The Impact of Immune Checkpoint-Inhibitors Therapy in Urinary Bladder Cancer
Abstract
Simple Summary
Abstract
1. Introduction
1.1. Inflammation and Cancer
1.2. Tumor Immune Microenvironment
1.3. Immune Checkpoints
2. Methods
3. Immunotherapy
3.1. Bacillus Calmette-Guerin Intravesical Therapy
3.2. Immune Checkpoints Inhibitors
3.3. Anti-PD-L1 Therapies
| Ref | Agents | Study | Target | Phase | n | PD-L1 Expression | PD-L1 Expression (n) | ORR% | FDA Approval for UC |
|---|---|---|---|---|---|---|---|---|---|
| [56] | Atezolizumab | Imvigor 210 | PD-L1 | II | 310 | IC ≥ 5% | IC ≥ 5%: 100 | 26% | 18 May 2016 |
| IC ≥ 1% | IC ≥ 1%: 207 | 18% | |||||||
| IC < 1% | IC < 1%: 103 | 8% | |||||||
| [56] | Durvalumab | Study 1108 | PD-L1 | I/II | 191 | PD-L1 ≥ 25% | PD-L1 ≥ 25%: 98 | 27.6% | 1 May 2017 |
| PD-L1 < 25% | PD-L1 < 25%: 79 | 5.1% | |||||||
| [49] | Avelumab | JAVELIN | PD-L1 | Ib | 249 | PD-L1 ≥ 5% | PD-L1 ≥ 5%: 63 | 24% | 9 May 2017 |
| [49] | Nivolumab | CheckMate 275 | PD-1 | II | 265 | PD-L1 > 5% | PD-L1 > 5%: 81 | 28.4% | 5 February 2017 |
| PD-L1 ≥ 1% | PD-L1 ≥ 1%: 122 | 23.8% | |||||||
| [56] | Pembrolizumab | Keynote-045 | PD-1 | III | 542 | CPS ≥ 10 | Pembrolizumab: 270 | 21.1% | 18 May 2017 |
| Chemotherapy: 272 | 11.4% |
3.4. Anti-PD-1 Therapies
3.5. Anti-CTLA-4 Therapies
4. Combined Therapy
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saginala, K.; Barsouk, A.; Aluru, J.S.; Rawla, P.; Padala, S.A.; Barsouk, A. Epidemiology of bladder cancer. Med. Sci. 2020, 8, 15. [Google Scholar] [CrossRef]
- Saad, F.T.; Hincal, E.; Kaymakamzade, B. Dynamics of immune checkpoints, immune system, and bcg in the treatment of superficial bladder cancer. Comput. Math. Methods Med. 2017, 2017, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Crabb, S.J.; Douglas, J. The latest treatment options for bladder cancer. Br. Med. Bull. 2018, 128, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2018, 144, 1941–1953. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Me, J.F.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Cumberbatch, M.G.K.; Noon, A.P.; EAU Young Academic Urologists—Urothelial Cancer Working Party. Epidemiology, aetiology and screening of bladder cancer. Transl. Androl. Urol. 2019, 8, 5–11. [Google Scholar] [CrossRef]
- Aghaalikhani, N.; Rashtchizadeh, N.; Shadpour, P.; Allameh, A.; Mahmoodi, M. Cancer stem cells as a therapeutic target in bladder cancer. J. Cell. Physiol. 2018, 234, 3197–3206. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, J.; Thurairaja, R.; Nair, R. Bladder cancer. Surg. Oxf. 2019, 37, 529–537. [Google Scholar] [CrossRef]
- Pierantoni, F.; Maruzzo, M.; Gardi, M.; Bezzon, E.; Gardiman, M.P.; Porreca, A.; Basso, U.; Zagonel, V. Immunotherapy and urothelial carcinoma: An overview and future prospectives. Crit. Rev. Oncol. 2019, 143, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Morales-Barrera, R.; Suárez, C.; González, M.; Valverde, C.; Serra, E.; Mateo, J.; Raventos, C.; Maldonado, X.; Morote, J.; Carles, J. The future of bladder cancer therapy: Optimizing the inhibition of the fibroblast growth factor receptor. Cancer Treat. Rev. 2020, 86, 102000. [Google Scholar] [CrossRef] [PubMed]
- Magers, M.J.; Lopez-Beltran, A.; Montironi, R.; Williamson, S.R.; Kaimakliotis, H.Z.; Cheng, L. Staging of bladder cancer. Histopathology 2018, 74, 112–134. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Zhao, K.; Wang, L.; Sun, E. The prognostic values of tumor-infiltrating neutrophils, lymphocytes and neutrophil/lymphocyte rates in bladder urothelial cancer. Pathol. Res. Pract. 2018, 214, 1074–1080. [Google Scholar] [CrossRef]
- Ma, Y.; Feng, X.-F.; Yang, W.-X.; You, C.-G. Exploring the pathological mechanism of bladder cancer based on tumor mutational burden analysis. BioMed. Res. Int. 2019, 2019, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Paner, G.P.; Stadler, W.M.; Hansel, D.E.; Montironi, R.; Lin, D.W.; Amin, M.B. Updates in the eighth edition of the tumor-node-metastasis staging classification for urologic cancers. Eur. Urol. 2018, 73, 560–569. [Google Scholar] [CrossRef]
- Qiu, S.; Deng, L.; Liao, X.; Nie, L.; Qi, F.; Jin, K.; Tu, X.; Zheng, X.; Li, J.; Liu, L.; et al. Tumor-associated macrophages promote bladder tumor growth through PI3K/AKT signal induced by collagen. Cancer Sci. 2019, 110, 2110–2118. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Walker, J.; Williams, J.A.; Bellmunt, J. The evolving role of PD-L1 testing in patients with metastatic urothelial carcinoma. Cancer Treat. Rev. 2020, 82, 101925. [Google Scholar] [CrossRef]
- Galdiero, M.R.; Marone, G.; Mantovani, A. Cancer inflammation and cytokines. Cold Spring Harb. Perspect. Biol. 2017, 10, a028662. [Google Scholar] [CrossRef] [PubMed]
- Shadpour, P.; Zamani, M.; Aghaalikhani, N.; Rashtchizadeh, N. Inflammatory cytokines in bladder cancer. J. Cell. Physiol. 2019, 234, 14489–14499. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Baby, D.; Rajguru, J.P.; Patil, P.B.; Thakkannavar, S.S.; Pujari, V.B. Inflammation and cancer. Ann. Afr. Med. 2019, 18, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Murata, M. Inflammation and cancer. Environ. Heal. Prev. Med. 2018, 23, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bottazzi, B.; Riboli, E.; Mantovani, A. Aging, inflammation and cancer. Semin. Immunol. 2018, 40, 74–82. [Google Scholar] [CrossRef] [PubMed]
- De Boer, E.C.; De Jong, W.H.; Steerenberg, P.A.; Aarden, L.A.; Tetteroo, E.; De Groot, E.R.; Van Der Meijden, A.P.M.; Vegt, P.D.J.; Debruyne, F.M.J.; Ruitenberg, E.J. Induction of urinary interleukin-1 (IL-1), IL-2, IL-6, and tumour necrosis factor during intravesical immunotherapy with bacillus Calmette-Guérin in superficial bladder cancer. Cancer Immunol. Immunother. 1992, 34, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Roh, J.S.; Sohn, D.H. Damage-associated molecular patterns in inflammatory diseases. Immune Netw. 2018, 18, e27. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, R.; Su, X.; Pan, Y.; Han, X.; Shao, C.; Shi, Y. Harnessing tumor-associated macrophages as aids for cancer immunotherapy. Mol. Cancer 2019, 18, 1–16. [Google Scholar] [CrossRef]
- Crispen, P.L.; Kusmartsev, S. Mechanisms of immune evasion in bladder cancer. Cancer Immunol. Immunother. 2019, 69, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Vlachostergios, P.J.; Faltas, B.M. The molecular limitations of biomarker research in bladder cancer. World J. Urol. 2018, 37, 837–848. [Google Scholar] [CrossRef]
- Annels, N.E.; Simpson, G.R.; Pandha, H. Modifying the non-muscle invasive bladder cancer immune microenvironment for optimal therapeutic response. Front. Oncol. 2020, 10, 175. [Google Scholar] [CrossRef] [PubMed]
- Lobo, J.; Jerónimo, C.; Henrique, R. Targeting the immune system and epigenetic landscape of urological tumors. Int. J. Mol. Sci. 2020, 21, 829. [Google Scholar] [CrossRef]
- Ornstein, M.C.; Diaz-Montero, C.M.; Rayman, P.; Elson, P.; Haywood, S.; Finke, J.H.; Kim, J.S.; Pavicic, P.G.; Lamenza, M.; Devonshire, S.; et al. Myeloid-derived suppressors cells (MDSC) correlate with clinicopathologic factors and pathologic complete response (pCR) in patients with urothelial carcinoma (UC) undergoing cystectomy. Urol. Oncol. Semin. Orig. Investig. 2018, 36, 405–412. [Google Scholar] [CrossRef]
- Sharifi, L.; Nowroozi, M.R.; Amini, E.; Arami, M.K.; Ayati, M.; Mohsenzadegan, M. A review on the role of M2 macrophages in bladder cancer; pathophysiology and targeting. Int. Immunopharmacol. 2019, 76, 105880. [Google Scholar] [CrossRef] [PubMed]
- Rubio, C.; Munera-Maravilla, E.; Lodewijk, I.; Suarez-Cabrera, C.; Karaivanova, V.; Ruiz-Palomares, R.; Paramio, J.M.; Dueñas, M. Macrophage polarization as a novel weapon in conditioning tumor microenvironment for bladder cancer: Can we turn demons into gods? Clin. Transl. Oncol. 2018, 21, 391–403. [Google Scholar] [CrossRef]
- Wołącewicz, M.; Hrynkiewicz, R.; Grywalska, E.; Suchojad, T.; Leksowski, T.; Roliński, J.; Niedźwiedzka-Rystwej, P. Immunotherapy in bladder cancer: Current methods and future perspectives. Cancers 2020, 12, 1181. [Google Scholar] [CrossRef]
- Park, R.; Winnicki, M.; Liu, E.; Chu, W.-M. Immune checkpoints and cancer in the immunogenomics era. Brief. Funct. Genom. 2018, 18, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Hsu, M.M.; Balar, A.V. PD-1/PD-L1 combinations in advanced urothelial cancer: Rationale and current clinical trials. Clin. Genitourin. Cancer 2019, 17, e618–e626. [Google Scholar] [CrossRef]
- Song, D.; Powles, T.; Shi, L.; Zhang, L.; Ingersoll, M.A.; Lu, Y. Bladder cancer, a unique model to understand cancer immunity and develop immunotherapy approaches. J. Pathol. 2019, 249, 151–165. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.; Plimack, E.R. Immunotherapy for urothelial carcinoma: Current evidence and future directions. Curr. Urol. Rep. 2018, 19, 109. [Google Scholar] [CrossRef] [PubMed]
- Massari, F.; Di Nunno, V.; Cubelli, M.; Santoni, M.; Fiorentino, M.; Montironi, R.; Cheng, L.; Lopez-Beltran, A.; Battelli, N.; Ardizzoni, A. Immune checkpoint inhibitors for metastatic bladder cancer. Cancer Treat. Rev. 2018, 64, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Sankin, A.; Narasimhulu, D.; John, P.; Gartrell, B.; Schoenberg, M.; Zang, X. The expanding repertoire of targets for immune checkpoint inhibition in bladder cancer: What lies beneath the tip of the iceberg, PD-L. Urol. Oncol. Semin. Orig. Investig. 2018, 36, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Katz, H.; Wassie, E.; Alsharedi, M. Checkpoint inhibitors: The new treatment paradigm for urothelial bladder cancer. Med. Oncol. 2017, 34, 170. [Google Scholar] [CrossRef]
- Dyck, L.; Mills, K.H. Immune checkpoints and their inhibition in cancer and infectious diseases. Eur. J. Immunol. 2017, 47, 765–779. [Google Scholar] [CrossRef]
- Picardo, S.L.; Doi, J.; Hansen, A.R. Structure and optimization of checkpoint inhibitors. Cancers 2019, 12, 38. [Google Scholar] [CrossRef]
- Burugu, S.; Dancsok, A.R.; Nielsen, T.O. Emerging targets in cancer immunotherapy. Semin. Cancer Biol. 2018, 52, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Riley, R.S.; June, C.H.; Langer, R.; Mitchell, M.J. Delivery technologies for cancer immunotherapy. Nat. Rev. Drug Discov. 2019, 18, 175–196. [Google Scholar] [CrossRef]
- Kimiz-Gebologlu, I.; Gulce-Iz, S.; Biray-Avci, C. Monoclonal antibodies in cancer immunotherapy. Mol. Biol. Rep. 2018, 45, 2935–2940. [Google Scholar] [CrossRef] [PubMed]
- Morales, A.; Eidinger, D.; Bruce, A. Intracavitary bacillus calmette-guerin in the treatment of superficial bladder tumors. J. Urol. 1976, 116, 180–182. [Google Scholar] [CrossRef]
- Liu, K.; Sun, E.; Lei, M.; Li, L.; Gao, J.; Nian, X.; Wang, L. BCG-induced formation of neutrophil extracellular traps play an important role in bladder cancer treatment. Clin. Immunol. 2019, 201, 4–14. [Google Scholar] [CrossRef]
- Kamat, A.M.; Li, R.; O’Donnell, M.A.; Black, P.C.; Roupret, M.; Catto, J.W.; Comperat, E.; Ingersoll, M.A.; Witjes, W.P.; McConkey, D.J.; et al. Predicting response to intravesical bacillus calmette-guérin immunotherapy: Are we there yet? A systematic review. Eur. Urol. 2018, 73, 738–748. [Google Scholar] [CrossRef] [PubMed]
- Butt, S.-U.; Malik, L. Role of immunotherapy in bladder cancer: Past, present and future. Cancer Chemother. Pharmacol. 2018, 81, 629–645. [Google Scholar] [CrossRef] [PubMed]
- Ślusarczyk, A.; Zapała, P.; Zapała, L.; Piecha, T.; Radziszewski, P. Prediction of BCG responses in non-muscle-invasive bladder cancer in the era of novel immunotherapeutics. Int. Urol. Nephrol. 2019, 51, 1089–1099. [Google Scholar] [CrossRef] [PubMed]
- Grimm, M.-O.; Bex, A.; De Santis, M.; Ljungberg, B.; Catto, J.W.; Rouprêt, M.; Hussain, S.A.; Bellmunt, J.; Powles, T.; Wirth, M.; et al. Safe use of immune checkpoint inhibitors in the multidisciplinary management of urological cancer: The European association of urology position. Eur. Urol. 2019, 76, 368–380. [Google Scholar] [CrossRef] [PubMed]
- Ghate, K.; Amir, E.; Kuksis, M.; Hernandez-Barajas, D.; Rodriguez-Romo, L.; Booth, C.M.; Vera-Badillo, F.E. PD-L1 expression and clinical outcomes in patients with advanced urothelial carcinoma treated with checkpoint inhibitors: A meta-analysis. Cancer Treat. Rev. 2019, 76, 51–56. [Google Scholar] [CrossRef]
- Rouanne, M.; Roumiguié, M.; Houédé, N.; Masson-Lecomte, A.; Colin, P.; Pignot, G.; Larré, S.; Xylinas, E.; Rouprêt, M.; Neuzillet, Y. Development of immunotherapy in bladder cancer: Present and future on targeting PD(L)1 and CTLA-4 pathways. World J. Urol. 2018, 36, 1727–1740. [Google Scholar] [CrossRef]
- Zhu, J.; Armstrong, A.J.; Friedlander, T.W.; Kim, W.; Pal, S.K.; George, D.J.; Zhang, T. Biomarkers of immunotherapy in urothelial and renal cell carcinoma: PD-L1, tumor mutational burden, and beyond. J. Immunother. Cancer 2018, 6, 4. [Google Scholar] [CrossRef]
- Ghatalia, P.; Plimack, E.R. Integration of immunotherapy into the treatment of advanced urothelial carcinoma. J. Natl. Compr. Cancer Netw. 2020, 18, 355–361. [Google Scholar] [CrossRef]
- Stenehjem, D.D.; Tran, D.; Nkrumah, M.A.; Gupta, S. PD1/PDL1 inhibitors for the treatment of advanced urothelial bladder cancer. OncoTargets Ther. 2018, 11, 5973–5989. [Google Scholar] [CrossRef]
- Petrylak, D.P.; Powles, T.; Bellmunt, J.; Braiteh, F.; Loriot, Y.; Morales-Barrera, R.; Burris, H.A.; Kim, J.W.; Ding, B.; Kaiser, C.; et al. Atezolizumab (MPDL3280A) monotherapy for patients with metastatic urothelial cancer. JAMA Oncol. 2018, 4, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Faiena, I.; Cummings, A.L.; Crosetti, A.M.; Pantuck, A.J.; Chamie, K.; Drakaki, A. Durvalumab: An investigational anti-PD-L1 monoclonal antibody for the treatment of urothelial carcinoma. Drug Des. Dev. Ther. 2018, 12, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Balar, A.V.; Castellano, D.; O’Donnell, P.H.; Grivas, P.; Vuky, J.; Powles, T.; Plimack, E.R.; Hahn, N.M.; de Wit, R.; Pang, L.; et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): A multicentre, single-arm, phase 2 study. Lancet Oncol. 2017, 18, 1483–1492. [Google Scholar] [CrossRef]
- Sundahl, N.; Vandekerkhove, G.; Decaestecker, K.; Meireson, A.; De Visschere, P.; Fonteyne, V.; De Maeseneer, D.; Reynders, D.; Goetghebeur, E.; Van Dorpe, J.; et al. Randomized phase 1 trial of pembrolizumab with sequential versus concomitant stereotactic body radiotherapy in metastatic urothelial carcinoma. Eur. Urol. 2019, 75, 707–711. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; McDermott, D.F. Ipilimumab in combination with nivolumab for the treatment of renal cell carcinoma. Expert Opin. Biol. Ther. 2018, 18, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Saad, P.; Kasi, A. Ipilimumab; Stat Pearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Shao, I.; Chang, Y.; Pang, S. Recent advances in upper tract urothelial carcinomas: From bench to clinics. Int. J. Urol. 2018, 26, 148–159. [Google Scholar] [CrossRef]
- Calles, A.; Aguado, G.; Sandoval, C.; Álvarez, R. The role of immunotherapy in small cell lung cancer. Clin. Transl. Oncol. 2019, 21, 961–976. [Google Scholar] [CrossRef]
- Muñoz-Unceta, N.; Burgueño, I.; Jiménez, E.; Paz-Ares, L. Durvalumab in NSCLC: Latest evidence and clinical potential. Ther. Adv. Med. Oncol. 2018, 10. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, W.; Huang, Y.; Cui, R.; Li, X.; Li, B. Evolving roles for targeting CTLA-4 in cancer immunotherapy. Cell. Physiol. Biochem. 2018, 47, 721–734. [Google Scholar] [CrossRef]
- Kim, T.J.; Cho, K.S.; Koo, K.C. Current status and future perspectives of immunotherapy for locally advanced or metastatic urothelial carcinoma: A comprehensive review. Cancers 2020, 12, 192. [Google Scholar] [CrossRef]
- Jenkins, R.W.; Barbie, D.A.; Flaherty, K.T. Mechanisms of resistance to immune checkpoint inhibitors. Br. J. Cancer 2018, 118, 9–16. [Google Scholar] [CrossRef]
- Bai, R.; Chen, N.; Li, L.; Du, N.; Bai, L.; Lv, Z.; Tian, H.; Cui, J. Mechanisms of cancer resistance to immunotherapy. Front. Oncol. 2020, 10, 1290. [Google Scholar] [CrossRef]
- Barrueto, L.; Caminero, F.; Cash, L.; Makris, C.; Lamichhane, P.; Deshmukh, R.R. Resistance to checkpoint inhibition in cancer immunotherapy. Transl. Oncol. 2020, 13, 100738. [Google Scholar] [CrossRef]
- O’Donnell, J.S.; Long, G.V.; Scolyer, R.A.; Teng, M.W.; Smyth, M.J. Resistance to PD1/PDL1 checkpoint inhibition. Cancer Treat. Rev. 2017, 52, 71–81. [Google Scholar] [CrossRef]
- Marincola, F.M.; Jaffee, E.M.; Hicklin, D.J.; Ferrone, S. Escape of human solid tumors from T–Cell recognition: Molecular mechanisms and functional significance. Adv. Immunol. 1999, 74, 181–273. [Google Scholar] [CrossRef]
- Galsky, M.D.; Arija, J.A.; Bamias, A.; Davis, I.D.; De Santis, M.; Kikuchi, E.; Garcia-Del-Muro, X.; De Giorgi, U.; Mencinger, M.; Izumi, K.; et al. Atezolizumab with or without chemotherapy in metastatic urothelial cancer (IMvigor130): A multicentre, randomised, placebo-controlled phase 3 trial. Lancet 2020, 395, 1547–1557. [Google Scholar] [CrossRef]
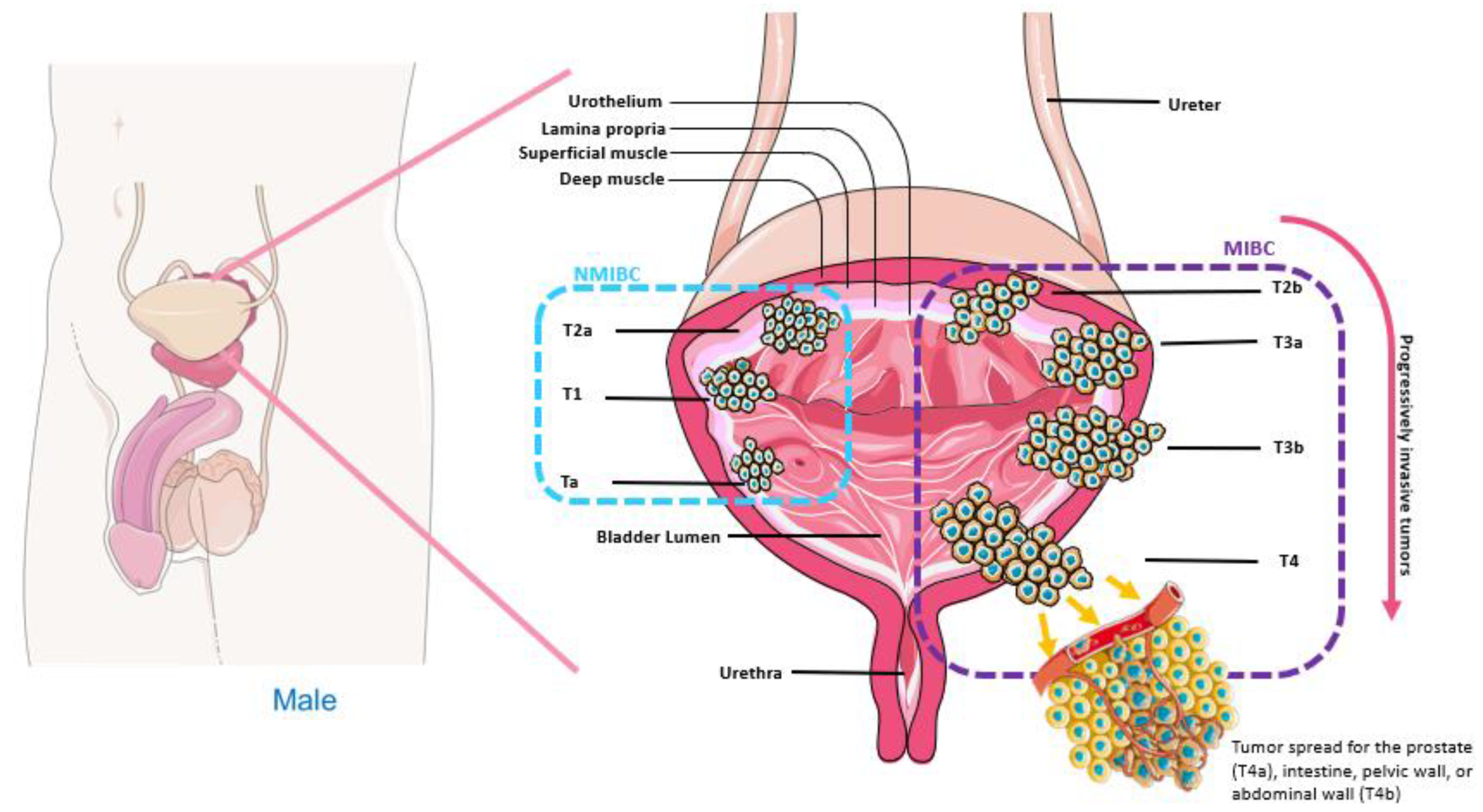

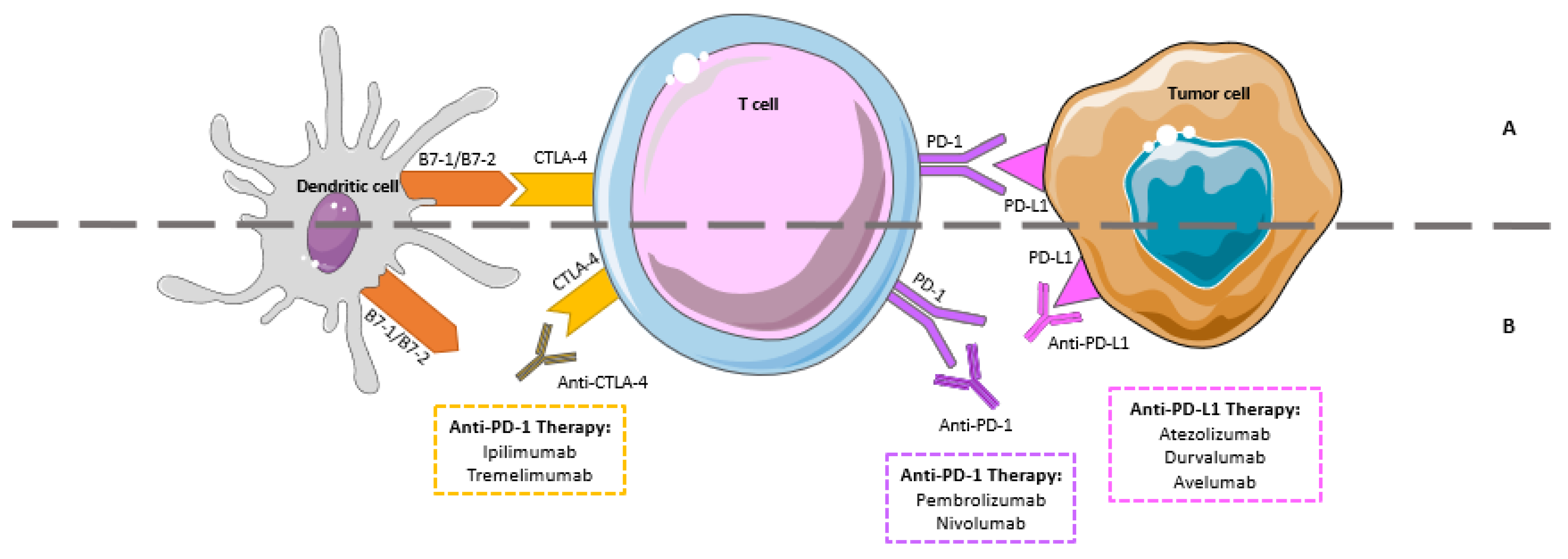
| Ref | Author | Year | Type of Study | Drug | Methods | Results |
|---|---|---|---|---|---|---|
| [27] | Annels N et al. | 2020 | R | BCG | Review of the BCG approach and its action on the tumor microenvironment in the NMIBC | Influence of the tumor microenvironment on BCG immunotherapy results; the interaction between cancer, immunity, and BCG |
| [32] | Wołącewicz M et al. | 2020 | R | Pembrolizumab Atezolizumab Nivolumab Durvalumab Avelumab Ipilimumab BCG | Review the current state of immunotherapy | Studies on the different immunotherapies approved by the FDA for BC demonstrate that therapies are well-tolerated and safe therapies |
| [35] | Song D et al. | 2019 | R | Pembrolizumab Atezolizumab Nivolumab Durvalumab Avelumab Ipilimumab BCG | Review the current state of immunotherapy | Intravesical instillation with BCG in the NMIBC and ICIs (anti-PD-1 and anti-PD-L1) in the advanced stage of UC is effective |
| [37] | Massari F et al. | 2018 | R | Combined Therapy | Review the current state of immunotherapy and combined therapies | ICIs have demonstrated a better safety profile compared to chemotherapy Progress studies that investigate the durability, safety, and tolerance of combined therapies and monotherapy in different contexts |
| [39] | Katz H et al. | 2017 | R | Pembrolizumab Atezolizumab Nivolumab Durvalumab Avelumab Ipilimumab | Review the current state of immunotherapy and combined therapies | Main studies with ICIs that led to their approval with UC therapy |
| [42] | Butt S et al. | 2018 | R | Pembrolizumab Atezolizumab Nivolumab Durvalumab Avelumab Ipilimumab BCG | Review the current state of immunotherapy | Studies with ICIs have shown promising activity in the treatment of BC Ongoing studies to investigate the best-tolerated dose in the context of monotherapy and combination therapy |
| [43] | Kim H et al. | 2018 | R | Pembrolizumab Atezolizumab Nivolumab Durvalumab Avelumab | Review of the current status of immune checkpoint inhibitors and analysis of the application potential of PD-1 / PD-L1 inhibitors in different contexts | Studies with ICIs have demonstrated lasting long-term responses and tolerable safety profiles Approximately 70 to 80% of patients may not respond to ICIS therapy |
| [44] | Balar A et al. | 2017 | CT | Pembrolizumab | All patients receive 200 mg ofPembrolizumab intravenously every three weeksClinical follow-up every six weeks PD-L1 expression was determined by IHC | After six months, 63% of patients had discontinued therapy Median duration of response has not been reached 42% of patients had disease progression 62% of patients had AE There were 18 deaths associated with therapy |
| [45] | Faiena I et al. | 2018 | R | Durvalumab | Review of Durvalumab therapy | Durvalumab is effective in advanced settings There are still patients who do not respond or show disease progression after initial therapy with Durvalumab Ongoing studies involving Durvalumab in the context of combination therapy |
| [46] | Rouanne M et al. | 2018 | R | Pembrolizumab Atezolizumab Nivolumab Durvalumab Avelumab | Review the current state of immunotherapy | Monotherapy with ICIs has shown good results as second-line therapy Many patients do not respond to monotherapy with anti-PD-1 therapy and anti-PD-L1 therapy Ongoing studies are evaluating ICIs in different contexts, namely, high-risk NMIBC and MIBC, in neoadjuvant and adjuvant treatment or as first-line therapy |
| [47] | Stenehjem D et al. | 2018 | R | Pembrolizumab Atezolizumab Nivolumab Durvalumab Avelumab | Review of the current status of immune checkpoint inhibitors and analysis of the application potential of PD-1/PD-L1 inhibitors in different contexts | The PD-1 and PD-L1 inhibitors have favorable efficacy and safety profiles The only Pembrolizumab demonstrated superiority compared to standard chemotherapy Atezolizumab and Pembrolizumab were well-tolerated in the first-line setting in patients ineligible for cisplatin |
| [48] | Necchi A et al. | 2018 | CT | Pembrolizumab | Patients received 200 mg of Pembrolizumab intravenously every three weeks (three cycles) PD-L1 expression was determined by IHC | 6% of patients discontinued therapy Neoadjuvant therapy with Pembrolizumab was successful in 42% of patients Some AE have been registered |
| [49] | Petrylak D et al. | 2018 | CT | Atezolizumab | Nine patients received 1200 mg of Atezolizumab, and 86 patients received 15 mg/kg, both intravenously, every three weeks (16 cycles) PD-L1 expression was determined by IHC | 1% of patients discontinued therapy 67% of patients had AE The therapy administered was well tolerated and provided long-term clinical benefits |
| [50] | Galsky M et al. | 2020 | CT | Combined Therapy | Group A: Atezolizumab with platinum-based chemotherapy Group B: Atezolizumab monotherapy Group C: Placebo with platinum-based chemotherapy In group A, patients received a dose of gemcitabine, carboplatin or cisplatin, two times per cycle In group B, 1200 mg of Atezolizumab per cycle was administered intravenously In group C, one dose per cycle Clinical evaluation every nine weeks | 52%, 54%, and 57% deaths e recorded in group A, B, and C, respectively In group A, 12% of patients had disease progression In group B, 37% of patients had disease progression In group C, 15% of patients had disease progression In all patients, 97% had AE |
| [51] | Suzman D et al. | 2019 | CT | Combined Therapy | Patients received 1200 mg Atezolizumab every three weeks Patients received 200 mg of Pembrolizumab every three weeks PD-L1 expression was determined by IHC | 82% of patients discontinued therapy with Atezolizumab AE associated with Atezolizumab have been reported one death associated with Atezolizumab has been reposted 50% of patients discontinued therapy with Pembrolizumab AE associated with Pembrolizumab has been reported 13 death associated with Atezolizumab have been reposted |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, A.L.; Abreu-Mendes, P.; Martins, D.; Mendes, F. The Impact of Immune Checkpoint-Inhibitors Therapy in Urinary Bladder Cancer. Onco 2021, 1, 3-22. https://doi.org/10.3390/onco1010002
Silva AL, Abreu-Mendes P, Martins D, Mendes F. The Impact of Immune Checkpoint-Inhibitors Therapy in Urinary Bladder Cancer. Onco. 2021; 1(1):3-22. https://doi.org/10.3390/onco1010002
Chicago/Turabian StyleSilva, Ana Lúcia, Pedro Abreu-Mendes, Diana Martins, and Fernando Mendes. 2021. "The Impact of Immune Checkpoint-Inhibitors Therapy in Urinary Bladder Cancer" Onco 1, no. 1: 3-22. https://doi.org/10.3390/onco1010002
APA StyleSilva, A. L., Abreu-Mendes, P., Martins, D., & Mendes, F. (2021). The Impact of Immune Checkpoint-Inhibitors Therapy in Urinary Bladder Cancer. Onco, 1(1), 3-22. https://doi.org/10.3390/onco1010002








