Physicochemical Properties of Tungsten Trioxide Photoanodes Fabricated by Wet Coating of Soluble, Particulate, and Mixed Precursors
Abstract
1. Introduction
1.1. Tungsten(VI) Oxide Layers Prepared by Top-Down Approach
1.2. Tungsten(VI) Oxide Layers by Bottom-Up Approach
1.3. Fabrication of WO3 Layers by Brick-and-Mortar Approach
1.4. Wet Coating Techniques–Deposition of Liquid Formulations onto Substrates
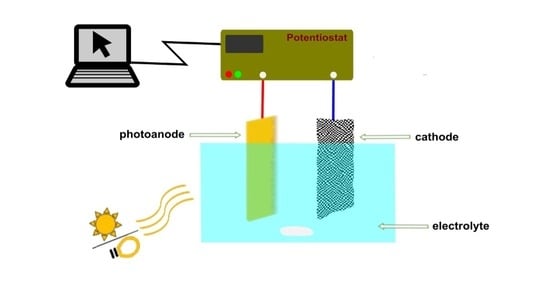
2. Materials and Methods
2.1. Materials
- Tungsten(VI) oxide, Sigma Aldrich, Billerica, MA, USA;
- Isobutyl alcohol p.a, Penta, Prague, Czech Republic;
- Tungstic acid (99%), Sigma Aldrich, Billerica, MA, USA;
- Tungsten fine powder, Sigma Aldrich, St. Louis, MO, USA;
- Hydrogen peroxide (15%), Penta, Prague, Czech Republic;
- Distilled water;
- Acetic acid (98%), Chemapol, Prague, Czech Republic;
- Dowanol® PM (1-methoxy-2-propanol, ≥99.5%), P–LAB, Prague, Czech Republic;
- Neodisher® LM3, Dr. Weigert, Hamburg, Germany;
- Perchloric acid (60% solution), Sigma Aldrich, Billerica, MA, USA;
- Hexanol (98%), Chemapol, Prague, Czech Republic;
- Ethanol, (absolute), Sigma Aldrich, Billerica, MA, USA;
- Isopropyl alcohol (≥98%), Sigma Aldrich, Billerica, MA, USA;
- Glycerol, Penta, Prague, Czech Republic;
- Tap water;
- Acetylated peroxotungstic acid (APTA).
2.2. Preparation of Precursor Stock Solution/Dispersions/Mixture
2.2.1. WO3 Nanoparticles Stock Dispersion:
2.2.2. APTA Stock Solution
2.2.3. WO3-APTA Stock Mixture
2.3. Coating
2.3.1. Substrate Preparation
2.3.2. Inkjet Printing
- Particulate ink: The coating ink was made by mixing 2 mL of each 20% WO3 stock dispersion and hexanol.
- Soluble precursor ink: The coating ink was made by mixing 20% APTA stock solution (0.6 g of powdered APTA in 2.4 g of isobutanol) in 1 mL of hexanol.
- Brick-and-mortar ink: The coating ink was made by mixing the WO3-APTA stock mixture and hexanol in a 1:1 ratio. For each prepared formulation, 2 mL was taken and injected into the cartridge. Then, two layers of the composition (1 cm2) were printed on the prepared glass substrates.
2.3.3. Meyer Rod Coating
2.4. Post-Deposition Treatment
2.5. Characterization
3. Results
3.1. Thermogravimetric Analysis
3.2. X-ray Diffraction
3.3. Inkjet Printer Sample Series
3.3.1. Particulate Ink


3.3.2. Soluble Precursor Ink


3.3.3. Brick-and-Mortar Ink


3.4. Meyer Rod-Coated Sample Series
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deng, Y.; Zhao, R. Advanced Oxidation Processes (AOPs) in Wastewater Treatment. Curr. Pollut. Rep. 2015, 1, 167–176. [Google Scholar] [CrossRef]
- Gautam, P.; Popat, A.; Lokhandwala, S. Advances & Trends in Advance Oxidation Processes and Their Applications. In Advanced Industrial Wastewater Treatment and Reclamation of Water: Comparative Study of Water Pollution Index during Pre-industrial, Industrial Period and Prospect of Wastewater Treatment for Water Resource Conservation; Roy, S., Garg, A., Garg, S., Tran, T.A., Eds.; Environmental Science and Engineering; Springer International Publishing: Cham, Switzwerland, 2022; pp. 45–69. ISBN 9783030838119. [Google Scholar]
- Coha, M.; Farinelli, G.; Tiraferri, A.; Minella, M.; Vione, D. Advanced Oxidation Processes in the Removal of Organic Substances from Produced Water: Potential, Configurations, and Research Needs. Chem. Eng. J. 2021, 414, 128668. [Google Scholar] [CrossRef]
- Dewil, R.; Mantzavinos, D.; Poulios, I.; Rodrigo, M.A. New Perspectives for Advanced Oxidation Processes. J. Environ. Manag. 2017, 195, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Cardenas, J.A.; Esteban-García, B.; Agüera, A.; Sánchez-Pérez, J.A.; Manzano-Agugliaro, F. Wastewater Treatment by Advanced Oxidation Process and Their Worldwide Research Trends. Int. J. Environ. Res. Public Health 2019, 17, 170. [Google Scholar] [CrossRef]
- Atalay, S.; Ersöz, G. Chapter 7—Hybrid Application of Advanced Oxidation Processes to Dyes’ Removal. In Green Chemistry and Water Remediation: Research and Applications; Sharma, S.K., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 209–238. [Google Scholar]
- Ma, D.; Yi, H.; Lai, C.; Liu, X.; Huo, X.; An, Z.; Li, L.; Fu, Y.; Li, B.; Zhang, M.; et al. Critical Review of Advanced Oxidation Processes in Organic Wastewater Treatment. Chemosphere 2021, 275, 130104. [Google Scholar] [CrossRef] [PubMed]
- Miklos, D.; Remy, C.; Jekel, M.; Linden, K.G.; Drewes, J.E.; Hübner, U. Evaluation of Advanced Oxidation Processes for Water and Wastewater Treatment—A Critical Review. Water Res. 2018, 139, 118–131. [Google Scholar] [CrossRef]
- Feijoo, S.; Yu, X.; Kamali, M.; Appels, L.; Dewil, R. Generation of Oxidative Radicals by Advanced Oxidation Processes (AOPs) in Wastewater Treatment: A Mechanistic, Environmental and Economic Review. Rev. Environ. Sci. Technol. 2023, 22, 205–248. [Google Scholar] [CrossRef]
- Moreira, F.C.; Boaventura, R.A.R.; Brillas, E.; Vilar, V.J.P. Electrochemical Advanced Oxidation Processes: A Review on Their Application to Synthetic and Real Wastewaters. Appl. Catal. B Environ. 2017, 202, 217–261. [Google Scholar] [CrossRef]
- Swaminathan, M.; Muruganandham, M.; Sillanpää, M. Advanced Oxidation Processes for Wastewater Treatment. Int. J. Photoenergy 2013, 2013, 683682. [Google Scholar] [CrossRef]
- Matilainen, A.; Sillanpää, M. Removal of Natural Organic Matter from Drinking Water by Advanced Oxidation Processes. Chemosphere 2010, 80, 351–365. [Google Scholar] [CrossRef]
- Stefan, M.I. (Ed.) Advanced Oxidation Processes for Water Treatment: Fundamentals and Applications; IWA Publishing: London, UK, 2018; ISBN 9781780407197. [Google Scholar]
- Zhang, X.; Yu, W.; Guo, Y.; Li, S.; Chen, Y.; Wang, H.; Bian, Z. Recent Advances in Photoelectrocatalytic Advanced Oxidation Processes: From Mechanism Understanding to Catalyst Design and Actual Applications. Chem. Eng. J. 2023, 455, 140801. [Google Scholar] [CrossRef]
- Zawadzki, P. Visible Light–Driven Advanced Oxidation Processes to Remove Emerging Contaminants from Water and Wastewater: A Review. Water Air Soil Pollut. 2022, 233, 374. [Google Scholar] [CrossRef]
- Oturan, M.A.; Aaron, J.-J. Advanced Oxidation Processes in Water/Wastewater Treatment: Principles and Applications. A Review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 2577–2641. [Google Scholar] [CrossRef]
- Muruganandham, M.; Suri, R.; Jafari, S.; Sillanpää, M.; Lee, G.; Wu, J.J.; Swaminathan, M. Recent Developments in Homogeneous Advanced Oxidation Processes for Water and Wastewater Treatment. Int. J. Photoenergy 2014, 2014, 821674. [Google Scholar] [CrossRef]
- Babu, D.S.; Srivastava, V.; Nidheesh, P.V.; Kumar, M.S. Detoxification of Water and Wastewater by Advanced Oxidation Processes. Sci. Total Environ. 2019, 696, 133961. [Google Scholar] [CrossRef]
- Chaplin, B.P. Critical Review of Electrochemical Advanced Oxidation Processes for Water Treatment Applications. Environ. Sci. Process. Impacts 2014, 16, 1182–1203. [Google Scholar] [CrossRef] [PubMed]
- Macwan, D.P.; Dave, P.N.; Chaturvedi, S. A Review on Nano-TiO2 Sol–Gel Type Syntheses and Its Applications. J. Mater. Sci. 2011, 46, 3669–3686. [Google Scholar] [CrossRef]
- Jia, Z.; La, L.B.T.; Zhang, W.C.; Liang, S.-X.; Jiang, B.; Xie, S.K.; Habibi, D.; Zhang, L.C. Strong Enhancement on Dye Photocatalytic Degradation by Ball-Milled TiO2: A Study of Cationic and Anionic Dyes. J. Mater. Sci. Technol. 2017, 33, 856–863. [Google Scholar] [CrossRef]
- Strieth-Kalthoff, F.; James, M.J.; Teders, M.; Pitzer, L.; Glorius, F. Energy Transfer Catalysis Mediated by Visible Light: Principles, Applications, Directions. Chem. Soc. Rev. 2018, 47, 7190–7202. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, P.G.V.; González, M.O.A.; de Oliveira Ferreira, P.; da Cunha Jácome Vidal, P.; Pereira, J.P.P.; Ferreira, H.R.; Oprime, P.C. Overview of Printing and Coating Techniques in the Production of Organic Photovoltaic Cells. Int. J. Energy Res. 2020, 44, 9912–9931. [Google Scholar] [CrossRef]
- Veprek, S.; Veprek-Heijman, M.G.J.; Karvankova, P.; Prochazka, J. Different Approaches to Superhard Coatings and Nanocomposites. Thin Solid Films 2005, 476, 1–29. [Google Scholar] [CrossRef]
- Camacho, S.Y.T.; Rey, A.M.; Hernández-Alonso, M.D.; Llorca, J.; Medina, F.; Contreras, S. Pd/TiO2-WO3 Photocatalysts for Hydrogen Generation from Water-Methanol Mixtures. Appl. Surf. Sci. 2018, 455, 570–580. [Google Scholar] [CrossRef]
- Ouyang, W.; Kuna, E.; Yepez, A.; Balu, A.M.; Romero, A.A.; Colmenares, J.C.; Luque, R. Mechanochemical Synthesis of TiO2 Nanocomposites as Photocatalysts for Benzyl Alcohol Photo-Oxidation. Nanomaterials 2016, 6, 93. [Google Scholar] [CrossRef] [PubMed]
- Shabdan, Y.; Markhabayeva, A.; Bakranov, N.; Nuraje, N. Photoactive Tungsten-Oxide Nanomaterials for Water-Splitting. Nanomaterials 2020, 10, 1871. [Google Scholar] [CrossRef] [PubMed]
- Coronado, J.M. A Historical Introduction to Photocatalysis. In Design of Advanced Photocatalytic Materials for Energy and Environmental Applications; Coronado, J.M., Fresno, F., Hernández-Alonso, M.D., Portela, R., Eds.; Springer: London, UK, 2013; pp. 1–4. ISBN 9781447150602. [Google Scholar]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Lee, J.-Y.; An, J.; Chua, C.K. Fundamentals and Applications of 3D Printing for Novel Materials. Appl. Mater. Today 2017, 7, 120–133. [Google Scholar] [CrossRef]
- Homola, T.; Ďurašová, Z.; Shekargoftar, M.; Souček, P.; Dzik, P. Optimization of TiO2 Mesoporous Photoanodes Prepared by Inkjet Printing and Low-Temperature Plasma Processing. Plasma Chem. Plasma Process. 2020, 40, 1311–1330. [Google Scholar] [CrossRef]
- Vidmar, T.; Topič, M.; Dzik, P.; Opara Krašovec, U. Inkjet Printing of Sol–Gel Derived Tungsten Oxide Inks. Sol. Energy Mater. Sol. Cells 2014, 125, 87–95. [Google Scholar] [CrossRef]
- Kumar, M.; Xiong, X.; Wan, Z.; Sun, Y.; Tsang, D.C.W.; Gupta, J.; Gao, B.; Cao, X.; Tang, J.; Ok, Y.S. Ball Milling as a Mechanochemical Technology for Fabrication of Novel Biochar Nanomaterials. Bioresour. Technol. 2020, 312, 123613. [Google Scholar] [CrossRef]
- Xu, C.; Sudipta, D.; De, S.; Balu, A.M.; Ojeda, M.; Luque, R. Mechanochemical Synthesis of Advanced Nanomaterials for Catalytic Applications. Chem. Commun. 2015, 51, 6698–6713. [Google Scholar] [CrossRef]
- Laranjo, M.T.; Ricardi, N.C.; Arenas, L.T.; Benvenutti, E.V.; de Oliveira, M.C.; Buchner, S.; Santos, M.J.L.; Costa, T.M.H. Influence of Ball Milling on Textural and Morphological Properties of TiO2 and TiO2/SiO2 Xerogel Powders Applied in Photoanodes for Solar Cells. J. Solid State Electrochem. 2016, 20, 1731–1741. [Google Scholar] [CrossRef]
- Gies, M.; Michel, F.; Lupó, C.; Schlettwein, D.; Becker, M.; Polity, A. Electrochromic Switching of Tungsten Oxide Films Grown by Reactive Ion-Beam Sputter Deposition. J. Mater. Sci. 2021, 56, 615–628. [Google Scholar] [CrossRef]
- Sheng, Y.; Yang, J.; Wang, F.; Liu, L.; Liu, H.; Yan, C.; Guo, Z. Sol-Gel Synthesized Hexagonal Boron Nitride/Titania Nanocomposites with Enhanced Photocatalytic Activity. Appl. Surf. Sci. 2019, 465, 154–163. [Google Scholar] [CrossRef]
- Saleem, Z.; Pervaiz, E.; Yousaf, M.U.; Niazi, M.B.K. Two-Dimensional Materials and Composites as Potential Water Splitting Photocatalysts: A Review. Catalysts 2020, 10, 464. [Google Scholar] [CrossRef]
- de Moura, D.S.; Pazinato, J.C.O.; Pereira, M.B.; Mertins, O.; Silva, E.R.; Garcia, I.T.S. Poly(Vinyl Alcohol) as a Structuring Agent for Peroxotungstic Acid. J. Mol. Liq. 2018, 269, 92–100. [Google Scholar] [CrossRef]
- Fang, Y.; Sun, X.; Cao, H. Influence of PEG Additive and Annealing Temperature on Structural and Electrochromic Properties of Sol–Gel Derived WO3 Films. J. Sol-Gel Sci. Technol. 2011, 59, 145–152. [Google Scholar] [CrossRef]
- Işık, D.; Ak, M.; Durucan, C. Structural, Electrochemical and Optical Comparisons of Tungsten Oxide Coatings Derived from Tungsten Powder-Based Sols. Thin Solid Films 2009, 518, 104–111. [Google Scholar] [CrossRef]
- Zhang, Y.; Yuan, J.; Le, J.; Song, L.; Hu, X. Structural and Electrochromic Properties of Tungsten Oxide Prepared by Surfactant-Assisted Process. Sol. Energy Mater. Sol. Cells 2009, 93, 1338–1344. [Google Scholar] [CrossRef]
- Nakajima, T.; Hagino, A.; Nakamura, T.; Tsuchiya, T.; Sayama, K. WO3 Nanosponge Photoanodes with High Applied Bias Photon-to-Current Efficiency for Solar Hydrogen and Peroxydisulfate Production. J. Mater. Chem. A Mater. Energy Sustain. 2016, 4, 17809–17818. [Google Scholar] [CrossRef]
- Ojeda, M.; Gaster, C.; Harris, C. Fabrication and Internal Functionalization of Highly Macroporous WO3 Thin Films. Preprints 2018, 2018100227. [Google Scholar] [CrossRef]
- Sadale, S.B.; Neumann-Spallart, M. Drop-Cast Tungsten Trioxide Semiconducting Films in Photoelectrocatalysis. J. Electroanal. Chem. 2020, 877, 114502. [Google Scholar] [CrossRef]
- Wang, P.; Wang, S.; Wang, H.; Wu, Z.; Wang, L. Recent Progress on Photo-Electrocatalytic Reduction of Carbon Dioxide. Part. Part. Syst. Charact. 2018, 35, 1700371. [Google Scholar] [CrossRef]
- Trojanowicz, M. Impact of Nanotechnology on Design of Advanced Screen-Printed Electrodes for Different Analytical Applications. Trends Anal. Chem. 2016, 84, 22–47. [Google Scholar] [CrossRef]
- Suresh, R.R.; Lakshmanakumar, M.; Arockia Jayalatha, J.B.B.; Rajan, K.S.; Sethuraman, S.; Krishnan, U.M.; Rayappan, J.B.B. Fabrication of Screen-Printed Electrodes: Opportunities and Challenges. J. Mater. Sci. 2021, 56, 8951–9006. [Google Scholar] [CrossRef]
- Rong, Y.; Ming, Y.; Ji, W.; Li, D.; Mei, A.; Hu, Y.; Han, H. Toward Industrial-Scale Production of Perovskite Solar Cells: Screen Printing, Slot-Die Coating, and Emerging Techniques. J. Phys. Chem. Lett. 2018, 9, 2707–2713. [Google Scholar] [CrossRef]
- Adly, N.; Bachmann, B.; Krause, K.J.; Offenhäusser, A.; Wolfrum, B.; Yakushenko, A. Three-Dimensional Inkjet-Printed Redox Cycling Sensor. RSC Adv. 2017, 7, 5473–5479. [Google Scholar] [CrossRef]
- Bachalo, W.D.; Bachalo, W.D. Measurements of Inkjet Droplet Size, Velocity, and Angle of Trajectory. In Inkjet Printing in Industry: Materials, Technologies, Systems, and Applications; Wiley: Hoboken, NJ, USA, 2022. [Google Scholar] [CrossRef]
- Cai, G.; Darmawan, P.; Cui, M.; Chen, J.; Wang, X.; Eh, A.L.-S.; Magdassi, S.; Lee, P.S. Inkjet-Printed All Solid-State Electrochromic Devices Based on NiO/WO3 Nanoparticle Complementary Electrodes. Nanoscale 2016, 8, 348–357. [Google Scholar] [CrossRef]
- Calvert, P. Inkjet Printing for Materials and Devices. Chem. Mater. 2001, 13, 3299–3305. [Google Scholar] [CrossRef]
- Cummins, G.; Desmulliez, M.P.Y. Inkjet Printing of Conductive Materials: A Review. Circuit World 2012, 38, 193–213. [Google Scholar] [CrossRef]
- da Costa, C.H.; Costa, C.; Pinheiro, C.B.; da Silva Henriques, I.D.; Laia, C.A.T. Inkjet Printing of Sol-Gel Synthesized Hydrated Tungsten Oxide Nanoparticles for Flexible Electrochromic Devices. ACS Appl. Mater. Interfaces 2012, 4, 1330–1340. [Google Scholar] [CrossRef]
- Hansora, D.; Cherian, D.; Mehrotra, R.; Jang, J.-W.; Lee, J.S. Fully Inkjet-Printed Large-Scale Photoelectrodes. Joule 2023, 7, 884–919. [Google Scholar] [CrossRef]
- Hui, R.; Wang, Z.; Yick, S.; Maric, R.; Ghosh, D. Fabrication of Ceramic Films for Solid Oxide Fuel Cells via Slurry Spin Coating Technique. J. Power Sources 2007, 172, 840–844. [Google Scholar] [CrossRef]
- Deepa, M.; Saxena, T.K.; Singh, D.P.; Sood, K.N.; Agnihotry, S.A. Spin Coated versus Dip Coated Electrochromic Tungsten Oxide Films: Structure, Morphology, Optical and Electrochemical Properties. Electrochim. Acta 2006, 51, 1974–1989. [Google Scholar] [CrossRef]
- Shendage, S.S.; Patil, V.L.; Vanalakar, S.A.; Patil, S.P.; Bhosale, J.L.; Kim, J.H.; Patil, P.S. Characterization and Gas Sensing Properties of Spin Coated WO3 Thin Films. Z. Phys. Chem. 2020, 234, 1819–1834. [Google Scholar] [CrossRef]
- Go, M.; Alam, A.; Choie, H.K.; Zhong, Z.; Lee, K.H.; Seo, Y.; Hwang, B.; Woo, K.; Kim, T.-W.; Lim, S. Meyer-Rod Coated 2D Single-Crystalline Copper Nanoplate Film with Intensive Pulsed Light for Flexible Electrode. Coat. World 2020, 10, 88. [Google Scholar] [CrossRef]
- Jung, M.; Kim, J.; Koo, H.; Lee, W.; Subramanian, V.; Cho, G. Roll-to-Roll Gravure with Nanomaterials for Printing Smart Packaging. J. Nanosci. Nanotechnol. 2014, 14, 1303–1317. [Google Scholar] [CrossRef]
- Bragg, L. The Diffraction of X-Rays. Br. J. Radiol. 1956, 29, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Schülli, T.U.; Leake, S.J. X-ray Nanobeam Diffraction Imaging of Materials. Curr. Opin. Solid State Mater. Sci. 2018, 22, 188–201. [Google Scholar] [CrossRef]
- Bunaciu, A.A.; Udriştioiu, E.G.; Aboul-Enein, H.Y. X-ray Diffraction: Instrumentation and Applications. Crit. Rev. Anal. Chem. 2015, 45, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Lider, V.V. X-ray Diffraction Topography Methods (Review). Phys. Solid State 2021, 63, 189–214. [Google Scholar] [CrossRef]
- Reimer, L. Scanning Electron Microscopy, 2nd ed.; Springer Series in Optical Sciences; Springer: Berlin/Heidelberg, Germany, 2013; ISBN 9783540639763. [Google Scholar]
- Pan, H.; Heagy, M.D. Bicarbonate Reduction with Semiconductor Photocatalysts: Study of Effect of Positive Hole Scavengers. MRS Commun. 2018, 8, 1173–1177. [Google Scholar] [CrossRef]
- Karimi Estahbanati, M.R.; Feilizadeh, M.; Attar, F.; Iliuta, M.C. Current Developments and Future Trends in Photocatalytic Glycerol Valorization: Process Analysis. React. Chem. Eng. 2021, 6, 197–219. [Google Scholar] [CrossRef]







| S. No | Technique | Advantages | Disadvantages |
|---|---|---|---|
| 1. | Screen Printer | Low cost [47,48] | One of the principal drawbacks of this method is the lack of flexibility in modifying the morphology and film thickness [49]. |
| 2. | Inkjet Printer | Inkjet printing offers several advantages, including non-contact, maskless, and combinatorial processing. It also consumes minimal materials and generates minimal waste [50,51]. | Nozzle clogging, wetting behaviour, and film homogeneity [52,53,54,55,56]. |
| 3. | Spin Coater | A primary factor contributing to the popularity of spin coating is its ease of handling and rapid processing [57]. | The utilization of spin-coating for automated fabrication is not feasible and lacks the capability to pattern substrates selectively. Furthermore, it has high material waste consumption [58,59]. |
| 4. | Meyer rod | The technique referred to as bar coating does not involve any additional processes such as pre-patterning of the substrate. Its purpose is to achieve a uniform, homogeneous coating with efficient processing [60]. | The thickness of the laminating layer varies and depends on the range of possible bar diameters [61]. |
| S. No | Type of Stock Mixture | Crystalline Phase | ||
|---|---|---|---|---|
| Monoclinic | Monoclinic Epsilon | Anorthic | ||
| 1. | WO3 raw | +++ * | + * | + |
| 2. | WO3 ground | ++ * | + | +++ |
| 3. | WO3 ground + fired at 300 °C | ++ | ++ | + |
| 4. | APTA fired at 500 °C | +++ | ||
| 5. | WO3 ground + APTA fired at 400 °C | +++ | + | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chittoory, V.K.L.R.; Filipsika, M.; Bartoš, R.; Králová, M.; Dzik, P. Physicochemical Properties of Tungsten Trioxide Photoanodes Fabricated by Wet Coating of Soluble, Particulate, and Mixed Precursors. Photochem 2024, 4, 111-127. https://doi.org/10.3390/photochem4010006
Chittoory VKLR, Filipsika M, Bartoš R, Králová M, Dzik P. Physicochemical Properties of Tungsten Trioxide Photoanodes Fabricated by Wet Coating of Soluble, Particulate, and Mixed Precursors. Photochem. 2024; 4(1):111-127. https://doi.org/10.3390/photochem4010006
Chicago/Turabian StyleChittoory, Valli Kamala Laxmi Ramya, Marketa Filipsika, Radim Bartoš, Marcela Králová, and Petr Dzik. 2024. "Physicochemical Properties of Tungsten Trioxide Photoanodes Fabricated by Wet Coating of Soluble, Particulate, and Mixed Precursors" Photochem 4, no. 1: 111-127. https://doi.org/10.3390/photochem4010006
APA StyleChittoory, V. K. L. R., Filipsika, M., Bartoš, R., Králová, M., & Dzik, P. (2024). Physicochemical Properties of Tungsten Trioxide Photoanodes Fabricated by Wet Coating of Soluble, Particulate, and Mixed Precursors. Photochem, 4(1), 111-127. https://doi.org/10.3390/photochem4010006