Roe Deer as a Model Species for Aerial Survey-Based Ungulate Population Estimation in Agricultural Habitats
Abstract
1. Introduction
2. Materials and Methods
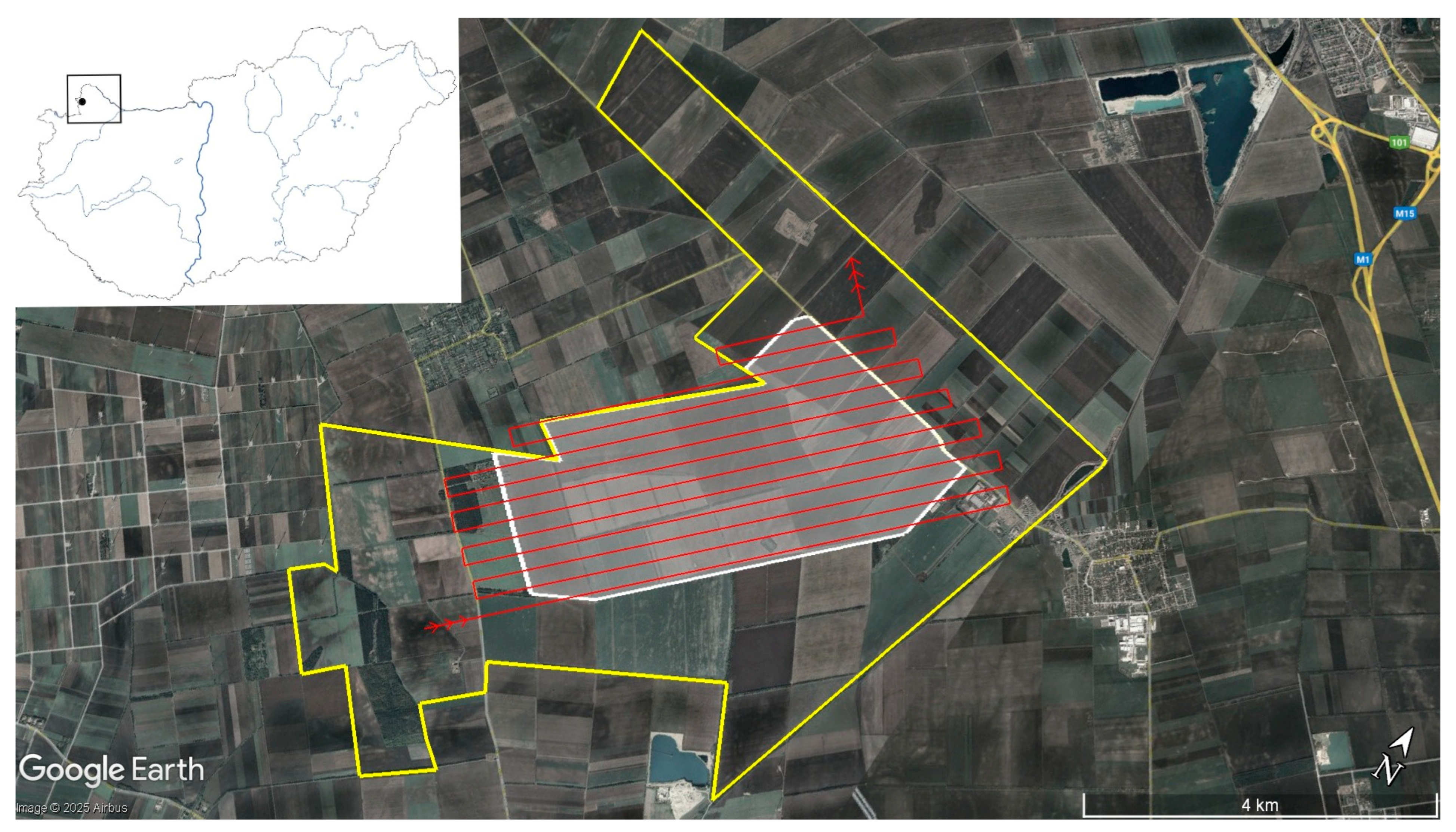
2.1. Study Area
2.2. Data Collection
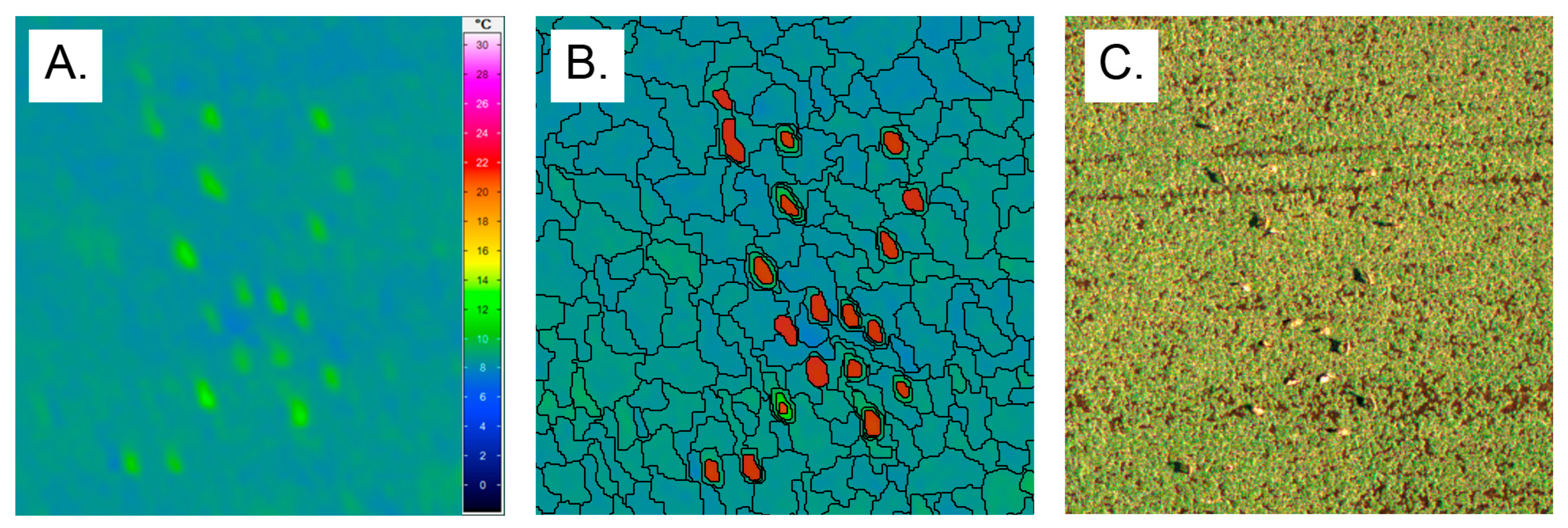
2.3. Image Processing and Classification
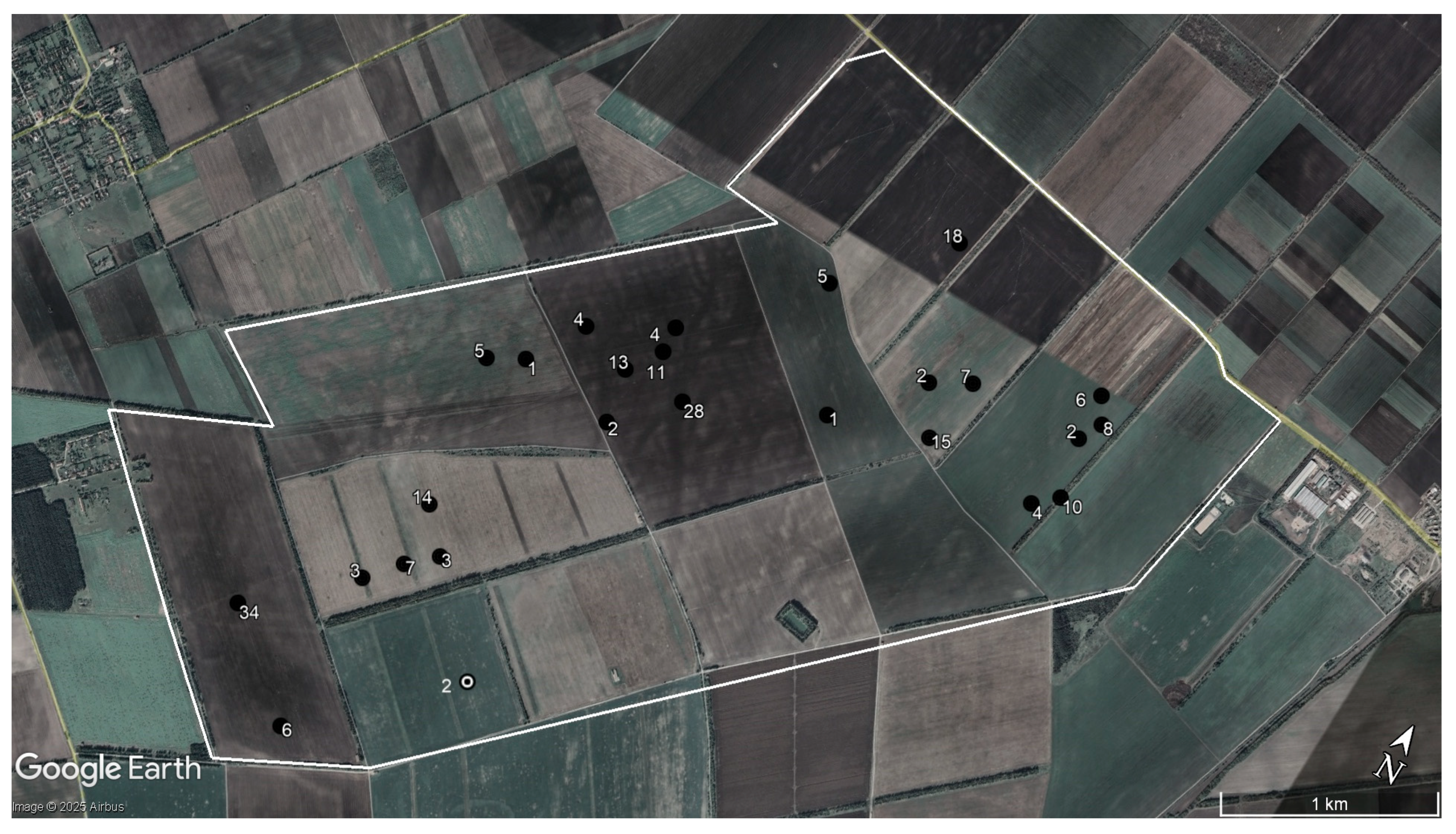
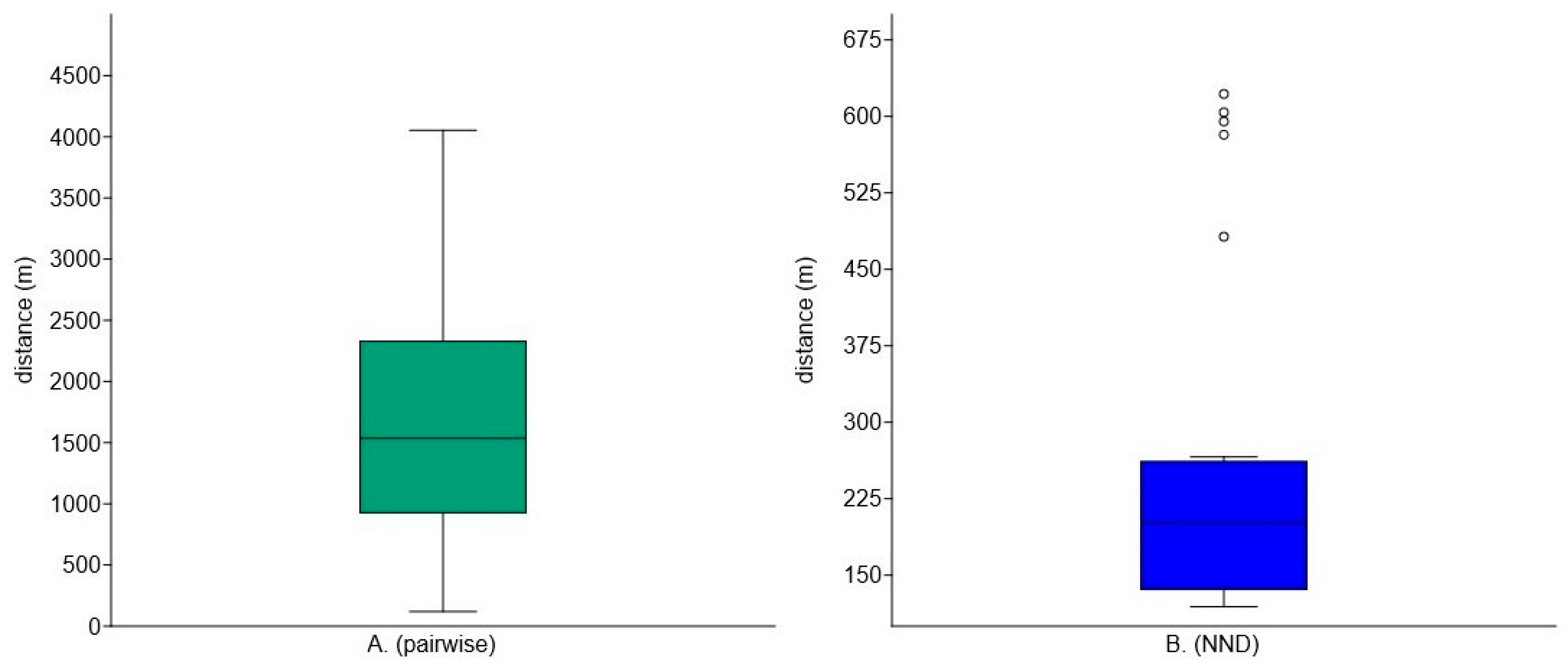
2.4. Data Analysis
- S1: entire area (100%).
- S2: odd transects (transects 1; 3; 5; 7; 9; 11; 13)—approximately 50%.
- S3: even transects (transects 2; 4; 6; 8; 10; 12)—approximately 50%.
- S4–S7: four different sampling combinations of approximately 25% each:
- ○
- S4: transects 1; 5; 9; 13.
- ○
- S5: transects 2; 6; 10.
- ○
- S6: transects 3; 7; 11.
- ○
- S7: transects 4; 8; 12.
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El Bilali, H.; Bassole, I.H.N.; Dambo, L.; Berjan, S. Climate change and food security. Agric. For. 2020, 66, 197–210. [Google Scholar] [CrossRef]
- Pascual-Rico, R.; Morales-Reyes, Z.; Aguilera-Alcalá, N.; Olszańska, A.; Sebastián-González, E.; Naidoo, R.; Moleón, M.; Botella, J.L.; von Wehrden, F.H.; Martín-López, B.; et al. Usually hated, sometimes loved: A review of wild ungulates’ contributions to people. Sci. Total Environ. 2021, 801, 149652. [Google Scholar] [CrossRef]
- Massei, G.; Kindberg, J.; Licoppe, A.; Gačić, D.; Šprem, N.; Kamler, J.; Baubet, E.; Hohmann, U.; Monaco, A.; Ozoliņš, J.; et al. Wild boar populations up, numbers of hunters down? A review of trends and implications for Europe. Pest. Manag. Sci. 2015, 71, 492–500. [Google Scholar] [CrossRef]
- Hone, J. How many feral pigs in Australia? An update. Aust. J. Zool. 2020, 67, 215–220. [Google Scholar] [CrossRef]
- Lewis, J.S.; Corn, J.L.; Mayer, J.J.; Jordan, T.R.; Farnsworth, M.L.; Burdett, C.L.; VerCauteren, K.C.; Sweeney, S.J.; Miller, R.S. Historical, current, and potential population size estimates of invasive wild pigs (Sus scrofa) in the United States. Biol. Invasions 2019, 21, 2373–2384. [Google Scholar] [CrossRef]
- VerCauteren, K.C. The Deer Boom: Discussions on Population Growth and Range Expansion of the White-Tailed Deer; USDA Wildlife Services—Staff Publications: Washington, DC, USA, 2003; p. 281. Available online: https://digitalcommons.unl.edu/icwdm_usdanwrc/281 (accessed on 29 June 2025).
- Burbaitė, L.; Csányi, S. Red deer population and harvest changes in Europe. Acta Zool. Litu. 2024, 20, 179–188. [Google Scholar] [CrossRef]
- Kiffner, C.; Lee, D.E. Population Dynamics of Browsing and Grazing Ungulates in the Anthropocene. In The Ecology of Browsing and Grazing II. Ecological Studies; Gordon, I., Prins, H., Eds.; Springer: Cham, Switzerland, 2019; Volume 239. [Google Scholar] [CrossRef]
- Reimoser, F.; Putman, R. Impacts of wild ungulates on vegetation: Costs and benefits. In Ungulate Management in Europe—Problems and Practices; Putman, R., Apollonio, M., Andersen, R., Eds.; Cambridge University Press: Cambridge, UK, 2011; pp. 144–191. [Google Scholar] [CrossRef]
- Marada, P.; Cukor, J.; Linda, R.; Vacek, Z.; Vacek, S.; Havránek, F. Extensive Orchards in the Agricultural Landscape: Effective Protection against Fraying Damage Caused by Roe Deer. Sustainability 2019, 11, 3738. [Google Scholar] [CrossRef]
- Bleier, N.; Kovács, I.; Schally, G.; Szemethy, L.; Csányi, S. Spatial and temporal characteristics of the damage caused by wild ungulates in maize (Zea mays L.) crops. Int. J. Pest Manag. 2016, 63, 92–100. [Google Scholar] [CrossRef]
- Tari, T.; Horváth, A. Temporospatial characteristics of wild boar rooting damage in maize fields. Rev. Agric. Rural Dev. 2022, 11, 121–125. [Google Scholar] [CrossRef]
- Bergmann, H.; Schulz, K.; Conraths, F.J.; Sauter-Louis, C. A Review of Environmental Risk Factors for African Swine Fever in European Wild Boar. Animals 2021, 11, 2692. [Google Scholar] [CrossRef]
- VerCauteren, K.C.; Lavelle, M.J.; Hygnstrom, S. From the Field: Fences and Deer-Damage Management: A Review of Designs and Efficacy. Wildl. Soc. Bull. 2006, 34, 191–200. [Google Scholar] [CrossRef]
- Geisser, H.; Reyer, H.U. Efficacy of hunting, feeding, and fencing to reduce crop damage by wild boars. J. Wildl. Manag. 2004, 68, 939–946. [Google Scholar] [CrossRef]
- Milner-Gulland, E.J.; Bunnefeld, N.; Proaktor, G. The Science of Sustainable Hunting. In Recreational Hunting, Conservation and Rural Livelihoods; Dickson, B., Hutton, B.J., Adams, W.M., Eds.; Blackwell Publishing Ltd.: Oxford, UK, 2009; pp. 73–93. [Google Scholar] [CrossRef]
- Jay-Russell, M.; Doyle, M.P. (Eds.) Food Safety Risks from Wildlife; Springer: Cham, Switzerland, 2015; p. 254. [Google Scholar] [CrossRef]
- Buckland, S.T.; Goudie, I.B.J.; Borchers, D.L. Wildlife Population Assessment: Past Developments and Future Directions. Biometrics 2000, 56, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Enetwild Consortium; Keuling, O.; Sange, M.; Acevedo, P.; Podgorski, T.; Smith, G.; Scandura, M.; Apollonio, M.; Ferroglio, E.; Body, G.; et al. Guidance on estimation of wild boar population abundance and density: Methods, challenges, possibilities. EFSA J. 2018, 15, EN-1449. [Google Scholar] [CrossRef]
- Engeman, R.M.; Massei, G.; Sage, M.; Gentle, M.N. Monitoring wild pig populations: A review of methods. Environ. Sci. Pollut. Res. 2013, 20, 8077–8091. [Google Scholar] [CrossRef]
- Zhai, Z.; Martínez, J.F.; Beltran, V.; Martínez, N.L. Decision support systems for agriculture 4.0: Survey and challenges. Comput. Electron. Agric. 2020, 170, 105256. [Google Scholar] [CrossRef]
- Gonzalez, L.F.; Montes, G.A.; Puig, E.; Johnson, S.; Mengersen, K.; Gaston, K.J. Unmanned Aerial Vehicles (UAVs) and Artificial Intelligence Revolutionizing Wildlife Monitoring and Conservation. Sensors 2016, 16, 97. [Google Scholar] [CrossRef]
- Obermoller, T.R.; Norton, A.S.; Michel, E.S.; Haroldson, B.S. Use of drones with thermal infrared to locate white-tailed deer neonates for capture. Wildl. Soc. Bull. 2021, 45, 682–689. [Google Scholar] [CrossRef]
- Niwa, H. Assessing the activity of deer and their influence on vegetation in a wetland using automatic cameras and low altitude remote sensing (LARS). Eur. J. Wildl. Res. 2021, 67, 3. [Google Scholar] [CrossRef]
- Wang, D.; Shao, Q.; Yue, H. Surveying Wild Animals from Satellites, Manned Aircraft and Unmanned Aerial Systems (UASs): A Review. Remote Sens. 2019, 11, 1308. [Google Scholar] [CrossRef]
- Converse, R.L.; Lippitt, C.D.; Koneff, M.D.; White, T.P.; Weinstein, B.G.; Gibbons, R.; Stewart, D.R.; Fleishman, A.B.; Butler, M.J.; Sesnie, S.E.; et al. Remote sensing and machine learning to improve aerial wildlife population surveys. Front. Conserv. Sci. 2024, 5, 1416706. [Google Scholar] [CrossRef]
- Christiansen, P.; Steen, K.A.; Jørgensen, R.N.; Karstoft, H. Automated detection and recognition of wildlife using thermal cameras. Sensors 2014, 14, 13778–13793. [Google Scholar] [CrossRef]
- Brown, J.; Qiao, Y.; Clark, C.; Lomax, S.; Rafique, K.; Sukkarieh, S. Automated aerial animal detection when spatial resolution conditions are varied. Comput. Electron. Agrc. 2022, 193, 106689. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, T.; Skidmore, A.K.; Lamprey, R.; Ngene, S. Bounding box versus point annotation: The impact on deep learning performance for animal detection in aerial images. ISPRS J. Photogramm. Remote Sens. 2025, 222, 99–111. [Google Scholar] [CrossRef]
- Faragó, S. A Lajta Project—Egy Tartamos Mezei vad és Ökoszisztéma Vizsgálat 20 Éve; Nyugat-magyarországi Egyetem Kiadó: Sopron, Hungary, 2012. [Google Scholar]
- eCognition, Ecognition Developer Reference Book (Ver. 8.9. 1); Trimble Germany GmbH: Munich, Germany, 2013; Available online: https://docs.ecognition.com/v9.5.0/Page%20collection/eCognition%20Suite%20Overview.htm (accessed on 29 June 2025).
- Baatz, M.; Schäpe, A. Multiresolution Segmentation: An optimization approach for high quality multi-scale image segmentation. In Angewandte Geogr. Informationsverarbeitung XII; Strobl, J., Blaschke, T., Eds.; Wichmann Verlag: Kalsruhe, Germany, 2000; pp. 12–23. [Google Scholar]
- Morellet, N.; Klein, F.; Solberg, E.; Andersen, R. The census and management of populations of ungulates in Europe. In Ungulate Management in Europe—Problems and Practices; Putman, R., Apollonio, M., Andersen, R., Eds.; Cambridge University Press: Cambridge, UK, 2011; pp. 106–143. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Vermeulen, C.; Lejeune, P.; Lisein, J.; Sawadogo, P.; Bouché, P. Unmanned Aerial Survey of Elephants. PLoS ONE 2013, 8, e54700. [Google Scholar] [CrossRef] [PubMed]
- LeResche, R.E.; Rausch, R.A. Accuracy and Precision of Aerial Moose Censusing. J. Wildl. Manag. 1974, 38, 175–182. [Google Scholar] [CrossRef]
- Schlossberg, S.; Chase, M.J.; Griffin, C.R. Testing the Accuracy of Aerial Surveys for Large Mammals: An Experiment with African Savanna Elephants (Loxodonta africana). PLoS ONE 2016, 11, e0164904. [Google Scholar] [CrossRef]
- Zabel, F.; Findlay, M.A.; White, P.J. Assessment of the accuracy of counting large ungulate species (red deer Cervus elaphus) with UAV-mounted thermal infrared cameras during night flights. Wildl. Biol. 2023, 2023, e01071. [Google Scholar] [CrossRef]
- Preston, T.M.; Wildhaber, M.L.; Green, N.S.; Albers, J.L.; Debenedetto, G.P. Enumerating white-tailed deer using unmanned aerial vehicles. Wildl. Soc. Bull. 2021, 45, 97–108. [Google Scholar] [CrossRef]
- Chrétien, L.P.; Théau, J.; Ménard, P. Visible and thermal infrared remote sensing for the detection of white-tailed deer using an unmanned aerial system. Wildl. Soc. Bull. 2016, 40, 181–191. [Google Scholar] [CrossRef]
- Lyu, H.; Qiu, F.; An, L.; Stow, D.; Lewison, R.; Bohnett, E. Deer survey from drone thermal imagery using enhanced faster R-CNN based on ResNets and FPN. Ecol. Inform. 2024, 79, 102383. [Google Scholar] [CrossRef]
- Roca, A.; Torre, G.; Giribet, J.I.; Castro, G.; Colombo, L.; Mas, I.; Pereira, J. Efficient endangered deer species monitoring with uav aerial imagery and deep learning. In Proceedings of the 2024 IEEE Biennial Congress of Argentina (ARGENCON), San Nicolás de los Arroyos, Argentina, 18–20 September 2024; pp. 1–8. [Google Scholar] [CrossRef]
- Franke, U.; Goll, B.; Hohmann, U.; Heurich, M. Aerial ungulate surveys with a combination of infrared and high–resolution natural colour images. Anim. Biodivers. Conserv. 2012, 35, 285–293. [Google Scholar] [CrossRef]
- Israel, M. A UAV-based roe deer fawn detection system. ISPRS Arch. 2011, 38, 51–55. [Google Scholar] [CrossRef]
- Cukor, J.; Bartoška, J.; Rohla, J.; Sova, J.; Machálek, A. Use of aerial thermography to reduce mortality of roe deer fawns before harvest. PeerJ 2019, 7, e6923. [Google Scholar] [CrossRef]
- Delisle, Z.J.; McGovern, P.G.; Dillman, B.G.; Swihart, R.K. Imperfect detection and wildlife density estimation using aerial surveys with infrared and visible sensors. Remote Sens. Ecol. Conserv. 2023, 9, 222–234. [Google Scholar] [CrossRef]
- Havens, K.J.; Sharp, E.J. Properties of Thermal Signatures—Chapter 9. In Thermal Imaging Techniques to Survey and Monitor Animals in the Wild; Havens, K.J., Sharp, E.J., Eds.; Academic Press: New York, NY, USA, 2007; pp. 143–169. [Google Scholar] [CrossRef]
- Khaemba, W.M. Spatial point pattern analysis of aerial survey data to assess clustering in wildlife distributions. Int. J. Appl. Earth Obs. Geoinf. 2001, 3, 139–145. [Google Scholar] [CrossRef]
- Kocsis, M.; Faragó, S. Az őz (Capreolus capreolus) évszakos területhasználata a Lajta-projektben. In Erdészeti, Környezettudományi, Természetvédelmi és Vadgazdálkodási Tudományos Konferencia–Konferencia Kiadvány; Lakatos, F., Kui, B., Eds.; NymE Kiadó: Sopron, Hungary, 2007; pp. 94–96. [Google Scholar]
- Tóth, B.; Bleier, N.; Schally, G.; Lehoczki, R.; Csányi, S. Otthonterület-becslési módszerek összehasonlítása az őz területhasználatának elemzésében. Vadbiológia 2014, 16, 51–62. [Google Scholar]
- Morellet, N.; Bonenfant, C.; Börger, L.; Ossi, F.; Cagnacci, F.; Heurich, M.; Kjellander, P.; Linnell, J.D.C.; Nicoloso, S.; Sustr, P.; et al. Seasonality, weather and climate affect home range size in roe deer across a wide latitudinal gradient within Europe. J. Anim. Ecol. 2013, 82, 1326–1339. [Google Scholar] [CrossRef]
- Náhlik, A.; Sándor, G.; Tari, T.; Király, G. Space Use and Activity Patterns of Red Deer in a Highly Forested and in a Patchy Forest-Agricultural Habitat. Acta silv. Lignaria Hung. 2009, 5, 109–118. [Google Scholar] [CrossRef]
- Náhlik, A.; Heffenträger, G.; Pócza, G.; Sándor, G.; Tari, T. Daytime movements of red deer disturbed by human activity. In Proceedings of the 8th International Deer Biology Congress and International Wildlife Management Symposium, Harbin, China, 27–31 July 2014; pp. 57–59. [Google Scholar]
- Tracey, J.P.; Fleming, P.J.S.; Melville, G.J. Accuracy of some aerial survey estimators: Contrasts with known numbers. Wildl. Res. 2008, 35, 377–384. [Google Scholar] [CrossRef]
- Stache, A.; Heller, E.; Hothorn, T.; Heurich, M. Activity patterns of European roe deer (Capreolus capreolus) are strongly influenced by individual behaviour. Folia Zool. 2013, 62, 67–75. [Google Scholar] [CrossRef]
- Tóth, G.; Katona, K. Comparison of Population Density Estimation Methods for Roe Deer (Capreolus capreolus). Diversity 2024, 16, 500. [Google Scholar] [CrossRef]
- Gerard, J.F.; Le Pendu, Y.; Maublanc, M.L.; Vincent, J.P.; Poulle, M.L.; Cibien, C. Large group formation in European Roe deer: An adaptive feature ? Revue d’Écologie 1995, 50, 391–401. [Google Scholar] [CrossRef]
- Pays, O.; Fortin, D.; Gassani, J.; Duchesne, J. Group Dynamics and Landscape Features Constrain the Exploration of Herds in Fusion-Fission Societies: The Case of European Roe Deer. PLoS ONE 2012, 7, e34678. [Google Scholar] [CrossRef]
- Pays, O.; Benhamou, S.; Helder, R.; Gerard, J.F. The dynamics of group formation in large mammalian herbivores: An analysis in the European roe deer. Anim. Behav. 2007, 74, 1429–1441. [Google Scholar] [CrossRef]



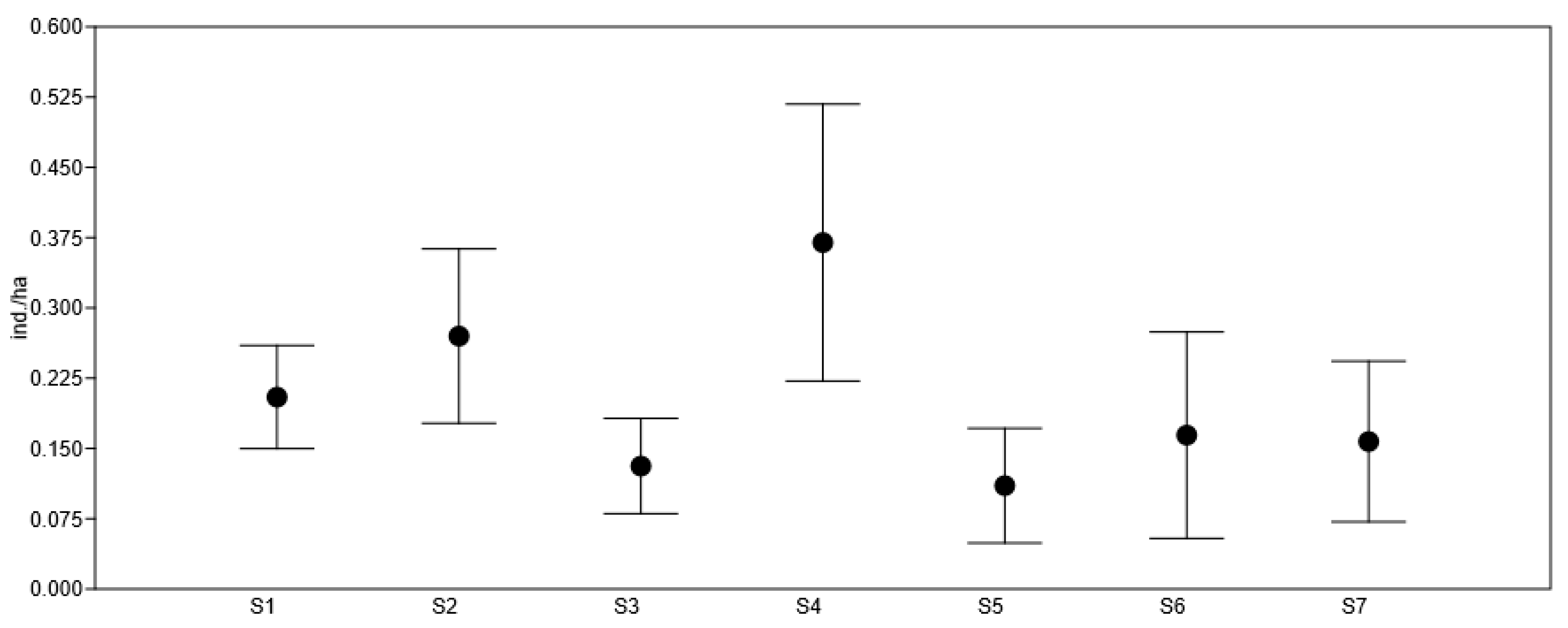
| S2 | S3 | S4 | S5 | S6 | S7 | |
|---|---|---|---|---|---|---|
| Estimated minimum population number | 187 ** | 74 ** | 229 * | 52 ** | 56 ** | 70 ** |
| Estimated mean population number | 281 * | 136 ** | 385 * | 115 ** | 171 ** | 164 ** |
| Estimated maximum population number | 374 * | 199 ** | 541 * | 177 ** | 285 * | 257 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tari, T.; Czimber, K.; Faragó, S.; Heffenträger, G.; Kalmár, S.; Kovács, G.; Sándor, G.; Náhlik, A. Roe Deer as a Model Species for Aerial Survey-Based Ungulate Population Estimation in Agricultural Habitats. Geomatics 2025, 5, 53. https://doi.org/10.3390/geomatics5040053
Tari T, Czimber K, Faragó S, Heffenträger G, Kalmár S, Kovács G, Sándor G, Náhlik A. Roe Deer as a Model Species for Aerial Survey-Based Ungulate Population Estimation in Agricultural Habitats. Geomatics. 2025; 5(4):53. https://doi.org/10.3390/geomatics5040053
Chicago/Turabian StyleTari, Tamás, Kornél Czimber, Sándor Faragó, Gábor Heffenträger, Sándor Kalmár, Gyula Kovács, Gyula Sándor, and András Náhlik. 2025. "Roe Deer as a Model Species for Aerial Survey-Based Ungulate Population Estimation in Agricultural Habitats" Geomatics 5, no. 4: 53. https://doi.org/10.3390/geomatics5040053
APA StyleTari, T., Czimber, K., Faragó, S., Heffenträger, G., Kalmár, S., Kovács, G., Sándor, G., & Náhlik, A. (2025). Roe Deer as a Model Species for Aerial Survey-Based Ungulate Population Estimation in Agricultural Habitats. Geomatics, 5(4), 53. https://doi.org/10.3390/geomatics5040053






