Improved Hand Function in Children with Cerebral Palsy with Repeat Doses of Group Based Hybrid Pediatric Constraint-Induced Movement Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Setting
2.2. Outcome Measures
2.3. Intervention
2.4. Data Analysis
3. Results
3.1. Subject Characteristics
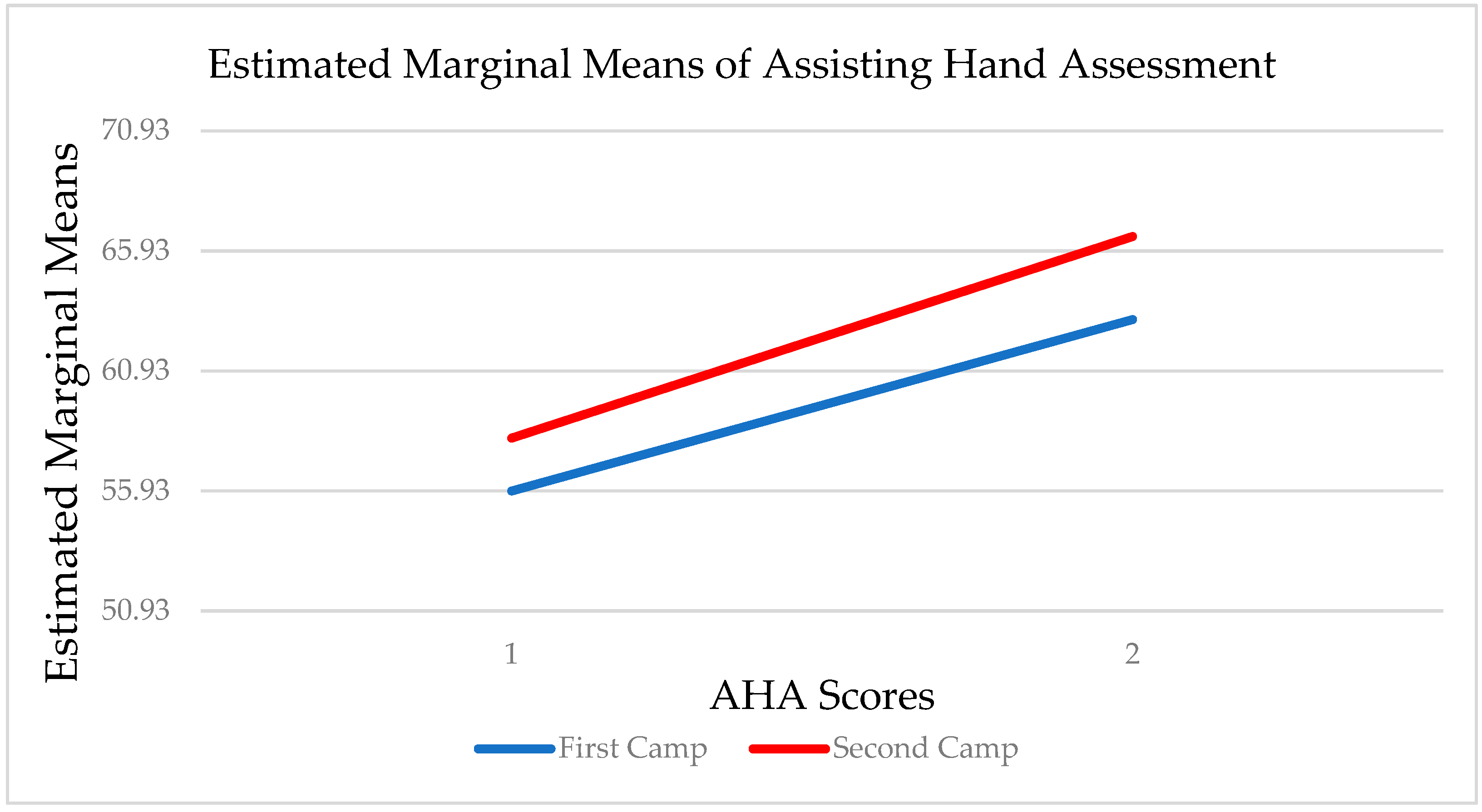
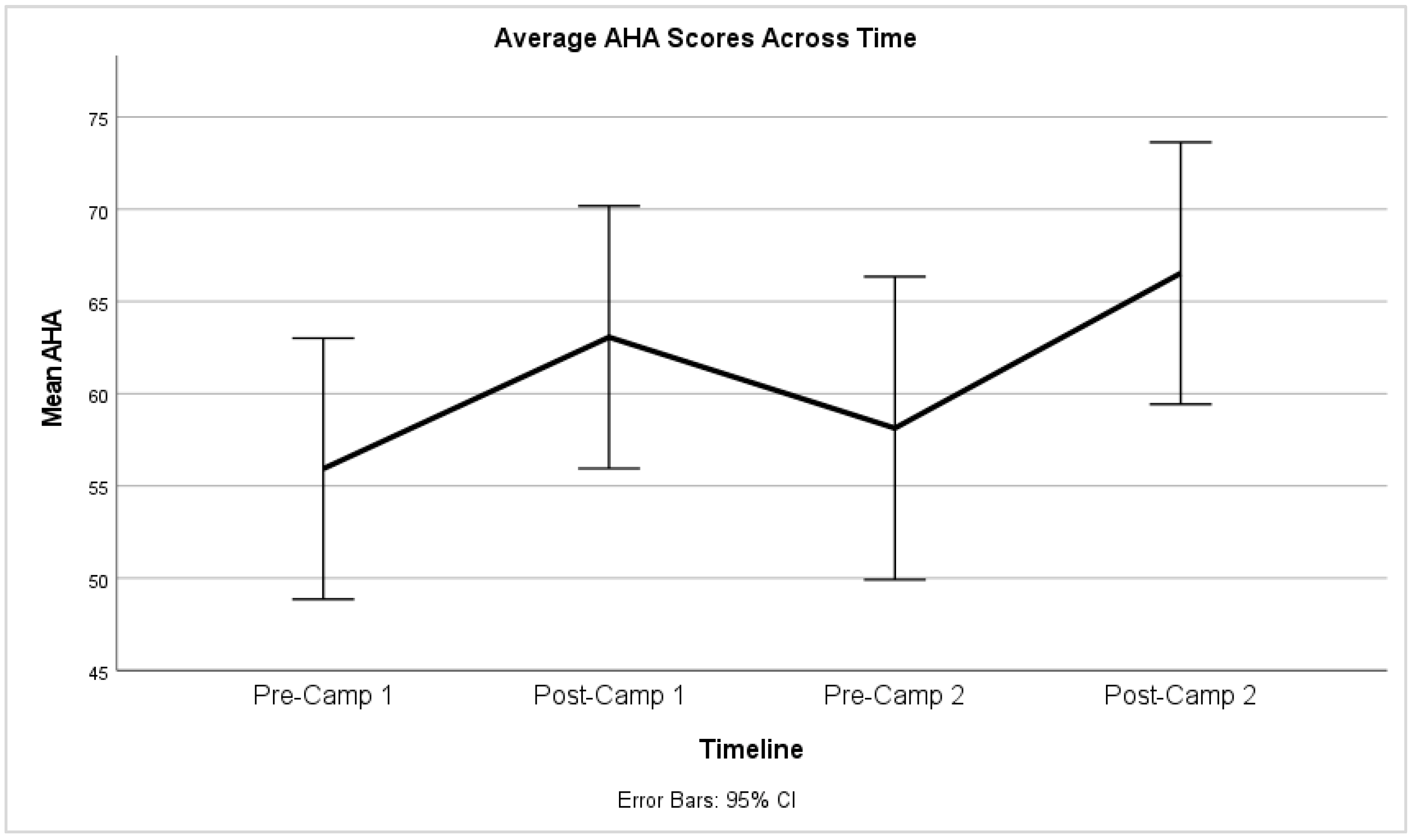
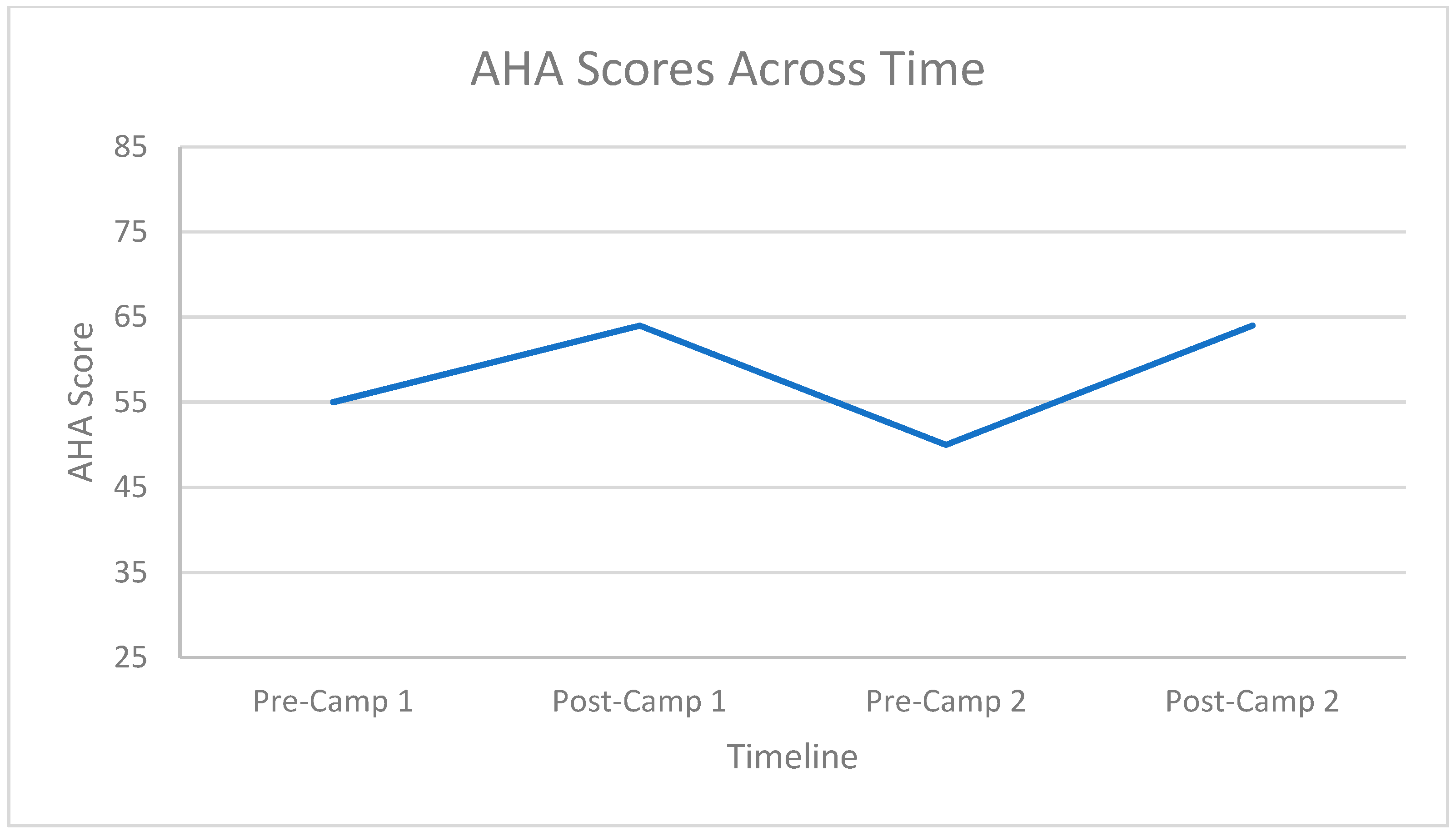
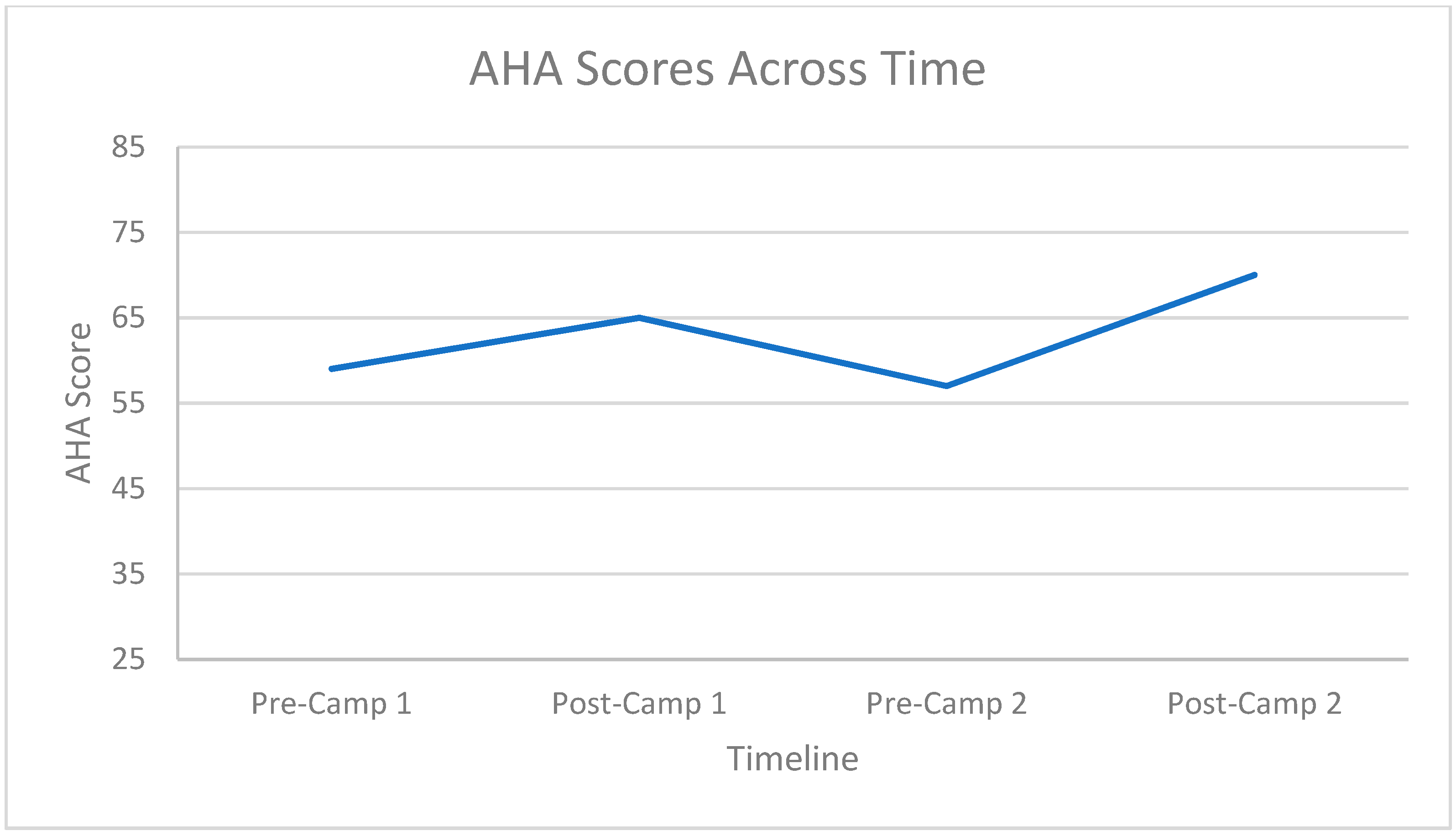
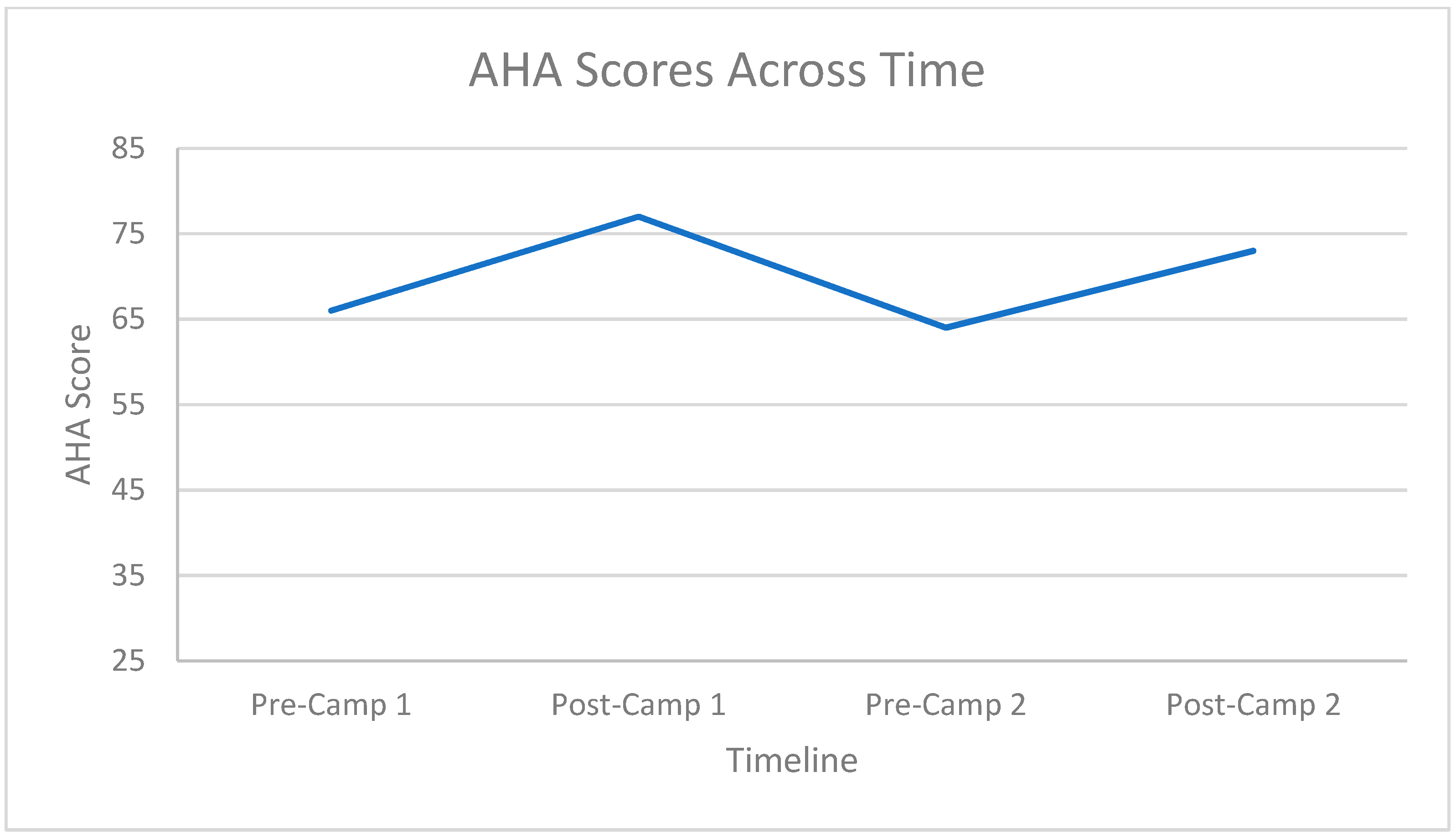
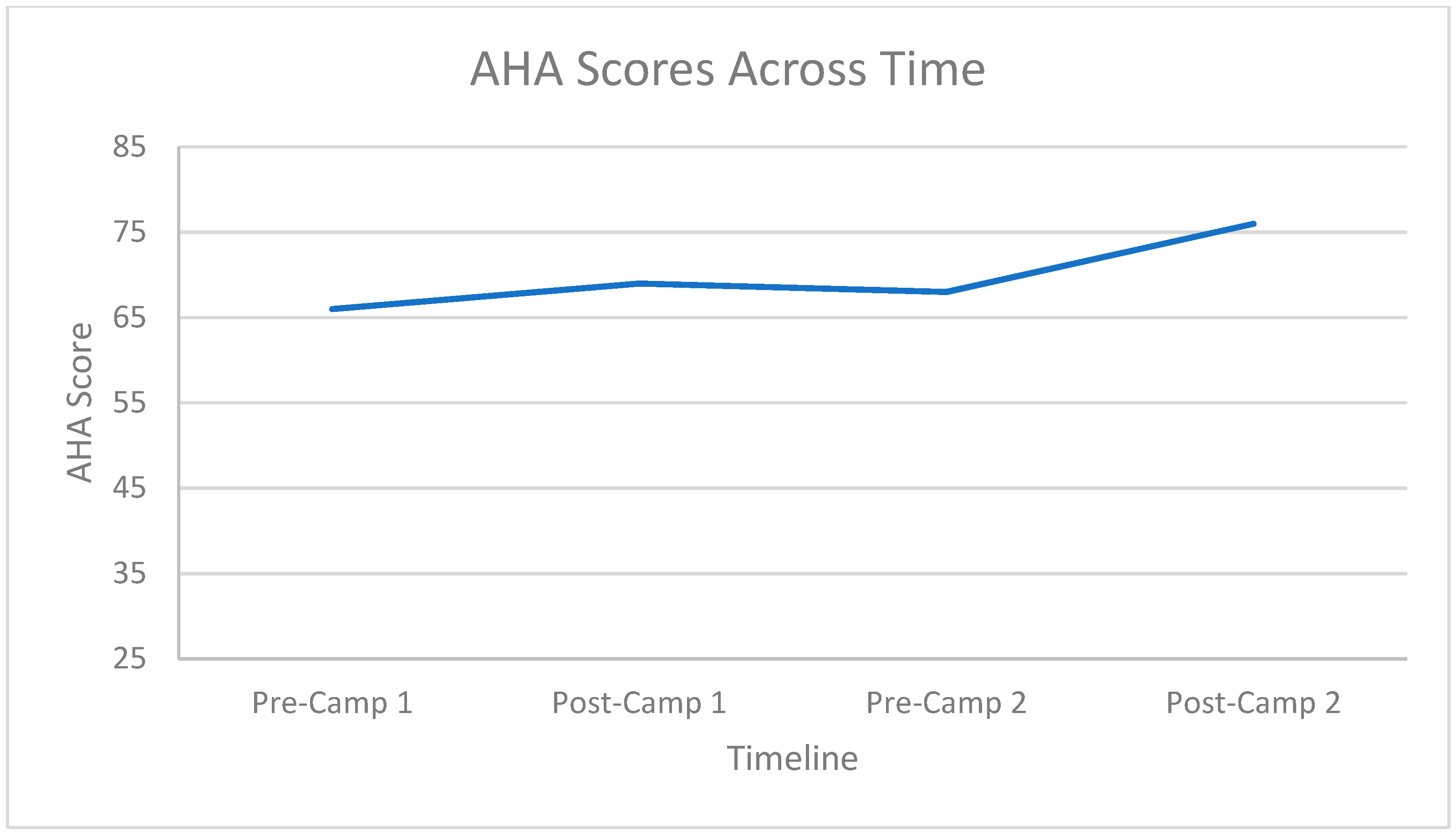
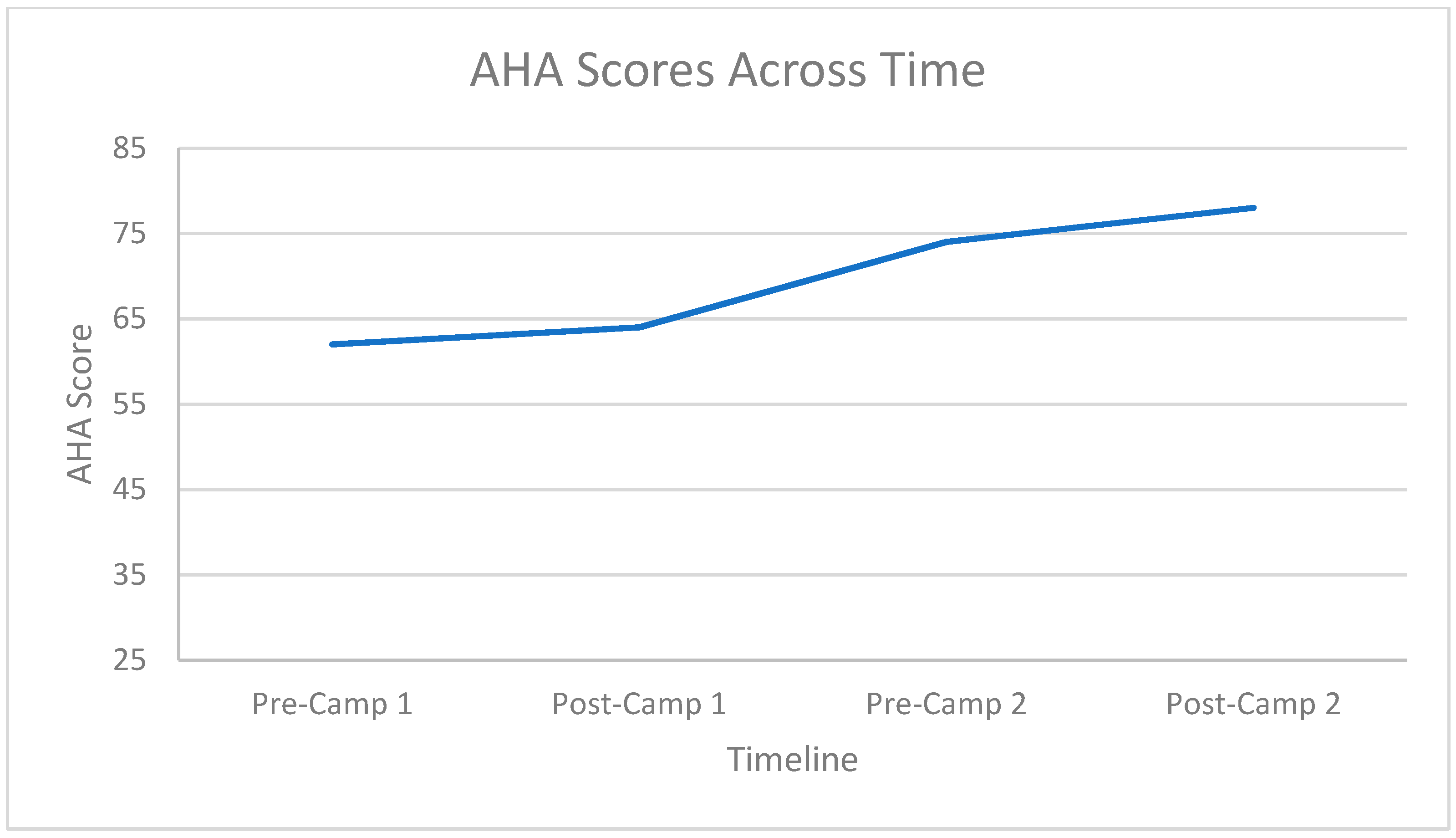
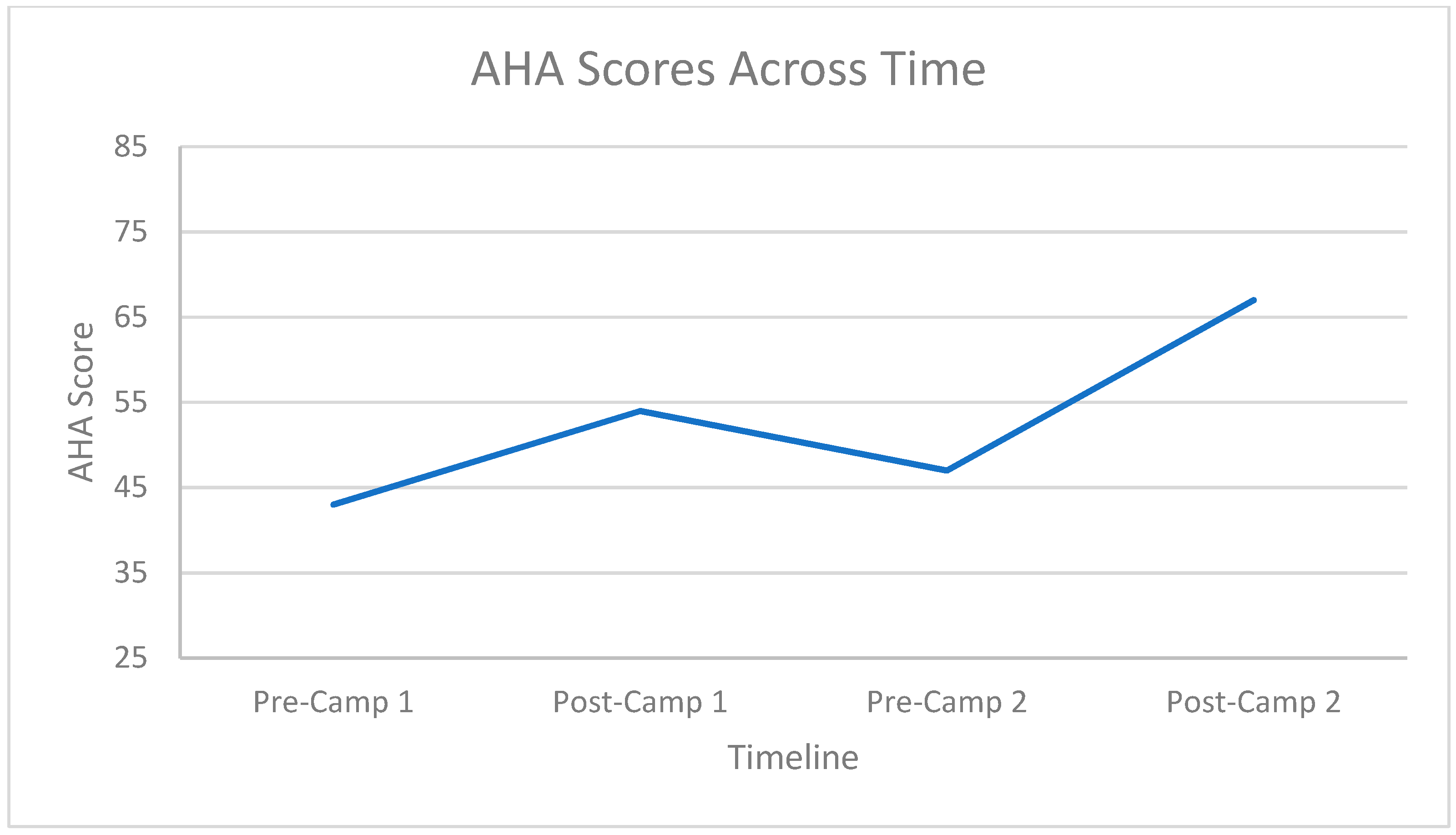
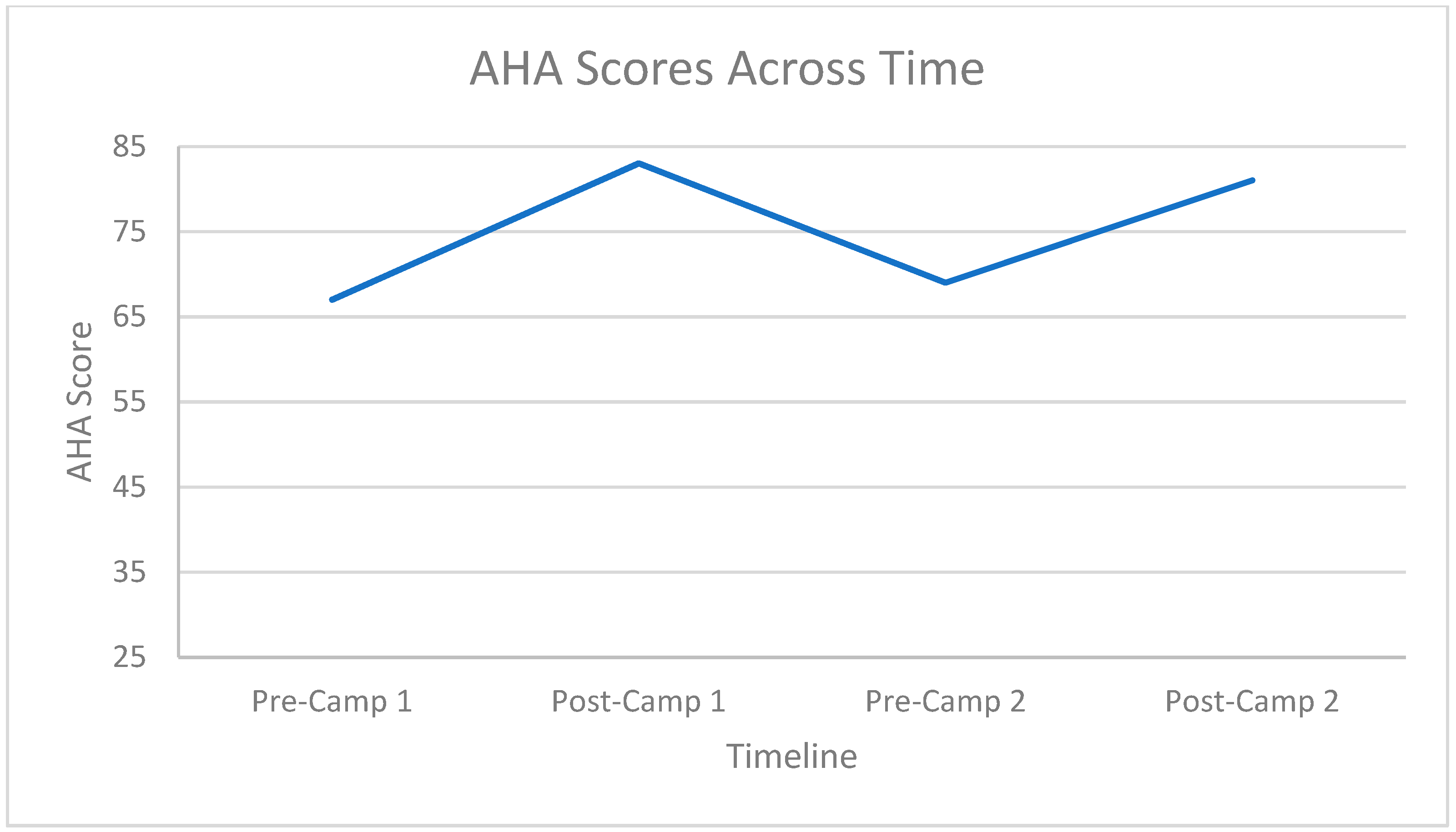
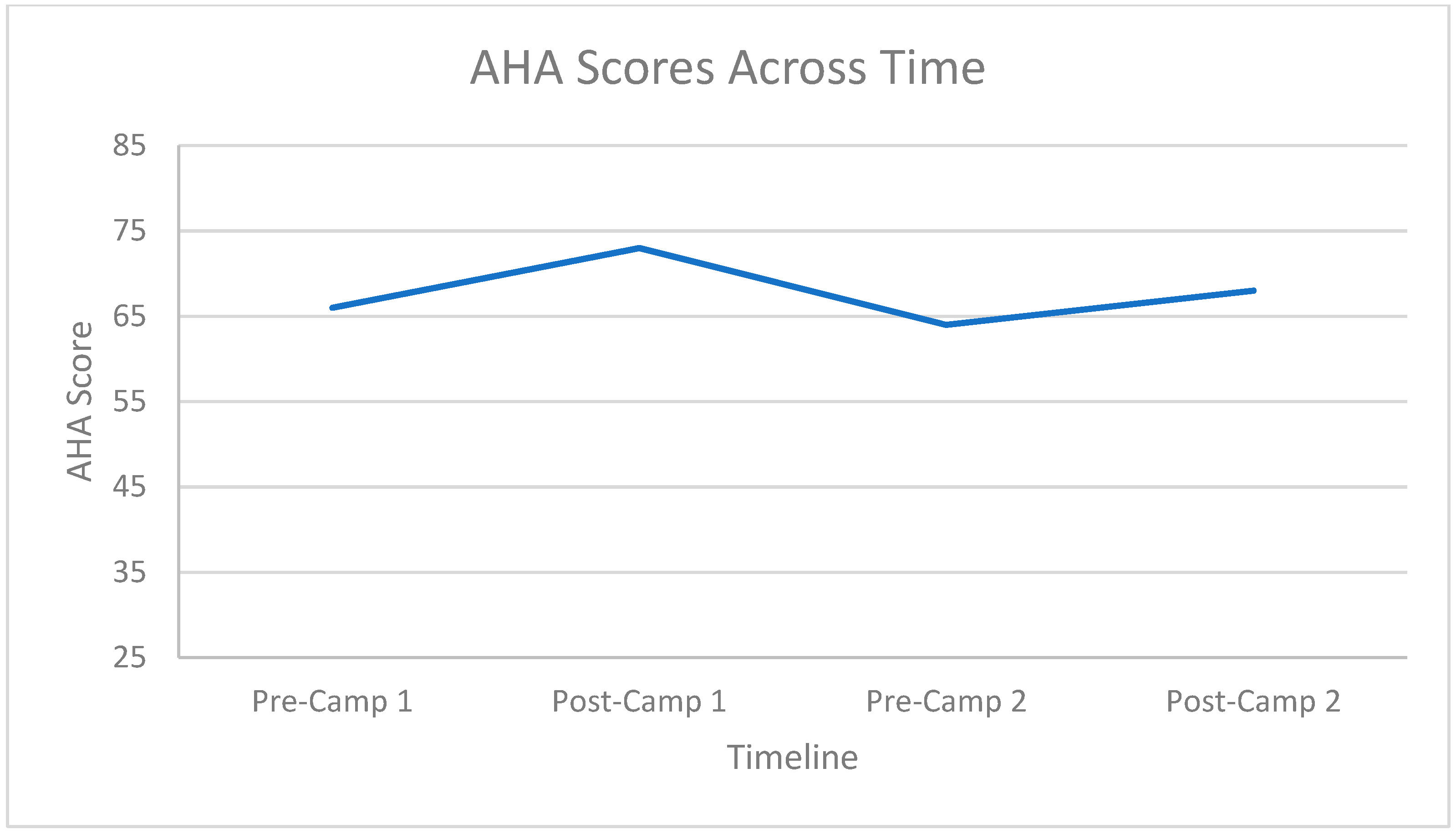
3.2. Hand Function Changes
3.3. Carryover Effect between Repeat Doses
4. Discussion
4.1. Limitations
4.2. Implications for Practice
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Centers for Disease Control and Prevention. Data and Statistics for Cerebral Palsy. Available online: https://www.cdc.gov/ncbddd/cp/data.html (accessed on 28 April 2021).
- Christensen, D.; Braun, K.V.N.; Doernberg, N.S.; Maenner, M.J.; Arneson, C.L.; Durkin, M.S.; Benedict, R.E.; Kirby, R.S.; Wingate, M.S.; Fitzgerald, R.; et al. Prevalence of cerebral palsy, co-occurring Autism Spectrum Disorders, and motor functioning–Autism and Developmental Disabilities Monitoring Network, USA, 2008. Dev. Med. Child. Neurol. 2014, 56, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, P.; Paneth, N.; Leviton, A.; Goldstein, M.; Bax, M. A Report: The Definition and Classification of Cerebral Palsy April 2006. Dev. Med. Child. Neurol. 2007, 49, 8–14. [Google Scholar]
- Ramey, S.L.; Coker-Bolt, P.; DeLuca, S.C. Handbook of Pediatric Constraint-Induced Movement Therapy (CIMT), 1st ed.; American Occupational Therapy Association, Inc.: Bethesda, MD, USA, 2013; pp. 19–54. [Google Scholar]
- Hoare, B.; Imms, C.; Carey, L.; Wasiak, J. Constraint-Induced Movement Therapy in the Treatment of the Upper Limb in Children with Hemiplegic Cerebral Palsy: A Cochrane Systematic Review. Clin. Rehabil. 2007, 21, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Blanton, S.; Wolf, S.L. An application of upper-extremity constraint-induced movement therapy in a patient with subacute stroke. Phys. Ther. 1999, 79, 847–853. [Google Scholar] [CrossRef]
- Nesin, S.M.; Sabitha, K.R.; Gupta, A.; Laxmi, T.R. Constraint induced movement therapy as a rehabilitative strategy for ischemic stroke—Linking neural plasticity with restoration of skilled movements. J. Stroke Cerebrovasc. Dis. 2019, 28, 1640–1653. [Google Scholar] [CrossRef]
- Chen, Y.; Pope, S.; Tyler, D.; Warren, G.L. Effectiveness of Constraint-Induced Movement Therapy on Upper-Extremity Function in Children with Cerebral Palsy: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Clin. Rehabil. 2014, 28, 939–953. [Google Scholar] [CrossRef]
- Sparrow, J.; Zhu, L.; Gajjar, A.; Mandrell, B.N.; Ness, K.K. Constraint-Induced Movement Therapy for Children with Brain Tumors. Pediatric Phys. Ther. 2017, 29, 55–61. [Google Scholar] [CrossRef]
- Komar, A.; Ashley, K.; Hanna, K.; Lavallee, J.; Woodhouse, J.; Bernstein, J.; Andres, M.; Reed, N. Retrospective Analysis of an Ongoing Group-Based Modified Constraint-Induced Movement Therapy Program for Children with Acquired Brain Injury. Phys. Occup. Ther. Pediatr. 2016, 36, 186–203. [Google Scholar] [CrossRef]
- Vaz, D.V.; Mancini, M.C.; do Amaral, M.F.; de Brito Brandão, M.; de França Drummond, A.; da Fonseca, S.T. Clinical Changes during an Intervention Based on Constraint-Induced Movement Therapy Principles on Use of the Affected Arm of a Child with Obstetric Brachial Plexus Injury: A Case Report. Occup. Ther. Int. 2010, 17, 159–167. [Google Scholar] [CrossRef]
- Chiu, H.-C.; Ada, L. Constraint-Induced Movement Therapy Improves Upper Limb Activity and Participation in Hemiplegic Cerebral Palsy: A Systematic Review. J. Physiother. 2016, 62, 130–137. [Google Scholar] [CrossRef]
- Hoare, B.J.; Wallen, M.A.; Thorley, M.N.; Jackman, M.L.; Carey, L.M.; Imms, C. Constraint-induced Movement Therapy in Children with Unilateral Cerebral Palsy. Cochrane Database Syst. Rev. 2019, 4, CD004149. [Google Scholar] [CrossRef] [PubMed]
- DeLuca, S.C.; Ramey, S.L.; Trucks, M.R.; Wallace, D.A. Multiple Treatments of Pediatric Constraint-Induced Movement Therapy (PCIMT): A Clinical Cohort Study. Am. J. Occup. Ther. 2015, 69, 6906180010p1–6906180010p9. [Google Scholar] [CrossRef] [PubMed]
- Eliasson, A.C.; Krumlinde-Sundholm, L.; Gordon, A.M.; Feys, H.; Klingels, K.; Aarts, P.B.M.; Rameckers, E.; Autti-Rämö, I.; Hoare, B. Guidelines for Future Research in Constraint-Induced Movement Therapy for Children with Unilateral Cerebral Palsy: An Expert Consensus. Dev. Med. Child. Neurol. 2014, 56, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Roberts, H.; Shierk, A.; Clegg, N.J.; Baldwin, D.; Smith, L.; Yeatts, P.; Delgado, M.R. Constraint Induced Movement Therapy Camp for Children with Hemiplegic Cerebral Palsy Augmented by Use of an Exoskeleton to Play Games in Virtual Reality. Phys. Occup. Ther. Pediatr. 2021, 41, 150–165. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.-C.; Hung, J.-W.; Tseng, C.-Y.; Huang, Y.-C. Group Constraint-Induced Movement Therapy for Children with Hemiplegic Cerebral Palsy: A Pilot Study. Am. J. Occup. Ther. 2013, 67, 201–208. [Google Scholar] [CrossRef][Green Version]
- Ko, E.J.; Sung, I.Y.; Moon, H.J.; Yuk, J.S.; Kim, H.-S.; Lee, N.H. Effect of Group-Task-Oriented Training on Gross and Fine Motor Function, and Activities of Daily Living in Children with Spastic Cerebral Palsy. Phys. Occup. Ther. Pediatr. 2020, 40, 18–30. [Google Scholar] [CrossRef]
- Eliasson, A.-C.; Krumlinde-Sundholm, L.; Rösblad, B.; Beckung, E.; Arner, M.; Örvall, A.-M.; Rosenbaum, P. The Manual Classification System (MACS) for Children with Cerebral Palsy: Scale Development and Evidence of Validity and Reliability. Dev. Med. Child. Neurol. 2006, 48, 549–554. [Google Scholar] [CrossRef]
- Krumlinde-Sundholm, L.; Holmefur, M.; Kottorp, A.; Eliasson, A.-C. The Assisting Hand Assessment: Current Evidence of Validity, Reliability, and Responsiveness to Change. Dev. Med. Child. Neurol. 2007, 49, 259–264. [Google Scholar] [CrossRef]
- Ryll, U.C.; Bastiaenen, H.G.; Eliasson, A.-C. Assisting Hand Assessment and Children’s Hand-Use Experience Questionnaire–Observed Versus Perceived Bimanual Performance in Children with Unilateral Cerebral Palsy. Phys. Occup. Ther. Pediatr. 2017, 37, 199–209. [Google Scholar] [CrossRef]
- Louwers, A.; Krumlinde-Sundholm, L.; Boeschoten, K.; Beelen, A. Reliability of the Assisting Hand Assessment in Adolescents. Dev. Med. Child. Neurol. 2017, 59, 926–932. [Google Scholar] [CrossRef]
- Boyd, R.; Sakzewski, L.; Ziviani, J.; Abbott, D.F.; Badawy, R.; Gilmore, R.; Provan, K.; Tournier, J.-D.; Macdonell, R.A.L.; Jackson, G.D. INCITE: A Randomized Trial Comparing Constraint Induced Movement Therapy and Bimanual Training in Children with Congenital Hemiplegia. BMC Neurol. 2010, 10, 4. [Google Scholar] [CrossRef] [PubMed]
- Gordon, A.M.; Cbinnan, A.; Gill, S.; Petra, E.; Hung, Y.; Charles, J. Both Constraint-induced Movement Therapy and Bimanual Training Lead to Improved Performance of Upper Extremity Function in Children with Hemiplegia. Dev. Med. Child. Neurol. 2008, 50, 957–958. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | Received Two Doses of pCIMT (n = 15) |
|---|---|
| Mean age, months | |
| Dose 1 | 91 |
| Dose 2 | 108 |
| Age range, months | 63–180 |
| Gender, n (%) | |
| Boys | 9 (60) |
| Girls | 6 (40) |
| Side of hemiparesis, n (%) | |
| Right | 7 (46) |
| Left | 8 (53) |
| MACS level, n (%) | |
| I (least impaired) | 3 (20) |
| II | 11 (73) |
| III | 1 (6) |
| Camp Frequency | Mean | Standard Deviation | |
|---|---|---|---|
| Pre-AHA | First Camp | 55.93 | 12.781 |
| Second Camp | 58.13 | 14.832 | |
| Total | 57.03 | 13.801 | |
| Post-AHA | First Camp | 63.07 | 12.853 |
| Second Camp | 66.53 | 12.822 | |
| Total | 64.80 | 12.837 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roberts, H.; Shierk, A.; Alfonso, A.J.; Yeatts, P.; DeJong, T.L.; Clegg, N.J.; Baldwin, D.; Delgado, M.R. Improved Hand Function in Children with Cerebral Palsy with Repeat Doses of Group Based Hybrid Pediatric Constraint-Induced Movement Therapy. Disabilities 2022, 2, 365-378. https://doi.org/10.3390/disabilities2020026
Roberts H, Shierk A, Alfonso AJ, Yeatts P, DeJong TL, Clegg NJ, Baldwin D, Delgado MR. Improved Hand Function in Children with Cerebral Palsy with Repeat Doses of Group Based Hybrid Pediatric Constraint-Induced Movement Therapy. Disabilities. 2022; 2(2):365-378. https://doi.org/10.3390/disabilities2020026
Chicago/Turabian StyleRoberts, Heather, Angela Shierk, Arianne J. Alfonso, Paul Yeatts, Trey L. DeJong, Nancy J. Clegg, Deborah Baldwin, and Mauricio R. Delgado. 2022. "Improved Hand Function in Children with Cerebral Palsy with Repeat Doses of Group Based Hybrid Pediatric Constraint-Induced Movement Therapy" Disabilities 2, no. 2: 365-378. https://doi.org/10.3390/disabilities2020026
APA StyleRoberts, H., Shierk, A., Alfonso, A. J., Yeatts, P., DeJong, T. L., Clegg, N. J., Baldwin, D., & Delgado, M. R. (2022). Improved Hand Function in Children with Cerebral Palsy with Repeat Doses of Group Based Hybrid Pediatric Constraint-Induced Movement Therapy. Disabilities, 2(2), 365-378. https://doi.org/10.3390/disabilities2020026







