Infrared Spectrum and UV-Induced Photochemistry of Matrix-Isolated Phenyl 1-Hydroxy-2-Naphthoate
Abstract
:1. Introduction
2. Experimental Details: Matrix Isolation Infrared and Photochemical Experiments
3. Computational Details
4. Results and Discussion
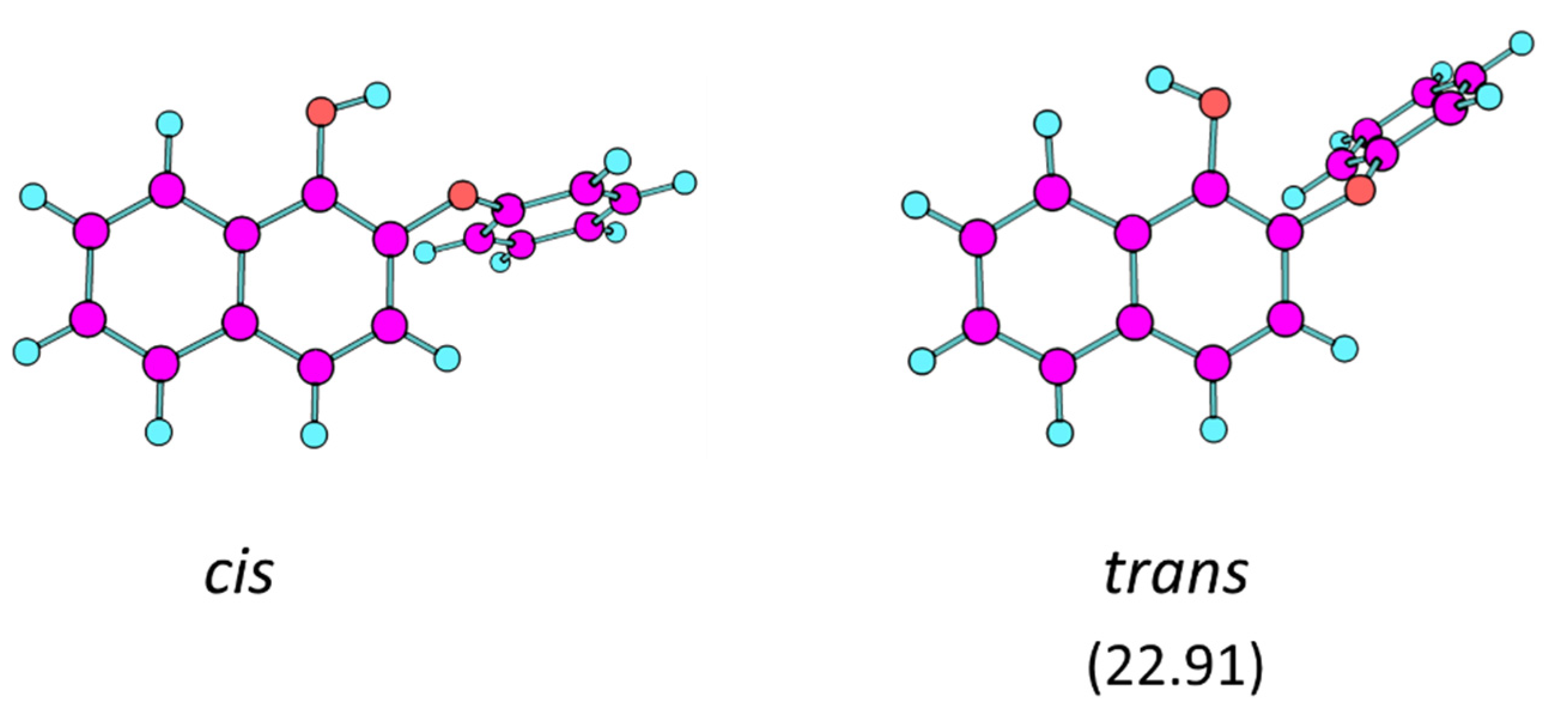
4.1. Geometries and Relative Energies of the PHN Conformers
4.2. Infrared Spectra of Matrix-Isolated PHN
4.2.1. 3500–2900 cm−1 Region
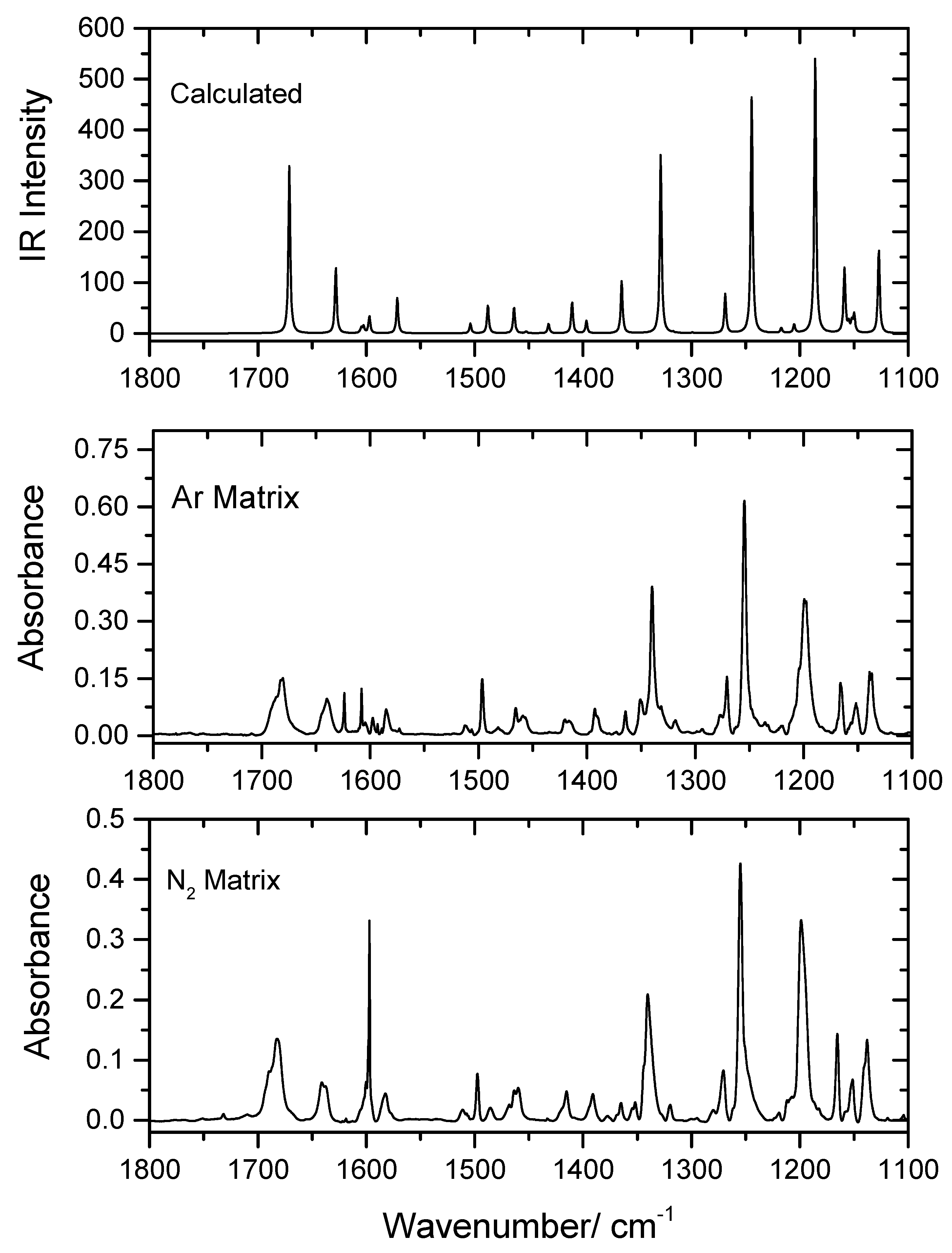
4.2.2. 1800–1100 cm−1 Region
4.2.3. 1100–450 cm−1 Region
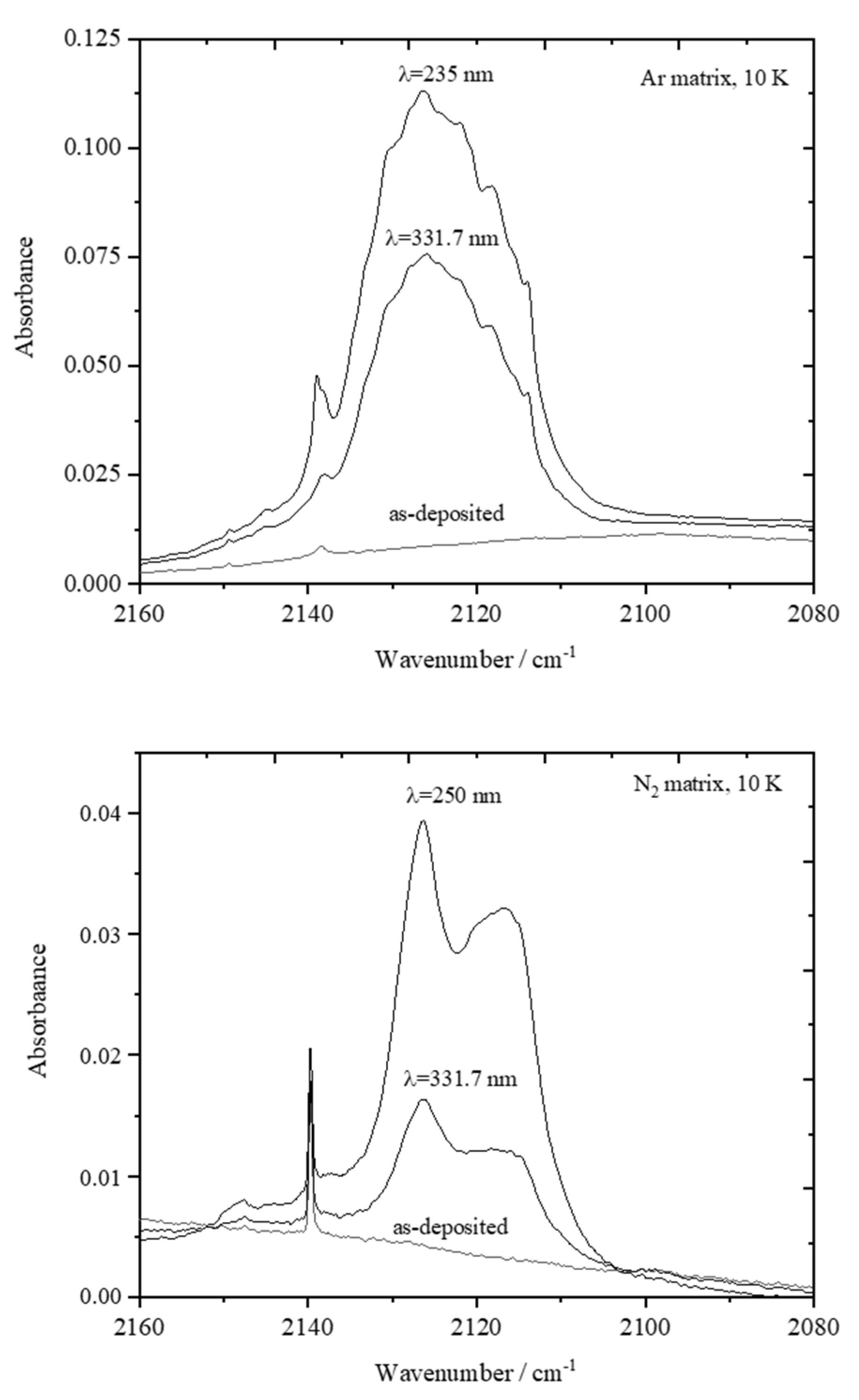
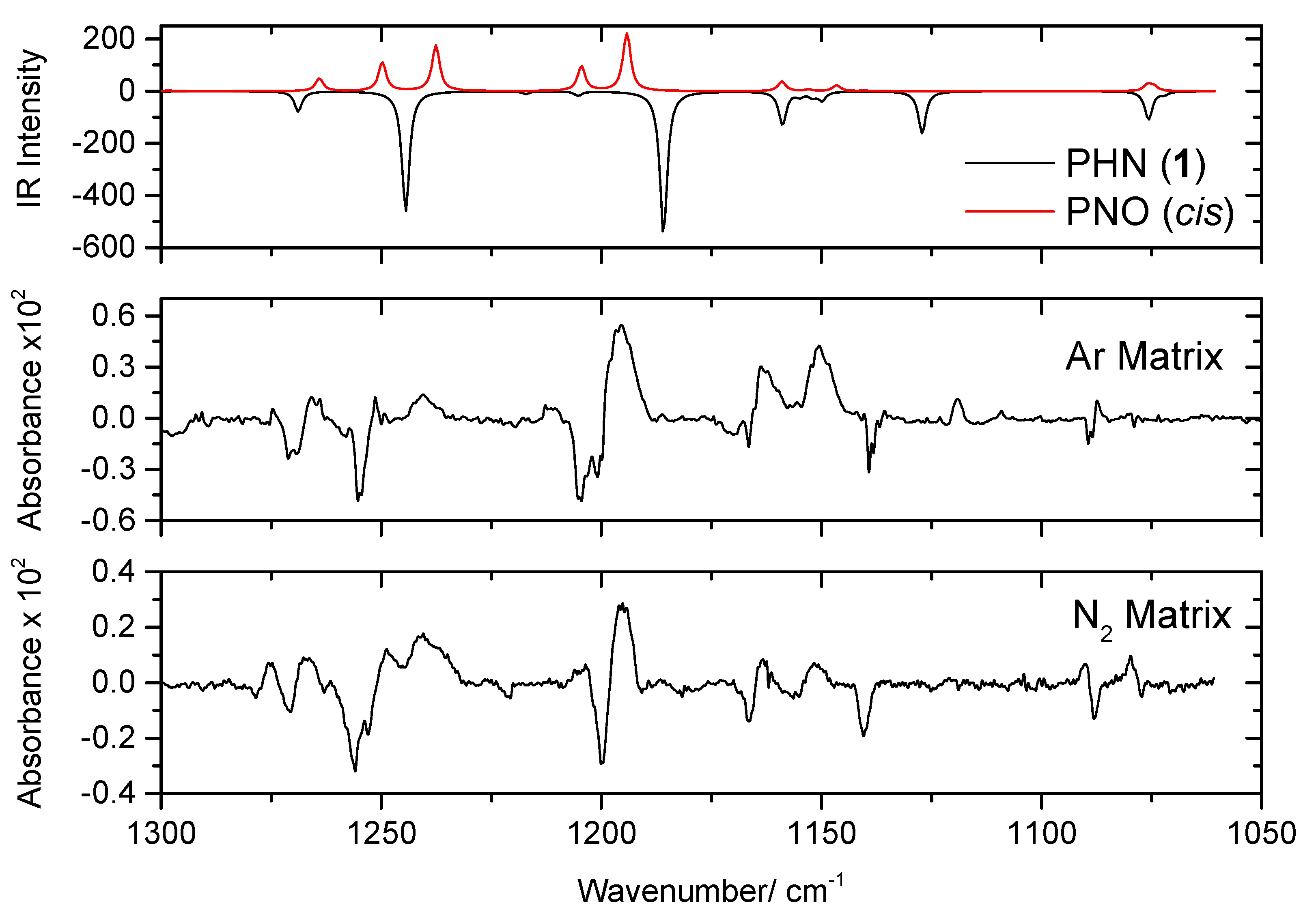
4.3. Narrowband UV-Induced Decarbonylation of PHN
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, J.-Y.; Jin, H.; Wang, G.-F.; Yu, P.-J.; Wu, S.-Y.; Zhu, Z.-G.; Li, Z.-H.; Tian, Y.-X.; Xu, W.; Zhang, J.-J.; et al. Liposomal Curcumin Targeting Endometrial Cancer Through the NF-κB Pathway. Inflamm. Res. 2011, 60, 851–859. [Google Scholar] [CrossRef]
- Morand, E.F.; Iskander, M.N. Napththalene Derivatives Which Inhibit the Cytokine or Biological Activity of Macrophage Migration Inhibitory Factor (mif). PCT International Application 2003. WO 2003104178A1, 18 December 2003. [Google Scholar]
- Baboshin, M.A.; Golovleva, L.A. Increase of 1-Hydroxy-2-naphthoic Acid Concentration as a Cause of Temporary Cessation of Growth for Arthrobacter sp. K3: Kinetic Analysis. Microbiology 2009, 78, 180–186. [Google Scholar] [CrossRef]
- Sastry, M.N.V.; Claessens, S.; Habonimana, P.P.; De Kimpe, N. Synthesis of the Natural Products 3-Hydroxymollugin and 3-Methoxymollugin. J. Org. Chem. 2010, 75, 2274–2280. [Google Scholar] [CrossRef]
- Meyers, A.I.; Avila, W.B. Chemistry of Aryloxazolines. Applications to the Synthesis of Lignan Lactone Derivatives. J. Org. Chem. 1981, 46, 3881–3886. [Google Scholar] [CrossRef]
- Zjawiony, J.; Peterson, J.R. An Improved Synthesis of Naphtho[2,3-d]-1,3-dioxole-5-methoxy-6-carboxylic acid. Org. Prep. Proced. Int. 1991, 23, 163–172. [Google Scholar] [CrossRef]
- Hauser, F.M.; Rhee, R.P. Syntheses of .alpha.- and .beta.-Sorigenin Methyl Ethers. J. Org. Chem. 1977, 42, 4155–4157. [Google Scholar] [CrossRef]
- Franck, R.W.; Bhat, V.; Subramaniam, C.S. The Sstereoselective Total Synthesis of the Natural Enantiomer of Olivin Trimethyl Ether. J. Am. Chem. Soc. 1986, 108, 2455–2457. [Google Scholar] [CrossRef]
- Woolfe, G.J.; Thistlethwaite, P.J. Excited-state Prototropic Reactivity in Salicylamide and Salicylanilide. J. Am. Chem. Soc. 1980, 102, 6917–6923. [Google Scholar] [CrossRef]
- Woolfe, G.J.; Thistlethwaite, P.J. Excited-state Prototropism in Esters of o-Hydroxy-2-naphthoic Acids. J. Am. Chem. Soc. 1981, 103, 3849–3854. [Google Scholar] [CrossRef]
- Law, K.Y.; Shoham, J. Photoinduced Proton Transfers in Methyl Salicylate and Methyl 2-hydroxy-3-naphthoate. J. Phys. Chem. 1994, 98, 3114–3120. [Google Scholar] [CrossRef]
- Bergmann, E.D.; Hirshberg, Y.; Pinchas, S. Ultra-violet Spectrum and Constitution of 3-Hydroxy-2-naphthoic Acid and Related Compounds. J. Chem. Soc. 1950, 2351–2356. [Google Scholar] [CrossRef]
- Naboikin, U.V.; Zadorozhnyi, B.A.; Pavlova, E.N. Peculiarities of the Luminescence of Ortho-Disubstituted Aromatic Hydrocarbons: II. Opt. Spectrosc. (Eng. Transl.) 1959, 6, 312. [Google Scholar]
- McCarthy, A.; Ruth, A.A. Fluorescence Excitation and Excited State Intramolecular Relaxation Dynamics of Jet-cooled Methyl-2-hydroxy-3-naphthoate. Chem. Phys. 2013, 425, 177–184. [Google Scholar] [CrossRef]
- Catalán, J.; del Valle, J.C.; Palomar, J.; Díaz, C.; de Paz, J.L.G. The Six-Membered Intramolecular Hydrogen Bond Position as a Switch for Inducing an Excited State Intramolecular Proton Transfer (ESIPT) in Esters of o-Hydroxynaphthoic Acids. J. Phys. Chem. A 1999, 103, 10921–10934. [Google Scholar] [CrossRef]
- Tobita, S.; Yamamoto, M.; Kurahayashi, N.; Tsukagoshi, R.; Nakamura, Y.; Shizuka, H. Effects of Electronic Structures on the Excited-state Intramolecular Proton Transfer of 1-Hydroxy-2-acetonaphthone and Related Compounds. J. Phys. Chem. A 1998, 102, 5206–5214. [Google Scholar] [CrossRef]
- Sıdır, İ.; Gülseven Sıdır, Y. Solvatochromism and Intramolecular Hydrogen-bonding Assisted Dipole Moment of Phenyl 1-Hydroxy-2-naphthoate in the Ground and Excited States. J. Mol. Liq. 2016, 221, 972–985. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09, Revision A.02; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Becke, A.D. Density-functional Exchange-energy Approximation with Correct Asymptotic Behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Lee, C.T.; Yang, W.T.; Parr, R.G. Development of the Colle-Salvetti Correlation-energy Formula into a Functional of the Electron Density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vosko, S.H.; Wilk, L.; Nusair, M. Accurate Spin-dependent Electron Liquid Correlation Energies for Local Spin Density Calculations: A Critical Analysis. Can. J. Phys. 1980, 58, 1200–1211. [Google Scholar] [CrossRef] [Green Version]
- McLean, A.D.; Chandler, G.S. Contracted Gaussian Basis Sets for Molecular Calculations. I. Second Row Atoms, Z=11–18. J. Chem. Phys. 1980, 72, 5639–5648. [Google Scholar] [CrossRef]
- Dennington, R.; Keith, T.; Milam, J. GaussView (Version 5.0); Semichem Inc.: Shawnee Mission, KS, USA, 2009. [Google Scholar]
- Fausto, R.; Batista de Carvalho, L.A.E.; Teixeira-Dias, J.J.C. Conformational Analysis of Carbonyl and Thiocarbonyl Ethyl Esters: The HC(=X)Y-CH2CH3 (X, Y = O or S) Internal Rotation. J. Comput. Chem. 1992, 13, 799–809. [Google Scholar] [CrossRef]
- Lopes, S.; Nikitin, T.; Fausto, R. Structural, Spectroscopic, and Photochemical Study of Ethyl Propiolate Isolated in Cryogenic Argon and Nitrogen Matrices. Spectrochim. Acta A 2020, 241, 118670. [Google Scholar] [CrossRef] [PubMed]
- Reva, I.D.; Stepanian, S.; Adamowicz, L.; Fausto, R. Combined FTIR Matrix Isolation and Ab Initio Studies of Pyruvic Acid: Proof for Existence of the Second Conformer. J. Phys. Chem. A 2001, 105, 4773–4780. [Google Scholar] [CrossRef] [Green Version]
- Apóstolo, R.F.G.; Bento, R.F.; Tarczay, G.; Fausto, R. The First Experimental Observation of the Higher-Energy Trans Conformer of Trifuoroacetic Acid. J. Mol. Struct. 2016, 1125, 288–295. [Google Scholar] [CrossRef]
- Avadanei, M.; Cozan, V.; Kuş, N.; Fausto, R. Structure and Photochemistry of N-Salicylidene-p-carboxyaniline Isolated in Solid Argon. J. Phys. Chem. A 2015, 119, 9121–9132. [Google Scholar] [CrossRef]
- Maçôas, E.M.S.; Fausto, R.; Lundell, J.; Pettersson, M.; Khriachtchev, L.; Räsänen, M. Conformational Analysis and Near-infrared-induced Rotamerization of Malonic Acid in an Argon Matrix. J. Phys. Chem. A 2000, 104, 11725–11732. [Google Scholar] [CrossRef] [Green Version]
- Beć, K.B.; Grabska, J.; Ozaki, Y.; Hawranek, J.P.; Huck, C.W. Influence of Non-fundamental Modes on Mid-infrared Spectra: Anharmonic DFT Study of Aliphatic Ethers. J. Phys. Chem. A 2017, 121, 1412–1424. [Google Scholar] [CrossRef]
- Wu, L.; Lambo, R.; Tan, Y.; Liu, A.-W.; Hu, S.-M. Infrared Spectroscopy of CO Isolated in Solid Nitrogen Matrix. Chin. J. Chem. Phys. 2014, 27, 5–8. [Google Scholar] [CrossRef] [Green Version]
- Jarmelo, S.; Reva, I.D.; Lapinski, L.; Nowak, M.J.; Fausto, R. Matrix-isolated Diglycolic Anhydride: Vibrational Spectra and Photochemical Reactivity. J. Phys. Chem. A 2008, 112, 11178–11189. [Google Scholar] [CrossRef] [Green Version]
- Giuliano, B.M.; Reva, I.; Fausto, R. Infrared Spectra and Photochemistry of Matrix-isolated Pyrrole-2-carbaldehyde. J. Phys. Chem. A 2010, 114, 2506–2517. [Google Scholar] [CrossRef] [Green Version]
- Breda, S.; Reva, I.; Fausto, R. UV-induced Unimolecular Photochemistry of Diketene Isolated in Cryogenic Inert Matrices. J. Phys. Chem. A 2012, 116, 2131–2140. [Google Scholar] [CrossRef] [PubMed]
- Lapinski, L.; Reva, I.; Gerega, A.; Nowak, M.J.; Fausto, R. UV-induced Transformations of Matrix-isolated 6-Azacytosine. J. Chem. Phys. 2018, 149, 104301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
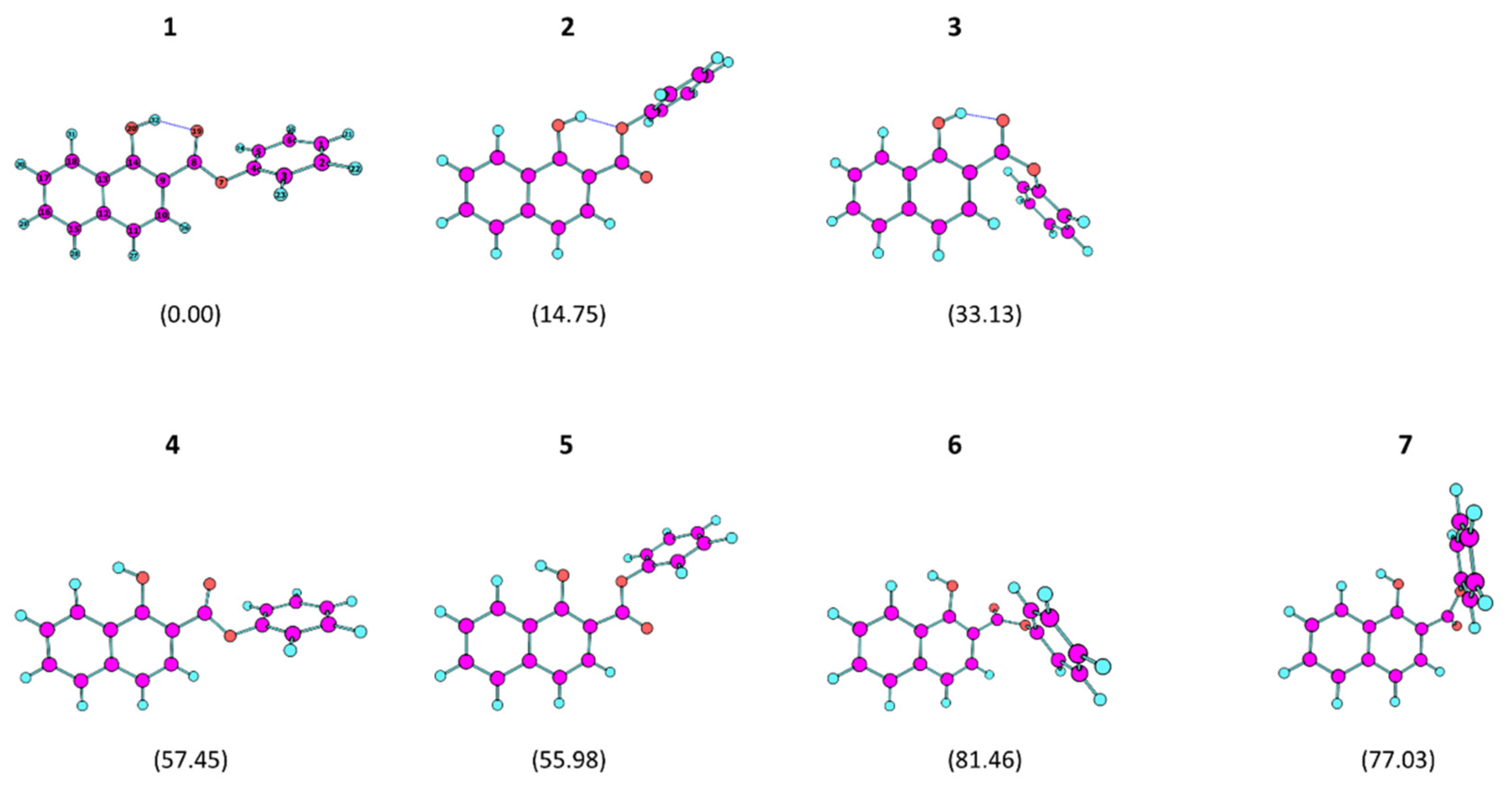

| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
|---|---|---|---|---|---|---|---|
| ΔE | 0.00 | 14.75 | 33.13 | 57.45 | 55.98 | 81.46 | 77.03 |
| ΔE(0) | 0.00 | 14.41 | 32.35 | 54.14 | 53.04 | 77.36 | 73.15 |
| ΔG° | 0.00 | 13.02 | 33.53 | 49.65 | 49.48 | 75.72 | 73.19 |
| p(298.15) | 99.5 | 0.5 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| μ | 1.75 | 1.25 | 3.80 | 3.41 | 3.89 | 5.55 | 6.17 |
| Dihedral Angle b | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| C–C–O–H | −0.1 | 0.1 | −3.4 | −172.5 | −171.1 | 164.3 | −170.6 |
| O=C–C–C | −0.2 | −179.8 | 6.6 | −10.8 | 162.5 | −59.6 | −132.9 |
| C–O–C=O | −0.7 | 0.7 | −118.2 | −2.1 | −3.3 | 157.1 | −153.1 |
| C–C–O–C | −68.8 | 76.6 | 25.7 | −63.7 | −57.5 | −52.3 | 44.6 |
| Conformer | C–O (Naphtol) | O–H | H…O | C=O | C–O (Carboxylic) | O…O | <(O–H…O) |
|---|---|---|---|---|---|---|---|
| 1 | 1.339 | 0.986 | 1.715 | 1.224 | 1.358 | 2.593 | 146.2 |
| 2 | 1.347 | 0.973 | 1.751 | 1.201 | 1.397 | 2.597 | 143.2 |
| 3 | 1.336 | 0.987 | 1.700 | 1.221 | 1.377 | 2.579 | 146.2 |
| Experimental | Calculated (Form 1) | |||
|---|---|---|---|---|
| Ar Matrix | N2 Matrix | νb | IIR | Approximate Description c |
| 3435/3375 | 3429/3375 | 3314 | 476.4 | νO–H |
| 3166 | 3167 | 3141 | 3.3 | νC–H ring2 |
| 3138 | 5.0 | νC–H ring3 | ||
| n.obs. | n.obs. | 3133 | 1.3 | νC–H ring1 |
| 3124 | 3131 | 3128 | 7.1 | νC–H ring1 |
| 3119 | 22.0 | νC–H ring1 | ||
| 3097 | 3102 | 3117 | 21.8 | νC–H ring3 |
| 3085 | 3087 | 3109 | 7.8 | νC–H ring1 |
| 3074 | 3078 | 3107 | 15.8 | νC–H ring2 |
| 3105 | 4.0 | νC–H ring3 | ||
| 3100 | 0.4 | νC–H ring1 | ||
| n.obs. | n.obs. | 3093 | 0.4 | νC–H ring3 |
| 1691/1686/1682/1680 | 1693/1689/1682/1681 | 1671 | 329.1 | νC=O |
| 1644/1639 | 1641/1638 | 1628 | 131.5 | ν rings2-3, δO–H |
| 1610 | 1619 | 1605 | 10.4 | ν rings2-3 |
| 1605 | 1606 | 1602 | 12.8 | ν ring1 |
| 1598 | 1600 | 1597 | 33.6 | ν ring1 |
| 1585 | 1582 | 1571 | 69.9 | ν rings2-3, δO–H |
| 1512/1506 | 1511/1507 | 1504 | 19.0 | ν rings2-3, δC–H rings2-3 |
| 1496 | 1498 | 1488 | 55.5 | ν ring1, δC–H ring1 |
| 1465 | 1469/1464 | 1464 | 51.6 | ν rings2-3, δC–H rings2-3, δO–H |
| 1459 | 1460 | 1453 | 3.4 | ν ring1, δC–H ring1 |
| 1434 | 1431 | 1432 | 18.6 | ν rings2-3, δC–H rings2-3, δO–H |
| 1420/1415 | 1420/1415 | 1410 | 62.1 | δC–H rings2-3, νC–O(H), δO–H |
| 1393/1390 | 1395/1391 | 1397 | 24.3 | δC–H rings2-3, νC–O(H), δO–H |
| 1373/1364 | 1369/1365 | 1364 | 103.0 | ν rings2-3, δO–H |
| 1351/1350/1340/1331 | 1354/1352/1343/1340 | 1328 | 354.9 | δO–H, νC–C, νC–O, νC–O(H), ν rings2-3 |
| 1318 | 1320 | 1317 | 1.4 | ν ring1, δC–H ring1 |
| 1298/1294 | 1300/1295 | 1299 | 1.4 | ν ring1, δC–H ring1 |
| 1276/1271 | 1280/1271 | 1269 | 78.2 | δO–H, δC–H ring3 |
| 1255 | 1255 | 1244 | 464.8 | νC–C, νC–O, δC–H rings2-3 |
| 1236/1234 | 1242 | 1217 | 10.0 | νC–O(H), δ rings2-3, δC–H rings2-3 |
| 1223/1219 | 1223/1219 | 1205 | 16.5 | δ rings2-3, δC–H rings2-3 |
| 1205/1204/1199/1198 | 1212/1207/1199/1195 | 1186 | 549.5 | νC–O–C asym. |
| 1166/1165 | 1165 | 1159 | 129.6 | δC–H ring1 |
| 1159 | 1158 | 1155 | 16.6 | δC–H ring1 |
| 1156 | 1152 | 1152 | 19.6 | δC–H ring3 |
| 1151 | 1151 | 1150 | 34.6 | δC–H rings2-3 |
| 1139/1137 | 1140/1138 | 1127 | 165.6 | δC–H rings2-3, δO–H, νC–O |
| 1088 | 1089 | 1076 | 110.6 | δ rings2-3, δC–H rings2-3, νC–O(H) |
| 1078/1074 | 1079/1073 | 1072 | 10.3 | δC–H ring1 |
| 1027 | 1025 | 1023 | 0.8 | ν ring3 |
| 1025 | 1022 | 1020 | 15.0 | δC–H ring1 |
| 1008/1002 | 1009/1002 | 997 | 9.3 | δ ring1 |
| n.obs. | n.obs. | 980 | 0.0 | γC–H ring3 |
| n.obs. | n.obs. | 977 | 0.1 | γC–H ring1 |
| 963 | 967 | 963 | 0.6 | γC–H rings2-3 |
| 955 | n.obs. | 958 | 0.1 | γC–H ring1 |
| 953 | 954 | 954 | 0.7 | γC–H rings2-3 |
| 931/930 | 933 | 923 | 24.5 | γC–H ring1 |
| 903/902 | 906 | 899 | 24.0 | γC–H ring1 |
| 880 | 879 | 870 | 3.1 | δ ring3 |
| 871 | 875 | 865 | 4.0 | γC–H rings2-3 |
| 845/840 | 846 | 830 | 13.0 | γC–H ring1, δC–O–C |
| 826 | n.obs. | 821 | 0.5 | γC–H ring1 |
| 822 | 825 | 814 | 9.2 | γC–H rings2-3 |
| 800/797 | 801 | 790 | 36.9 | γC–H rings2-3 |
| 794/792 | 792 | 786 | 3.1 | δO–C=O, δ rings1-2-3 |
| 774 | 777 | 777 | 122.2 | τO–H |
| 754 | 755 | 757 | 12.2 | γC=O |
| 745/743/742/740 | 746 | 737 | 38.6 | γC–H ring1 |
| 734/731 | 735 | 726 | 4.7 | γC–H rings2-3, γC=O |
| 723 | 723 | 717 | 14.9 | δ rings2-3 |
| 687 | 691 | 684 | 25.2 | γC–H ring1 |
| 660 | 661 | 656 | 5.9 | γC–O(H) |
| 632 | 631 | 628 | 0.8 | δ rings1-2-3 |
| 616 | 617 | 616 | 0.7 | δ ring1 |
| 605 | 604 | 599 | 4.4 | δ rings2-3 |
| 576 | 578 | 577 | 8.1 | τ rings2-3 |
| 552 | 551 | 550 | 15.2 | Skeletal deformation |
| 527 | 528 | 525 | 1.1 | Skeletal deformation |
| 504/501 | 503 | 500 | 7.5 | τ ring1 |
| 488 | 488 | 483 | 8.1 | δ rings2-3 |
| 479 | 481 | 479 | 0.4 | τ rings2-3 |
| 422 | 422 | 423 | 6.1 | τ ring3 |
| n.obs. | n.obs. | 412 | 0.2 | τ ring1 |
| n.obs. | n.obs. | 408 | 4.7 | δC–O(ph) |
| n.i. | n.i. | 387 | 1.3 | Skeletal deformation |
| 345 | 12.4 | νH...O | ||
| 277 | 1.1 | Skeletal deformation | ||
| 268 | 2.2 | Skeletal deformation | ||
| 220 | 2.6 | rings2-3 sym. butterfly | ||
| 214 | 0.7 | τ ring1 | ||
| 157 | 1.5 | δC–C=O | ||
| 142 | 0.5 | rings2-3 asym. butterfly | ||
| 99 | 0.1 | γC–C(ester) | ||
| 74 | 0.2 | τC–C | ||
| 56 | 0.7 | γC–O(ph) | ||
| 28 | 0.5 | τC–O | ||
| 15 | 0.1 | τO–C | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sıdır, İ.; Góbi, S.; Gülseven Sıdır, Y.; Fausto, R. Infrared Spectrum and UV-Induced Photochemistry of Matrix-Isolated Phenyl 1-Hydroxy-2-Naphthoate. Photochem 2021, 1, 10-25. https://doi.org/10.3390/photochem1010002
Sıdır İ, Góbi S, Gülseven Sıdır Y, Fausto R. Infrared Spectrum and UV-Induced Photochemistry of Matrix-Isolated Phenyl 1-Hydroxy-2-Naphthoate. Photochem. 2021; 1(1):10-25. https://doi.org/10.3390/photochem1010002
Chicago/Turabian StyleSıdır, İsa, Sándor Góbi, Yadigar Gülseven Sıdır, and Rui Fausto. 2021. "Infrared Spectrum and UV-Induced Photochemistry of Matrix-Isolated Phenyl 1-Hydroxy-2-Naphthoate" Photochem 1, no. 1: 10-25. https://doi.org/10.3390/photochem1010002
APA StyleSıdır, İ., Góbi, S., Gülseven Sıdır, Y., & Fausto, R. (2021). Infrared Spectrum and UV-Induced Photochemistry of Matrix-Isolated Phenyl 1-Hydroxy-2-Naphthoate. Photochem, 1(1), 10-25. https://doi.org/10.3390/photochem1010002










