Abstract
A variety of 1-(1,4-dihydroxynaphtalen-2-yl) ketones was synthesized using the photo-Friedel–Crafts acylation of 1,4-naphthoquinone with aldehydes. Subsequent oxidation using silver oxide readily furnished the corresponding 2-acylated 1,4 naphthoquinones. Notably, these naphthoquinone derivatives underwent spontaneous partial reduction upon storage. The synthesized compounds were subjected to antimicrobial screening. High inhibition effects on Staphylococcus aureus were found for the majority of compounds, which makes them interesting for potential future medicinal applications.
1. Introduction
Acylated 1,4-naphthohydroquinones and their naphthoquinone equivalents are known for their broad biological activities (Figure 1). Compounds I and IV, for example, showed antibiotic properties [,,], while derivatives II, III and V demonstrated cytotoxic activities instead [,,].
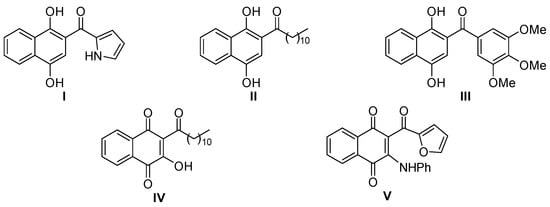
Figure 1.
Examples of bioactive acylated naphthoquinones and naphthohydroquinones.
The photochemical acylation or ‘photo-Friedel–Crafts acylation’ of quinones with aldehydes represents a rapid pathway for the construction of acylated 1,4-naphthohydroquinones [], although the term ‘photo-Friedel–Crafts’ reaction has also been suggested for other transformations [,,]. This versatile photoreaction was initially discovered in 1888, when solutions of the starting materials were exposed to natural sunlight for long periods of time []. Since its rediscovery as a simple synthesis procedure by Kraus et al. in 1992 [], several improved synthesis protocols have been developed. Friedrich and co-workers found that irradiations of 1,4-naphthoquinone with aliphatic aldehydes with UVB light significantly shortened the reaction time []. In 2011, Benites et al. described solar exposure experiments for the heteroacylation of 1,4-quinones []. A major improvement was the replacement of benzene with trifluorotoluene (TFT) as a much more sustainable solvent by Mitchell and co-workers []. Subsequent oxidation, most commonly using silver (I) oxide, readily yields the corresponding naphthoquinones []. The photoacylation of quinones is now routinely used in the discovery of biologically active compounds [,,,,,,,,]. The aim of this study was to synthesize libraries of acylated naphthohydroquinones and naphthoquinones and to study their antimicrobial activities.
2. Results and Discussion
2.1. Optimization Studies
Initially, the currently known reaction protocols were further improved in terms of their convenience in producing larger quantities of the desired acylation products. To achieve this, several parameters, i.e., choice of organic solvent, wavelength, glass type and light sources, were examined experimentally. The photoaddition of 1,4-naphthoquinone (1) with excess amounts of butyraldehyde (2a) was selected as a model system (Scheme 1), as 2a and the corresponding acylation product 3a are not prone to Norrish-type follow-up reactions under the chosen experimental conditions [,]. Irradiations were carried out in a Rayonet photochemical chamber reactor, reaction progress was monitored by TLC and conversions and compositions were determined by 1H-NMR analysis of the crude products. In line with previous observations [], no reaction was observed in the dark.
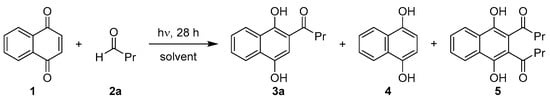
Scheme 1.
Photoacylation of 1,4-naphthoquinone with butyraldehyde as a model system for process optimization.
2.1.1. Solvent Optimization
A series of photoreactions of 1 (1 mmol) and 2a (5 mmol) in different degassed organic solvents (50 mL) was conducted with UVB light (300 ± 25 nm) in a Pyrex Schlenk flask (Table 1). In acetone, exhaustive irradiation for 28 h was necessary until TLC analysis indicated the near-complete consumption of 1 (entry 1). All subsequent irradiations were thus performed for the same duration. In most cases, the corresponding acylated naphthohydroquinone 3a was readily obtained in isolated yields of 51–76%.

Table 1.
Experimental results of the solvent optimization study (300 ± 25 nm, Pyrex, 28 h).
The experimental results revealed a dependency of chemoselectivity, i.e., photoacylation to 3a vs. photoreduction to 4, on the organic solvent used. The most selective conversions occurred in acetone, acetonitrile and trifluorotoluene (entries 1–3). In contrast, photoreduction of naphthoquinone to 4 was observed in alcoholic solvents (entries 5–7) []. In line with observations made by Mitchell et al. [], the polar photoacylation product 3a precipitated during irradiation in trifluorotoluene and was collected by successive filtrations. The product 3a, which strongly absorbs within the UVB range, was thus largely removed from the reaction mixture, hence reducing light-filtering effects, and enabling complete conversion []. The photochemical activation mode, i.e., direct excitation vs. photosensitization, also depended on the solvent system, and triplet sensitization was assumed to operate in acetone []. A similar sensitization pathway was proposed in the presence of benzophenone []. While acetone and acetonitrile produced higher yields, their flammability and toxicity make them less attractive for large-scale syntheses [].
2.1.2. Wavelength Optimization
Glass has a profound impact on light transmission []. To find the optimum wavelength for irradiation, the emission of the light sources and the glass type of the reaction vessel were thus investigated. A range of additional photoacylations involving the 1/2a model system were consequently performed for 28 h in a Pyrex (λ ≥ 300 nm) or quartz (λ ≥ 200 nm) vessel with different UV as well as visible lamps (Table 2). Almost no reaction was observed upon irradiation with visible light in TFT (entry 1), whereas irradiation with UVA light gave complete conversion and an isolated yield for 3a of 55% (entry 5). Quartz-filtered UVB light in acetone also showed photoreduction to 4 (entry 6). Likewise, irradiations with UVC light in quartz vessels furnished by-products 4 and 5 in both acetone and acetonitrile (entries 7 and 8). Bisacylation products similar to 5 have been occasionally described, but their formation remains largely unclear to this day [,].

Table 2.
Experimental results of the wavelength optimization study.
Based on these results, the optimal preparative irradiation parameters were TFT as the solvent, UVA light and Pyrex as the reaction vessel material. Under these conditions, selective and complete conversion was achieved, and the precipitated photoproduct could be easily isolated by successive filtration and subsequent drying. Although irradiation with UVB light produced a slightly higher yield of 3a, it may initiate degradation reactions for longer-chained aliphatic aldehydes or experience strong filtering for aromatic aldehydes or their respective photoacylation products, respectively [,].
2.2. Photoacylations
In order to produce adequate amounts of material for subsequent oxidations and screening in a single run, the amount of 1 was increased 7-fold and the irradiation time was shortened to 15 h. To further suppress undesired interferences, the amount of aldehyde was further reduced to 3.6 equivalents. Applying these modified conditions, 1,4-naphthoquinone (7 mmol) was irradiated in the presence of various aldehydes 2a–k (25 mmol) for 15 h in 140 mL of TFT (Scheme 2 and Table 3). In almost all cases, the desired colored photoacylation products readily precipitated and were isolated by consecutive filtration in yields of 22–57%. The collected amounts were sufficient for further investigations but may be increased upon further optimization. Irradiation with isobutyraldehyde (2d) and benzaldehyde (2h) gave rather complex mixtures, presumably due to competing photo-oxidations [], and consequently demanded purification by column chromatography instead (entries 4 and 8). All photoproducts exhibited a characteristic sharp singlet peak between 13 and 14 ppm in their 1H-NMR spectra, representing the newly formed hydroxy group at C-1 locked in an intramolecular hydrogen bond with the acyl-carbonyl group. Analysis of the liquid waste streams revealed the presence of residual photoacylation products 3 and minor by-products, particularly bisacylation products similar to 5, but no attempts were made to isolate these compounds. Importantly, no degradants from Norrish-type cleavage reactions could be detected for the aliphatic aldehydes and their photoacylation products 3a–g. Photoacylations involving benzaldehydes showed variable yields, which suggests a correlation with the stability of their respective acyl-radical intermediates [].
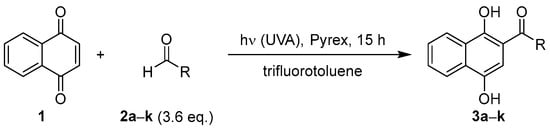
Scheme 2.
Photoacylation of 1,4-naphthoquinone with various aldehydes.

Table 3.
Experimental results of photoacylations of 1,4-naphthoquinone with various aldehydes.
Selected transformations were furthermore performed in natural sunlight [,,]. Test tubes containing degassed solutions of 1 and aldehydes 2a, e and f were illuminated for 4 days under partially sunny conditions and furnished somewhat lower yields to those obtained with artificial UVA light of 43–47%.
2.3. Oxidations
The photoacylation products 3a–j were readily oxidized with freshly prepared silver (I) oxide in diethyl ether and in the presence of sodium sulfate as a drying agent (Scheme 3) []. The acylated naphthoquinones 6a–j were obtained as colorful solids in yields of 72–98% (Table 4). The identity of the compounds was confirmed by the absence of the hydroxy-group peaks in their 1H-NMR and the presence of three carbonyl singlet peaks between 180 and 200 ppm in their 13C-NMR spectra. Upon storage, spontaneous partial reduction of the acylated naphthoquinones to their corresponding naphthohydroquinones was noticed in the solid state. All compounds were thus stored in dry amber flasks under nitrogen. Importantly, these spontaneous reductions pose a significant challenge to the usability of acylated 1,4-naphthoquinone derivatives and must be considered when synthesizing, analyzing or utilizing these compounds.
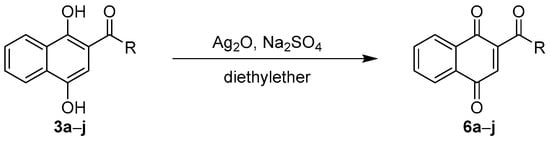
Scheme 3.
Oxidation to acylated naphthoquinones.

Table 4.
Experimental results of oxidations of acylated 1,4-naphthoquinones.
Complete reductions were also observed during GC-MS analyses and the same chromatograms and spectra to those of their corresponding photoproducts 3a–j were recorded. This thermal reduction was likely caused by the presence of water vapor as known from other MS studies of quinones [].
2.4. Antimicrobial Activity Testing
Due to the known antimicrobial activity of naphthoquinones and their derivatives, all acylated naphthohydroquinones and selected naphthoquinone analogues synthetized were subjected to antibiotic activity screening []. The disc diffusion method versus suitable control antibiotics was chosen using the following five bacteria [,]: Staphylococcus aureus and Enterococcus faecium as Gram-positive strains and Escherichia coli, Pseudomonas aeruginosa and Klebsiella pneumoniae as Gram-negative strains, respectively. Initial screening was performed with 220 μg doses to identify any hit compounds. All compounds showed strong inhibition of S. aureus, which justified further dilution studies (Table 5).

Table 5.
Antimicrobial activity of photoacylation and oxidation products against S. aureus.
At concentrations of 100 μg and 10 μg, the dodecanoyl derivatives 3g and 4g were found inactive (entries 7 and 17). After a further reduction to 5 μg, compounds 3j, 6a, 6c and 6f also failed to show any activity (entries 10, 12, 14 and 16). For all other bacteria screened, isolated weak to moderate activities were observed for some of the compounds. In particular, the short-chained derivatives 3a, 3b, 3e, 6a and 6c also inhibited E. faecium and E. coli. Only naphthoquinone 6c additionally inhibited K. pneumoniae, while naphthohydroquinones 3c and 3j showed moderate activities against E. faecium as well. In contrast, none of the substances tested showed any activity against P. aeruginosa. These results confirm the potential of acylated naphthohydroquinones, in particular, to inhibit bacterial growth. However, more research needs to be conducted to gain a deeper understanding of their efficacy and suitability as antibiotics.
3. Materials and Methods
3.1. General Information
All chemicals were purchased from Sigma-Aldrich or Carl Roth and were used as received. Irradiation experiments were carried out in a Rayonet RPR-200 photochemical chamber reactor (Southern New England Ultraviolet Company, Branford, CT, USA) equipped with 16 × 8 W UVA (350 ± 25 nm), UVB (300 ± 25 nm), visible light (cool white, 400–700 nm) or UVC (254 nm) fluorescent or germicidal tubes. Pyrex (λ ≥ 300 nm) or quartz (λ ≥ 200 nm) Schlenk flasks with capacities of 60 and 180 mL were used as reaction vessels. A cold finger was inserted into the flask to maintain the reaction temperature below 25 °C. The reaction mixtures were degassed with N2 through a sidearm for approx. 5 min before capping the reaction vessel. Photoreactions were monitored by thin-layer chromatography (TLC) or 1H-NMR spectroscopy. Solar exposures were conducted in Pyrex test tubes at Building A of Hochschule Fresenius in Idstein, Germany (50°22′ N, 8°27′ E), in May 2024.
3.2. Photoacylations
3.2.1. General Procedure for Photoacylations with Artificial Light
A solution of 1,4 naphthoquinone (1, 7 mmol) and aldehyde (2a–k, 25 mmol) in 140 mL of trifluorotoluene was prepared in a Pyrex Schlenk flask. The mixture was degassed for 5 min with nitrogen and irradiated for 15 h with UVA light (16 × 8 W Ushio F8T5BL, Tokyo, Japan). Any precipitated photoproduct 3a–k was filtered off and the liquid filtrate was evaporated to dryness. The semisolid to oily residue was sonicate with little cyclohexane until a precipitation was formed. After resting, the solid was filtered off. The trituration process was repeated until no more precipitate was obtained. The combined solid material was dried in vacuum. When no precipitation was formed, the crude reaction mixture was evaporated to dryness and the oily residues were subjected to column chromatography using a mixture of cyclohexane and ethyl acetate (4:1) as mobile phase.
3.2.2. General Procedure for Solar Photoacylations in Sunlight
A solution of 1,4 naphthoquinone (1, 6 mmol) and aldehyde (2a, e or f, 25 mmol) in 120 mL of trifluorotoluene was spread over 6 Pyrex test tubes. Each solution was degassed with nitrogen for 5 min, the tubes were capped and exposed to sunlight for 4 days. In all three cases, precipitates formed during illumination. The products were isolated by successive filtration as described above.
All photoacylation products 3a–k are known and their spectroscopic details match previously described data [,,,,]. Characteristic spectroscopic details of 3a–k are compiled in the Supplementary Materials.
3.3. Oxidations
3.3.1. Synthesis of Silver (I) Oxide []
A solution of 10 g of sodium hydroxide in 100 mL of hot water was added to a solution of 30 g of silver nitrate in 100 mL of hot water. The brown precipitate of silver (I) oxide was washed with 5 × 50 mL of warm water and once with 50 mL of ethanol by decantation. The solid was filtered off, washed with ethanol and dried under vacuum.
3.3.2. General Procedure for Oxidation
A solution of 3a–j (1.8 mmol) in dry diethyl ether (50 mL) was prepared in a flask covered with aluminum foil. Silver (I) oxide (3 mmol) and anhydrous sodium sulfate (25 mmol) were added, and the suspension was stirred rapidly overnight. The slurry was subsequently filtered over a pad of Celite® and the liquid filtrate was evaporated to dryness to obtain compounds 6a–j as colorful solids.
All oxidation products 6a–j are known and their spectroscopic data match previously reported data [,,,,]. Characteristic spectroscopic details of 6a–j can be found in the Supplementary Materials.
3.4. Antimicrobial Activity Testing
General Procedure for Bioscreening
A stock solution was prepared by dissolving the selected compound in 1 mL of ultrapure acetone in a plastic Eppendorf tube (using a sterilized pipette tip). Agar plates were then prepared, and the bacteria—Escherichia coli, Staphylococcus aureus, Enterococcus faecium, Klebsiella pneumoniae or Pseudomonas aeruginosa—were applied via the spread plate technique. A total of 20 µL of the stock solutions were applied to small, sterile filter discs. Negative control discs were prepared by pipetting 20 µL of acetone onto the respective filter discs. All discs were left to dry in labelled glass Petri dishes with their lids on. Once the filter discs were dry and the bacteria suspension had settled into the agar, the discs were placed onto the plates using sterilized tweezers. Each plate consisted of four ‘test discs’, one negative control disc and one antibiotic disc (positive control). Fosfomycin (200 µg), vancomycin (30 µg) and nalidixic acid (30 µg) discs were used as reference antibiotics on S. aureus, E. faecium and E. coli and K. pneumoniae and P. aeruginosa, respectively.
4. Conclusions
In conclusion, a library of 2-acylated 1,4-hydroxynaphthoquinones was generated in moderate yields via the photo-Friedel–Crafts acylation of 1,4-naphthoquinone and readily available aldehydes. The easy procedure makes this methodology attractive for scale-up and examples of technical-scale photoacylations in concentrated sunlight have already been reported [,]. Subsequent thermal oxidation furnished the corresponding 2-acylated 1,4-naphthoquinones in high to excellent yields. As both processes have been separately realized under continuous-flow conditions [,,], they may be subsequently combined in series through telescoping []. Antibiotic screening conducted with most of the compounds synthetized revealed strong activity against S. aureus, justifying further medicinal chemistry studies.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/photochem4040031/s1, General methods, experimental setups and spectroscopic data. Figure S1. UVA photoacylations in the Rayonet reactor prior to (left) and after irradiation (center and right). Figure S2. Solar photoacylations prior to (left) and after (center and right) exposure.
Author Contributions
A.M. (Alexis Mercier), A.M. (Alizée Monet) and M.A.Y. conducted the research and collected the data; A.M. (Alexis Mercier) drafted the manuscript; M.I.H. supervised and assisted with the biological screening; M.O. secured the funding, supervised the chemical research and wrote the final manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Hochschule Fresenius through the Forschungsförderung 1 program 2023.
Data Availability Statement
All data is available on request from the corresponding author.
Acknowledgments
A.Me. thanks the Franco-German Youth Office OFAJ-DFJW for a scholarship, A.Mo. thanks the ERASMUS+ program for a Student Mobility of Traineeship grant and M.A.Y. thanks the Ministry of Higher Education and Scientific Research of Iraq for a Ph.D. scholarship. The authors thank Ferdinand Friedrichs, Delphine Nayrat, Mario Funke, Marco Bernhard and Maximilian Greif for their technical support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hase, J.; Nishimura, T. Antibacterial Properties of Naphthoquinones. I. Syntheses and Antibacterial Properties of Acylnaphthoquinones. J. Pharm. Soc. Jpn. 1955, 75, 203–207. [Google Scholar] [CrossRef]
- Hase, J.; Nishimura, T. Antibacterial Properties of Naphthoquinones. II. Syntheses and Antibacterial Properties of Acylnaphthoquinones. J. Pharm. Soc. Jpn. 1955, 75, 207–209. [Google Scholar] [CrossRef][Green Version]
- Araya, G.; Benites, J.; Reyes, J.S.; Marcoleta, A.E.; Valderrama, J.A.; Lagos, R.; Monasterio, O. Inhibition of Escherichia coli and Bacillus subtilis FtsZ Polymerization and Bacillus subtilis Growth by Dihydroxynaphtyl Aryl Ketones. Front. Microbiol. 2019, 10, 1225. [Google Scholar] [CrossRef]
- Pedroza, D.A.; De Leon, F.; Varela-Ramirez, A.; Lema, C.; Aguilera, R.J.; Mito, S. The cytotoxic Effect of 2-Acylated-1,4-naphthohydroquinones on Leukemia/Lymphoma Cells. Bioorg. Med. Chem. 2014, 22, 842–847. [Google Scholar] [CrossRef]
- Benites, J.; Valderrama, J.A.; Ríos, D.; Lagos, R.; Monasterio, O.; Calderon, P.B. Inhibition of Cancer Cell Growth and Migration by Dihydroxynaphthyl Aryl Ketones. Mol. Cell. Toxicol. 2016, 12, 237–242. [Google Scholar] [CrossRef]
- Benites, J.; Valderrama, J.A.; Contreras, Á.; Enríquez, C.; Pino-Rios, R.; Yáñez, O.; Calderon, P.B. Discovery of New 2-Phenylamino-3-acyl-1,4-naphthoquinones as Inhibitors of Cancer Cells Proliferation: Searching for Intra-Cellular Targets Playing a Role in Cancer Cells Survival. Molecules 2023, 28, 4323. [Google Scholar] [CrossRef]
- Oelgemöller, M.; Mattay, J. The “Photochemical Friedel-Crafts Acylation” of Quinones: From the Beginnings of Organic Photochemistry to Modern Solar Chemical Applications. In CRC Handbook of Organic Photochemistry and Photobiology, 2nd ed.; Horspool, W.M., Lenci, F., Eds.; CRC Press: Boca Raton, FL, USA, 2004; Chapter 88; pp. 1–45. [Google Scholar] [CrossRef]
- Yonemitsu, O. Electron Transfer Photochemistry. Yakugaku Zasshi—J. Pharm. Soc. Jpn. 1982, 102, 716–734. [Google Scholar] [CrossRef][Green Version]
- Martens, J.; Praefcke, K.; Schulze, U. Intramolekulare Photo-Friedel-Crafts-Reaktionen; eine neues Syntheseprinzip für Heterocyclen. Synthesis 1976, 532–533. [Google Scholar] [CrossRef]
- Bryce-Smith, D.; Deshpande, R.; Gilbert, A.; Grzonka, J. Acid-catalysis of Photochemical Reactions. Chem. Commun. 1970, 561–562. [Google Scholar] [CrossRef]
- Klinger, H. Ueber die Einwirkung des Sonnenlichts auf organische Verbindungen. Justus Liebigs Ann. Chem. 1888, 249, 137–146. [Google Scholar] [CrossRef]
- Kraus, G.A.; Kirihara, M. Quinone Photochemistry. A General Synthesis of Acylhydroquinones. J. Org. Chem. 1992, 57, 3256–3257. [Google Scholar] [CrossRef]
- Friedrichs, F.; Murphy, B.; Nayrat, D.; Ahner, T.; Funke, M.; Ryan, M.; Lex, J.; Mattay, J.; Oelgemöller, M. An improved Procedure for the Photoacylation of 1,4-Naphthoquinone with Aliphatic Aldehydes. Synlett 2008, 3137–3140. [Google Scholar] [CrossRef]
- Benites, J.; Rios, D.; Díaz, P.; Valderrama, J.A. The Solar-chemical Photo-Friedel–Crafts Heteroacylation of 1,4-Quinones. Tetrahedron Lett. 2011, 52, 609–611. [Google Scholar] [CrossRef]
- Mitchell, L.J.; Lewis, W.; Moody, C.J. Solar Photochemistry: Optimisation of the Photo Friedel–Crafts Acylation of Naphthoquinones. Green Chem. 2013, 15, 2830–2842. [Google Scholar] [CrossRef]
- Spruit, C.J.P. Carbonyl-substituted Naphthoquinones. Part I. Methyl Ketones Unsubstituted in the Side Chain. Recl. Trav. Chim. Pays-Bas 1947, 66, 655–672. [Google Scholar] [CrossRef]
- Arenas, P.; Peña, A.; Ríos, D.; Benites, J.; Muccioli, G.G.; Calderon, P.B.; Valderrama, J.A. Eco-Friendly Synthesis and Antiproliferative Evaluation of Some Oxygen Substituted Diaryl Ketones. Molecules 2013, 18, 9818–9832. [Google Scholar] [CrossRef]
- Benites, J.; Valderrama, J.A.; Ramos, M.; Valenzuela, M.; Guerrero-Castilla, A.; Muccioli, G.G.; Calderon, P.B. Half-Wave Potentials and In Vitro Cytotoxic Evaluation of 3-Acylated 2,5-Bis(phenylamino)-1,4-benzoquinones on Cancer Cells. Molecules 2019, 24, 1780. [Google Scholar] [CrossRef]
- Gutierrez, E.; Benites, J.; Valderrama, J.A.; Calderon, P.B.; Verrax, J.; Nova, E.; Villanelo, F.; Maturana, D.; Escobar, C.; Lagos, R.; et al. Binding of Dihydroxynaphthyl Aryl Ketones to Tubulin Colchicine Site Inhibits Microtubule Assembly. Biochem. Biophys. Res. Commun. 2015, 466, 418–425. [Google Scholar] [CrossRef]
- Kraus, G.A.; Mengwasser, J. Quinones as Key Intermediates in Natural Products Synthesis. Syntheses of Bioactive Xanthones from Hypericum perforatum. Molecules 2009, 14, 2857–2861. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Idhayadhulla, A.; Lee, Y.R.; Wee, Y.-J.; Kim, S.H. Anti-tyrosinase, Antioxidant, and Antibacterial Activities of Novel 5-Hydroxy-4-acetyl-2,3-dihydronaphtho[1,2-b]furans. Eur. J. Med. Chem. 2014, 86, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Majhi, S. Applications of Norrish Type I and II Reactions in the total Synthesis of Natural Products: A Review. Photochem. Photobiol. Sci. 2021, 20, 1357–1378. [Google Scholar] [CrossRef]
- Albini, A. Norrish’ Type I and II Reactions and their Role in the Building of Photochemical Science. Photochem. Photobiol. Sci. 2021, 20, 161–181. [Google Scholar] [CrossRef]
- Leshina, T.; Polyakov, N. The Mechanism of Photoreduction of Quinones by Alcohols from Proton CIDNP Data in high and low Magnetic Fields. J. Phys. Chem. 1990, 94, 4379–4382. [Google Scholar] [CrossRef]
- Maruyama, K.; Miyagi, Y. Photo-induced Condensation Reaction of p-Quinones with Aldehydes. Bull. Chem. Soc. Jpn. 1974, 47, 1303–1304. [Google Scholar] [CrossRef]
- Bunce, N.J.; Ridley, J.E.; Zerner, M.C. On the Excited States of p-Quinones and an Interpretation of the Photocycloaddition of p-Quinones to Alkenes. Theor. Chim. Acta 1977, 45, 283–300. [Google Scholar] [CrossRef]
- Kraus, G.A.; Liu, P. Benzophenone-Mediated Conjugate Additions of Aromatic Aldehydes to Quinones. Tetrahedron Lett. 1994, 35, 7723–7726. [Google Scholar] [CrossRef]
- Capello, C.; Fischer, U.; Hungerbühler, K. What is a Green Solvent? A Comprehensive Framework for the Environmental Assessment of Solvents. Green Chem. 2007, 9, 927–934. [Google Scholar] [CrossRef]
- Montalti, M.; Credi, A.; Prodi, L.; Gandolfi, M.T. Light Sources and Filters. In Handbook of Photochemistry, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2006; Chapter 11b; pp. 595–600. [Google Scholar] [CrossRef]
- Oelgemöller, M.; Schiel, C.; Fröhlich, R.; Mattay, J. The “Photo-Friedel-Crafts Acylation” of 1,4-Naphthoquinones. Eur. J. Org. Chem. 2002, 2465–2474. [Google Scholar] [CrossRef]
- Zhu, L.; Tang, Y.; Chen, Y.; Cronin, T. Wavelength-dependent Photolysis of C3-C7 Aldehydes in the 280–330 nm Region. Spectrosc. Lett. 2009, 42, 467–478. [Google Scholar] [CrossRef]
- Meng, Q.-X.; Sakaguchi, Y.; Hayashi, H. Effects of Substitution and Excited Wavelength on the Photochemistry of Benzaldehydes studied by CIDEP. Mol. Phys. 1997, 90, 15–23. [Google Scholar] [CrossRef]
- Vanoye, L.; Favre-Réguillon, A.; Aloui, A.; Philippe, R.; de Bellefon, C. Insights in the Aerobic Oxidation of Aldehydes. RSC Adv. 2013, 3, 18931–18937. [Google Scholar] [CrossRef]
- Vinogradov, M.G.; Nikishin, G.I. The Chemistry of Acyl Radicals in Solution. Russ. Chem. Rev. 1971, 40, 916–932. [Google Scholar] [CrossRef]
- De Leon, F.; Kalagara, S.; Navarro, A.A.; Mito, S. Synthesis of 6-Acyl-5,8-quinolinediols by Photo-Friedel–Crafts Acylation using Sunlight. Tetrahedron Lett. 2013, 54, 3147–3149. [Google Scholar] [CrossRef]
- Zeller, K.-P. Mass Spectra of Quinones. In The Chemistry of the Quinonoid Compounds Part 1; Patai, S., Ed.; John Wiley & Sons Ltd.: London, UK, 1974; Chapter 5; pp. 231–256. [Google Scholar] [CrossRef]
- Mone, N.S.; Bhagwat, S.A.; Sharma, D.; Chaskar, M.; Patil, R.H.; Zamboni, P.; Nawani, N.N.; Satpute, S.K. Naphthoquinones and Their Derivatives: Emerging Trends in Combating Microbial Pathogens. Coatings 2021, 11, 434. [Google Scholar] [CrossRef]
- Cushnie, T.P.T.; Cushnie, B.; Echeverría, J.; Fowsantear, W.; Thammawat, S.; Dodgson, J.L.A.; Law, S.; Clow, S.M. Bioprospecting for Antibacterial Drugs: A Multidisciplinary Perspective on Natural Product Source Material, Bioassay Selection and avoidable Pitfalls. Pharm. Res. 2020, 37, 125. [Google Scholar] [CrossRef]
- Kemp, A.; Durand, M.; Wall, D.; Szieber, P.; Hermanns, M.I.; Oelgemöller, M. Synthesis of 1H-Isoindolin-1-ones via a Simple Photodecarboxylative Addition and Evaluation of their Antibiotic Activity. Photochem. Photobiol. Sci. 2024, 23, 1353–1360. [Google Scholar] [CrossRef]
- Jha, R.K.; Upadhyay, A.; Kanika Jain, S.; KA, N.; Kumar, S. Light-Driven Carbon−Carbon Coupling of α-sp3−CH of Aliphatic Alcohols with sp2−CH Bond of 1,4-Naphthoquinones. Org. Lett. 2022, 24, 7605–7610. [Google Scholar] [CrossRef]
- Yaseen, M.A.; Mumtaz, S.; Hunter, R.L.; Wall, D.; Belluau, V.; Robertson, M.J.; Oelgemöller, M. Continuous-Flow Photochemical Transformations of 1,4-Naphthoquinones and Phthalimides in a Concentrating Solar Trough Reactor. Aust. J. Chem. 2020, 73, 1149–1157, Erratum in: Aust. J. Chem. 2020, 73, 1301. [Google Scholar] [CrossRef]
- Helferich, B.; Klein, W. Zur Synthese von Disacchariden IV. Zwei Tetra-acetyl-β-d-glucosen. Liebigs Ann. Chem. 1926, 450, 219–229. [Google Scholar] [CrossRef]
- Green, I.R. The Synthesis of 2-Acetyl-1,4-naphthoquinone: A Multi-step Synthesis. J. Chem. Educ. 1982, 59, 698–699. [Google Scholar] [CrossRef]
- Sunassee, S.N.; Veale, C.G.L.; Shunmoogam-Gounden, N.; Osoniyi, O.; Hendricks, D.T.; Caira, M.R.; de la Mare, J.-A.; Edkins, A.L.; Pinto, A.V.; da Silva, E.N., Jr.; et al. Cytotoxicity of Lapachol, β-Lapachone and Related Synthetic 1,4-Naphthoquinones Against Oesophageal Cancer Cells. Eur. J. Med. Chem. 2013, 62, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Schiel, C.; Oelgemöller, M.; Mattay, J. Photoacylation of Electron rich Quinones: An Application of the Photo-Friedel-Crafts Reaction. Synthesis 2001, 1275–1279. [Google Scholar] [CrossRef]
- Schiel, C.; Oelgemöller, M.; Ortner, J.; Mattay, J. Green Photochemistry: The Solar-chemical Photo-Friedel-Crafts Acylation of Quinones. Green Chem. 2001, 3, 224–228. [Google Scholar] [CrossRef]
- Khan, H.; Rajesh, V.M.; Ravva, M.K.; Sen, S. Optimization of Blue LED Photo-Flow Synthesis in Continuous Flow Reactors Using Design of Experiments (DoE): Efficient Synthesis of Diverse Diaryl Ketones. Chem. Eng. J. 2024, 501, 157657. [Google Scholar] [CrossRef]
- Derikvand, F.; Bigi, F.; Maggi, R.; Piscopo, C.G.; Sartori, G. Oxidation of Hydroquinones to Benzoquinones with Hydrogen Peroxide using Catalytic Amount of Silver Oxide under Batch and Continuous-flow Conditions. J. Catal. 2010, 271, 99–103. [Google Scholar] [CrossRef]
- Otake, Y.; Nakamura, H.; Fuse, S. Recent Advances in the Integrated Micro-flow Synthesis Containing Photochemical Reactions. Tetrahedron Lett. 2018, 59, 1691–1697. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).