Highly Specialized Textiles with Antimicrobial Functionality—Advances and Challenges
Abstract
1. Introduction
1.1. General Background
- -
- biostats, biocides (antibacterial, antifungal, antiviral), barriers, and antibiofilm;
- -
- textiles with bound or leaching antimicrobial finishing;
- -
- textiles made of natural (cotton, wool, silk, linen) or synthetic fibers (PP, PE, PES) or blends (cotton/elastane, cotton/PES, wool/acrylic);
- -
- textiles able to release compounds with biologic activity;
- -
- wearable and washing resistant.
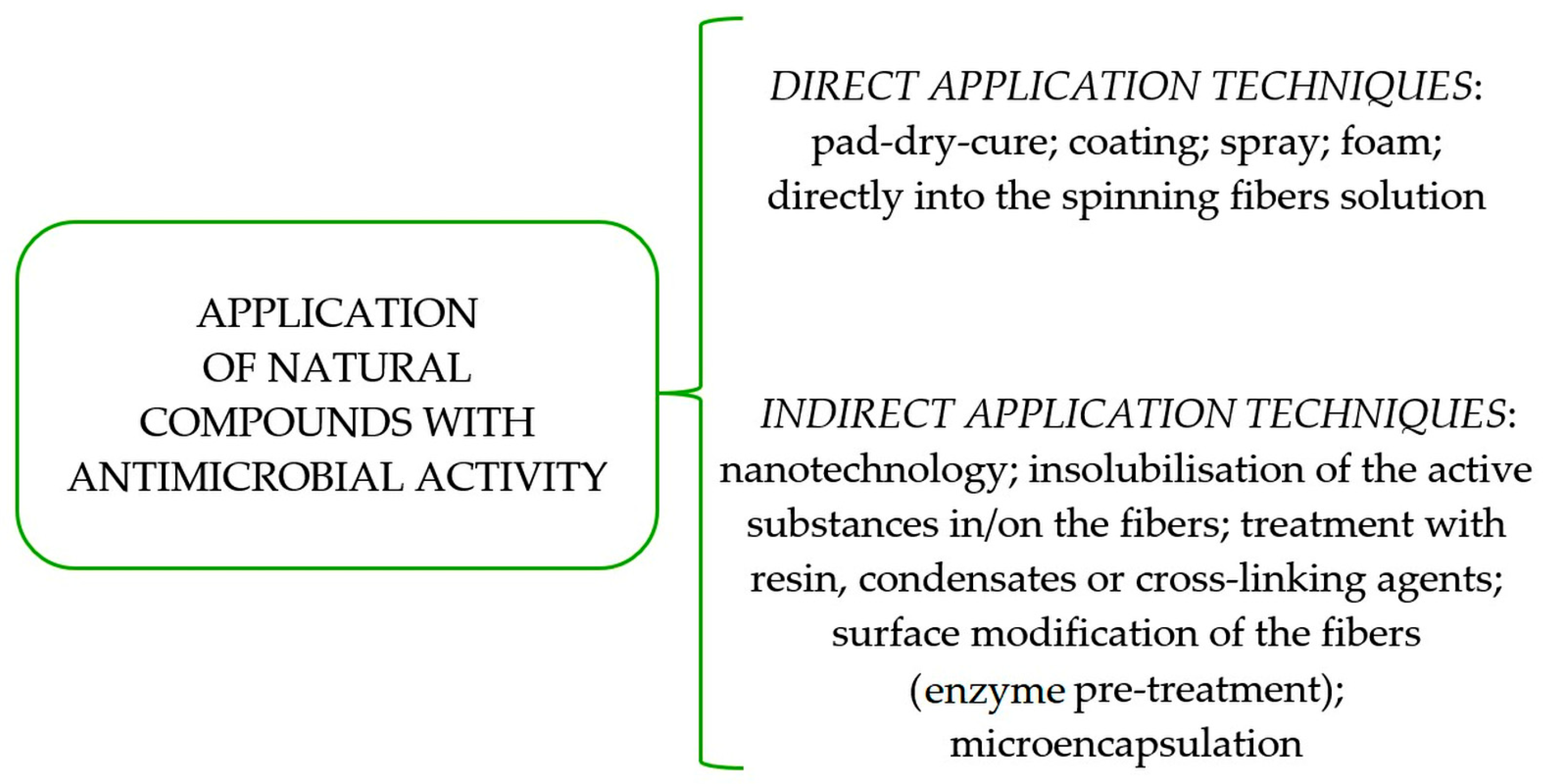
1.2. Processing Techniques
2. Synthetic Antimicrobial Agents for Textile Finishing
3. Natural Compounds with Biocide Activity Applied to Antimicrobial Textiles
- -
- UV protection properties (conferred by using lignin extracts, natural dye extracts);
- -
- Antioxidant properties (conferred by using natural dye extracts);
- -
- Antimicrobial properties (conferred by using chitosan, lignin, cyclodextrins, essential oils).
3.1. Chitosan
- -
- 1,2,3,4-butanetetracarboxylic acid (BTCA) and citric acid (CA), when cellulose fibers are considered;
- -
- organic anhydrides, such as succinic and phthalic ones, for grafting chitosan on wool fabrics;
- -
- citric acid in combination with oxidizing agents having reduced toxicity, such as potassium permanganate and sodium hypophosphite, for an effective cross-linking between chitosan and textile substrates—cotton cellulose, wool fabrics).
3.2. Lignin
3.3. Cyclodextrins
3.4. Sericin
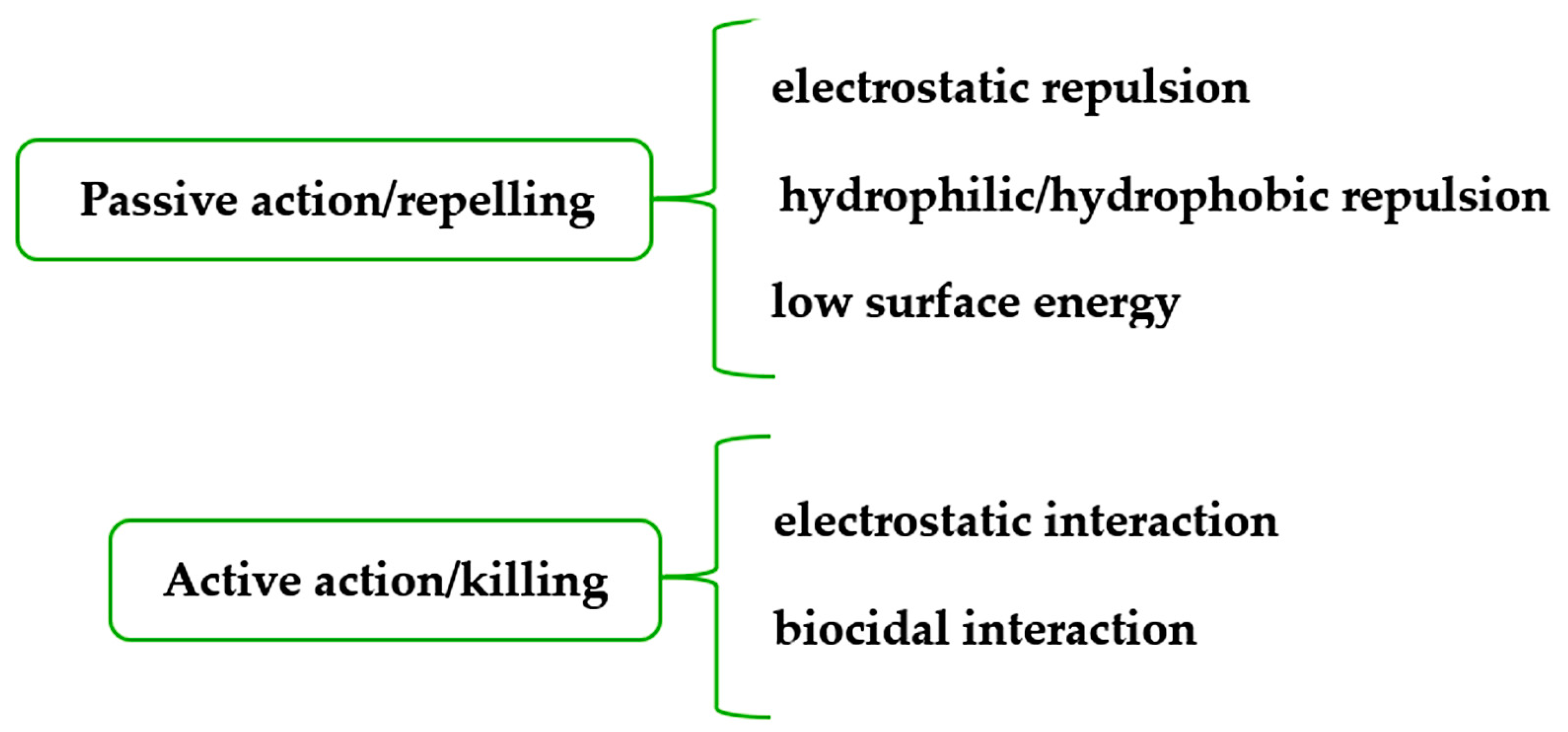
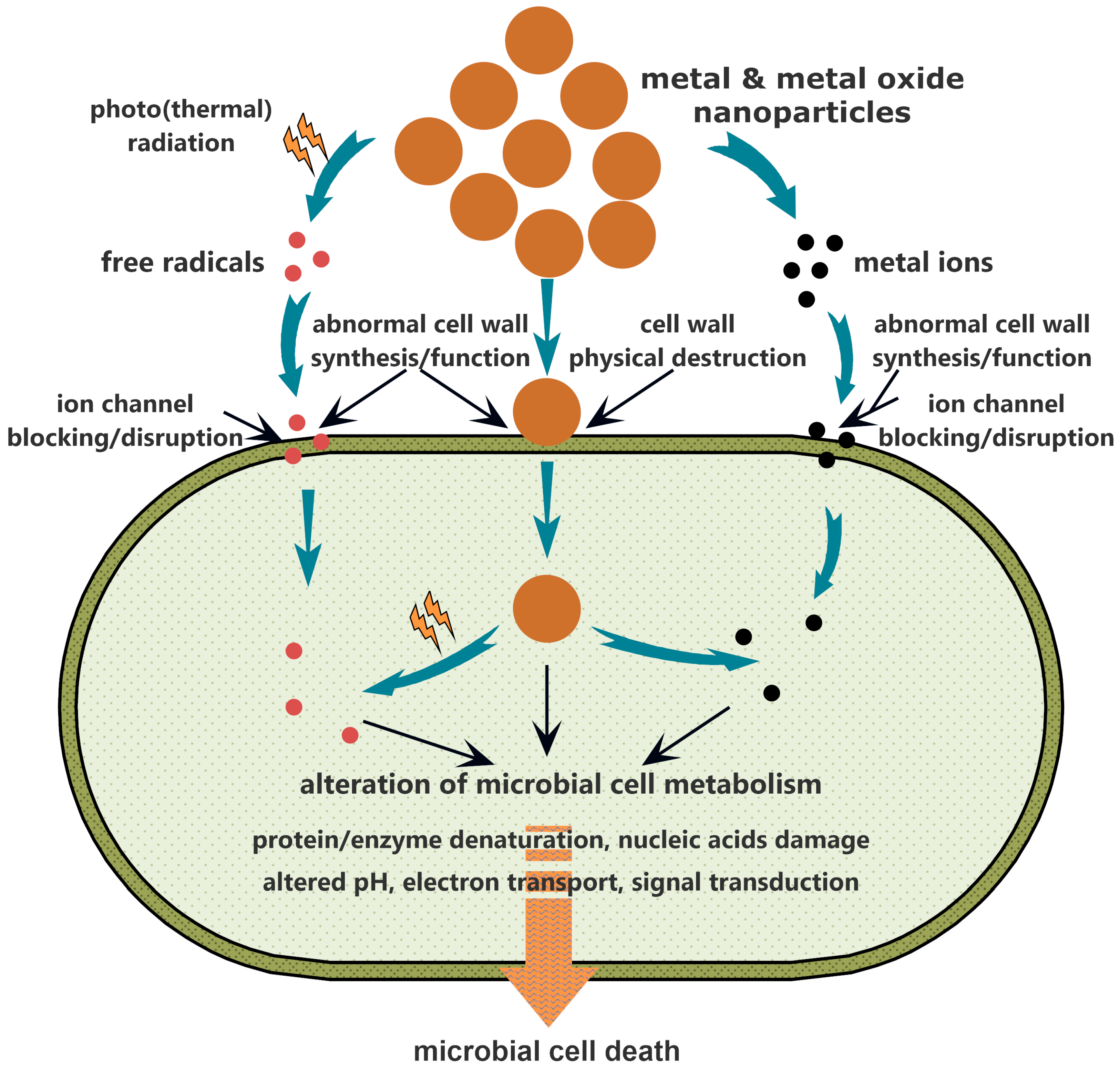
4. Metal and Metal Oxide Nanoparticles
5. Challenges in Antimicrobial Textiles Manufacturing
- qualitative tests—AATCC TM147, AATCC TM30 (American Association of Textile Chemists and Colorists Test Method), ISO 20645, ISO 11721 (International Organization for Standardization) and SN 195920, SN 195921 (Swiss standard);
- quantitative tests—AATCC TM100, ISO 20743, SN 195924, JIS L 1902 (Japanese industry standards) and ASTM E 2149 (or its modification) [156].
- -
- the use of natural plant fibers with intrinsic antimicrobial activity, raw or modified [158];
- -
- employ of biopolymers with intrinsic antimicrobial activity (i.e., chitosan) as both support and antimicrobial finishing, and with multiple functionality [159];
- -
- combining various antimicrobial compounds in order to enhance the effect in the final product; for example, plant extracts and plant-derived molecules with biologic activity have been encapsulated in chitosan particles that were subsequently used as antimicrobial finishing for cotton fabrics [160];
- -
- use of complex antimicrobial formulations including metals, metal oxides, and other nanoparticles (Ag, TiO2, silica), natural compounds (curcumin, Aloe vera), and binders;
- -
- increasing the compatibility between the textile substrate and the antimicrobial finishing in order to achieve materials with enhance stability and wearability;
- -
- a constant concern to maintain the production cost of most of these materials in the affordable range for the public—this can be achieved through an increased funding of research, both from public and private funds, and a more active involvement of the business community in healthcare and environmental protection.
6. Conclusions and Future Trends
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Novi, V.T.; Gonzalez, A.; Brockgreitens, J.; Abbas, A. Highly Efficient and Durable Antimicrobial Nanocomposite Textiles. Sci. Rep. 2022, 12, 17332. [Google Scholar] [CrossRef] [PubMed]
- Roychoudhury, S.; Das, A.; Sengupta, P.; Dutta, S.; Roychoudhury, S.; Choudhury, A.P.; Fuzayel Ahmed, A.B.; Bhattacharjee, S.; Slama, P. Viral Pandemics of the Last Four Decades: Pathophysiology, Health Impacts and Perspectives. Int. J. Environ. Res. Public Health 2020, 17, 9411. [Google Scholar] [CrossRef] [PubMed]
- Piret, J.; Boivin, G. Pandemics Throughout History. Front. Microbiol. 2021, 11, 631736. [Google Scholar] [CrossRef] [PubMed]
- Antimicrobial Textiles Market, Global Industry Size Forecast. Available online: https://www.marketsandmarkets.com/Market-Reports/antimicrobial-textile-market-254286152.html (accessed on 21 March 2023).
- Halepoto, H.; Gong, T.; Memon, H. A Bibliometric Analysis of Antibacterial Textiles. Sustainability 2022, 14, 11424. [Google Scholar] [CrossRef]
- Miraftab, M. High Performance Medical Textiles: An Overview; Woodhead Publishing Limited: Sawston, UK, 2014; ISBN 9780857099075. [Google Scholar]
- Morais, D.S.; Guedes, R.M.; Lopes, M.A. Antimicrobial Approaches for Textiles: From Research to Market. Materials 2016, 9, 498. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Dejarnette, S.; Chen, W.; Scholle, F.; Wang, Q.; Ghiladi, R.A. Color-Variable Dual-Dyed Photodynamic Antimicrobial Polyethylene Terephthalate (PET)/Cotton Blended Fabrics. Photochem. Photobiol. Sci. 2023, 1–18. [Google Scholar] [CrossRef]
- Antunes, J.; Matos, K.; Carvalho, S.; Cavaleiro, A.; Cruz, S.M.A.; Ferreira, F. Carbon-Based Coatings in Medical Textiles Surface Functionalisation: An Overview. Processes 2021, 9, 1997. [Google Scholar] [CrossRef]
- Zanoaga, M.; Tanasa, F. Antimicrobial Reagents as Functional Finishing for Textiles Intended for Biomedical Applications. I. Synthetic Organic Compounds. Chem. J. Mold. 2017, 9, 14–32. [Google Scholar] [CrossRef]
- Tanasa, F.; Zanoaga, M. Antimicrobial Reagents as Functional Finishing for Textiles Intended for Biomedical Applications. II. Metals and Metallic Compounds: Silver. In IFMBE Proceedings; Springer: Berlin/Heidelberg, Germany, 2016; Volume 55, pp. 305–308. [Google Scholar] [CrossRef]
- Zanoaga, M.; Tanasa, F. Antimicrobial Reagents as Functional Finishing for Textiles Intended for Biomedical Applications. III. Other Metals and Metallic Compounds. In IFMBE Proceedings; Springer: Berlin/Heidelberg, Germany, 2016; Volume 55, pp. 309–314. [Google Scholar]
- Riaz, S.; Ashraf, M. Recent Advances in Development of Antimicrobial Textiles. In Advances in Functional Finishing of Textiles; Springer: Singapore, 2020; pp. 129–168. [Google Scholar] [CrossRef]
- Ibrahim, A.; Laquerre, J.-É.; Forcier, P.; Deregnaucourt, V.; Decaens, J.; Vermeersch, O.; Ibrahim, A.; Laquerre, J.-É.; Forcier, P.; Deregnaucourt, V.; et al. Antimicrobial Agents for Textiles: Types, Mechanisms and Analysis Standards. In Textiles for Functional Applications; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Jin, L.; Zhou, F.; Wu, S.; Cui, C.; Sun, S.; Li, G.; Chen, S.; Ma, J. Development of Novel Segmented-Pie Microfibers from Copper-Carbon Nanoparticles and Polyamide Composite for Antimicrobial Textiles Application. Text. Res. J. 2021, 92, 3–14. [Google Scholar] [CrossRef]
- Suh, I.-Y.; Kim, Y.-J.; Zhao, P.; Cho, D.S.; Kang, M.; Huo, Z.-Y.; Kim, S.-W. Self-Powered Microbial Blocking Textile Driven by Triboelectric Charges. Nano Energy 2023, 110, 108343. [Google Scholar] [CrossRef]
- Saha, J.; Mondal, M.I.H. Antimicrobial Textiles from Natural Resources: Types, Properties and Processing. In Antimicrobial Textiles from Natural Resources; Woodhead Publishing: Sawston, UK, 2021; pp. 1–43. [Google Scholar] [CrossRef]
- Gulati, R.; Sharma, S.; Sharma, R.K. Antimicrobial Textile: Recent Developments and Functional Perspective. Polym. Bull. 2022, 79, 5747–5771. [Google Scholar] [CrossRef]
- Favatela, M.F.; Otarola, J.; Ayala-Peña, V.B.; Dolcini, G.; Perez, S.; Torres Nicolini, A.; Alvarez, V.A.; Lassalle, V.L. Development and Characterization of Antimicrobial Textiles from Chitosan-Based Compounds: Possible Biomaterials against SARS-CoV-2 Viruses. J. Inorg. Organomet. Polym. Mater. 2022, 32, 1473–1486. [Google Scholar] [CrossRef] [PubMed]
- Assylbekova, G.; Alotaibi, H.F.; Yegemberdiyeva, S.; Suigenbayeva, A.; Sataev, M.; Koshkarbaeva, S.; Abdurazova, P.; Sakibayeva, S.; Prokopovich, P. Sunlight Induced Synthesis of Silver Nanoparticles on Cellulose for the Preparation of Antimicrobial Textiles. J. Photochem. Photobiol. 2022, 11, 100134. [Google Scholar] [CrossRef]
- Naebe, M.; Haque, A.N.M.A.; Haji, A. Plasma-Assisted Antimicrobial Finishing of Textiles: A Review. Engineering 2022, 12, 145–163. [Google Scholar] [CrossRef]
- Nortjie, E.; Basitere, M.; Moyo, D.; Nyamukamba, P. Extraction Methods, Quantitative and Qualitative Phytochemical Screening of Medicinal Plants for Antimicrobial Textiles: A Review. Plants 2022, 11, 2011. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.I.; Shvalya, V.; Cvelbar, U.; Silva, R.; Marques-Oliveira, R.; Remião, F.; Felgueiras, H.P.; Padrão, J.; Zille, A. Stabilization of Silver Nanoparticles on Polyester Fabric Using Organo-Matrices for Controlled Antimicrobial Performance. Polymers 2022, 14, 1138. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, Z.; Meng, Y.; Song, Y.; Li, L.; Yu, M.; Li, J. Electron Beam Irradiation Grafting of Metal-Organic Frameworks onto Cotton to Prepare Antimicrobial Textiles. RSC Adv. 2023, 13, 1853–1861. [Google Scholar] [CrossRef]
- Yim, S.; Cheung, J.W.; Cheng, I.Y.; Ho, L.W.; Szeto, S.S.; Chan, P.; Lam, Y.; Kan, C. Longitudinal Study on the Antimicrobial Performance of a Polyhexamethylene Biguanide (PHMB)-Treated Textile Fabric in a Hospital Environment. Polymers 2023, 15, 1203. [Google Scholar] [CrossRef]
- Morris, H.; Murray, R. Medical Textiles. In Textile Progress; Taylor & Francis: Abingdon, UK, 2020; Volume 52, pp. 1–127. [Google Scholar]
- Britannica. Microbiology: Definition, History, & Microorganisms. Available online: https://www.britannica.com/science/microbiology (accessed on 28 April 2023).
- Tania, I.S.; Ali, M.; Arafat, M.T. Processing Techniques of Antimicrobial Textiles. In Antimicrobial Textiles from Natural Resources; Woodhead Publishing: Sawston, UK, 2021; pp. 189–215. [Google Scholar] [CrossRef]
- Gao, Y.; Cranston, R. Recent Advances in Antimicrobial Treatments of Textiles. Text. Res. J. 2008, 78, 60–72. [Google Scholar] [CrossRef]
- Sarathi, P.; Thilagavathi, G. Synthesis and characterization of titanium dioxide nano-particles and their applications to textiles for microbe resistance. J. Text. Appar. Technol. Manag. 2009, 6, 138525674. [Google Scholar]
- Mohamed, F.A.; Abd El-Megied, S.A.; Bashandy, M.S.; Ibrahim, H.M. Synthesis, Application and Antibacterial Activity of New Reactive Dyes Based on Thiazole Moiety. Pigment Resin Technol. 2018, 47, 246–254. [Google Scholar] [CrossRef]
- Tanasă, F.; Nechifor, M.; Teacă, C.A.; Stanciu, M.C. Physical Methods for the Modification of the Natural Fibers Surfaces. In Surface Treatment Methods of Natural Fibres and their Effects on Biocomposites; Woodhead Publishing: Sawston, UK, 2022; pp. 125–146. ISBN 9780128218631. [Google Scholar]
- Yuan, X.; Yin, W.; Ke, H.; Wei, Q.; Huang, Z.; Chen, D. Properties and Application of Multi-Functional and Structurally Colored Textile Prepared by Magnetron Sputtering. J. Ind. Text. 2022, 51, 1295–1311. [Google Scholar] [CrossRef]
- Shahidi, S.; Ghoranneviss, M. Plasma Sputtering for Fabrication of Antibacterial and Ultraviolet Protective Fabric. Cloth. Text. Res. J. 2015, 34, 37–47. [Google Scholar] [CrossRef]
- Ma, C.; Nikiforov, A.; De Geyter, N.; Dai, X.; Morent, R.; Ostrikov, K. Future Antiviral Polymers by Plasma Processing. Prog. Polym. Sci. 2021, 118, 101410. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathi, V.; Thilagavathi, G. Development of Plasma Enhanced Antiviral Surgical Gown for Healthcare Workers. Fash. Text. 2015, 2, 4. [Google Scholar] [CrossRef]
- Singh, N.; Sheikha, J. Microencapsulation and Its Application in Production of Functional Textiles. Indian J. Fibre Text. Res. 2020, 45, 495–509. [Google Scholar] [CrossRef]
- Yip, J.; Luk, M.Y.A. Microencapsulation Technologies for Antimicrobial Textiles. In Antimicrobial Textiles; Woodhead Publishing: Sawston, UK, 2016; pp. 19–46. ISBN 9780081005859. [Google Scholar]
- Systematic, B.A.; Podgornik, B.B.; Šandri, S. Microencapsulation for Functional Textile Coatings With. Coatings 2021, 11, 1371. [Google Scholar]
- Rivero, P.J.; Goicoechea, J. Sol-Gel Technology for Antimicrobial Textiles. In Antimicrobial Textiles; Woodhead Publishing: Sawston, UK, 2016; pp. 47–72. ISBN 9780081005859. [Google Scholar]
- Abramova, A.V.; Abramov, V.O.; Bayazitov, V.M.; Voitov, Y.; Straumal, E.A.; Lermontov, S.A.; Cherdyntseva, T.A.; Braeutigam, P.; Weiße, M.; Günther, K. A Sol-Gel Method for Applying Nanosized Antibacterial Particles to the Surface of Textile Materials in an Ultrasonic Field. Ultrason. Sonochem. 2020, 60, 104788. [Google Scholar] [CrossRef] [PubMed]
- Pakdel, E.; Daoud, W.A.; Wang, X. Assimilating the Photo-Induced Functions of TiO2-Based Compounds in Textiles: Emphasis on the Sol-Gel Process. Text. Res. J. 2015, 85, 1404–1428. [Google Scholar] [CrossRef]
- Bu, Y.; Zhang, S.; Cai, Y.; Yang, Y.; Ma, S.; Huang, J.; Yang, H.; Ye, D.; Zhou, Y.; Xu, W.; et al. Fabrication of Durable Antibacterial and Superhydrophobic Textiles via in Situ Synthesis of Silver Nanoparticle on Tannic Acid-Coated Viscose Textiles. Cellulose 2019, 26, 2109–2122. [Google Scholar] [CrossRef]
- Rodríguez-Tobías, H.; Morales, G.; Grande, D. Comprehensive Review on Electrospinning Techniques as Versatile Approaches toward Antimicrobial Biopolymeric Composite Fibers. Mater. Sci. Eng. C 2019, 101, 306–322. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Sherman, S.; Filocamo, S.; Steckl, A.J. Long-Term Antimicrobial Effect of Nisin Released from Electrospun Triaxial Fiber Membranes. Acta Biomater. 2017, 53, 242–249. [Google Scholar] [CrossRef]
- Dong, A.; Wang, Y.J.; Gao, Y.; Gao, T.; Gao, G. Chemical Insights into Antibacterial N-Halamines. Chem. Rev. 2017, 117, 4806–4862. [Google Scholar] [CrossRef]
- Zain, N.M.; Akindoyo, J.O.; Beg, M.D.H. Synthetic Antimicrobial Agent and Antimicrobial Fabrics: Progress and Challenges. IIUM Eng. J. 2018, 19, 10–29. [Google Scholar] [CrossRef]
- Li, Z.; Chen, J.; Cao, W.; Wei, D.; Zheng, A.; Guan, Y. Permanent Antimicrobial Cotton Fabrics Obtained by Surface Treatment with Modified Guanidine. Carbohydr. Polym. 2018, 180, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ren, X.; Liang, J. Antimicrobial Modification Review. BioResources 2015, 10, 1964–1985. [Google Scholar]
- Dhillon, G.S.; Kaur, S.; Pulicharla, R.; Brar, S.K.; Cledón, M.; Verma, M.; Surampalli, R.Y. Triclosan: Current Status, Occurrence, Environmental Risks and Bioaccumulation Potential. Int. J. Environ. Res. Public Health 2015, 12, 5657–5684. [Google Scholar] [CrossRef]
- Kumar, S.B. Chlorhexidine Mouthwash—A Review. J. Pharm. Sci. Res. 2017, 9, 1450–1452. [Google Scholar]
- Xue, Y.; Xiao, H.; Zhang, Y. Antimicrobial Polymeric Materials with Quaternary Ammonium and Phosphonium Salts. Int. J. Mol. Sci. 2015, 16, 3626–3655. [Google Scholar] [CrossRef] [PubMed]
- Ergene, C.; Yasuhara, K.; Palermo, E.F. Biomimetic Antimicrobial Polymers: Recent Advances in Molecular Design. Polym. Chem. 2018, 9, 2407–2427. [Google Scholar] [CrossRef]
- Huang, K.S.; Yang, C.H.; Huang, S.L.; Chen, C.Y.; Lu, Y.Y.; Lin, Y.S. Recent Advances in Antimicrobial Polymers: A Mini-Review. Int. J. Mol. Sci. 2016, 17, 1578. [Google Scholar] [CrossRef] [PubMed]
- Gerba, C.P. Quaternary Ammonium Biocides: Efficacy in Application. Appl. Environ. Microbiol. 2015, 81, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Niu, L.; Ma, S.; Li, J.; Tay, F.R.; Chen, J. hua Quaternary Ammonium-Based Biomedical Materials: State-of-the-Art, Toxicological Aspects and Antimicrobial Resistance. Prog. Polym. Sci. 2017, 71, 53–90. [Google Scholar] [CrossRef]
- El-Newehy, M.H.; Meera, M.A.; Aldalbahi, A.K.; Thamer, B.M.; Mahmoud, Y.A.G.; El-Hamshary, H. Biocidal Polymers: Synthesis, Characterization and Antimicrobial Activity of Bis-Quaternary Onium Salts of Poly(Aspartate-Co-Succinimide). Polymers 2020, 13, 23. [Google Scholar] [CrossRef]
- Foksowicz-Flaczyk, J.; Walentowska, J.; Przybylak, M.; Maciejewski, H. Multifunctional Durable Properties of Textile Materials Modified by Biocidal Agents in the Sol-Gel Process. Surf. Coat. Technol. 2016, 304, 160–166. [Google Scholar] [CrossRef]
- Sharon, E.; Sharabi, R.; Eden, A.; Zabrovsky, A.; Ben-Gal, G.; Sharon, E.; Pietrokovski, Y.; Houri-Haddad, Y.; Beyth, N. Antibacterial Activity of Orthodontic Cement Containing Quaternary Ammonium Polyethylenimine Nanoparticles Adjacent to Orthodontic Brackets. Int. J. Environ. Res. Public Health 2018, 15, 606. [Google Scholar] [CrossRef]
- Daoud, F.C.; Coppry, M.; Moore, N.; Rogues, A.M. Do Triclosan Sutures Modify the Microbial Diversity of Surgical Site Infections? A Systematic Review and Meta-Analysis. Microorganisms 2022, 10, 927. [Google Scholar] [CrossRef]
- Ahmed, I.; Boulton, A.J.; Rizvi, S.; Carlos, W.; Dickenson, E.; Smith, N.A.; Reed, M. The Use of Triclosan-Coated Sutures to Prevent Surgical Site Infections: A Systematic Review and Meta-Analysis of the Literature. BMJ Open 2019, 9, e029727. [Google Scholar] [CrossRef]
- Subramani, K.; Seo, H.N.; Dougherty, J.; Chaudhry, K.; Bollu, P.; Rosenthal, K.S.; Zhang, J.F. In Vitro Evaluation of Antimicrobial Activity of Chlorhexidine Hexametaphosphate Nanoparticle Coatings on Orthodontic Elastomeric Chains. Mater. Res. Express 2020, 7, 075401. [Google Scholar] [CrossRef]
- Wood, N.J.; Jenkinson, H.F.; Davis, S.A.; Mann, S.; O’Sullivan, D.J.; Barbour, M.E. Chlorhexidine Hexametaphosphate Nanoparticles as a Novel Antimicrobial Coating for Dental Implants. J. Mater. Sci. Mater. Med. 2015, 26, 201. [Google Scholar] [CrossRef] [PubMed]
- Jones, I.A.; Joshi, L.T. Biocide Use in the Antimicrobial Era: A Review. Molecules 2021, 26, 2276. [Google Scholar] [CrossRef]
- Cheng, X.; Li, R.; Du, J.; Sheng, J.; Ma, K.; Ren, X.; Huang, T.S. Antimicrobial Activity of Hydrophobic Cotton Coated with N-Halamine. Polym. Adv. Technol. 2015, 26, 99–103. [Google Scholar] [CrossRef]
- Demir, B.; Cerkez, I.; Worley, S.D.; Broughton, R.M.; Huang, T.S. N-Halamine-Modified Antimicrobial Polypropylene Nonwoven Fabrics for Use against Airborne Bacteria. ACS Appl. Mater. Interfaces 2015, 7, 1752–1757. [Google Scholar] [CrossRef] [PubMed]
- Ren, T.; Dormitorio, T.V.; Qiao, M.; Huang, T.S.; Weese, J. N-Halamine Incorporated Antimicrobial Nonwoven Fabrics for Use against Avian Influenza Virus. Vet. Microbiol. 2018, 218, 78–83. [Google Scholar] [CrossRef]
- Li, R.; Sheng, J.; Cheng, X.; Li, J.; Ren, X.; Huang, T.S. Biocidal Poly (Vinyl Alcohol) Films Incorporated with N-Halamine Siloxane. Compos. Commun. 2018, 10, 89–92. [Google Scholar] [CrossRef]
- Zhou, C.E.; Kan, C.W.; Matinlinna, J.P.; Tsoi, J.K.H. Regenerable Antibacterial Cotton Fabric by Plasma Treatment with Dimethylhydantoin: Antibacterial Activity against S. Aureus. Coatings 2017, 7, 11. [Google Scholar] [CrossRef]
- de Morais, M.C.; de Oliveira Lima, E.; Perez-Castillo, Y.; de Sousa, D.P. Synthetic Cinnamides and Cinnamates: Antimicrobial Activity, Mechanism of Action, and In Silico Study. Molecules 2023, 28, 1918. [Google Scholar] [CrossRef]
- Imai, M.; Yokoe, H.; Tsubuki, M.; Takahashi, N. Growth Inhibition of Human Breast and Prostate Cancer Cells by Cinnamic Acid Derivatives and Their Mechanism of Action. Biol. Pharm. Bull. 2019, 42, 1134–1139. [Google Scholar] [CrossRef]
- Cai, R.; Miao, M.; Yue, T.; Zhang, Y.; Cui, L.; Wang, Z.; Yuan, Y. Antibacterial Activity and Mechanism of Cinnamic Acid and Chlorogenic Acid against Alicyclobacillus Acidoterrestris Vegetative Cells in Apple Juice. Int. J. Food Sci. Technol. 2019, 54, 1697–1705. [Google Scholar] [CrossRef]
- Robertson, J.; Gizdavic-Nikolaidis, M.; Swift, S. Investigation of Polyaniline and a Functionalised Derivative as Antimicrobial Additives to Create Contamination Resistant Surfaces. Materials 2018, 11, 436. [Google Scholar] [CrossRef] [PubMed]
- Sanchez Ramirez, D.O.; Varesano, A.; Carletto, R.A.; Vineis, C.; Perelshtein, I.; Natan, M.; Perkas, N.; Banin, E.; Gedanken, A. Antibacterial Properties of Polypyrrole-Treated Fabrics by Ultrasound Deposition. Mater. Sci. Eng. C 2019, 102, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Zare, E.N.; Agarwal, T.; Zarepour, A.; Pinelli, F.; Zarrabi, A.; Rossi, F.; Ashrafizadeh, M.; Maleki, A.; Shahbazi, M.A.; Maiti, T.K.; et al. Electroconductive Multi-Functional Polypyrrole Composites for Biomedical Applications. Appl. Mater. Today 2021, 24, 101117. [Google Scholar] [CrossRef]
- Wang, C.Y.; Makvandi, P.; Zare, E.N.; Tay, F.R.; Niu, L. Advances in Antimicrobial Organic and Inorganic Nanocompounds in Biomedicine. Adv. Ther. 2020, 3, 2000024. [Google Scholar] [CrossRef]
- Kachuk, D.S.; Mishchenko, E.V.; Venger, E.A.; Popovych, T.A. Biocidal Protection of Textile Materials. J. Chem. Technol. 2022, 30, 240–252. [Google Scholar] [CrossRef]
- Yıldırım, F.F.; Avinc, O.; Yavas, A.; Sevgisunar, G. Sustainable Antifungal and Antibacterial Textiles Using Natural Resources; Springer: Berlin/Heidelberg, Germany, 2020; ISBN 9783030385415. [Google Scholar]
- Pawłowska, A.; Stepczyńska, M. Natural Biocidal Compounds of Plant Origin as Biodegradable Materials Modifiers. J. Polym. Environ. 2022, 30, 1683–1708. [Google Scholar] [CrossRef]
- Walentowska, J.; Foksowicz-Flaczyk, J. Thyme Essential Oil for Antimicrobial Protection of Natural Textiles. Int. Biodeterior. Biodegrad. 2013, 84, 407–411. [Google Scholar] [CrossRef]
- Singh, N.; Sahu, O. Sustainable Cyclodextrin in Textile Applications; Elsevier Ltd.: Amsterdam, The Netherlands, 2018; ISBN 9780081024911. [Google Scholar]
- Liakos, I.; Rizzello, L.; Hajiali, H.; Brunetti, V.; Carzino, R.; Pompa, P.P.; Athanassiou, A.; Mele, E. Fibrous Wound Dressings Encapsulating Essential Oils as Natural Antimicrobial Agents. J. Mater. Chem. B 2015, 3, 1583–1589. [Google Scholar] [CrossRef]
- Tawiah, B.; Badoe, W.; Fu, S. Advances in the Development of Antimicrobial Agents for Textiles: The Quest for Natural Products. Review. Fibres Text. East. Eur. 2016, 24, 136–149. [Google Scholar] [CrossRef]
- Rather, L.J.; Shabbir, M.; Li, Q.; Mohammad, F. Coloration, UV Protective, and Antioxidant Finishing of Wool Fabric Via Natural Dye Extracts: Cleaner Production of Bioactive Textiles. Environ. Prog. Sustain. Energy 2019, 38, 13187. [Google Scholar] [CrossRef]
- Shahid-Ul-Islam; Shahid, M.; Mohammad, F. Green Chemistry Approaches to Develop Antimicrobial Textiles Based on Sustainable Biopolymers—A Review. Ind. Eng. Chem. Res. 2013, 52, 5245–5260. [Google Scholar] [CrossRef]
- Liu, Z.; Luo, Y.; Zhao, X.; Zheng, K.; Wu, M.; Wang, L. A Natural Antibacterial Agent Based on Modified Chitosan by Hinokitiol for Antibacterial Application on Cotton Fabric. Cellulose 2022, 29, 2731–2742. [Google Scholar] [CrossRef]
- Ali, S.W.; Purwar, R.; Joshi, M.; Rajendran, S. Antibacterial Properties of Aloe Vera Gel-Finished Cotton Fabric. Cellulose 2014, 21, 2063–2072. [Google Scholar] [CrossRef]
- Shahid-ul-Islam; Butola, B. S. Recent Advances in Chitosan Polysaccharide and Its Derivatives in Antimicrobial Modification of Textile Materials. Int. J. Biol. Macromol. 2019, 121, 905–912. [Google Scholar] [CrossRef]
- Benltoufa, S.; Miled, W.; Trad, M.; Slama, R.B.; Fayala, F. Chitosan Hydrogel-coated Cellulosic Fabric for Medical End-Use: Antibacterial Properties, Basic Mechanical and Comfort Properties. Carbohydr. Polym. 2020, 227, 115352. [Google Scholar] [CrossRef]
- Ferrero, F.; Periolatto, M. Antimicrobial Finish of Textiles by Chitosan UV-Curing. J. Nanosci. Nanotechnol. 2012, 12, 4803–4810. [Google Scholar] [CrossRef]
- Lobo, F.C.M.; Franco, A.R.; Fernandes, E.M.; Reis, R.L. An Overview of the Antimicrobial Properties of Lignocellulosic Materials. Molecules 2021, 26, 1749. [Google Scholar] [CrossRef]
- Sunthornvarabhas, J.; Liengprayoon, S.; Lerksamran, T.; Buratcharin, C.; Suwonsichon, T.; Vanichsriratana, W.; Sriroth, K. Utilization of Lignin Extracts from Sugarcane Bagasse as Bio-Based Antimicrobial Fabrics. Sugar Tech 2019, 21, 355–363. [Google Scholar] [CrossRef]
- Sunthornvarabhas, J.; Liengprayoon, S.; Suwonsichon, T. Antimicrobial Kinetic Activities of Lignin from Sugarcane Bagasse for Textile Product. Ind. Crops Prod. 2017, 109, 857–861. [Google Scholar] [CrossRef]
- Andreaus, J.; Dalmolin, M.C.; De Oliveira, I.B.; Barcellos, I.O. Aplicação de Ciclodextrinas Em Processos Têxteis. Quim. Nova 2010, 33, 929–937. [Google Scholar] [CrossRef]
- Utzeri, G.; Matias, P.M.C.; Murtinho, D.; Valente, A.J.M. Cyclodextrin-Based Nanosponges: Overview and Opportunities. Front. Chem. 2022, 10, 859406. [Google Scholar] [CrossRef]
- Rukmani, A.; Sundrarajan, M. Inclusion of Antibacterial Agent Thymol on β-Cyclodextrin-Grafted Organic Cotton. J. Ind. Text. 2012, 42, 132–144. [Google Scholar] [CrossRef]
- Sundrarajan, M.; Rukmani, A. Biopolishing and Cyclodextrin Derivative Grafting on Cellulosic Fabric for Incorporation of Antibacterial Agent Thymol. J. Text. Inst. 2013, 104, 188–196. [Google Scholar] [CrossRef]
- Ma, J.; Fan, J.; Xia, Y.; Kou, X.; Ke, Q.; Zhao, Y. Preparation of Aromatic β-Cyclodextrin Nano/Microcapsules and Corresponding Aromatic Textiles: A Review. Carbohydr. Polym. 2023, 308, 120661. [Google Scholar] [CrossRef]
- Rajendran, R.; Balakumar, C.; Sivakumar, R.; Amruta, T.; Devaki, N. Extraction and Application of Natural Silk Protein Sericin from Bombyx Mori as Antimicrobial Finish for Cotton Fabrics. J. Text. Inst. 2012, 103, 458–462. [Google Scholar] [CrossRef]
- Karatzani, A. The Use of Metal Threads in the Decoration of Late and Post-Byzantine Embroidered Church Textiles and Post-Byzantine Embroidered Church Textiles. Cah. Balk. 2021, 48, 233–253. [Google Scholar] [CrossRef]
- Schneegass, S.; Amft, O. (Eds.) Smart Textiles. Fundamentals, Design, and Interaction; Springer International Publishing: Berlin/Heidelberg, Germany, 2017; ISBN 9783319501239. [Google Scholar]
- Rajendran, S. (Ed.) Advanced Textiles for Wound Care, 2nd ed.; Woodhead Publishing: Sawston, UK, 2018; ISBN 9780081021927. [Google Scholar]
- Elmogahzy, Y.E. Engineering Textiles: Integrating the Design and Manufacture of Textile Products, 2nd ed.; Woodhead Publishing: Sawston, UK, 2020; ISBN 9780081024898. [Google Scholar]
- Thilagavathi, G.; Rathinamoorthy, R. (Eds.) Odour in Textiles. Generation and Control; CRC Press: Boca Raton, FL, USA, 2022; ISBN 9780367693367. [Google Scholar]
- Sabba, D. (Ed.) Nanotechnology in Smart Textiles; Arcler Press: Burlington, ON, Canada, 2019; ISBN 9781773616490. [Google Scholar]
- Joshi, M. (Ed.) Nanotechnology in Textiles: Advances and Developments in Polymer Nanocomposites; Jenny Stanford Publishing: Singapore, 2020; ISBN 9789814800815. [Google Scholar]
- Militky, J.; Periyasamy, A.P.; Venkataraman, M. (Eds.) Textiles and Their Use in Microbial Protection: Focus on COVID-19 and Other Viruses; CRC Press: Boca Raton, FL, USA, 2021; ISBN 9780367691059. [Google Scholar]
- Ul-Islam, S.; Butola, B.S. (Eds.) Nanomaterials in the Wet Processing of Textiles; Scrivener Publishing: Beverly, MA, USA, 2018; ISBN 978-1-119-45984-2. [Google Scholar]
- Franco, D.; Calabrese, G.; Guglielmino, S.P.P.; Conoci, S. Metal-Based Nanoparticles: Antibacterial Mechanisms and Biomedical Application. Microorganisms 2022, 10, 1778. [Google Scholar] [CrossRef]
- Chakraborty, N.; Jha, D.; Roy, I.; Kumar, P.; Gaurav, S.S. Nanobiotics against Antimicrobial Resistance: Harnessing the Power of Nanoscale Materials and Technologies. J. Nanobiotechnology 2022, 20, 375. [Google Scholar] [CrossRef]
- Frei, A.; Verderosa, A.D.; Elliott, A.G.; Zuegg, J.; Blaskovich, M.A.T. Metals to Combat Antimicrobial Resistance. Nat. Rev. Chem. 2023, 7, 202–224. [Google Scholar] [CrossRef]
- Ma, X.; Zhou, S.; Xu, X.; Du, Q. Copper-Containing Nanoparticles: Mechanism of Antimicrobial Effect and Application in Dentistry-a Narrative Review. Front. Surg. 2022, 9, 905892. [Google Scholar] [CrossRef]
- Mendes, C.R.; Dilarri, G.; Forsan, C.F.; Sapata, V.d.M.R.; Lopes, P.R.M.; de Moraes, P.B.; Montagnolli, R.N.; Ferreira, H.; Bidoia, E.D. Antibacterial Action and Target Mechanisms of Zinc Oxide Nanoparticles against Bacterial Pathogens. Sci. Rep. 2022, 12, 2658. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.M.; Simon, B.; Tom, B. Recent Advances in TiO2-Functionalized Textile Surfaces. Surf. Interfaces 2021, 22, 100890. [Google Scholar] [CrossRef]
- Guisbiers, G. (Ed.) Antimicrobial Activity of Nanoparticles, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2023; ISBN 9780128216378. [Google Scholar]
- Fernandes, M.; Padrão, J.; Ribeiro, A.I.; Fernandes, R.D.V.; Melro, L.; Nicolau, T.; Mehravani, B.; Alves, C.; Rodrigues, R.; Zille, A. Polysaccharides and Metal Nanoparticles for Functional Textiles: A Review. Nanomaterials 2022, 12, 1006. [Google Scholar] [CrossRef]
- Andra, S.; Balu, S.K.; Jeevanandam, J.; Muthalagu, M. Emerging nanomaterials for antibacterial textile fabrication. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2021, 394, 1355–1382. [Google Scholar] [CrossRef] [PubMed]
- Rujido-Santos, I.; Herbello-Hermelo, P.; Barciela-Alonso, M.C.; Bermejo-Barrera, P.; Moreda-Piñeiro, A. Metal Content in Textile and (Nano)Textile Products. Int. J. Environ. Res. Public Health 2022, 19, 944. [Google Scholar] [CrossRef]
- Dolez, P.I.; Benaddi, H. Toxicity Testing of Textiles. Adv. Charact. Test. Text. 2018, 151–188. [Google Scholar] [CrossRef]
- Mahmud, S.; Pervez, N.; Taher, M.A.; Mohiuddin, K.; Liu, H.H. Multifunctional Organic Cotton Fabric Based on Silver Nanoparticles Green Synthesized from Sodium Alginate. Text. Res. J. 2020, 90, 1224–1236. [Google Scholar] [CrossRef]
- Nam, S.; Selling, G.W.; Hillyer, M.B.; Condon, B.D.; Rahman, M.S.; Chang, S.C. Brown Cotton Fibers Self-Produce Ag Nanoparticles for Regenerating Their Antimicrobial Surfaces. ACS Appl. Nano Mater. 2021, 4, 13112–13122. [Google Scholar] [CrossRef]
- Ribeiro, A.I.; Senturk, D.; Silva, K.K.; Modic, M.; Cvelbar, U.; Dinescu, G.; Mitu, B.; Nikiforov, A.; Leys, C.; Kuchakova, I.; et al. Antimicrobial Efficacy of Low Concentration PVP-Silver Nanoparticles Deposited on DBD Plasma-Treated Polyamide 6,6 Fabric. Coatings 2019, 9, 581. [Google Scholar] [CrossRef]
- Khe, Y.; Sv, M.; Aa, S.; Jz, J.; Fm, T.; Ssh, R.; Renat, L. Antibacterial effect of cotton fabric treated with silver nanoparticles of different sizes and shapes. Int. J. Nanomater. Nanotechnol. Nanomed. 2019, 5, 016–023. [Google Scholar] [CrossRef]
- Jiang, L.; Zhou, Y.; Guo, Y.; Jiang, Z.; Chen, S.; Ma, J. Preparation of Silver Nanoparticle Functionalized Polyamide Fibers with Antimicrobial Activity and Electrical Conductivity. J. Appl. Polym. Sci. 2019, 136, 47584. [Google Scholar] [CrossRef]
- Mast, J.; Van Miert, E.; Siciliani, L.; Cheyns, K.; Blaude, M.N.; Wouters, C.; Waegeneers, N.; Bernsen, R.; Vleminckx, C.; Van Loco, J.; et al. Application of Silver-Based Biocides in Face Masks Intended for General Use Requires Regulatory Control. Sci. Total Environ. 2023, 870, 161889. [Google Scholar] [CrossRef]
- Tania, I.S.; Ali, M.; Azam, M.S. Mussel-Inspired Deposition of Ag Nanoparticles on Dopamine-Modified Cotton Fabric and Analysis of Its Functional, Mechanical and Dyeing Properties. J. Inorg. Organomet. Polym. Mater. 2021, 31, 4065–4076. [Google Scholar] [CrossRef]
- Dhineshbabu, N.R.; Rajendran, V. Antibacterial Activity of Hybrid Chitosan-Cupric Oxide Nanoparticles on Cotton Fab. IET Nanobiotechnology 2016, 10, 13–19. [Google Scholar] [CrossRef]
- Hasan, R. Production of Antimicrobial Textiles by Using Copper Oxide Nanoparticles. Int. J. Contemp. Res. Rev. 2018, 9, 20195–20202. [Google Scholar] [CrossRef] [PubMed]
- Vasantharaj, S.; Sathiyavimal, S.; Saravanan, M.; Senthilkumar, P.; Gnanasekaran, K.; Shanmugavel, M.; Manikandan, E.; Pugazhendhi, A. Synthesis of Ecofriendly Copper Oxide Nanoparticles for Fabrication over Textile Fabrics: Characterization of Antibacterial Activity and Dye Degradation Potential. J. Photochem. Photobiol. B Biol. 2019, 191, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Román, L.E.; Villalva, C.; Uribe, C.; Paraguay-Delgado, F.; Sousa, J.; Vigo, J.; Vera, C.M.; Gómez, M.M.; Solís, J.L. Textiles Functionalized with Copper Oxides: A Sustainable Option for Prevention of COVID-19. Polymers 2022, 14, 3066. [Google Scholar] [CrossRef]
- Asmat-Campos, D.; de Oca-Vásquez, G.M.; Rojas-Jaimes, J.; Delfín-Narciso, D.; Juárez-Cortijo, L.; Nazario-Naveda, R.; Batista Meneses, D.; Pereira, R.; de la Cruz, M.S. Cu2O Nanoparticles Synthesized by Green and Chemical Routes, and Evaluation of Their Antibacterial and Antifungal Effect on Functionalized Textiles. Biotechnol. Rep. 2023, 37, e00785. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Beddow, J.; Mee, C.; Maryniak, L.; Joyce, E.M.; Mason, T.J. Cytotoxicity Study of Textile Fabrics Impregnated With CuO Nanoparticles in Mammalian Cells. Int. J. Toxicol. 2017, 36, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Román, L.E.; Amézquita, M.J.; Uribe, C.L.; Maurtua, D.J.; Costa, S.A.; Costa, S.M.; Keiski, R.; Solís, J.L.; Gómez, M.M. In Situ Growth of CuO Nanoparticles onto Cotton Textiles. Adv. Nat. Sci. Nanosci. Nanotechnol. 2020, 11, 25009. [Google Scholar] [CrossRef]
- Shaheen, T.I.; Fouda, A.; Salem, S.S. Integration of Cotton Fabrics with Biosynthesized CuO Nanoparticles for Bactericidal Activity in the Terms of Their Cytotoxicity Assessment. Ind. Eng. Chem. Res. 2021, 60, 1553–1563. [Google Scholar] [CrossRef]
- Tharchanaa, S.B.; Anupriyanka, T.; Shanmugavelayutham, G. Ecofriendly Surface Modification of Cotton Fabric to Enhance the Adhesion of CuO Nanoparticles for Antibacterial Activity. Mater. Technol. 2022, 37, 3222–3230. [Google Scholar] [CrossRef]
- Petkova, P.; Francesko, A.; Perelshtein, I.; Gedanken, A.; Tzanov, T. Simultaneous Sonochemical-Enzymatic Coating of Medical Textiles with Antibacterial ZnO Nanoparticles. Ultrason. Sonochem. 2016, 29, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, S.; Rezaee, H.; Rashidi, A.; Ghoranneviss, M. In Situ Synthesis of ZnO Nanoparticles on Plasma Treated Cotton Fabric Utilizing Durable Antibacterial Activity. J. Nat. Fibers 2018, 15, 639–647. [Google Scholar] [CrossRef]
- Fiedot-Toboła, M.; Ciesielska, M.; Maliszewska, I.; Rac-Rumijowska, O.; Suchorska-Woźniak, P.; Teterycz, H.; Bryjak, M. Deposition of Zinc Oxide on Different Polymer Textiles and Their Antibacterial Properties. Materials 2018, 11, 707. [Google Scholar] [CrossRef]
- Palaniappan, G. Study on the Antimicrobial Efficacy of Fabrics Finished with Nano Zinc Oxide Particles. J. Textile Sci. Eng. 2020, 10. [Google Scholar]
- Irfan, M.; Naz, M.Y.; Saleem, M.; Tanawush, M.; Głowacz, A.; Glowacz, W.; Rahman, S.; Mahnashi, M.H.; Alqahtani, Y.S.; Alyami, B.A.; et al. Statistical Study of Nonthermal Plasma-Assisted ZnO Coating of Cotton Fabric through Ultrasonic-Assisted Green Synthesis for Improved Self-Cleaning and Antimicrobial Properties. Materials 2021, 14, 6998. [Google Scholar] [CrossRef]
- Tania, I.S.; Ali, M.; Akter, M. Fabrication, Characterization, and Utilization of ZnO Nanoparticles for Stain Release, Bacterial Resistance, and UV Protection on Cotton Fabric. J. Eng. Fiber. Fabr. 2022, 17, 114238. [Google Scholar] [CrossRef]
- Abdel Salam, K.A.; Ibrahim, N.A.; Maamoun, D.; Abdel Salam, S.H.; Fathallah, A.I.; Abdelrahman, M.S.; Mashaly, H.; Hassabo, A.G.; Khattab, T.A. Anti-Microbial Finishing of Polyamide Fabric Using Titanium Dioxide Nanoparticles. J. Text. Color. Polym. Sci. 2023, 20, 171–174. [Google Scholar] [CrossRef]
- Kale, R.D.; Meena, C.R. Synthesis of Titanium Dioxide Nanoparticles and Application on Nylon Fabric Using Layer by Layer Technique for Antimicrobial Property. Adv. Appl. Sci. Res. 2012, 3, 3073–3080. [Google Scholar]
- Rabiei, H.; Farhang Dehghan, S.; Montazer, M.; Khaloo, S.S.; Koozekonan, A.G. UV Protection Properties of Workwear Fabrics Coated with TiO2 Nanoparticles. Front. Public Health 2022, 10, 929095. [Google Scholar] [CrossRef]
- Tudu, B.K.; Sinhamahapatra, A.; Kumar, A. Surface Modification of Cotton Fabric Using TiO2 Nanoparticles for Self-Cleaning, Oil-Water Separation, Antistain, Anti-Water Absorption, and Antibacterial Properties. ACS Omega 2020, 5, 7850–7860. [Google Scholar] [CrossRef] [PubMed]
- Riaz, S.; Ashraf, M.; Aziz, H.; Younus, A.; Umair, M.; Salam, A.; Iqbal, K.; Hussain, M.T.; Hussain, T. Cationization of TiO2 Nanoparticles to Develop Highly Durable Multifunctional Cotton Fabric. Mater. Chem. Phys. 2022, 278, 125573. [Google Scholar] [CrossRef]
- Alvarez-Amparán, M.A.; Martínez-Cornejo, V.; Cedeño-Caero, L.; Hernandez-Hernandez, K.A.; Cadena-Nava, R.D.; Alonso-Núñez, G.; Moyado, S.F. Characterization and Photocatalytic Activity of TiO2 Nanoparticles on Cotton Fabrics, for Antibacterial Masks. Appl. Nanosci. 2022, 12, 4019–4032. [Google Scholar] [CrossRef] [PubMed]
- Riaz, S.; Ashraf, M.; Hussain, T.; Hussain, M.T.; Rehman, A.; Javid, A.; Iqbal, K.; Basit, A.; Aziz, H. Functional Finishing and Coloration of Textiles with Nanomaterials. Color. Technol. 2018, 134, 327–346. [Google Scholar] [CrossRef]
- Abu-Qdais, H.A.; Abu-Dalo, M.A.; Hajeer, Y.Y. Impacts of Nanosilver-Based Textile Products Using a Life Cycle Assessment. Sustainability 2021, 13, 3436. [Google Scholar] [CrossRef]
- Ramzan, U.; Majeed, W.; Hussain, A.A.; Qurashi, F.; Qamar, S.U.R.; Naeem, M.; Uddin, J.; Khan, A.; Al-Harrasi, A.; Razak, S.I.A.; et al. New Insights for Exploring the Risks of Bioaccumulation, Molecular Mechanisms, and Cellular Toxicities of AgNPs in Aquatic Ecosystem. Water 2022, 14, 2192. [Google Scholar] [CrossRef]
- Owen, L.; Laird, K. Development of a Silver-Based Dual-Function Antimicrobial Laundry Additive and Textile Coating for the Decontamination of Healthcare Laundry. J. Appl. Microbiol. 2021, 130, 1012–1022. [Google Scholar] [CrossRef]
- Shahidi, S.; Jamali, A.; Dalal Sharifi, S.; Ghomi, H. In-Situ Synthesis of CuO Nanoparticles on Cotton Fabrics Using Spark Discharge Method to Fabricate Antibacterial Textile. J. Nat. Fibers 2018, 15, 870–881. [Google Scholar] [CrossRef]
- Shahid-ul-Islam; Butola, B.S.; Kumar, A. Green Chemistry Based In-Situ Synthesis of Silver Nanoparticles for Multifunctional Finishing of Chitosan Polysaccharide Modified Cellulosic Textile Substrate. Int. J. Biol. Macromol. 2020, 152, 1135–1145. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Cai, Y.; Chen, X.; Ke, Y. Silver-based nanocomposite for fabricating high performance value-added cotton. Cellulose 2021, 29, 723–750. [Google Scholar] [CrossRef] [PubMed]
- Haase, H.; Jordan, L.; Keitel, L.; Keil, C.; Mahltig, B. Comparison of Methods for Determining the Effectiveness of Antibacterial Functionalized Textiles. PLoS ONE 2017, 12, e0188304. [Google Scholar] [CrossRef]
- Ščasníková, K.; Sibilová, A.; Bánovská, Z. Comparison of quantitative methods for determining the antibacterial effectiveness of non-woven textiles. Fibres Text. 2023, 29, 38–44. [Google Scholar] [CrossRef]
- Boudreau, M.D.; Imam, M.S.; Paredes, A.M.; Bryant, M.S.; Cunningham, C.K.; Felton, R.P.; Jones, M.Y.; Davis, K.J.; Olson, G.R. Differential Effects of Silver Nanoparticles and Silver Ions on Tissue Accumulation, Distribution, and Toxicity in the Sprague Dawley Rat Following Daily Oral Gavage Administration for 13 Weeks. Toxicol. Sci. 2016, 150, 131–160. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Mendoza, L.; Guamba, E.; Miño, K.; Romero, M.P.; Levoyer, A.; Alvarez-Barreto, J.F.; Machado, A.; Alexis, F. Antimicrobial Properties of Plant Fibers. Molecules 2022, 27, 7999. [Google Scholar] [CrossRef]
- Tien, N.D.; Lyngstadaas, S.P.; Mano, J.F.; Blaker, J.J.; Haugen, H.J. Recent Developments in Chitosan-Based Micro/Nanofibers for Sustainable Food Packaging, Smart Textiles, Cosmeceuticals, and Biomedical Applications. Molecules 2021, 26, 2683. [Google Scholar] [CrossRef]
- Antunes, J.C.; Domingues, J.M.; Miranda, C.S.; Silva, A.F.G.; Homem, N.C.; Amorim, M.T.P.; Felgueiras, H.P. Bioactivity of Chitosan-Based Particles Loaded with Plant-Derived Extracts for Biomedical Applications: Emphasis on Antimicrobial Fiber-Based Systems. Mar. Drugs 2021, 19, 359. [Google Scholar] [CrossRef]
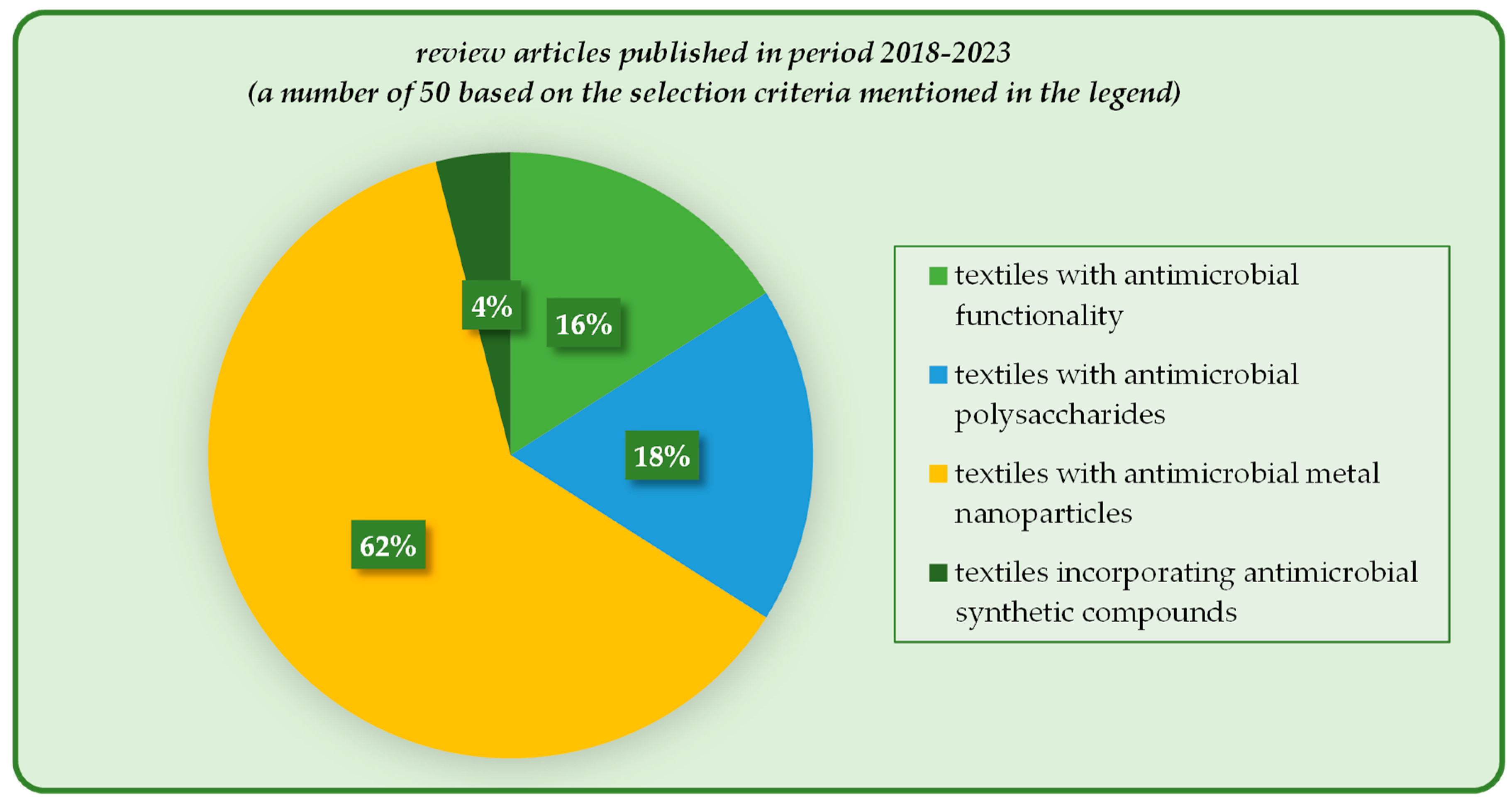
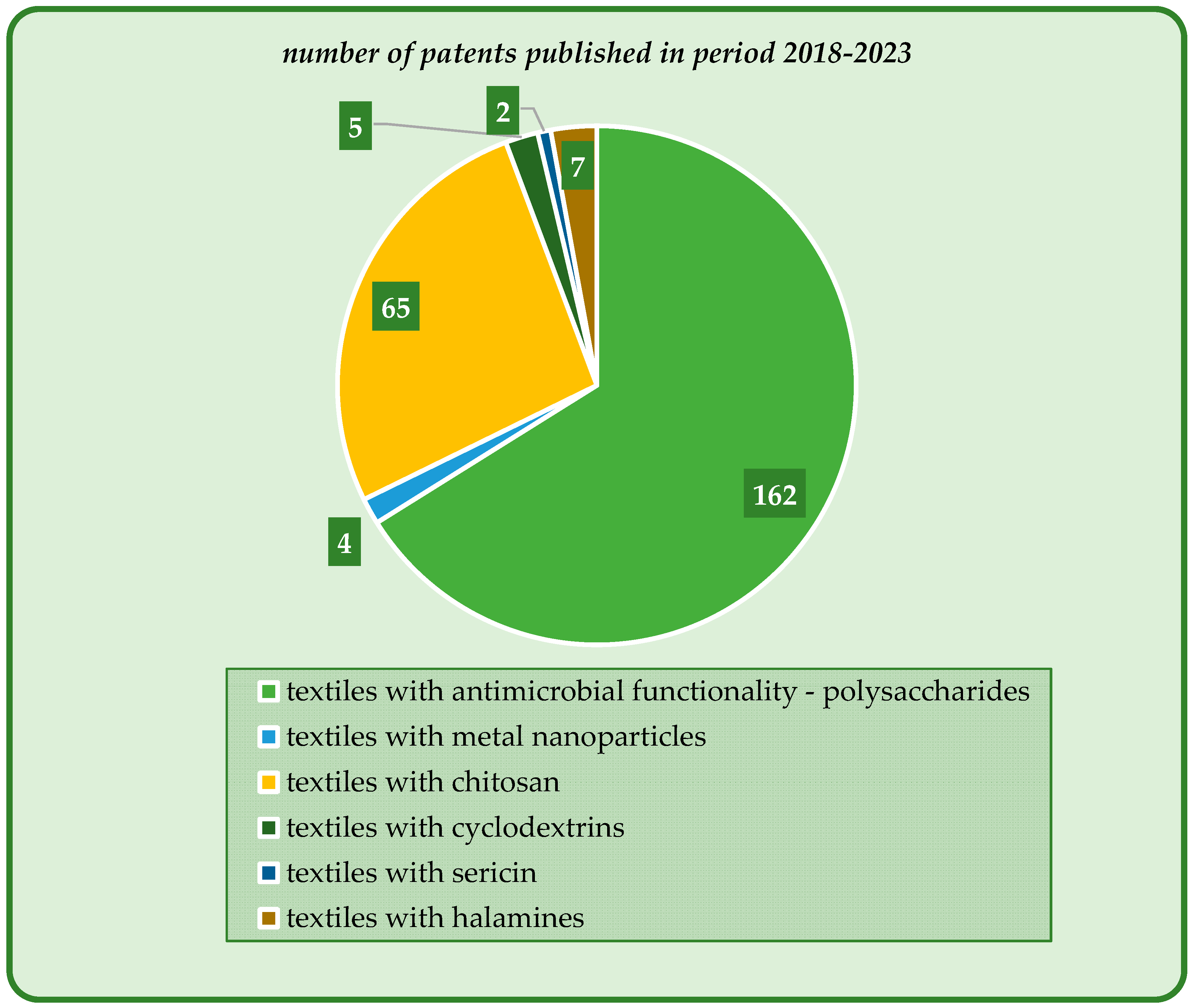

| Antimicrobial Agent | Properties and Applications | Antimicrobial Mechanism | Ref. |
|---|---|---|---|
| Quaternary ammonium compounds Polymeric materials having onium salts (quaternary ammonium and/or phosphonium salts) Quaternary ammonium polyethylenimine | - Healthcare, household products, surface preservation, food industry, pharmaceutical/cosmetic (preservation) - Highly effective as antimicrobial agents in orthodontic cements to introduce antibacterial activity toward S. mutants and L. casei | The long, lipophilic alkyl chain of the quaternary ammonium compounds perforates cell membranes, and produces the release of cytoplasmic components, autolysis and cell death of the microbial strain | [52,55,56,57,58,59] |
| Halogenated phenols Triclosan | - Antiseptic, disinfectant, fungicide, pesticide, antimicrobial, antiseptic, preservative - Antimicrobial activity against many types of Gram-positive and Gram-negative non- spore-forming bacteria, some fungi - Clinical settings, consumer products (cosmetics, cleaning products, paint, plastic materials, toys) - Durable antifungal finishing of cotton fabrics | Inhibits the active site of enoyl-acyl carrier protein reductase enzyme, which is essential to the fatty acids synthesis of bacteria and the building of the cell membrane | [10,58,60,61] |
| Chlorhexidine Hexametaphosphate salt of chlorhexidine (as nanoparticles) Polyhexamethylene biguanide (PHMB) | - Preoperative skin cleansing preparations, hand disinfectants, and oral mouth rinses - Efficient antimicrobial agent against gram-negative and -positive bacteria and yeasts. - Biomedical materials and consumer products - Antimicrobial efficacy against MRSA and P. aeruginosa, in both planktonic and biofilm growth conditions - Healthcare uniforms - Nonspecific antimicrobial properties and remained efficient (>99% against S. aureus and K. pneumoniae) after use for 5 months | Chlorhexidine inhibits membrane-bound ATPase, based on cell membrane disruption and leakage of intracellular constituents, a rapid process with most damage occurring within 20 s of exposure The positively charged biguanidines bind to negatively charged phosphate group of the bacterial cell wall or virus envelope, breaking the membrane integrity, which leads to cell lysis and subsequent cell death | [25,62,63,64] |
| N-halamines | - Antimicrobial activity against a broad spectrum of microorganisms, rechargeability, nontoxicity to humans - Medical devices, water purification, hospitals, antibacterial modification of cotton fabrics - Antimicrobial activity against aerosolized bacteria - Air filtration technology - Biocidal properties against S. aureus and E. coli - Food packaging and biomedical applications | The direct transfer of oxidative halogens to a cell after contact resulting in oxidation of the amino acids in the cell membrane and inactivation the microorganism | [46,49,65,66,67,68] |
| 5,5-dimethylhydantoin | Cotton fabric with regenerable antibacterial properties against S. aureus | Coating dimethylhydantoin on cotton fabric (by pad–dry–plasma–cure process) followed by chlorination inhibits the bacteria | [69] |
| Cinnamic acid derivatives | Pharmacological, antifungal, and antibacterial action | Plasma membrane disruption, nucleic acid and protein damage, and the induction of intracellular reactive oxygen species | [70,71,72] |
| Polyaniline and its derivatives | - Bacteria-resistant surfaces against S. aureus and E. coli - Wall and room-door coatings in hospitals | Different oxidation states of polyaniline and presence of functional groups | [73] |
| Polypyrrole (nanoparticles) | Antimicrobial treatment against S. aureus and E. coli of polyester fabrics | The positive charges (=NH+) in the polypyrrole backbone that are created by dopant compounds | [74,75] |
| Polythiophenes | Antimicrobial compounds able to kill bacteria selectively by damaging negatively charged cell envelopes | Cationic charges with capacity to create huge amounts of singlet oxygen that interact with organism | [76] |
| Fiber/Textile Type | Preparation | Morphology/Content | Microbial Strains | Applicative Characteristics | Refs. |
|---|---|---|---|---|---|
| Plain weave 100% bleached organic cotton fabric | Dip and dry coating | 49.23 mg/kg, 73.28 mg/kg; eventually embedded in alginate matrix | Gram-positive Staphylococcus aureus ATCC 6538/Gram-negative Escherichia coli ATCC 873937 | Antibacterial and UV protection; superior coloration effect; leakage of about 2.04 mg/kg/cycle during first fifteenth washing cycle | [120] |
| Brown cotton fiber | In situ one-step process under heating | 8−21 nm spherical particles; 12.8 µg/kg weight fraction formation of Ag NPs | Gram-positive Staphylococcus aureus ATCC 6538/Gram-negative Pseudomonas aeruginosa ATCC 9027 | Stable antibacterial activity for 50 cycles of laundering; good dispersion; potential applications in sportswear, underwear, and medical textiles | [121] |
| Commercial polyamide 6,6 fabric | PVP-AgNP dispersions deposited on PA66 with/without DBD plasma pretreatment | 20 nm PVP-AgNP colloids | Gram-positive Staphylococcus aureus ATCC 6538/Gram-negative Escherichia coli ATCC 25922 | Plasma-treated polyamide fabric maintains antimicrobial activity even at very low Ag concentration after five washing cycles | [122] |
| Cellulosic/cotton fabrics | Photochemical reduction in Na–CMC solutions | 2–8 nm/5–35 nm spherical polydisperse/monodisperse nanoparticles | Staphylococcus epidermidis/Candida albicans | Antifungal effect; prevents odor formation | [123] |
| Polyamide 6 fibers | Electroless plating method; fibers pretreated with a dopamine/CuSO4/H2O2 system | Average particle size of 223 nm; surface continuous and compact silver layer | Escherichia coli AATCC 11229/Staphylococcus aureus AATCC 6538 | Antimicrobial efficiency of 99.9% and 100% against E. coli and S. aureus decrease to 83.5% and 87.9%, respectively after 1 h/2 h of ultrasonic washing; potential use as antibacterial/conductive textiles | [124] |
| Commercial prewashed PES fabric | Spray coating of PES with layers of chitosan or HMDSO before and after AgNP deposition | Quasi-spherical particles of 20–30 nm with relative uniform distribution | Staphylococcus aureus/Escherichia coli | Fast and cost-effective method; controlled release of silver; antimicrobial effect reduced by washing; applications in medical textiles | [23] |
| Reusable and single use face masks | Testing of commercial face masks from a safe-by-design perspective | Silver detected in both the external and the internal layer under both ionic and nanoparticulate form; mostly near-spherical particles of 13 to 155 nm | viral pathogens | Evaluation of content, type and in situ localization of silver-based biocides face; safety of silver-containing masks | [125] |
| Scoured and bleached 100% cotton fabric of plain weave structure | In situ deposition of Ag nanoparticles on cotton fabrics premodified with dopamine | Medium size of 33–43 nm | Staphylococcus aureus/Escherichia coli | Dopamine is effective in nanoparticles immobilization; ~98% remanent activity after twenty wash cycles | [126] |
| Fiber/Textile Type | Preparation | Morphology/Content | Microbial Strains | Applicative Characteristics | Refs. |
|---|---|---|---|---|---|
| Bleached and mercerized cotton fabric (100%) | Pure and hybrid CuO/colloidal chitosan nanosol sonochemically prepared; cotton coating by pad–dry–cure method | Spherical morphology with irregular formation; medium size of 58 nm | Staphylococcus aureus/Escherichia coli | Improved antibacterial activity for hybrid coatings after ten wash cycles | [127] |
| Fine–medium-weight 100% cotton woven fabric | Ex situ by wet chemical method/pad–dry–cure method | Spherical shape; size of 60–75 nm | Staphylococcus aureus/Escherichia coli | Antimicrobial activity decreases at laundering (S. aureus: 74.36%/12.05% after 10/20 cycles; E. coli: 69.54%/9.85% after 10/20 cycles washes; potential healthcare and hygiene uses | [128] |
| Cotton fabrics | Green synthesis with R. tuberosa leaf extract | Polydisperse nanorods ranging from 20 to 100 nm | Staphylococcus aureus; Escherichia coli; Klebsiella pneumoniae | Prevention of fabrics microbial damage; bioremediation of industrial dyes | [129] |
| Polyester/cotton 65/35 blend fabric (PES/CO) | In situ impregnation by the pad–dry/pad–dry/pad–thermofix process | Sizes of about 3 nm and 20 nm | antiviral species: SARS-CoV-2_COV2019 ITALY/INMI1 and Human Corona Virus 229E strain ATCC VR-740; Escherichia coli ATCC 25922 strains | 99.93%; 99.96% inactivation efficiency (30; 60 min exposure) against SARS-CoV-2; 99% efficiency on E. coli growth after 20 wash cycles; reusable face masks with antiviral/antibacterial properties and reduced environmental contamination | [130] |
| 70% cotton and 30% polyester mixed textiles | In situ and ex situ green and chemical syntheses | Green route: spherical morphology with sizes of 2.4 ± 0.5 nm; chemical route: no defined geometry with average size of 75 ± 28 nm | E. coli ATCC No. 8739/S. aureus ATCC No. 6538 bacteria; Aspergillus brasiliensis ATCC No. 16 404 fungus | In situ method and 734 ppm Cu2O gives better antifungal effects; high potential against aspergillosis | [131] |
| Rolls of cotton, plain unbleached woven cotton | In situ sonochemical method; “throwing the stones” (TTS) technology with preformed colloids and ultrasound impregnation | Homogeneous layer of ~40 nm nanoparticles on cotton fibers (0.9% w/w CuO) | HDF/HepG2 cells | Low toxicity (>95% HDF cell viability); nanoparticles do not penetrate the skin barrier; potential uses as antimicrobial fabrics for bed sheets, curtains, and laboratory coats | [132] |
| 100% cotton fabric | In situ by exhaust dyeing method | Small nanoparticles of different sizes and shapes randomly distributed on fiber surfaces | Escherichia coli | Still efficient after 20 washes, could be an economic alternative for antimicrobial textiles | [133] |
| Fabric samples | CuO biosynthesis with Aspergillus terreus strain AF-1; ex situ pad–dry–cure method | Homogeneous distributions of spherical, 11–47 nm nanoparticles; 6.1% elemental composition | Bacillus subtilis ATCC 6633, Staphylococcus aureus ATCC 6538, Escherichia coli ATCC 8739, and Pseudomonas aeruginosa ATCC 9027 | Green synthesis; potential uses in healthcare and hygiene products | [134] |
| Cotton fabric | Plasma pretreated cotton fabric; ex situ coating by pad–dry–cure method | Fabric roughness gradually rises with increases in plasma treatment time; 40 nm sized CuO nanoparticles | Bacillus subtilis, Staphylococcus aureus, Salmonella typhimurium, Klebsiella pneumoniae | Potential uses in various biomedical applications | [135] |
| Fiber/Textile Type | Preparation | Morphology/Content | Microbial Strains | Applicative Characteristics | Refs. |
|---|---|---|---|---|---|
| Bleached woven cotton fabric (100%; 144 g/m2) | Single-step sonoenzymatic process | 30–120 nm Zn nanoparticles | Staphylococcus aureus; Escherichia coli | Nanoparticles agglomeration regardless the enzyme used; 33.4% Zn retained on fabrics after ten washing cycles; potential antibacterial medical textiles | [136] |
| 100% plain woven cotton fabrics | Plasma pretreated cotton woven fabric; in situ, sonochemically | Spherical shape with 20–90 nm diameter | Staphylococcus aureus | Stability improves by cotton fabric prefunctionalization with plasma; Zn content goes from 5.63% to 5.41% after five washing cycles | [137] |
| Polyamide 6 (PA), polyethylene terephthalate (PET) and polypropylene (PP) textiles | Chemical bath deposition; washing; thermal stabilization; hydrothermal formation of nano/microrods | Irregular needles, flower-like agglomerates and nano/microrods | Escherichia coli; Staphylococcus aureus | Significant antibacterial activity, particularly in the case of PA/ZnO and Gram-negative bacteria; potential uses in everyday life applications | [138] |
| 100% cotton yarns and polyester/cotton (67/33) blend yarns | Exhaust–dry–cure method | Sizes ranging between 30 and 90 nm | Staphylococcus aureus; Escherichia coli | Antimicrobial efficacy of samples increases at blends, higher yarn twists and lower particle sizes | [139] |
| 100% cellulose cotton | Pad–dry–cure method assisted by open-air plasma modification; green sonochemically nanoparticle synthesis with Psidium guajava Linn (guava) plant extract | Hexagonal nanoparticles of about 41 nm agglomerated into larger clusters | Staphylococcus aureus; Escherichia coli | Open-air plasma treatment enhances nanoparticle adsorption; self-cleaning activity of 94% after five washing cycles | [140] |
| Gray cotton fabric (100% cotton) of plain weave structure | Pad–dry–cure method and thermo-fixation with sonochemically synthesized ZnO nanoparticles | Nearly spherical nanoparticles with an average size of 40–100 nm; 0.5%, 1%, and 2% ZnO content | Staphylococcus aureus; Escherichia coli | 86% reduction of microorganisms after 15 washes; uses as multifunctional textiles with antimicrobial, self-cleaning, and UV protective properties | [141] |
| Fiber/Textile Type | Preparation | Morphology/Content | Microbial Strains | Applicative Characteristics | Refs. |
|---|---|---|---|---|---|
| Polyamide 66 cloth in plain weave | Pad–dry–cure process | 700 nm particles | Aspergillus niger NRRL-A326 (fungus)/Staphylococcus aureus ATCC 6538-P (G+)/Escherichia coli ATCC 25933 (G)/Candida albicans ATCC 10231 (yeast) | Hydrophobic; photocatalytic self-cleaning activity; UV protection activity; potential applications in air filters, outdoor textiles, furniture, and medical textiles | [142] |
| Nylon 66 knitted fabrics | Synthesized by sol–gel method; subsequently applied by layer by layer (LBL) technique | Medium size of 40–60 nm; tendency to aggregation | Staphylococcus aureus (NCTC 3750)/Escherichia coli (AATCC-10148) | Potential applications in optics, biosensing, separation membranes and technical textile | [143] |
| Cotton–polyester twill fabric (70–30%) | In situ coating | Average diameter size of 98 nm | - | UV protective properties | [144] |
| Cotton fabric | Immersion in a mixture of perfluorodecyl triethoxysilane and TiO2NPs solution | Medium size of 50 nm; uniform coating | E. coli | Water repellency; self-cleaning; oil–water separation; stain resistance; antibacterial properties | [145] |
| Plain woven cotton mercerized fabric | Pad–dry–cure method; functionalization with trimethyl[3-trimethoxysilyl) propyl] ammonium chloride to enhance the affinity | Particles of 30 nm; 4% dried TiO2 NPs by weight | S. aureus/E. coli | Dye degradation; antibacterial properties; multifunctional cotton fabric for outdoor, industrial and medical applications | [146] |
| Cotton fabrics lab coat and indiolino fabrics | In situ impregnation; sonochemically, hydrothermal and solvothermal synthesis of TiO2 particles | Homogeneous distribution on the cotton fabric surface | Escherichia coli/Bacillus pumilus | Sonosynthesis with Ti isopropoxide as precursor enhances the bactericidal activity; self-cleaning properties; potential use for face masks | [147] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanasa, F.; Teaca, C.-A.; Nechifor, M.; Ignat, M.; Duceac, I.A.; Ignat, L. Highly Specialized Textiles with Antimicrobial Functionality—Advances and Challenges. Textiles 2023, 3, 219-245. https://doi.org/10.3390/textiles3020015
Tanasa F, Teaca C-A, Nechifor M, Ignat M, Duceac IA, Ignat L. Highly Specialized Textiles with Antimicrobial Functionality—Advances and Challenges. Textiles. 2023; 3(2):219-245. https://doi.org/10.3390/textiles3020015
Chicago/Turabian StyleTanasa, Fulga, Carmen-Alice Teaca, Marioara Nechifor, Maurusa Ignat, Ioana Alexandra Duceac, and Leonard Ignat. 2023. "Highly Specialized Textiles with Antimicrobial Functionality—Advances and Challenges" Textiles 3, no. 2: 219-245. https://doi.org/10.3390/textiles3020015
APA StyleTanasa, F., Teaca, C.-A., Nechifor, M., Ignat, M., Duceac, I. A., & Ignat, L. (2023). Highly Specialized Textiles with Antimicrobial Functionality—Advances and Challenges. Textiles, 3(2), 219-245. https://doi.org/10.3390/textiles3020015