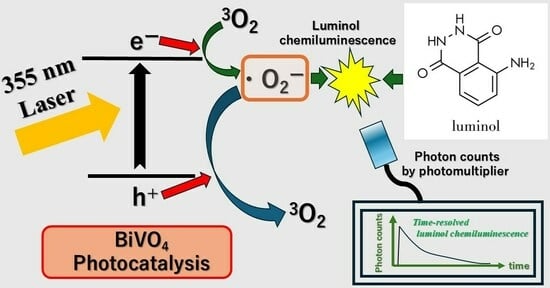
Time-Resolved Chemiluminescence of Luminol Formed by 355 nm Laser-Irradiated BiVO4 Photocatalysis: Effects of the Addition of Alcohols and Ag Ions
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Time-Resolved Chemiluminescence of Luminol Induced by the 532 nm Laser Irradiation of Rose Bengal and the Luminol Solution: The Time-Resolved Luminol Chemilulinescence Induced by Singlet Oxygen
3.2. Time-Resolved Chemiluminescence of Luminol Induced by the 355 nm Laser Irradiation of a BiVO4 and Luminol Suspension and Time-Resolved Luminol Chemilulinescence Induced by the Active Oxygen Species by 355 nm Laser-Irradiated Luminol and BiVO4 Photocatalyisis Suspensions
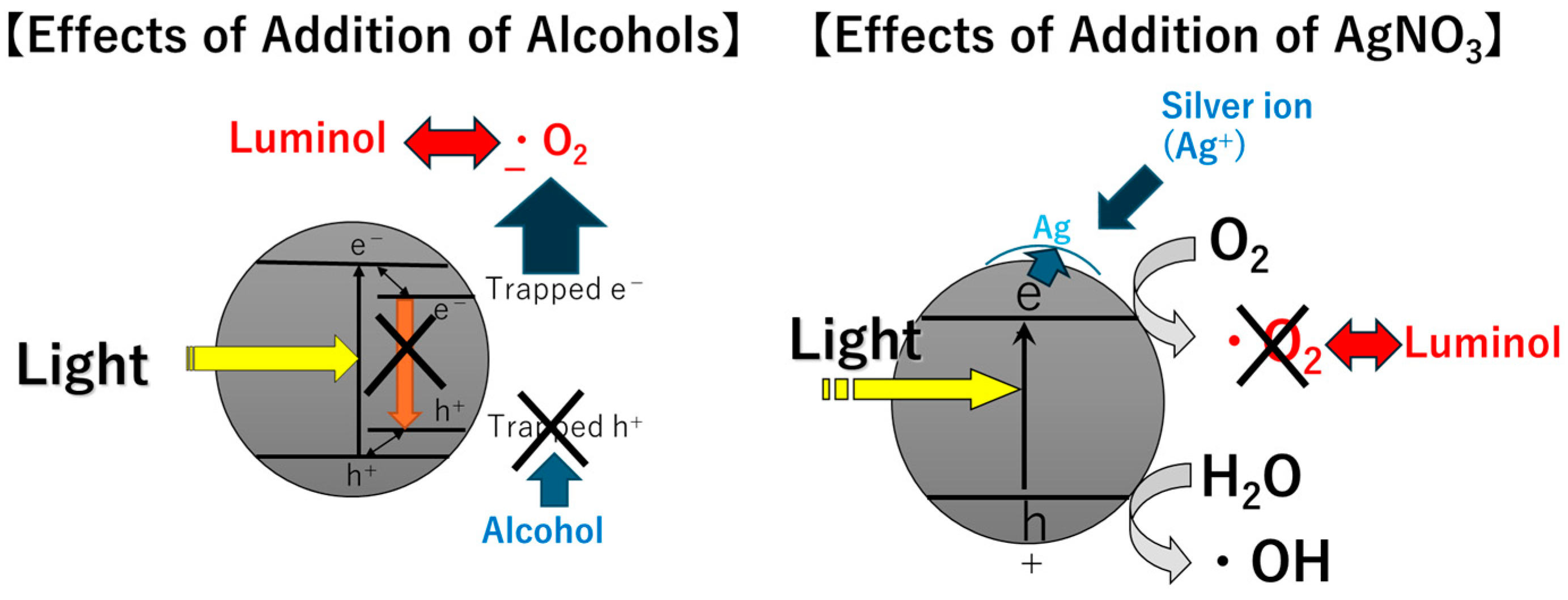
3.3. Effects of the Addition of Alcohols
3.4. Effects of the Addition of Silver Ions
3.5. Discussions
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Roda, A.; Guardigli, M. Analytical chemiluminescence and bioluminescence: Latest achievements and new horizons. Anal. Bioanal. Chem. 2022, 402, 69–76. [Google Scholar] [CrossRef]
- Garcia-Campana, A.; Lara, F.J. Trends in the analytical applications of chemiluminescence in the liquid phase. Anal. Bioanal. Chem. 2007, 387, 165–169. [Google Scholar] [CrossRef]
- Yue, L.; Liu, Y.T. Mechanistic Insight into pH-Dependent Luminol Chemiluminescence in Aqueous Solution. J. Phys. Chem. B. 2020, 124, 7682–7693. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Song, G.; Lin, J.-M. Reactive oxygen species and their chemiluminescence-detection methods. Trends Anal. Chem. 2006, 25, 985–994. [Google Scholar] [CrossRef]
- Ishibashi, K.; Fujishima, A.; Watanabe, T.; Hashimoto, K. Generation and Deactivation Processes of Superoxide Formed on TiO2 Film Illuminated by Very Weak UV Light in Air or Water. J. Phys. Chem. B 2000, 104, 4934–4938. [Google Scholar] [CrossRef]
- Hirakawa, T.; Nakaoka, Y.; Nishino, J.; Nosaka, Y. Primary Passages for Various TiO2 Photocatalysts Studied by Means of Luminol Chemiluminescent Probe. J. Phys. Chem. B 1999, 103, 4399–4403. [Google Scholar] [CrossRef]
- Shirai, N.; Matsuda, Y.; Sasaki, K. Visualization of short-lived reactive species in liquid in contact with atmospheric-pressure plasma by chemiluminescence of luminol. Appl. Phys. Express 2018, 11, 026201. [Google Scholar] [CrossRef]
- Shirai, N.; Suga, G.; Sasaki, K. Correlation between gas-phase OH density and intensity of luminol chemiluminescence in liquid interacting with atmospheric-pressure plasma. J. Phys. D Appl. Phys. 2019, 52, 39LT02. [Google Scholar] [CrossRef]
- Wu, X.-Z.; Lingyue, M.; Akiyama, K. Chemiluminescence study of active oxygen species produced by TiO2 photocatalytic reaction. Luminescence 2005, 20, 36–40. [Google Scholar] [CrossRef]
- Wu, X.-Z.; Akiyama, K.; Min, L. Time-resolved chemiluminescence of luminol induced by TiO2 photocatalytic reactions. Bull. Chem. Soc. Jpn. 2005, 78, 1149–1153. [Google Scholar] [CrossRef]
- Min, L.; Wu, X.-Z.; Tetsuya, S.; Inoue, H. Time-resolved chemiluminescence study of the TiO2 photocatalytic reaction and its induced active oxygen species. Luminescence 2007, 22, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Min, L.; Wu, X.-Z. Chemiluminescence from luminol solution after illumination of 355 nm pulse laser. Luminescence 2009, 24, 400–408. [Google Scholar] [CrossRef]
- Min, L.; Chen, X.; Wu, X.-Z. Comparison of chemiluminescence from luminol solution and luminol-TiO2 suspension after illumination of a 355 nm pulse laser. Luminescence 2010, 25, 355–359. [Google Scholar] [CrossRef]
- Malathi, A.; Madhavan, J.; Ashokkumar, M.; Arunachalam, P. A review on BiVO4 photocatalyst: Activity enhancement methods for solar photocatalytic applications. Appl. Catal. A General. 2018, 555, 47–74. [Google Scholar]
- Zhang, J.; Nosaka, Y. Generation of OH radicals and oxidation mechanism in photocatalysis of WO3 and BiVO4 powders. J. Photochem. Photobiol. A Chem. 2015, 303–304, 53–58. [Google Scholar] [CrossRef]
- Kohtani, S.; Tomohiro, M.; Tokumura, K.; Nakagaki, R. Photooxidation reactions of polycyclic aromatic hydrocarbons over pure and Ag-loaded BiVO4 photocatalysts. Appl. Catal. B Environ. 2005, 28, 265–272. [Google Scholar] [CrossRef]
- Terao, S.; Murakami, Y. Formation of OH Radicals on BiVO4–TiO2 Nanocomposite Photocatalytic Film under Visible-Light Irradiation: Roles of Photocatalytic Reduction Channels. Reactions 2024, 5, 98–110. [Google Scholar] [CrossRef]
- Vasil’ev, R.F.; Tsaplev, Y.B. Light-created chemiluminescence. Russ. Chem. Rev. 2006, 75, 989–1002. [Google Scholar] [CrossRef]
- Shi, W.; Wang, H.; Huang, Y. Luminol–silver nitrate chemiluminescence enhancement induced by cobalt ferrite nanoparticles. Luminescence 2011, 26, 547–552. [Google Scholar] [CrossRef]
- Hrakawa, T.; Yawata, K.; Nosaka, Y. Photocatalytic reactivity for •O2− and OH radical dot radical formation in anatase and rutile TiO2 suspension as the effect of H2O2 addition. Appl. Catal. A Gen. 2007, 325, 105–111. [Google Scholar] [CrossRef]
- Hirakawa, T.; Nosaka, Y. Properties of O2•− and OH• Formed in TiO2 Aqueous Suspensions by Photocatalytic Reaction and the Influence of H2O2 and Some Ions TiO2 luminol. Langumuir 2002, 18, 3247–3254. [Google Scholar] [CrossRef]
- Nosaka, Y.; Daimon, T.; Nosaka, Y.A.; Murakami, Y. Singlet oxygen formation in photocatalytic TiO2 aqueous suspension. Phys. Chem. Chem. Phys. 2004, 6, 2917–2918. [Google Scholar] [CrossRef]
- Xu, X.; Sun, Y.; Fan, Z.; Zhao, D.; Xiong, S.; Zhang, B.; Zhou, S.; Liu, G. Mechanisms for ·O2- and ·OH Production on Flowerlike BiVO4 Photocatalysis Based on Electron Spin Resonance. Front. Chem. 2018, 6, 00064. [Google Scholar] [CrossRef]
- Luo, Z.; Yan, Y.; Spinney, R.; Dionyssiou, D.D.; Villamena, F.A.; Xiao, R.; Vione, D. Environmental implications of superoxide radicals: From natural processes to engineering applications. Water Res. 2024, 261, 122023. [Google Scholar] [CrossRef]
- Daimon, T.; Nosaka, Y. Formation and Behavior of Singlet Molecular Oxygen in TiO2 Photocatalysis Studied by Detection of Near-Infrared Phosphorescence. J. Phys. Chem. C 2007, 111, 4420–4424. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamazaki, T.; Murakami, Y. Time-Resolved Chemiluminescence of Luminol Formed by 355 nm Laser-Irradiated BiVO4 Photocatalysis: Effects of the Addition of Alcohols and Ag Ions. Photochem 2024, 4, 518-526. https://doi.org/10.3390/photochem4040033
Yamazaki T, Murakami Y. Time-Resolved Chemiluminescence of Luminol Formed by 355 nm Laser-Irradiated BiVO4 Photocatalysis: Effects of the Addition of Alcohols and Ag Ions. Photochem. 2024; 4(4):518-526. https://doi.org/10.3390/photochem4040033
Chicago/Turabian StyleYamazaki, Tatsuya, and Yoshinori Murakami. 2024. "Time-Resolved Chemiluminescence of Luminol Formed by 355 nm Laser-Irradiated BiVO4 Photocatalysis: Effects of the Addition of Alcohols and Ag Ions" Photochem 4, no. 4: 518-526. https://doi.org/10.3390/photochem4040033
APA StyleYamazaki, T., & Murakami, Y. (2024). Time-Resolved Chemiluminescence of Luminol Formed by 355 nm Laser-Irradiated BiVO4 Photocatalysis: Effects of the Addition of Alcohols and Ag Ions. Photochem, 4(4), 518-526. https://doi.org/10.3390/photochem4040033