation in ovarian cancer. Cancers 2024, 16, 474. [Google Scholar] [CrossRef] [PubMed]
- Merenstein, D.; Pot, B.; Leyer, G.; Ouwehand, A.C.; Preidis, G.A.; Elkins, C.A.; Hill, C.; Lewis, Z.T.; Shane, A.L.; Zmora, N.; et al. Emerging issues in probiotic safety: 2023 perspectives. Gut Microbes 2023, 15, 2185034. [Google Scholar] [CrossRef]
- Martinez, R.C.R.; Bedani, R.; Saad, S.M.I. Scientific evidence for health effects attributed to the consumption of probiotics and prebiotics: An update for current perspectives and future challenges. Br. J. Nutr. 2015, 114, 1993–2015. [Google Scholar] [CrossRef] [PubMed]
- Flesch, A.T.; Tonial, S.T.; Contu, P.d.C.; Damin, D.C. Perioperative synbiotics administration decreases postoperative infections in patients with colorectal cancer: A randomized, double-blind clinical trial. Rev. Col. Bras. Cir. 2017, 44, 567–573. [Google Scholar] [CrossRef]
- Krebs, B. Prebiotic and synbiotic treatment before colorectal surgery-randomised double blind trial. Coll. Antropol. 2016, 40, 35–40. [Google Scholar]
- Farshi Radvar, F.; Mohammad-Zadeh, M.; Mahdavi, R.; Andersen, V.; Nasirimotlagh, B.; Faramarzi, E.; Lotfi Yagin, N. Effect of synbiotic supplementation on matrix metalloproteinase enzymes, quality of life and dietary intake and weight changes in rectal cancer patients undergoing neoadjuvant chemoradiotherapy. Med. J. Nutrition Metab. 2020, 13, 225–235. [Google Scholar] [CrossRef]
- Nascimento, M.; Caporossi, C.; Eduardo Aguilar-Nascimento, J.; Michelon Castro-Barcellos, H.; Teixeira Motta, R.; Reis Lima, S. Efficacy of synbiotics to reduce symptoms and rectal inflammatory response in acute radiation proctitis: A randomized, double-blind, placebo-controlled pilot trial. Nutr. Cancer 2020, 72, 602–609. [Google Scholar] [CrossRef]
- Sugawara, G.; Nagino, M.; Nishio, H.; Ebata, T.; Takagi, K.; Asahara, T.; Nomoto, K.; Nimura, Y. Perioperative synbiotic treatment to prevent postoperative infectious complications in biliary cancer surgery: A randomized controlled trial. Ann. Surg. 2006, 244, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Yano, M.; Motoori, M.; Kishi, K.; Miyashiro, I.; Ohue, M.; Ohigashi, H.; Asahara, T.; Nomoto, K.; Ishikawa, O. Impact of perioperative administration of synbiotics in patients with esophageal cancer undergoing esophagectomy: A prospective randomized controlled trial. Surgery 2012, 152, 832–842. [Google Scholar] [CrossRef]
- Sugimoto, T.; Atobe, S.; Kado, Y.; Takahashi, A.; Motoori, M.; Sugimura, K.; Miyata, H.; Yano, M.; Tanaka, K.; Doki, Y.; et al. Gut microbiota associated with the mitigation effect of synbiotics on adverse events of neoadjuvant chemotherapy in patients with esophageal cancer: A retrospective exploratory study. J. Med. Microbiol. 2023, 72, 001723. [Google Scholar] [CrossRef]
- Motoori, M.; Yano, M.; Miyata, H.; Sugimura, K.; Saito, T.; Omori, T.; Fujiwara, Y.; Miyoshi, N.; Akita, H.; Gotoh, K. Randomized study of the effect of synbiotics during neoadjuvant chemotherapy on adverse events in esophageal cancer patients. Clin. Nutr. 2017, 36, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Manifar, S.; Koopaie, M.; Jahromi, Z.M.; Kolahdooz, S. Effect of synbiotic mouthwash on oral mucositis induced by radiotherapy in oral cancer patients: A double-blind randomized clinical trial. Support. Care Cancer 2023, 31, 31. [Google Scholar] [CrossRef]
- Koopaie, M.; Vaziri, S.; Manifar, S.; Younespour, S.; Kolahdooz, S. Efficacy of synbiotic mouthwash on salivary TLR2 levels and oral mucositis in head and neck cancer patients undergoing radiotherapy: A randomized clinical trial. Support. Care Cancer 2025, 33, 481. [Google Scholar] [CrossRef]
- Sommacal, H.M.; Bersch, V.P.; Vitola, S.P.; Osvaldt, A.B. Perioperative synbiotics decrease postoperative complications in periampullary neoplasms: A randomized, double-blind clinical trial. Nutr. Cancer 2015, 67, 457–462. [Google Scholar] [CrossRef]
- Monshikarimi, A.; Ostadrahimi, A.; Asghari Jafarabadi, M.; EivaziZiaei, J.; Barzeghari, A.; Esfahani, A.; Payahoo, L.; Aamazadeh, F.; Farrin, N. Does combination of Lactobacillus rhamnosus Heriz I and beta glucan improve quality of life in women with breast cancer receiving chemotherapy? A randomized double-blind placebo-controlled clinical trial. Nutr. Food Sci. 2020, 50, 569–578. [Google Scholar] [CrossRef]
- Tirgar, A.; Rezaei, M.; Ehsani, M.; Salmani, Z.; Rastegari, A.; Jafari, E.; Khandani, B.K.; Nakhaee, N.; Khaksari, M.; Moazed, V. Exploring the synergistic effects of vitamin D and synbiotics on cytokines profile, and treatment response in breast cancer: A pilot randomized clinical trial. Sci. Rep. 2024, 14, 21372. [Google Scholar] [CrossRef]
- Khazaei, Y.; Basi, A.; Fernandez, M.L.; Foudazi, H.; Bagherzadeh, R.; Shidfar, F. The effects of synbiotics supplementation on reducing chemotherapy-induced side effects in women with breast cancer: A randomized placebo-controlled double-blind clinical trial. BMC Complement. Med. Ther. 2023, 23, 339. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, H.; Wong, A. Accounting for the health risk of probiotics. Heliyon 2024, 10, e27908. [Google Scholar] [CrossRef]
- Doron, S.; Snydman, D.R. Risk and Safety of Probiotics. Clin. Infect. Dis. 2015, 60, S129–S134. [Google Scholar] [CrossRef] [PubMed]
- Ciernikova, S.; Mego, M.; Semanova, M.; Wachsmannova, L.; Adamcikova, Z.; Stevurkova, V.; Drgona, L.; Zajac, V. Probiotic survey in cancer patients treated in the outpatient department in a comprehensive cancer center. Integr. Cancer Ther. 2017, 16, 188–195. [Google Scholar] [CrossRef]
- Redman, M.G.; Ward, E.J.; Phillips, R.S. The efficacy and safety of probiotics in people with cancer: A systematic review. Ann. Oncol. 2014, 25, 1919–1929. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.; Ye, H.; Zhou, X.; Wang, J.; Zhang, L.; Sun, W.; Duan, C.; Fan, M.; Zhou, W.; Bi, C.; et al. Evaluating the health risk of probiotic supplements from the perspective of antimicrobial resistance. Microbiol. Spectr. 2025, 13, e0001924. [Google Scholar] [CrossRef] [PubMed]
- Daniali, M.; Nikfar, S.; Abdollahi, M. Antibiotic resistance propagation through probiotics. Expert. Opin. Drug Metab. Toxicol. 2020, 16, 1207–1215. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tafla, T.; Balasubramanian, A.; Iyer, J.K. Exploring the Potential of Biotics in Cancer Prevention and Treatment—Mechanisms, Experimental, and Clinical Insights. Onco 2025, 5, 41. https://doi.org/10.3390/onco5030041
Tafla T, Balasubramanian A, Iyer JK. Exploring the Potential of Biotics in Cancer Prevention and Treatment—Mechanisms, Experimental, and Clinical Insights. Onco. 2025; 5(3):41. https://doi.org/10.3390/onco5030041
Chicago/Turabian StyleTafla, Tia, Abinaya Balasubramanian, and Janaki K. Iyer. 2025. "Exploring the Potential of Biotics in Cancer Prevention and Treatment—Mechanisms, Experimental, and Clinical Insights" Onco 5, no. 3: 41. https://doi.org/10.3390/onco5030041
APA StyleTafla, T., Balasubramanian, A., & Iyer, J. K. (2025). Exploring the Potential of Biotics in Cancer Prevention and Treatment—Mechanisms, Experimental, and Clinical Insights. Onco, 5(3), 41. https://doi.org/10.3390/onco5030041





