The Role of Tissue Factor-Positive Microparticles in Gynecological Cancer-Associated Disseminated Intravascular Coagulation: Molecular Mechanisms and Clinical Implications
Abstract
1. Introduction
2. Background on DIC in Cancer
3. Tumor-Derived TF-Positive Microparticles
4. Mechanisms of Coagulation Activation
5. Inhibitors of TF and TF-Positive Microparticle Pathways
5.1. Anticoagulants (LMWH and DOACs) and Emerging Strategies in TF-Driven Coagulopathy in Cancer
5.2. Tissue Factor–Targeted Antibodies and ADCs
5.3. TF Pathway Inhibitors (Ixolaris, rNAPc2)
5.4. Inhibitors of Tissue Factor and TF-Positive Microparticles: Current and Investigational Therapies in Gynecologic Cancers
6. Clinical Implications
6.1. Biomarkers and Diagnostics
6.2. Risk Stratification
6.3. Prognosis
6.4. Monitoring and Management
6.5. Pregnancy and Coagulopathy
7. Challenges and Future Directions
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sallah, S.; Wan, J.Y.; Nguyen, N.P.; Hanrahan, L.R.; Sigounas, G. Disseminated intravascular coagulation in solid tumors: Clinical and pathologic study. Thromb. Haemost. 2001, 86, 828–833. [Google Scholar] [CrossRef] [PubMed]
- Hisada, Y.; Mackman, N. Tissue factor and extracellular vesicles: Activation of coagulation and impact on survival in cancer. Cancers 2021, 13, 3839. [Google Scholar] [CrossRef] [PubMed]
- Hell, L.; Thaler, J.; Martinod, K.; Ay, C.; Posch, F.; Wagner, D.D.; Pabinger, I. OC-16—Neutrophil extracellular traps and tissue factor-bearing microvesicles: A liaison dangereuse causing overt DIC in cancer patients? Thromb. Res. 2016, 140 (Suppl. S1), S174–S175. [Google Scholar] [CrossRef]
- Zhu, B.; Gu, H.; Mao, Z.; Beeraka, N.M.; Zhao, X.; Anand, M.P.; Zheng, Y.; Zhao, R.; Li, S.; Manogaran, P.; et al. Global burden of gynaecological cancers in 2022 and projections to 2050. J. Glob. Health 2024, 14, 04155. [Google Scholar] [CrossRef]
- Kataki, A.C.; Tiwari, P.; Thilagavthi, R.; Krishnatreya, M. Epidemiology of gynaecological cancers. In Fundamentals in Gynaecologic Malignancy; Kataki, A.C., Barmon, D., Eds.; Springer Nature: Singapore, 2022; pp. 1–8. ISBN 978-981-19-5859-5. [Google Scholar]
- Han, X.; Guo, B.; Li, Y.; Zhu, B. Tissue factor in tumor microenvironment: A systematic review. J. Hematol. Oncol. 2014, 7, 54. [Google Scholar] [CrossRef]
- Cui, C.-J.; Wang, G.-J.; Yang, S.; Huang, S.-K.; Qiao, R.; Cui, W. Tissue Factor-bearing MPs and the risk of venous thrombosis in cancer patients: A meta-analysis. Sci. Rep. 2018, 8, 1675. [Google Scholar] [CrossRef] [PubMed]
- Falanga, A.; Russo, L.; Milesi, V. The coagulopathy of cancer. Curr. Opin. Hematol. 2014, 21, 423–429. [Google Scholar] [CrossRef]
- Unar, A.; Bertolino, L.; Patauner, F.; Gallo, R.; Durante-Mangoni, E. Pathophysiology of disseminated intravascular coagulation in sepsis: A clinically focused overview. Cells 2023, 12, 2120. [Google Scholar] [CrossRef]
- Gourley, C.; Farley, J.; Provencher, D.M.; Pignata, S.; Mileshkin, L.; Harter, P.; Maenpaa, J.; Kim, J.-W.; Pujaide-Lauraine, E.; Glasspool, R.M.; et al. Gynecologic Cancer InterGroup (GCIG) consensus review for ovarian and primary peritoneal low-grade serous carcinomas. Int. J. Gynecol. Cancer 2014, 24, S9–S13. [Google Scholar] [CrossRef]
- Kurman, R.J.; Shih, I.-M. The origin and pathogenesis of epithelial ovarian cancer: A proposed unifying theory. Am. J. Surg. Pathol. 2010, 34, 433–443. [Google Scholar] [CrossRef]
- Hamzah, A.B.A.; Choo, Y.M.; Hassali, M.A.; Saleem, F.; Verma, A.K. Disseminated intravascular coagulation and excessive fibrinolysis (DIC XFL) syndrome in prostate cancer: A rare complicated disorder. J. Clin. Diagn. Res. 2017, 11, XD01–XD02. [Google Scholar] [CrossRef] [PubMed]
- Vencken, P.M.L.H.; Ewing, P.C.; Zweemer, R.P. Epithelioid trophoblastic tumour: A case report and review of the literature. J. Clin. Pathol. 2006, 59, 1307–1308. [Google Scholar] [CrossRef]
- Voulgaris, E.; Pentheroudakis, G.; Vassou, A.; Pavlidis, N. Disseminated intravascular coagulation (DIC) and non-small cell lung cancer (NSCLC): Report of a case and review of the literature. Lung Cancer 2009, 64, 247–249. [Google Scholar] [CrossRef]
- Nishimura, R.; Koizumi, T.; Yokotani, T.; Taniguchi, R.; Morisue, K.; Yoshimura, M.; Hiranmoy, D.; Yamaguchi, S.; Nakagawa, T.; Hasegawa, K.; et al. Molecular heterogeneity of hCGbeta--related glycoproteins and the clinical relevance in trophoblastic and non-trophoblastic tumors. Int. J. Gynaecol. Obstet. 1998, 60 (Suppl. S1), S29–S32. [Google Scholar] [CrossRef]
- Unar, A.; Bertolino, L.; Patauner, F.; Gallo, R.; Durante-Mangoni, E. Decoding Sepsis-Induced Disseminated Intravascular Coagulation: A Comprehensive Review of Existing and Emerging Therapies. J. Clin. Med. 2023, 12, 6128. [Google Scholar] [CrossRef] [PubMed]
- Hell, L.; Däullary, T.; Burghart, V.; Mauracher, L.-M.; Grilz, E.; Moser, B.; Kramer, G.; Schmid, J.A.; Ay, C.; Pabinger, I.; et al. Extracellular Vesicle-Associated Tissue Factor Activity in Prostate Cancer Patients with Disseminated Intravascular Coagulation. Cancers 2021, 13, 1487. [Google Scholar] [CrossRef] [PubMed]
- Levi, M. Clinical characteristics of disseminated intravascular coagulation in patients with solid and hematological cancers. Thromb. Res. 2018, 164 (Suppl. S1), S77–S81. [Google Scholar] [CrossRef] [PubMed]
- Hisada, Y.; Mackman, N. Cancer cell-derived tissue factor-positive extracellular vesicles: Biomarkers of thrombosis and survival. Curr. Opin. Hematol. 2019, 26, 349–356. [Google Scholar] [CrossRef]
- Featherby, S.; Madkhali, Y.; Maraveyas, A.; Ettelaie, C. Apixaban Suppresses the Release of TF-Positive Microvesicles and Restrains Cancer Cell Proliferation through Directly Inhibiting TF-fVIIa Activity. Thromb. Haemost. 2019, 119, 1419–1432. [Google Scholar] [CrossRef]
- Svensson, K.J.; Kucharzewska, P.; Christianson, H.C.; Sköld, S.; Löfstedt, T.; Johansson, M.C.; Mörgelin, M.; Bengzon, J.; Ruf, W.; Belting, M. Hypoxia triggers a proangiogenic pathway involving cancer cell microvesicles and PAR-2-mediated heparin-binding EGF signaling in endothelial cells. Proc. Natl. Acad. Sci. USA 2011, 108, 13147–13152. [Google Scholar] [CrossRef]
- Press, J.Z.; Reyes, M.; Pitteri, S.J.; Pennil, C.; Garcia, R.; Goff, B.A.; Hanash, S.M.; Swisher, E.M. Microparticles from ovarian carcinomas are shed into ascites and promote cell migration. Int. J. Gynecol. Cancer 2012, 22, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Lami, V.; Pancani, R.; Sbrana, A.; Neri, T.; Boccaccio, C.; Cipriano, A.; Gattini, M.; Celi, A.; Nieri, D. Circulating, extracellular vesicle-associated tissue factor in cancer patients with and without venous thromboembolism. In Pulmonary Embolism; European Respiratory Society: Lausanne, Switzerland, 2023; p. PA2246. [Google Scholar]
- Claussen, C.; Rausch, A.-V.; Lezius, S.; Amirkhosravi, A.; Davila, M.; Francis, J.L.; Hisada, Y.M.; Mackman, N.; Bokemeyer, C.; Schmalfeldt, B.; et al. Microvesicle-associated tissue factor procoagulant activity for the preoperative diagnosis of ovarian cancer. Thromb. Res. 2016, 141, 39–48. [Google Scholar] [CrossRef]
- Cohen, J.G.; Prendergast, E.; Geddings, J.E.; Walts, A.E.; Agadjanian, H.; Hisada, Y.; Karlan, B.Y.; Mackman, N.; Walsh, C.S. Evaluation of venous thrombosis and tissue factor in epithelial ovarian cancer. Gynecol. Oncol. 2017, 146, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Steidel, C.; Ender, F.; Rody, A.; von Bubnoff, N.; Gieseler, F. Biologically Active Tissue Factor-Bearing Larger Ectosome-Like Extracellular Vesicles in Malignant Effusions from Ovarian Cancer Patients: Correlation with Incidence of Thrombosis. Int. J. Mol. Sci. 2021, 22, 790. [Google Scholar] [CrossRef] [PubMed]
- Koizume, S.; Ito, S.; Yoshioka, Y.; Kanayama, T.; Nakamura, Y.; Yoshihara, M.; Yamada, R.; Ochiya, T.; Ruf, W.; Miyagi, E.; et al. High-level secretion of tissue factor-rich extracellular vesicles from ovarian cancer cells mediated by filamin-A and protease-activated receptors. Thromb. Haemost. 2016, 115, 299–310. [Google Scholar] [CrossRef]
- Glassman, D.; Bateman, N.W.; Lee, S.; Zhao, L.; Yao, J.; Tan, Y.; Ivan, C.; Rangel, K.M.; Zhang, J.; Conrads, K.A.; et al. Molecular correlates of venous thromboembolism (VTE) in ovarian cancer. Cancers 2022, 14, 1496. [Google Scholar] [CrossRef]
- Moufarrij, S.; Havrilesky, L.; Jewell, E.L. Universal thromboprophylaxis in ovarian cancer patients before and after surgery? Gynecol. Oncol. 2023, 176, A1–A2. [Google Scholar] [CrossRef]
- Geddings, J.E.; Hisada, Y.; Boulaftali, Y.; Getz, T.M.; Whelihan, M.; Fuentes, R.; Dee, R.; Cooley, B.C.; Key, N.S.; Wolberg, A.S.; et al. Tissue factor-positive tumor microvesicles activate platelets and enhance thrombosis in mice. J. Thromb. Haemost. 2016, 14, 153–166. [Google Scholar] [CrossRef]
- Yokota, N.; Koizume, S.; Miyagi, E.; Hirahara, F.; Nakamura, Y.; Kikuchi, K.; Ruf, W.; Sakuma, Y.; Tsuchiya, E.; Miyagi, Y. Self-production of tissue factor-coagulation factor VII complex by ovarian cancer cells. Br. J. Cancer 2009, 101, 2023–2029. [Google Scholar] [CrossRef]
- Macleod, H.; Copty, N.; Doherty, D.; Weiss, L.; Fouhy, E.; Power, R.; Ryan, N.; Saeed, K.; ORourke, E.; Faryal, R.; et al. Direct oral anticoagulants are comparable to low molecular weight heparin at sustaining the circulating extracellular vesicle and inflammatory profiles of cancer associated thrombosis patients: An observational pilot study. Cancer Med. 2025, 14, e70920. [Google Scholar] [CrossRef]
- Vitale, F.V.; Longo-Sorbello, G.S.; Rotondo, S.; Ferrau, F. Understanding and treating solid tumor-related disseminated intravascular coagulation in the “era” of targeted cancer therapies. SAGE Open Med. 2017, 5, 2050312117749133. [Google Scholar] [CrossRef] [PubMed]
- Morris, P.E.; Steingrub, J.S.; Huang, B.Y.; Tang, S.; Liu, P.M.; Rhode, P.R.; Wong, H.C. A phase I study evaluating the pharmacokinetics, safety and tolerability of an antibody-based tissue factor antagonist in subjects with acute lung injury or acute respiratory distress syndrome. BMC Pulm. Med. 2012, 12, 5. [Google Scholar] [CrossRef]
- Marcucci, F.; Caserta, C.A.; Romeo, E.; Rumio, C. Antibody-Drug Conjugates (ADC) Against Cancer Stem-Like Cells (CSC)-Is There Still Room for Optimism? Front. Oncol. 2019, 9, 167. [Google Scholar] [CrossRef] [PubMed]
- Camarda, F.; Paderno, M.; Cannizzaro, M.C.; Nero, C.; Sabatucci, I.; Fucà, G.; Musacchio, L.; Salutari, V.; Scambia, G.; Lorusso, D. Antibody drug conjugates in recurrent or metastatic cervical cancer: A focus on tisotumab vedotin state of art. Ther. Adv. Med. Oncol. 2024, 16, 17588359241277648. [Google Scholar] [CrossRef]
- Markham, A. Tisotumab vedotin: First approval. Drugs 2021, 81, 2141–2147. [Google Scholar] [CrossRef]
- Hong, D.S.; Concin, N.; Vergote, I.; de Bono, J.S.; Slomovitz, B.M.; Drew, Y.; Arkenau, H.-T.; Machiels, J.-P.; Spicer, J.F.; Jones, R.; et al. Tisotumab vedotin in previously treated recurrent or metastatic cervical cancer. Clin. Cancer Res. 2020, 26, 1220–1228. [Google Scholar] [CrossRef]
- Heublein, S.; Egger, M.; Zhu, J.; Berger, L.; Mayr, D.; Schindlbeck, C.; Kuhn, C.; Hofmann, S.S.; Schuetz, F.; Jeschke, U.; et al. Evaluation of the anti-Thomsen-Friedenreich antibodies Nemod-TF1 and Nemod-TF2 as prognostic markers in breast cancer. Breast Cancer Res. Treat. 2020, 179, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Almogren, A.; Abdullah, J.; Ghapure, K.; Ferguson, K.; Glinsky, V.V.; Rittenhouse-Olson, K. Anti-Thomsen-Friedenreich-Ag (anti-TF-Ag) potential for cancer therapy. Front. Biosci. 2012, 4, 840–863. [Google Scholar] [CrossRef]
- Ma, L.; Wang, G.; Liu, S.; Bi, F.; Liu, M.; Wang, G. Intramuscular Expression of Plasmid-Encoded FVII-Fc Immunoconjugate for Tumor Immunotherapy by Targeting Tumoral Blood Vessels and Cells. Front. Oncol. 2021, 11, 638591. [Google Scholar] [CrossRef]
- Carneiro-Lobo, T.C.; Schaffner, F.; Disse, J.; Ostergaard, H.; Francischetti, I.M.B.; Monteiro, R.Q.; Ruf, W. The tick-derived inhibitor Ixolaris prevents tissue factor signaling on tumor cells. J. Thromb. Haemost. 2012, 10, 1849–1858. [Google Scholar] [CrossRef]
- Francischetti, I.M.B.; Toomer, K.; Zhang, Y.; Jani, J.; Siddiqui, Z.; Brotman, D.J.; Hooper, J.E.; Kickler, T.S. Upregulation of pulmonary tissue factor, loss of thrombomodulin and immunothrombosis in SARS-CoV-2 infection. EClinicalMedicine 2021, 39, 101069. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Yue, J.; Mao, M.; Liu, Q.; Zhou, J.; Yang, J. Recombinant nematode anticoagulant protein c2 inhibits cell invasion by decreasing uPA expression in NSCLC cells. Oncol. Rep. 2015, 33, 1815–1822. [Google Scholar] [CrossRef][Green Version]
- Jaiswal, R.; Sedger, L.M. Intercellular Vesicular Transfer by Exosomes, Microparticles and Oncosomes—Implications for Cancer Biology and Treatments. Front. Oncol. 2019, 9, 125. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Yau, M.-K.; Lim, J.; Wu, K.-C.; Xu, W.; Suen, J.Y.; Fairlie, D.P. A Potent Antagonist of Protease-Activated Receptor 2 That Inhibits Multiple Signaling Functions in Human Cancer Cells. J. Pharmacol. Exp. Ther. 2018, 364, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Schaffner, F.; Ruf, W. Tissue factor and PAR2 signaling in the tumor microenvironment. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 1999–2004. [Google Scholar] [CrossRef]
- Mousa, S.A.; Bozarth, J.; Barrett, J.S. Pharmacodynamic properties of the low molecular weight heparin, tinzaparin: Effect of molecular weight distribution on plasma tissue factor pathway inhibitor in healthy human subjects. J. Clin. Pharma 2003, 43, 727–734. [Google Scholar] [CrossRef]
- Mousa, S.A. Low-molecular-weight heparin in thrombosis and cancer. Semin. Thromb. Hemost. 2004, 30 (Suppl. S1), 25–30. [Google Scholar] [CrossRef]
- Pérez-Ruiz, A.; Montes, R.; Carrasco, P.; Rocha, E. Effects of a low molecular weight heparin, bemiparin, and unfractionated heparin on hemostatic properties of endothelium. Clin. Appl. Thromb. Hemost. 2002, 8, 65–71. [Google Scholar] [CrossRef]
- Ibrahim, E.; Norris, L.A.; Abu Saadeh, F. Update on extended prophylaxis for venous thromboembolism following surgery for gynaecological cancers. Thromb. Update 2021, 2, 100038. [Google Scholar] [CrossRef]
- Ketch, P.W.; Dowdy, S.C.; McBane, R.D.; Michael Straughn, J.; Boitano, T.K.L. Direct oral anticoagulants (DOACs) for postoperative venous thromboembolism prophylaxis in patients with gynecologic malignancies: A quality mini-review. Gynecol. Oncol. Rep. 2024, 56, 101508. [Google Scholar] [CrossRef]
- Vignoli, A.; Marchetti, M.; Balducci, D.; Barbui, T.; Falanga, A. Differential effect of the low-molecular-weight heparin, dalteparin, and unfractionated heparin on microvascular endothelial cell hemostatic properties. Haematologica 2006, 91, 207–214. [Google Scholar] [PubMed]
- Featherby, S.; Xiao, Y.P.; Ettelaie, C.; Nikitenko, L.L.; Greenman, J.; Maraveyas, A. Low molecular weight heparin and direct oral anticoagulants influence tumour formation, growth, invasion and vascularisation by separate mechanisms. Sci. Rep. 2019, 9, 6272. [Google Scholar] [CrossRef] [PubMed]
- Longo de Oliveira, A.L.M.; de Oliveira Pereira, R.F.; Agati, L.B.; Ribeiro, C.M.; Kawamura Suguiura, G.Y.; Cioni, C.H.; Bermudez, M.; Pirani, M.B.; Caffaro, R.A.; Castelli, V.; et al. Rivaroxaban Versus Enoxaparin for Thromboprophylaxis After major Gynecological Cancer Surgery: The VALERIA Trial: Venous thromboembolism prophylAxis after gynecoLogical pElvic cancer surgery with RIvaroxaban versus enoxAparin (VALERIA trial). Clin. Appl. Thromb. Hemost. 2022, 28, 10760296221132556. [Google Scholar] [CrossRef]
- Osaki, S.; Kawai, S.; Ito, M.; Otani, S.; Ichikawa, R.; Torii, Y.; Takahashi, H.; Toyama, H.; Ozaki, Y.; Fujii, T. Preliminary therapeutic outcomes of using direct oral anticoagulants to treat venous thromboembolism in gynecological cancer patients. Fujita Med. J. 2019, 5, 67–71. [Google Scholar] [CrossRef]
- Coleman, R.L.; Lorusso, D.; Gennigens, C.; González-Martín, A.; Randall, L.; Cibula, D.; Lund, B.; Woelber, L.; Pignata, S.; Forget, F.; et al. Efficacy and safety of tisotumab vedotin in previously treated recurrent or metastatic cervical cancer (innovaTV 204/GOG-3023/ENGOT-cx6): A multicentre, open-label, single-arm, phase 2 study. Lancet Oncol. 2021, 22, 609–619. [Google Scholar] [CrossRef]
- Vergote, I.; Van Nieuwenhuysen, E.; O’Cearbhaill, R.E.; Westermann, A.; Lorusso, D.; Ghamande, S.; Collins, D.C.; Banerjee, S.; Mathews, C.A.; Gennigens, C.; et al. Tisotumab Vedotin in Combination with Carboplatin, Pembrolizumab, or Bevacizumab in Recurrent or Metastatic Cervical Cancer: Results From the innovaTV 205/GOG-3024/ENGOT-cx8 Study. J. Clin. Oncol. 2023, 41, 5536–5549. [Google Scholar] [CrossRef] [PubMed]
- Cuende, J.; Liénart, S.; Dedobbeleer, O.; van der Woning, B.; De Boeck, G.; Stockis, J.; Huygens, C.; Colau, D.; Somja, J.; Delvenne, P.; et al. Monoclonal antibodies against GARP/TGF-β1 complexes inhibit the immunosuppressive activity of human regulatory T cells in vivo. Sci. Transl. Med. 2015, 7, 284ra56. [Google Scholar] [CrossRef]
- Breij, E.C.W.; de Goeij, B.E.C.G.; Verploegen, S.; Schuurhuis, D.H.; Amirkhosravi, A.; Francis, J.; Miller, V.B.; Houtkamp, M.; Bleeker, W.K.; Satijn, D.; et al. Data from An Antibody–Drug Conjugate That Targets Tissue Factor Exhibits Potent Therapeutic Activity against a Broad Range of Solid Tumors; American Association for Cancer Research: Philadelphia, PA, USA, 2023. [Google Scholar] [CrossRef]
- Madariaga, A.; Sanchez-Bayona, R.; Herrera, F.G.; Ramirez, P.T.; González Martín, A. Outcomes and endpoints of relevance in gynecologic cancer clinical trials. Int. J. Gynecol. Cancer 2023, 33, 323–332. [Google Scholar] [CrossRef]
- Madariaga, A.; Coleman, R.L.; González Martín, A. Novel therapies leading to a new landscape in gynecologic tumors. Int. J. Gynecol. Cancer 2023, 33, 321–322. [Google Scholar] [CrossRef]
- Monteiro, R.Q.; Rezaie, A.R.; Bae, J.-S.; Calvo, E.; Andersen, J.F.; Francischetti, I.M.B. Ixolaris binding to factor X reveals a precursor state of factor Xa heparin-binding exosite. Protein Sci. 2008, 17, 146–153. [Google Scholar] [CrossRef]
- Monteiro, R.Q.; Rezaie, A.R.; Ribeiro, J.M.C.; Francischetti, I.M.B. Ixolaris: A factor Xa heparin-binding exosite inhibitor. Biochem. J. 2005, 387, 871–877. [Google Scholar] [CrossRef] [PubMed]
- Nazareth, R.A.; Tomaz, L.S.; Ortiz-Costa, S.; Atella, G.C.; Ribeiro, J.M.C.; Francischetti, I.M.B.; Monteiro, R.Q. Antithrombotic properties of Ixolaris, a potent inhibitor of the extrinsic pathway of the coagulation cascade. Thromb. Haemost. 2006, 96, 7–13. [Google Scholar] [CrossRef]
- Lee, A.Y.Y.; Vlasuk, G.P. Recombinant nematode anticoagulant protein c2 and other inhibitors targeting blood coagulation factor VIIa/tissue factor. J. Intern. Med. 2003, 254, 313–321. [Google Scholar] [CrossRef]
- Bozzo, J. rNAPc2. Drugs Future 2006, 31, 0028. [Google Scholar] [CrossRef]
- Frédérick, R.; Pochet, L.; Charlier, C.; Masereel, B. Modulators of the coagulation cascade: Focus and recent advances in inhibitors of tissue factor, factor VIIa and their complex. Curr. Med. Chem. 2005, 12, 397–417. [Google Scholar] [CrossRef] [PubMed]
- Rath, N.; Olson, M.F. Rho-associated kinases in tumorigenesis: Re-considering ROCK inhibition for cancer therapy. EMBO Rep. 2012, 13, 900–908. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Trillo, A.; Pena, C.; García, S.; Pérez-Pampín, E.; Rodríguez-López, M.; Mera-Varela, A.; González, A.; Conde, C. ROCK inhibition with Y-27632 reduces joint inflammation and damage in serum-induced arthritis model and decreases in vitro osteoclastogenesis in patients with early arthritis. Front. Immunol. 2022, 13, 858069. [Google Scholar] [CrossRef]
- Ogata, S.; Morishige, K.-I.; Sawada, K.; Hashimoto, K.; Mabuchi, S.; Kawase, C.; Ooyagi, C.; Sakata, M.; Kimura, T. Fasudil inhibits lysophosphatidic acid-induced invasiveness of human ovarian cancer cells. Int. J. Gynecol. Cancer 2009, 19, 1473–1480. [Google Scholar] [CrossRef]
- Barcelo, J.; Samain, R.; Sanz-Moreno, V. Preclinical to clinical utility of ROCK inhibitors in cancer. Trends Cancer 2023, 9, 250–263. [Google Scholar] [CrossRef]
- Musacchio, L.; Caruso, G.; Pisano, C.; Cecere, S.C.; Di Napoli, M.; Attademo, L.; Tambaro, R.; Russo, D.; Califano, D.; Palaia, I.; et al. PARP inhibitors in endometrial cancer: Current status and perspectives. Cancer Manag. Res. 2020, 12, 6123–6135. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, X.; Zhang, X.; Xiao, Z.; Zhang, C.; Liu, X.; Xu, J.; Li, D.; Shen, Y. A homozygous RNF220 mutation leads to male infertility with small-headed sperm. Gene 2019, 688, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Taylor, K.N.; Eskander, R.N. PARP inhibitors in epithelial ovarian cancer. Recent Pat. Anticancer Drug Discov. 2018, 13, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Lau, C.-H.; Seow, K.-M.; Chen, K.-H. The molecular mechanisms of actions, effects, and clinical implications of PARP inhibitors in epithelial ovarian cancers: A systematic review. Int. J. Mol. Sci. 2022, 23, 8125. [Google Scholar] [CrossRef] [PubMed]
- Seimiya, H. Cancer therapy by PARP inhibitors. Nippon Rinsho 2015, 73, 1330–1335. [Google Scholar]
- Langer, F.; Spath, B.; Haubold, K.; Holstein, K.; Marx, G.; Wierecky, J.; Brümmendorf, T.H.; Dierlamm, J.; Bokemeyer, C.; Eifrig, B. Tissue factor procoagulant activity of plasma microparticles in patients with cancer-associated disseminated intravascular coagulation. Ann. Hematol. 2008, 87, 451–457. [Google Scholar] [CrossRef]
- Bonifay, A.; Mackman, N.; Hisada, Y.; Sachetto, A.T.A.; Hau, C.; Gray, E.; Hogwood, J.; Aharon, A.; Badimon, L.; Barile, L.; et al. Comparison of assays measuring extracellular vesicle tissue factor in plasma samples: Communication from the ISTH SSC Subcommittee on Vascular Biology. J. Thromb. Haemost. 2024, 22, 2910–2921. [Google Scholar] [CrossRef]
- Archibald, S.J.; Hisada, Y.; Bae-Jump, V.L.; Mackman, N. Evaluation of a new bead-based assay to measure levels of human tissue factor antigen in extracellular vesicles in plasma. Res. Pract. Thromb. Haemost. 2022, 6, e12677. [Google Scholar] [CrossRef]
- Rasila, K.K.; Burger, R.A.; Smith, H.; Lee, F.C.; Verschraegen, C. Angiogenesis in gynecological oncology—Mechanism of tumor progression and therapeutic targets. Int. J. Gynecol. Cancer 2005, 15, 710–726. [Google Scholar] [CrossRef]
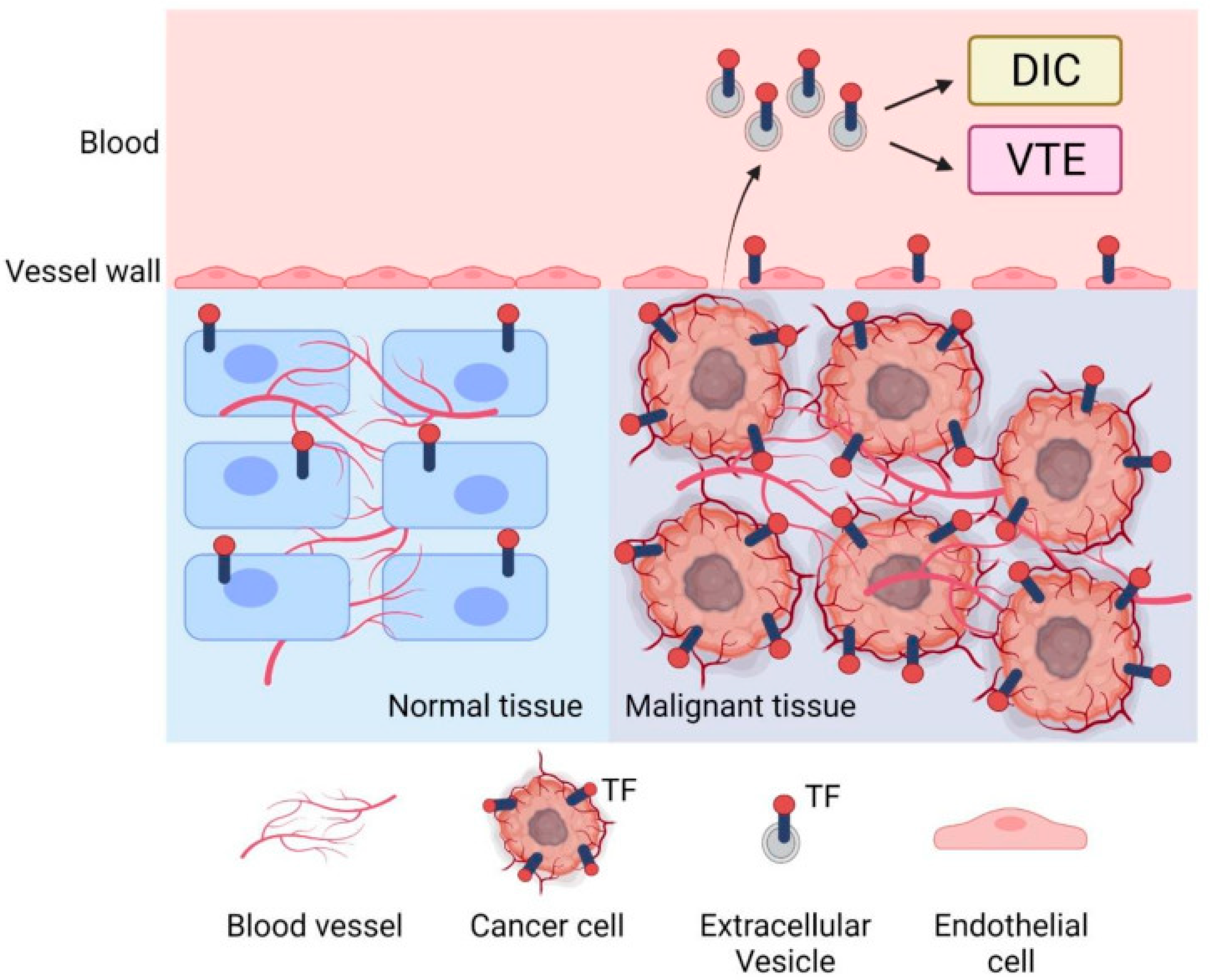
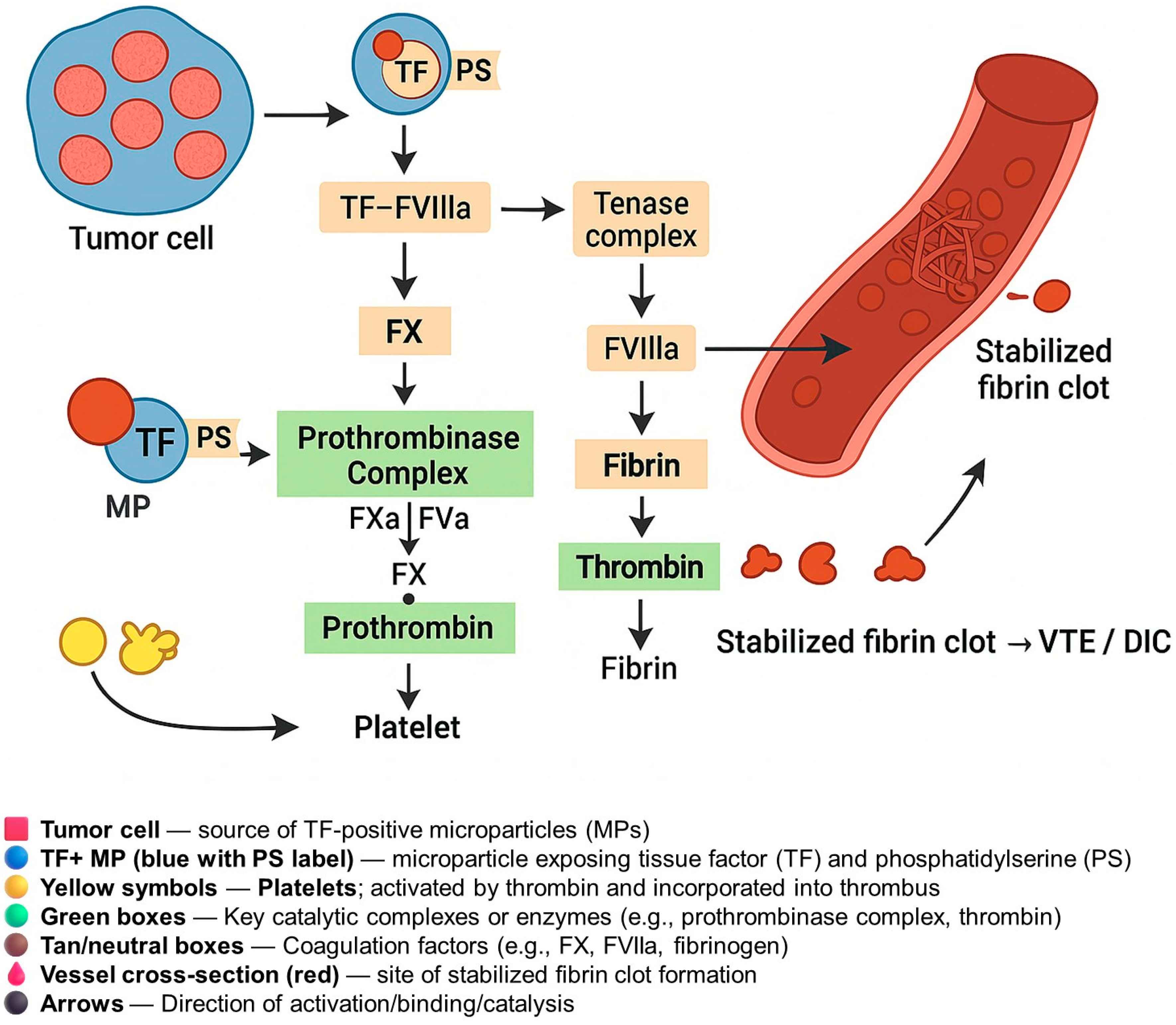
| Cancer Type | Histological Subtype | Sample/EV Source | TF Assay or Method | Key Findings | References |
|---|---|---|---|---|---|
| Ovarian carcinoma | High-grade serous carcinoma (HGSC) | Ascitic fluid; MPs via ultracentrifugation and flow cytometry | EpCAM+ MPs | Millions of EpCAM+ tumor-derived MPs were detected in ascites. These MPs promoted ovarian cancer cell migration and metastasis. | [22] |
| Advanced HGSC | Ascitic fluid EVs; high-speed centrifugation | TF activity (Zymuphen assay) | Isolated large TF+ EVs had very high TF activity (median 80 pg/mL). A total of 35% of patients had VTE; all but one VTE patient had EV–TF levels above median (p < 0.02). High TF+ MP levels correlated with thrombosis risk. EVs also activated the ERK pathway. | [26] | |
| Clear cell carcinoma (CCC) | Ovarian cancer cell lines; hypoxic EV release in vitro | TF Western blot and activity assay | CCC cells secreted large amounts of TF-rich EVs under hypoxia, facilitated by filamin-A and PAR signaling. These EVs had high procoagulant activity. | [27] | |
| Mixed subtypes | Plasma EVs from ovarian cancer patients | TF antigen/MP–TF activity assays | Elevated TF+ EV levels correlated with increased VTE risk and adverse clinical outcomes. Functional assays showed superior predictive value. | [28] | |
| Not specified | Plasma samples from high-risk gynecologic cancer patients | TF expression and MP profiling | TF-expressing MPs proposed as biomarkers for thrombotic risk. Data supports risk-adapted prophylactic anticoagulation strategies. | [29] |
| Agent/Class | Mechanism of Action (TF/MP Specific) | Target/Pathway | Clinical Status | Relevance to Gynecologic Cancers | Key Limitations/Challenges | Key References |
|---|---|---|---|---|---|---|
| LMWH (e.g., enoxaparin, bemiparin, tinzaparin) | Enhances TFPI release → inhibits TF-FVIIa complex and FXa; suppresses endothelial TF expression; reduces TF procoagulant activity; interferes with thrombin generation | Downstream coagulation; TF pathway inhibition via TFPI | Approved for VTE prophylaxis/treatment in cancer (incl. gynecologic) | Standard of care for VTE prophylaxis post pelvic/abdominal surgery; evidence of MP-TF activity reduction; limited data on DIC control | Bleeding risk (esp. in high-risk surgical/oncology patients); no confirmed direct anti-tumor effect; subcutaneous administration burden | [48,49,50,51,52,53] |
| DOACs (e.g., apixaban, rivaroxaban, edoxaban) | Direct FXa inhibition → downstream of TF-FVIIa; apixaban shown to reduce TF+ MP release; suppresses TF-FVIIa-PAR2 signaling; reduces MP-TF procoagulant activity in preclinical models | Downstream coagulation; MP-TF pathway modulation | Approved for cancer-associated VTE (in selected patients) | Alternative to LMWH for VTE prophylaxis/treatment in gynecologic cancer; trials support efficacy, patient convenience; emerging evidence for MP-TF modulation | Increased bleeding risk (esp. GI); limited data in gynecologic cancer subtypes; need for individualized selection | [20,52,54,55,56] |
| Tisotumab Vedotin (TV) | Anti-TF antibody-drug conjugate (ADC); binds tumor TF → internalization → MMAE cytotoxic release; mediates ADCC, ADCP, inhibits TF-PAR2 signaling; may reduce MP-TF procoagulant activity | Tumor TF; TF-PAR2 signaling | FDA-approved (recurrent/metastatic cervical cancer); trials ongoing in ovarian, endometrial cancer | Approved for r/m cervical cancer post-chemotherapy; promising in other gynecologic tumors with high TF expression | Ocular toxicity (requires prophylaxis), neuropathy, limited efficacy in non-responders, modest ORR (~24%); biomarker need for patient selection | [36,38,57,58] |
| Anti-TF mAbs (e.g., ALT-836) | Binds TF → blocks TF-FVIIa complex formation → prevents FX activation, reduces thrombin generation; may inhibit TF-mediated tumor signaling and MP-TF procoagulant activity | TF/TF-FVIIa | Phase I (non-oncology); preclinical oncology models | Preclinical studies suggest antithrombotic, anti-invasive potential; being explored in solid tumors (incl. gynecologic) | Bleeding at higher doses, no cancer clinical trial data yet, delivery and biomarker challenges | [59,60] |
| Ixolaris (tick salivary protein) | Binds FX/FXa → sequesters TF-FVIIa-FX complex → prevents initiation of coagulation and reduces MP-TF activity | TF/TF-FVIIa/FX | Preclinical (animal models, no human trials) | Inhibits MP-TF activity and tumor growth in preclinical ovarian cancer models; potential complement to other targeted therapies | No human trials; delivery challenges; bleeding risk; heterogeneity of TF expression in gynecologic tumors | [61,62,63,64,65] |
| rNAPc2 (recombinant nematode anticoagulant protein c2) | Binds FX/FXa → forms complex that inhibits TF-FVIIa → blocks coagulation initiation at extrinsic pathway | TF-FVIIa/FX/FXa | Clinical trials (non-gynecologic settings; no direct gynecologic data) | Theoretically targets TF-mediated processes involved in tumor growth, angiogenesis, and metastasis; no direct evidence in gynecologic models | No gynecologic trials; delivery and translational barriers; potential bleeding risk; heterogeneity of TF expression | [66,67,68] |
| ROCK inhibitors (e.g., Y-27632) | Inhibit RhoA/ROCK pathway → ↓ actomyosin contractility → ↓ cytoskeletal dynamics → ↓ MP formation and MP-TF release | RhoA/ROCK; cytoskeletal regulation; MP biogenesis | Preclinical (no gynecologic-specific clinical trials) | Reduces cancer cell invasion/migration in ovarian models (fasudil); theoretical potential to reduce MP-TF release and metastasis in gynecologic cancers | Lack of gynecologic-specific data; delivery challenges; off-target effects; systemic toxicity; need for isoform-selective inhibitors | [69,70,71,72] |
| PARP inhibitors (e.g., I-191) | No direct inhibition of MP-TF; indirectly modulates MP-TF via effects on transcription, chromatin remodeling, apoptosis, and tumor microenvironment | DNA repair pathways (PARP1/2); homologous recombination deficiency (HRD); transcriptional regulation | Approved (ovarian, endometrial, cervical: specific contexts) | Significant role in HRD/BRCA+ ovarian cancer; emerging in endometrial/cervical; indirect influence on TF/MP-TF via broader cellular effects | Resistance (e.g., BRCA reversion); toxicity (hematologic, fatigue); high cost; limited direct MP-TF data | [73,74,75,76,77] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qureshi, M.; Alam, M.T.; Unar, A. The Role of Tissue Factor-Positive Microparticles in Gynecological Cancer-Associated Disseminated Intravascular Coagulation: Molecular Mechanisms and Clinical Implications. Onco 2025, 5, 33. https://doi.org/10.3390/onco5030033
Qureshi M, Alam MT, Unar A. The Role of Tissue Factor-Positive Microparticles in Gynecological Cancer-Associated Disseminated Intravascular Coagulation: Molecular Mechanisms and Clinical Implications. Onco. 2025; 5(3):33. https://doi.org/10.3390/onco5030033
Chicago/Turabian StyleQureshi, Muqaddas, Muhammad Tanveer Alam, and Ahsanullah Unar. 2025. "The Role of Tissue Factor-Positive Microparticles in Gynecological Cancer-Associated Disseminated Intravascular Coagulation: Molecular Mechanisms and Clinical Implications" Onco 5, no. 3: 33. https://doi.org/10.3390/onco5030033
APA StyleQureshi, M., Alam, M. T., & Unar, A. (2025). The Role of Tissue Factor-Positive Microparticles in Gynecological Cancer-Associated Disseminated Intravascular Coagulation: Molecular Mechanisms and Clinical Implications. Onco, 5(3), 33. https://doi.org/10.3390/onco5030033







