Screening Activity of Brain Cancer-Derived Factors on Primary Human Brain Pericytes
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Primary Pericyte Cell Culture
2.2. Immunocytochemistry and Inflammatory Treatments
2.3. Cytometric Bead Array to Study Secreted Factors
2.4. Using Fluorescent Carboxylated Beads to Study Pericyte Phagocytosis
2.5. Imaging Methods to Capture and Quantify Light and Fluorescent Images
2.6. Statistical Analysis
3. Results
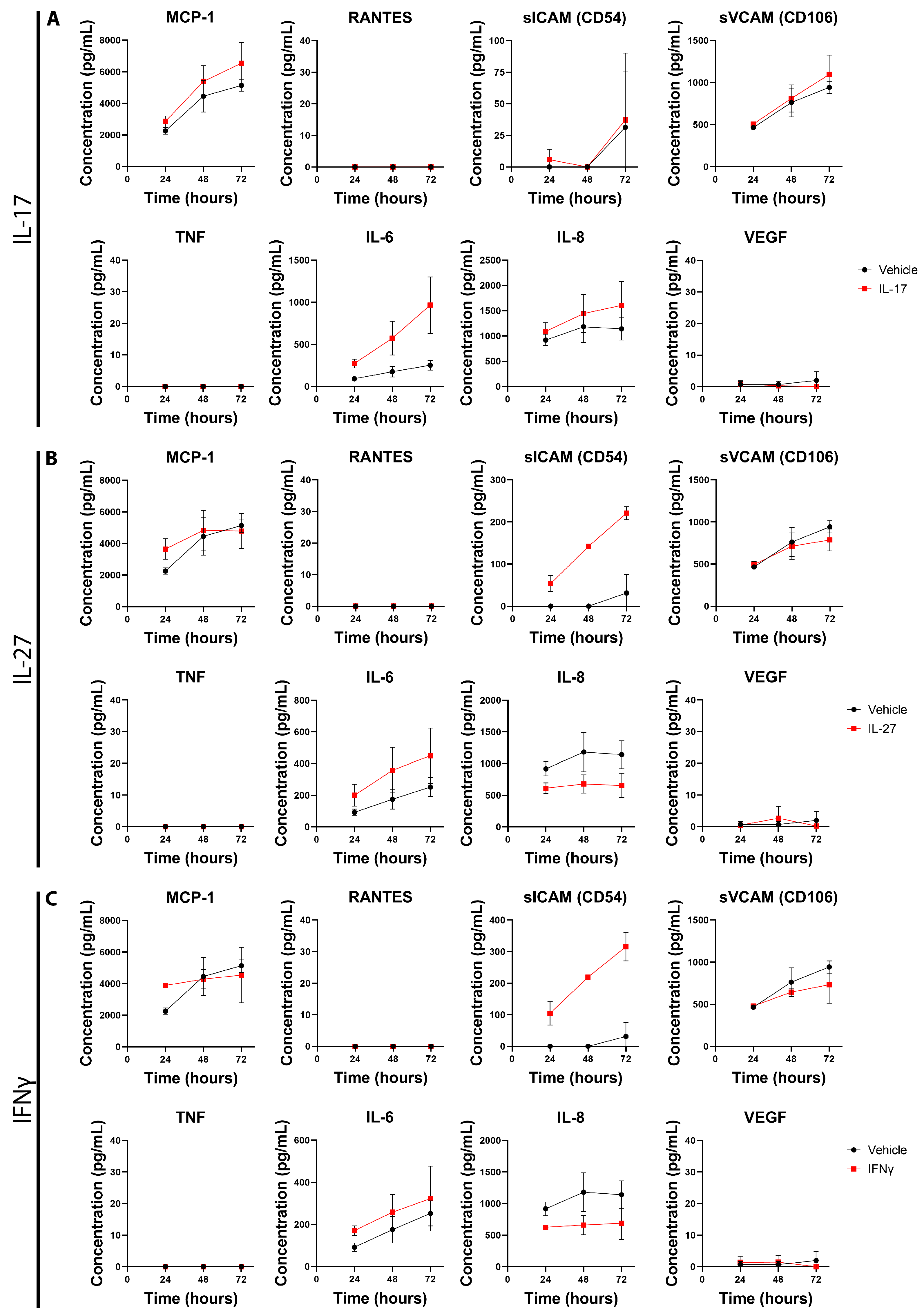
3.1. Brain Pericytes Responded to Classical Inflammatory Cytokines, TGFβ Superfamily Cytokines, IL-17, and IL-27
3.2. TGFβ and GDF-15 Induce SMAD2/3 Signalling and Inhibit C/EBP-δ Activity in Brain Pericytes
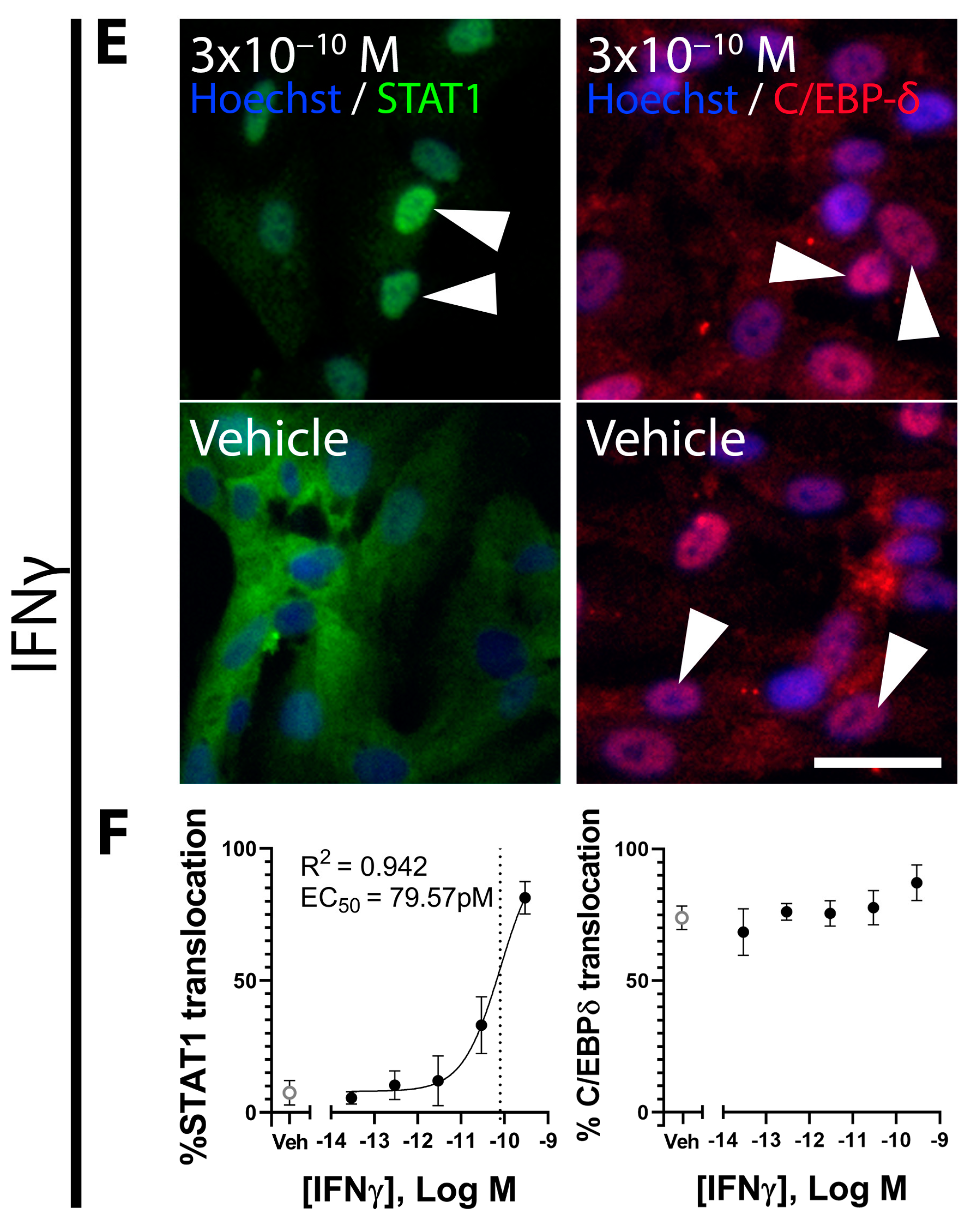
3.3. Inflammatory Factors IL-17, IL-27, and IFNγ Induce Changes in Primary Pericyte Inflammatory Phenotype
3.4. IFNγ and IL-27 Reduce Phagocytosis in Primary Pericytes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hanif, F.; Muzaffar, K.; Perveen, K.; Malhi, S.M.; Simjee, S.U. Glioblastoma Multiforme: A Review of its Epidemiology and Pathogenesis through Clinical Presentation and Treatment. Asian Pac. J. Cancer Prev. 2017, 18, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Linkous, A.G.; Yazlovitskaya, E.M. Angiogenesis in glioblastoma multiforme: Navigating the maze. Anti-Cancer Agents Med. Chem. 2011, 11, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Darmanis, S.; Sloan, S.A.; Croote, D.; Mignardi, M.; Chernikova, S.; Samghababi, P.; Zhang, Y.; Neff, N.; Kowarsky, M.; Caneda, C.; et al. Single-Cell RNA-Seq Analysis of Infiltrating Neoplastic Cells at the Migrating Front of Human Glioblastoma. Cell Rep. 2017, 21, 1399–1410. [Google Scholar] [CrossRef] [PubMed]
- Robilliard, L.D.; Yu, J.; Anchan, A.; Finlay, G.; Angel, C.E.; Graham, E.S. Comprehensive Assessment of Secreted Immuno-Modulatory Cytokines by Serum-Differentiated and Stem-like Glioblastoma Cells Reveals Distinct Differences between Glioblastoma Phenotypes. Int. J. Mol. Sci. 2022, 23, 14164. [Google Scholar] [CrossRef]
- Anchan, A.; Martin, O.; Hucklesby, J.J.W.; Finlay, G.; Johnson, R.H.; Robilliard, L.D.; O’Carroll, S.J.; Angel, C.E.; Graham, E.S. Analysis of Melanoma Secretome for Factors That Directly Disrupt the Barrier Integrity of Brain Endothelial Cells. Int. J. Mol. Sci. 2020, 21, 8193. [Google Scholar] [CrossRef]
- Pantic Bisevac, J.; Stanojevic, I.; Mijuskovic, Z.; Banovic, T.; Djukic, M.; Vojvodic, D. High Interleukin 27 Production is Associated with Early Clinical Stage and Localized Disease in Patients with Melanoma. J. Med. Biochem. 2016, 35, 443–450. [Google Scholar] [CrossRef]
- Rustenhoven, J.; Jansson, D.; Smyth, L.C.; Dragunow, M. Brain Pericytes as Mediators of Neuroinflammation. Trends Pharmacol. Sci. 2017, 38, 291–304. [Google Scholar] [CrossRef]
- Stark, K.; Eckart, A.; Haidari, S.; Tirniceriu, A.; Lorenz, M.; Von Brühl, M.L.; Gärtner, F.; Khandoga, A.G.; Legate, K.R.; Pless, R.; et al. Capillary and arteriolar pericytes attract innate leukocytes exiting through venules and ‘instruct’ them with pattern-recognition and motility programs. Nat. Immunol. 2013, 14, 41–51. [Google Scholar] [CrossRef]
- Alban, T.J.; Bayik, D.; Otvos, B.; Rabljenovic, A.; Leng, L.; Jia-Shiun, L.; Roversi, G.; Lauko, A.; Momin, A.A.; Mohammadi, A.M.; et al. Glioblastoma Myeloid-Derived Suppressor Cell Subsets Express Differential Macrophage Migration Inhibitory Factor Receptor Profiles That Can Be Targeted to Reduce Immune Suppression. Front. Immunol. 2020, 11, 1191. [Google Scholar] [CrossRef]
- Roth, P.; Junker, M.; Tritschler, I.; Mittelbronn, M.; Dombrowski, Y.; Breit, S.N.; Tabatabai, G.; Wick, W.; Weller, M.; Wischhusen, J. GDF-15 contributes to proliferation and immune escape of malignant gliomas. Clin. Cancer Res. 2010, 16, 3851–3859. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Bao, Z.; Zhang, X.; Li, F.; Lai, T.; Cao, C.; Chen, Z.; Li, W.; Shen, H.; Ying, S. Effectiveness and safety of PD-1/PD-L1 inhibitors in the treatment of solid tumors: A systematic review and meta-analysis. Oncotarget 2017, 8, 59901–59914. [Google Scholar] [CrossRef] [PubMed]
- Smyth, L.C.D.; Rustenhoven, J.; Park, T.I.H.; Schweder, P.; Jansson, D.; Heppner, P.A.; O’Carroll, S.J.; Mee, E.W.; Faull, R.L.M.; Curtis, M.; et al. Unique and shared inflammatory profiles of human brain endothelia and pericytes. J. Neuroinflammation 2018, 15, 138. [Google Scholar] [CrossRef]
- O’Carroll, S.J.; Kho, D.T.; Wiltshire, R.; Nelson, V.; Rotimi, O.; Johnson, R.; Angel, C.E.; Graham, E.S. Pro-inflammatory TNFaα and IL-1β differentially regulate the inflammatory phenotype of brain microvascular endothelial cells. J. Neuroinflammation 2015, 12, 131. [Google Scholar] [CrossRef] [PubMed]
- Rustenhoven, J.; Scotter, E.L.; Jansson, D.; Kho, D.T.; Oldfield, R.L.; Bergin, P.S.; Mee, E.W.; Faull, R.L.M.; Curtis, M.A.; Graham, S.E.; et al. An anti-inflammatory role for C/EBPδ in human brain pericytes. Sci. Rep. 2015, 5, 12132. [Google Scholar] [CrossRef]
- Rustenhoven, J.; Aalderink, M.; Scotter, E.L.; Oldfield, R.L.; Bergin, P.S.; Mee, E.W.; Graham, E.S.; Faull, R.L.M.; Curtis, M.A.; Park, T.I.H.; et al. TGF-beta1 regulates human brain pericyte inflammatory processes involved in neurovasculature function. J. Neuroinflammation 2016, 13, 37. [Google Scholar] [CrossRef] [PubMed]
- Thanabalasundaram, G.; Schneidewind, J.; Pieper, C.; Galla, H.J. The impact of pericytes on the blood-brain barrier integrity depends critically on the pericyte differentiation stage. Int. J. Biochem. Cell Biol. 2011, 43, 1284–1293. [Google Scholar] [CrossRef]
- von Roemeling, C.A.; Wang, Y.; Qie, Y.; Yuan, H.; Zhao, H.; Liu, X.; Yang, Z.; Yang, M.; Deng, W.; Bruno, K.A.; et al. Therapeutic modulation of phagocytosis in glioblastoma can activate both innate and adaptive antitumour immunity. Nat. Commun. 2020, 11, 1508. [Google Scholar] [CrossRef]
- Bruna, A.; Darken, R.S.; Rojo, F.; Ocaña, A.; Peñuelas, S.; Arias, A.; Paris, R.; Tortosa, A.; Mora, J.; Baselga, J.; et al. High TGFb;-Smad Activity Confers Poor Prognosis in Glioma Patients and Promotes Cell Proliferation Depending on the Methylation of the PDGF-B Gene. Cancer Cell 2007, 11, 147–160. [Google Scholar] [CrossRef]
- Roy, L.-O.; Poirier, M.-B.; Fortin, D. Differential Expression and Clinical Significance of Transforming Growth Factor-Beta Isoforms in GBM Tumors. Int. J. Mol. Sci. 2018, 19, 1113. [Google Scholar] [CrossRef]
- Peñuelas, S.; Anido, J.; Prieto-Sánchez, R.M.; Folch, G.; Barba, I.; Cuartas, I.; García-Dorado, D.; Poca, M.A.; Sahuquillo, J.; Baselga, J.; et al. TGF-beta; Increases Glioma-Initiating Cell Self-Renewal through the Induction of LIF in Human Glioblastoma. Cancer Cell 2009, 15, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, S.S.; Paliogianni, F.; Yamada, H.; Balow, J.E.; Boumpas, D.T. Effect of transforming growth factor-β on early and late activation events in human T cells. J. Immunol. 1993, 150, 3109–3118. [Google Scholar] [CrossRef] [PubMed]
- Wick, W.; Platten, M.; Weller, M. Glioma Cell Invasion: Regulation of Metalloproteinase Activity by TGF-β. J. Neuro-Oncol. 2001, 53, 177–185. [Google Scholar] [CrossRef]
- Olsen, O.E.; Skjærvik, A.; Størdal, B.F.; Sundan, A.; Holien, T. TGF-β contamination of purified recombinant GDF15. PLoS ONE 2017, 12, e0187349. [Google Scholar] [CrossRef]
- Sasahara, A.; Tominaga, K.; Nishimura, T.; Yano, M.; Kiyokawa, E.; Noguchi, M.; Noguchi, M.; Kanauchi, H.; Ogawa, T.; Minato, H.; et al. An autocrine/paracrine circuit of growth differentiation factor (GDF) 15 has a role for maintenance of breast cancer stemlike cells. Oncotarget 2017, 8, 24869–24881. [Google Scholar] [CrossRef] [PubMed]
- Modi, A.; Purohit, P.; Roy, D.; Vishnoi, J.R.; Pareek, P.; Elhence, P.; Singh, P.; Sharma, S.; Sharma, P.; Misra, S. FOXM1 mediates GDF-15 dependent stemness and intrinsic drug resistance in breast cancer. Mol. Biol. Rep. 2022, 49, 2877–2888. [Google Scholar] [CrossRef] [PubMed]
- Hutter, G.; Theruvath, J.; Graef, C.M.; Zhang, M.; Schoen, M.K.; Manz, E.M.; Bennett, M.L.; Olson, A.; Azad, T.D.; Sinha, R.; et al. Microglia are effector cells of CD47-SIRPα antiphagocytic axis disruption against glioblastoma. Proc. Natl. Acad. Sci. USA 2019, 116, 997–1006. [Google Scholar] [CrossRef]
- Held-Feindt, J.; Hattermann, K.; Müerköster, S.S.; Wedderkopp, H.; Knerlich-Lukoschus, F.; Ungefroren, H.; Mehdorn, H.M.; Mentlein, R. CX3CR1 promotes recruitment of human glioma-infiltrating microglia/macrophages (GIMs). Exp. Cell Res. 2010, 316, 1553–1566. [Google Scholar] [CrossRef]
- Cunha, M.M.; Pereira, A.B.M.; Lino, R.C.; da Silva, P.R.; Andrade-Silva, L.E.; de Vito, F.B.; de Souza, H.M.; Silva-Vergara, M.L.; Rogério, A.P. Effects of combination of Cryptococcus gattii and IFN-γ, IL-4 or IL-27 on human bronchial epithelial cells. Immunobiology 2023, 228, 152312. [Google Scholar] [CrossRef]
- Xiong, Y.; Li, Y.; Cui, X.; Zhang, L.; Yang, X.; Liu, H. ADAP restraint of STAT1 signaling regulates macrophage phagocytosis in immune thrombocytopenia. Cell. Mol. Immunol. 2022, 19, 898–912. [Google Scholar] [CrossRef]
- Meng, Y.; Chen, C.; Liu, Y.; Tian, C.; Li, H.H. Angiotensin II Regulates Dendritic Cells through Activation of NF-κB/p65, ERK1/2 and STAT1 Pathways. Cell. Physiol. Biochem. 2017, 42, 1550–1558. [Google Scholar] [CrossRef] [PubMed]
- Thota, B.; Arimappamagan, A.; Kandavel, T.; Shastry, A.H.; Pandey, P.; Chandramouli, B.A.; Hegde, A.S.; Kondaiah, P.; Santosh, V. STAT-1 expression is regulated by IGFBP-3 in malignant glioma cells and is a strong predictor of poor survival in patients with glioblastoma: Laboratory investigation. J. Neurosurg. JNS 2014, 121, 374–383. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Li, X.; Su, J.; Wang Gong, F.; Lu, J.; Wei, Y. STAT1 determines aggressiveness of glioblastoma both in vivo and in vitro through wnt/β-catenin signalling pathway. Cell Biochem. Funct. 2020, 38, 630–641. [Google Scholar] [CrossRef] [PubMed]
- Ling, H.; Recklies, A.D. The chitinase 3-like protein human cartilage glycoprotein 39 inhibits cellular responses to the inflammatory cytokines interleukin-1 and tumour necrosis factor-alpha. Biochem. J. 2004, 380, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.G.; Hartl, D.; Lee, G.R.; Koller, B.; Matsuura, H.; Da Silva, C.A.; Sohn, M.H.; Cohn, L.; Homer, R.J.; Kozhich, A.A.; et al. Role of breast regression protein 39 (BRP-39)/chitinase 3-like-1 in Th2 and IL-13–induced tissue responses and apoptosis. J. Exp. Med. 2009, 206, 1149–1166. [Google Scholar] [CrossRef]
- Amatya, N.; Garg, A.V.; Gaffen, S.L. IL-17 Signaling: The Yin and the Yang. Trends Immunol. 2017, 38, 310–322. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McCullough, S.; Albers, E.; Anchan, A.; Yu, J.; Connor, B.; Graham, E.S. Screening Activity of Brain Cancer-Derived Factors on Primary Human Brain Pericytes. Onco 2024, 4, 381-396. https://doi.org/10.3390/onco4040027
McCullough S, Albers E, Anchan A, Yu J, Connor B, Graham ES. Screening Activity of Brain Cancer-Derived Factors on Primary Human Brain Pericytes. Onco. 2024; 4(4):381-396. https://doi.org/10.3390/onco4040027
Chicago/Turabian StyleMcCullough, Samuel, Eliene Albers, Akshata Anchan, Jane Yu, Bronwen Connor, and E. Scott Graham. 2024. "Screening Activity of Brain Cancer-Derived Factors on Primary Human Brain Pericytes" Onco 4, no. 4: 381-396. https://doi.org/10.3390/onco4040027
APA StyleMcCullough, S., Albers, E., Anchan, A., Yu, J., Connor, B., & Graham, E. S. (2024). Screening Activity of Brain Cancer-Derived Factors on Primary Human Brain Pericytes. Onco, 4(4), 381-396. https://doi.org/10.3390/onco4040027





