Marine-Derived Therapeutics for the Management of Glioblastoma: A Case Series and Comprehensive Review of the Literature
Simple Summary
Abstract
1. Introduction
2. Case Series
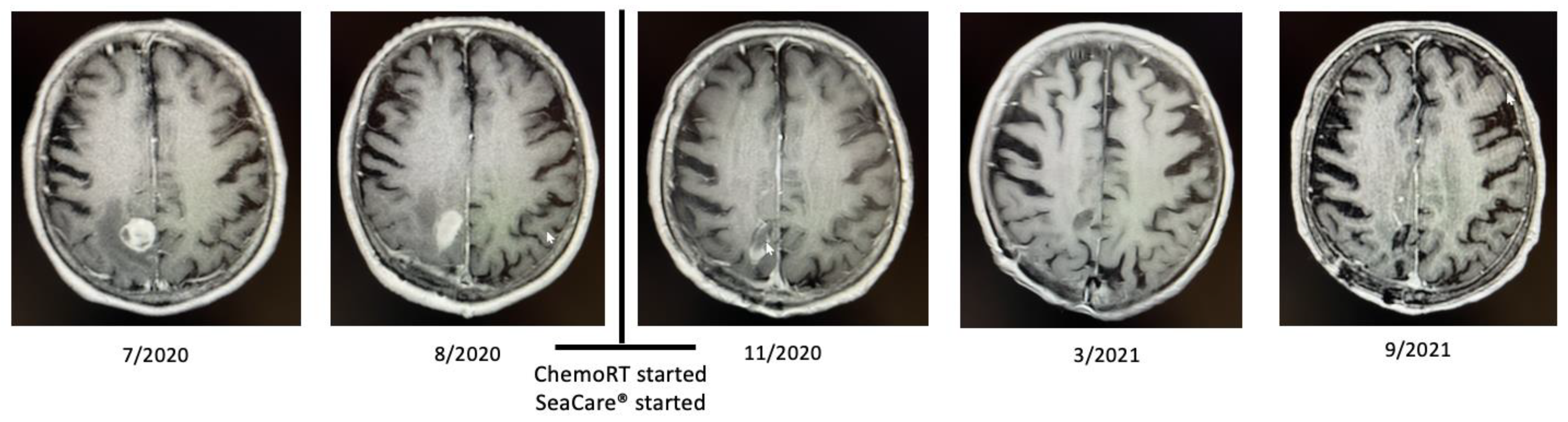
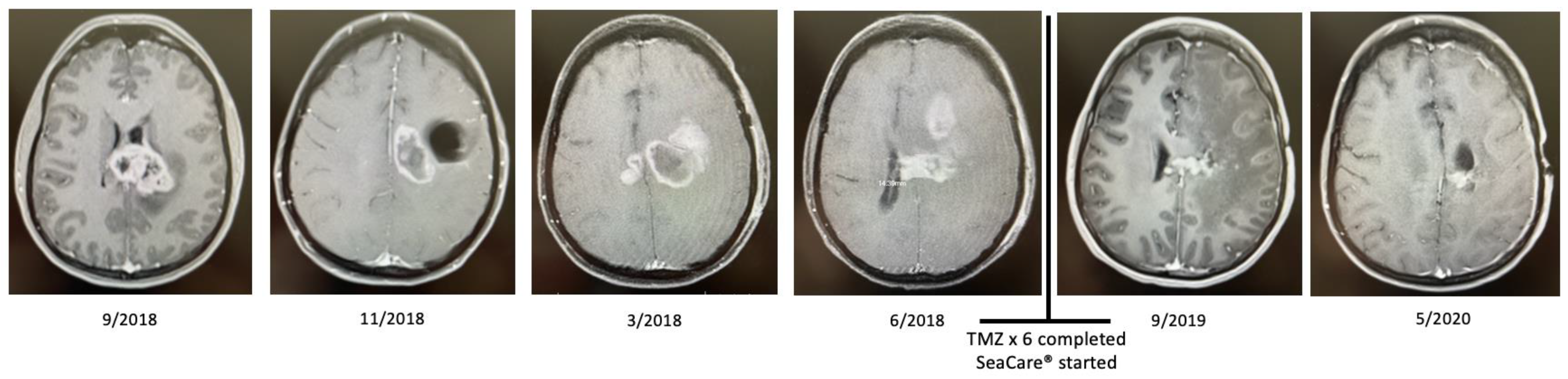
2.1. Patient 1
2.2. Patient 2
3. Discussion
3.1. Targeting the Tumor Immune Microenvironment in Glioblastoma and Immunomodulation of Tumor-Associated Macrophages
3.2. Tumor Immune Microenvironment Modulation and Other Anticancer Properties of Marine-Derived Therapeutics
3.2.1. Sea Cucumber
3.2.2. Seagrass
3.2.3. Sea Squirt
3.2.4. Sea Urchin
3.3. Clinical Experience with Majority Sea Cucumber Blend Therapeutics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kotecha, R.; Odia, Y.; Khosla, A.A.; Ahluwalia, M.S. Key Clinical Principles in the Management of Glioblastoma. JCO Oncol. Pract. 2023, 19, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Zuo, W.; Kwok, H.F. Development of Marine-Derived Compounds for Cancer Therapy. Mar. Drugs 2021, 19, 342. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Taillibert, S.; Kanner, A.; Read, W.; Steinberg, D.; Lhermitte, B.; Toms, S.; Idbaih, A.; Ahluwalia, M.S.; Fink, K.; et al. Effect of Tumor-Treating Fields Plus Maintenance Temozolomide vs Maintenance Temozolomide Alone on Survival in Patients With Glioblastoma: A Randomized Clinical Trial. JAMA 2017, 318, 2306–2316. [Google Scholar] [CrossRef]
- Zitvogel, L.; Tesniere, A.; Kroemer, G. Cancer despite immunosurveillance: Immunoselection and immunosubversion. Nat. Rev. Immunol. 2006, 6, 715–727. [Google Scholar] [CrossRef]
- Boles, K.S.; Barchet, W.; Diacovo, T.; Cella, M.; Colonna, M. The tumor suppressor TSLC1/NECL-2 triggers NK-cell and CD8+ T-cell responses through the cell-surface receptor CRTAM. Blood 2005, 106, 779–786. [Google Scholar] [CrossRef]
- Lin, Y.; Xu, J.; Lan, H. Tumor-associated macrophages in tumor metastasis: Biological roles and clinical therapeutic applications. J. Hematol. Oncol. 2019, 12, 76. [Google Scholar] [CrossRef] [PubMed]
- Xuan, W.; Lesniak, M.S.; James, C.D.; Heimberger, A.B.; Chen, P. Context-Dependent Glioblastoma-Macrophage/Microglia Symbiosis and Associated Mechanisms. Trends Immunol. 2021, 42, 280–292. [Google Scholar] [CrossRef]
- Yin, J.; Kim, S.S.; Choi, E.; Oh, Y.T.; Lin, W.; Kim, T.H.; Sa, J.K.; Hong, J.H.; Park, S.H.; Kwon, H.J.; et al. ARS2/MAGL signaling in glioblastoma stem cells promotes self-renewal and M2-like polarization of tumor-associated macrophages. Nat. Commun. 2020, 11, 2978. [Google Scholar] [CrossRef]
- Kashfi, K.; Kannikal, J.; Nath, N. Macrophage Reprogramming and Cancer Therapeutics: Role of iNOS-Derived NO. Cells 2021, 10, 3194. [Google Scholar] [CrossRef]
- Na, Y.R.; Yoon, Y.N.; Son, D.I.; Seok, S.H. Cyclooxygenase-2 inhibition blocks M2 macrophage differentiation and suppresses metastasis in murine breast cancer model. PLoS ONE 2013, 8, e63451. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yang, B.; Huang, J.; Lin, Y.; Xiang, T.; Wan, J.; Li, H.; Chouaib, S.; Ren, G. Cyclooxygenase-2 in tumor-associated macrophages promotes breast cancer cell survival by triggering a positive-feedback loop between macrophages and cancer cells. Oncotarget 2015, 6, 29637–29650. [Google Scholar] [CrossRef] [PubMed]
- Mussbacher, M.; Derler, M.; Basilio, J.; Schmid, J.A. NF-kappaB in monocytes and macrophages—An inflammatory master regulator in multitalented immune cells. Front. Immunol. 2023, 14, 1134661. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ding, H.; Li, Z.; Peng, Y.; Tan, H.; Wang, C.; Huang, G.; Li, W.; Ma, G.; Wei, W. Exploration and functionalization of M1-macrophage extracellular vesicles for effective accumulation in glioblastoma and strong synergistic therapeutic effects. Signal Transduct. Target Ther. 2022, 7, 74. [Google Scholar] [CrossRef]
- Mantovani, A.; Allavena, P.; Marchesi, F.; Garlanda, C. Macrophages as tools and targets in cancer therapy. Nat. Rev. Drug Discov. 2022, 21, 799–820. [Google Scholar] [CrossRef] [PubMed]
- Jaynes, J.M.; Sable, R.; Ronzetti, M.; Bautista, W.; Knotts, Z.; Abisoye-Ogunniyan, A.; Li, D.; Calvo, R.; Dashnyam, M.; Singh, A.; et al. Mannose receptor (CD206) activation in tumor-associated macrophages enhances adaptive and innate antitumor immune responses. Sci. Transl. Med. 2020, 12, eaax6337. [Google Scholar] [CrossRef]
- Beatty, G.L.; Torigian, D.A.; Chiorean, E.G.; Saboury, B.; Brothers, A.; Alavi, A.; Troxel, A.B.; Sun, W.; Teitelbaum, U.R.; Vonderheide, R.H.; et al. A phase I study of an agonist CD40 monoclonal antibody (CP-870,893) in combination with gemcitabine in patients with advanced pancreatic ductal adenocarcinoma. Clin. Cancer Res. 2013, 19, 6286–6295. [Google Scholar] [CrossRef]
- Fitzgerald, K.A.; Kagan, J.C. Toll-like Receptors and the Control of Immunity. Cell 2020, 180, 1044–1066. [Google Scholar] [CrossRef]
- Leblond, M.M.; Peres, E.A.; Helaine, C.; Gerault, A.N.; Moulin, D.; Anfray, C.; Divoux, D.; Petit, E.; Bernaudin, M.; Valable, S. M2 macrophages are more resistant than M1 macrophages following radiation therapy in the context of glioblastoma. Oncotarget 2017, 8, 72597–72612. [Google Scholar] [CrossRef]
- Meng, Y.; Beckett, M.A.; Liang, H.; Mauceri, H.J.; van Rooijen, N.; Cohen, K.S.; Weichselbaum, R.R. Blockade of tumor necrosis factor alpha signaling in tumor-associated macrophages as a radiosensitizing strategy. Cancer Res. 2010, 70, 1534–1543. [Google Scholar] [CrossRef]
- Cortes, J.; Vahdat, L.; Blum, J.L.; Twelves, C.; Campone, M.; Roché, H.; Bachelot, T.; Awada, A.; Paridaens, R.; Goncalves, A.; et al. Phase II study of the halichondrin B analog eribulin mesylate in patients with locally advanced or metastatic breast cancer previously treated with an anthracycline, a taxane, and capecitabine. J. Clin. Oncol. 2010, 28, 3922–3928. [Google Scholar] [CrossRef] [PubMed]
- Schöffski, P.; Chawla, S.; Maki, R.G.; Italiano, A.; Gelderblom, H.; Choy, E.; Grignani, G.; Camargo, V.; Bauer, S.; Rha, S.Y.; et al. Eribulin versus dacarbazine in previously treated patients with advanced liposarcoma or leiomyosarcoma: A randomised, open-label, multicentre, phase 3 trial. Lancet 2016, 387, 1629–1637. [Google Scholar] [CrossRef] [PubMed]
- Pro, B.; Advani, R.; Brice, P.; Bartlett, N.L.; Rosenblatt, J.D.; Illidge, T.; Matous, J.; Ramchandren, R.; Fanale, M.; Connors, J.M.; et al. Five-year results of brentuximab vedotin in patients with relapsed or refractory systemic anaplastic large cell lymphoma. Blood 2017, 130, 2709–2717. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, S.; O’Connor, O.A.; Pro, B.; Trümper, L.; Iyer, S.; Advani, R.; Bartlett, N.L.; Christensen, J.H.; Morschhauser, F.; Domingo-Domenech, E.; et al. The ECHELON-2 Trial: 5-year results of a randomized, phase III study of brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma. Ann. Oncol. 2022, 33, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Connors, J.M.; Jurczak, W.; Straus, D.J.; Ansell, S.M.; Kim, W.S.; Gallamini, A.; Younes, A.; Alekseev, S.; Illés, Á.; Picardi, M.; et al. Brentuximab Vedotin with Chemotherapy for Stage III or IV Hodgkin’s Lymphoma. N. Engl. J. Med. 2017, 378, 331–344. [Google Scholar] [CrossRef]
- Sehn, L.H.; Herrera, A.F.; Flowers, C.R.; Kamdar, M.K.; McMillan, A.; Hertzberg, M.; Assouline, S.; Kim, T.M.; Kim, W.S.; Ozcan, M.; et al. Polatuzumab Vedotin in Relapsed or Refractory Diffuse Large B-Cell Lymphoma. J. Clin. Oncol. 2020, 38, 155–165. [Google Scholar] [CrossRef]
- Yu, E.Y.; Petrylak, D.P.; O’Donnell, P.H.; Lee, J.L.; van der Heijden, M.S.; Loriot, Y.; Stein, M.N.; Necchi, A.; Kojima, T.; Harrison, M.R.; et al. Enfortumab vedotin after PD-1 or PD-L1 inhibitors in cisplatin-ineligible patients with advanced urothelial carcinoma (EV-201): A multicentre, single-arm, phase 2 trial. Lancet Oncol. 2021, 22, 872–882. [Google Scholar] [CrossRef]
- Du, H.; Bao, Z.; Hou, R.; Wang, S.; Su, H.; Yan, J.; Tian, M.; Li, Y.; Wei, W.; Lu, W.; et al. Transcriptome sequencing and characterization for the sea cucumber Apostichopus japonicus (Selenka, 1867). PLoS ONE 2012, 7, e33311. [Google Scholar] [CrossRef]
- Bordbar, S.; Anwar, F.; Saari, N. High-value components and bioactives from sea cucumbers for functional foods—A review. Mar. Drugs 2011, 9, 1761–1805. [Google Scholar] [CrossRef]
- Nigrelli, R.F. The effects of holothurin on fish, and mice with Sarcoma 180. Zool. Sci. Contrib. N. Y. Zool. Soc. 1952, 37, 89–90. [Google Scholar] [CrossRef]
- Khotimchenko, Y. Pharmacological Potential of Sea Cucumbers. Int. J. Mol. Sci. 2018, 19, 1342. [Google Scholar] [CrossRef] [PubMed]
- Puspitasari, Y.E.; De Bruyne, T.; Foubert, K.; Aulanni’am, A.a.; Pieters, L.; Hermans, N.; Tuenter, E. Holothuria triterpene glycosides: A comprehensive guide for their structure elucidation and critical appraisal of reported compounds. Phytochem. Rev. 2022, 21, 1315–1358. [Google Scholar] [CrossRef]
- Yuan, L.; Huang, X.; Zhou, K.; Zhu, X.; Huang, B.; Qiu, S.; Cao, K.; Xu, L. Sea cucumber extract TBL-12 inhibits the proliferation, migration, and invasion of human prostate cancer cells through the p38 mitogen-activated protein kinase and intrinsic caspase apoptosis pathway. Prostate 2019, 79, 826–839. [Google Scholar] [CrossRef] [PubMed]
- Gjorgjevski, M.; Hannen, R.; Carl, B.; Li, Y.; Landmann, E.; Buchholz, M.; Bartsch, J.W.; Nimsky, C. Molecular profiling of the tumor microenvironment in glioblastoma patients: Correlation of microglia/macrophage polarization state with metalloprotease expression profiles and survival. Biosci. Rep. 2019, 39, BSR20182361. [Google Scholar] [CrossRef]
- Xue, Q.; Cao, L.; Chen, X.Y.; Zhao, J.; Gao, L.; Li, S.Z.; Fei, Z. High expression of MMP9 in glioma affects cell proliferation and is associated with patient survival rates. Oncol. Lett. 2017, 13, 1325–1330. [Google Scholar] [CrossRef]
- Zhou, W.; Yu, X.; Sun, S.; Zhang, X.; Yang, W.; Zhang, J.; Zhang, X.; Jiang, Z. Increased expression of MMP-2 and MMP-9 indicates poor prognosis in glioma recurrence. Biomed. Pharmacother. 2019, 118, 109369. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, Z.D.; Xue, Y.; Wang, J.F.; Li, H.; Tang, Q.J.; Wang, Y.M.; Dong, P.; Xue, C.H. Ds-echinoside A, a new triterpene glycoside derived from sea cucumber, exhibits antimetastatic activity via the inhibition of NF-κB-dependent MMP-9 and VEGF expressions. J. Zhejiang Univ. Sci. B 2011, 12, 534–544. [Google Scholar] [CrossRef]
- Zhao, Q.; Xue, Y.; Liu, Z.D.; Li, H.; Wang, J.F.; Li, Z.J.; Wang, Y.M.; Dong, P.; Xue, C.H. Differential effects of sulfated triterpene glycosides, holothurin A1, and 24-dehydroechinoside A, on antimetastasic activity via regulation of the MMP-9 signal pathway. J. Food Sci. 2010, 75, H280–H288. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Kaur, G.; Hamid, H.; Abdullah, T.; Ali, M.; Niwa, M.; Alam, M.S. Terminoside A, a new triterpene glycoside from the bark of Terminalia arjuna inhibits nitric oxide production in murine macrophages. J. Asian Nat. Prod. Res. 2003, 5, 137–142. [Google Scholar] [CrossRef]
- Wu, S.Y.; Wu, A.T.; Yuan, K.S.; Liu, S.H. Brown Seaweed Fucoidan Inhibits Cancer Progression by Dual Regulation of mir-29c/ADAM12 and miR-17-5p/PTEN Axes in Human Breast Cancer Cells. J. Cancer 2016, 7, 2408–2419. [Google Scholar] [CrossRef]
- Li, X.; Chen, L.; Peng, X.; Zhan, X. Progress of tumor-associated macrophages in the epithelial-mesenchymal transition of tumor. Front. Oncol. 2022, 12, 911410. [Google Scholar] [CrossRef]
- Liu, J.; Li, C.; Zhang, L.; Liu, K.; Jiang, X.; Wang, X.; Yang, L.; Liang, W.; Liu, K.; Hu, J.; et al. Association of tumour-associated macrophages with cancer cell EMT, invasion, and metastasis of Kazakh oesophageal squamous cell cancer. Diagn. Pathol. 2019, 14, 55. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fu, X.; Duan, D.; Liu, X.; Xu, J.; Gao, X. Extraction and Identification of Phlorotannins from the Brown Alga, Sargassum fusiforme (Harvey) Setchell. Mar. Drugs 2017, 15, 49. [Google Scholar] [CrossRef] [PubMed]
- Catarino, M.D.; Amarante, S.J.; Mateus, N.; Silva, A.M.S.; Cardoso, S.M. Brown Algae Phlorotannins: A Marine Alternative to Break the Oxidative Stress, Inflammation and Cancer Network. Foods 2021, 10, 1478. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.R.; Lee, M.S.; Shin, T.S.; Hua, H.; Jang, B.C.; Choi, J.S.; Byun, D.S.; Utsuki, T.; Ingram, D.; Kim, H.R. Phlorofucofuroeckol A inhibits the LPS-stimulated iNOS and COX-2 expressions in macrophages via inhibition of NF-κB, Akt, and p38 MAPK. Toxicol. In Vitro 2011, 25, 1789–1795. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.Y.; Guo, J.; Hu, X.M.; Zhao, S.Q.; Li, S.L.; Wang, J. An in vivo anti-tumor effect of eckol from marine brown algae by improving the immune response. Food Funct. 2019, 10, 4361–4371. [Google Scholar] [CrossRef]
- Oh, Y.; Shim, K.B.; Ahn, C.B.; Kim, S.S.; Je, J.Y. Sea Squirt (Halocynthia roretzi) Hydrolysates Induce Apoptosis in Human Colon Cancer HT-29 Cells through Activation of Reactive Oxygen Species. Nutr. Cancer 2019, 71, 118–127. [Google Scholar] [CrossRef]
- Zhu, Y.; Han, S.; Li, J.; Gao, H.; Dong, B. Aqueous Extract of Sea Squirt (Halocynthia roretzi) with Potent Activity against Human Cancer Cells Acts Synergistically with Doxorubicin. Mar. Drugs 2022, 20, 284. [Google Scholar] [CrossRef]
- Lichter, W.; Wallham, L.L.; Van Der Worf, B.A.; Middle Brook, R.E.; Sigal, M.M.; Weinheimer, A.J. Food Drugs from the Sea. Proc. Mar. Tech. Soc. 1972, 173, 117–127. [Google Scholar]
- Rinehart, K.L. Antitumor compounds from tunicates. Med. Res. Rev. 2000, 20, 1–27. [Google Scholar] [CrossRef]
- Erba, E.; Bergamaschi, D.; Bassano, L.; Damia, G.; Ronzoni, S.; Faircloth, G.T.; D’Incalci, M. Ecteinascidin-743 (ET-743), a natural marine compound, with a unique mechanism of action. Eur. J. Cancer 2001, 37, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Scotto, K.W. ET-743: More than an innovative mechanism of action. Anticancer. Drugs 2002, 13 (Suppl. S1), S3–S6. [Google Scholar] [PubMed]
- Corey, E.J.; Gin, D.Y.; Kania, R.S. Enantioselective Total Synthesis of Ecteinascidin 743. J. Am. Chem. Soc. 1996, 118, 9202–9203. [Google Scholar] [CrossRef]
- Demetri, G.D.; von Mehren, M.; Jones, R.L.; Hensley, M.L.; Schuetze, S.M.; Staddon, A.; Milhem, M.; Elias, A.; Ganjoo, K.; Tawbi, H.; et al. Efficacy and Safety of Trabectedin or Dacarbazine for Metastatic Liposarcoma or Leiomyosarcoma After Failure of Conventional Chemotherapy: Results of a Phase III Randomized Multicenter Clinical Trial. J. Clin. Oncol. 2016, 34, 786–793. [Google Scholar] [CrossRef]
- NCI. FDA Approves Trabectedin to Treat Two Types of Soft Tissue Sarcoma. Available online: https://www.cancer.gov/news-events/cancer-currents-blog/2015/fda-trabectedin-sarcoma#:~:text=On%20October%2023%2C%20the%20Food,tissue%20sarcomas%3A%20liposarcoma%20and%20leiomyosarcoma (accessed on 30 November 2023).
- Molla, M.H.R.; Aljahdali, M.O. Marine-derived sea urchin compounds as potential anti-cancer drug candidate against colorectal cancer: In silico and in vitro studies. Saudi J. Biol. Sci. 2023, 30, 103748. [Google Scholar] [CrossRef]
- Xu, Y.; Ryu, S.; Lee, Y.K.; Lee, H.J. Brassicasterol from Edible Aquacultural Hippocampus abdominalis Exerts an Anti-Cancer Effect by Dual-Targeting AKT and AR Signaling in Prostate Cancer. Biomedicines 2020, 8, 370. [Google Scholar] [CrossRef] [PubMed]
- Khalil, E.A.; Swelim, H.; El-Tantawi, H.; Abdellatif, A. Sea urchin (Diadema savignyi) extract as a novel protective agent against cisplatin induced neurotoxicity in rats. BMC Pharmacol. Toxicol. 2023, 24, 11. [Google Scholar] [CrossRef]
- Chakraborty, K.; Francis, P. Stomopneulactone D from long-spined sea urchin Stomopneustes variolaris: Anti-inflammatory macrocylic lactone attenuates cyclooxygenase-2 expression in lipopolysaccharide-activated macrophages. Bioorg. Chem. 2020, 103, 104140. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. A Trial of TBL12 Sea Cucumber Extract in Patients with Untreated Asymptomatic Myeloma (NCT01302366). Available online: https://clinicaltrials.gov/study/NCT01302366 (accessed on 30 November 2023).
| Drug | Marine Source | Indication (Year Approved) | Reference |
|---|---|---|---|
| Eribulin | Sea sponge (Halichondria) | Breast cancer (2010), liposarcoma (2016) | [21] [22] |
| Brentuxumab vedotin | Sea slug (Dolabella) | ALCL (2011) PTCL (2017) | [23] [24] |
| cHL | [25] | ||
| Polatuzumab vedotin | Sea slug (Dolabella) | NHL (2019) | [26] |
| Enfortumab vedotin | Sea slug (Dolabella) | Urothelial carcinoma (2023) | [27] |
| Trabectedin | Sea squirt (Ecteinascidia) | Soft tissue sarcoma (2015) | [28] |
| Name | Common Name | Phylum | Class | Order | Family | Genus | Species | Actual % |
|---|---|---|---|---|---|---|---|---|
| Sea Cucumber | Black Teatfish | Echinodermata | Holothuria | Holothuriida | Holothuriidae | Holothuria | H. nobilis | 45 |
| Sandfish | Echinodermata | Holothuria | Holothuriida | Holothuriidae | Holothuria | H. scabra | 40 | |
| Sargassum (whole plant) | Sea Weed | Heterokontophyta | Phaeophyceae | Fucales | Sagassaceae | Sargassum | S. pallidum | 5 |
| Sea Sponge | Sea Squirt | Chordata | Ascidiacea | Pleurogona | Styelidae | Styela | S. clava | 5 |
| Sea Urchin | Purple Sea Urchin | Echinodermata | Echinoidea | Camarodonta | Toxopneustidae | Heliocidaris | H. erythrogramma | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karbhari, N.; Khagi, S. Marine-Derived Therapeutics for the Management of Glioblastoma: A Case Series and Comprehensive Review of the Literature. Onco 2024, 4, 369-380. https://doi.org/10.3390/onco4040026
Karbhari N, Khagi S. Marine-Derived Therapeutics for the Management of Glioblastoma: A Case Series and Comprehensive Review of the Literature. Onco. 2024; 4(4):369-380. https://doi.org/10.3390/onco4040026
Chicago/Turabian StyleKarbhari, Nishika, and Simon Khagi. 2024. "Marine-Derived Therapeutics for the Management of Glioblastoma: A Case Series and Comprehensive Review of the Literature" Onco 4, no. 4: 369-380. https://doi.org/10.3390/onco4040026
APA StyleKarbhari, N., & Khagi, S. (2024). Marine-Derived Therapeutics for the Management of Glioblastoma: A Case Series and Comprehensive Review of the Literature. Onco, 4(4), 369-380. https://doi.org/10.3390/onco4040026






