Recipe for the One-Pot Synthesis of C-/O-Doped Luminescent Boron Nitride Quantum Dots with Tunable Optical Properties for Bioapplications
Abstract
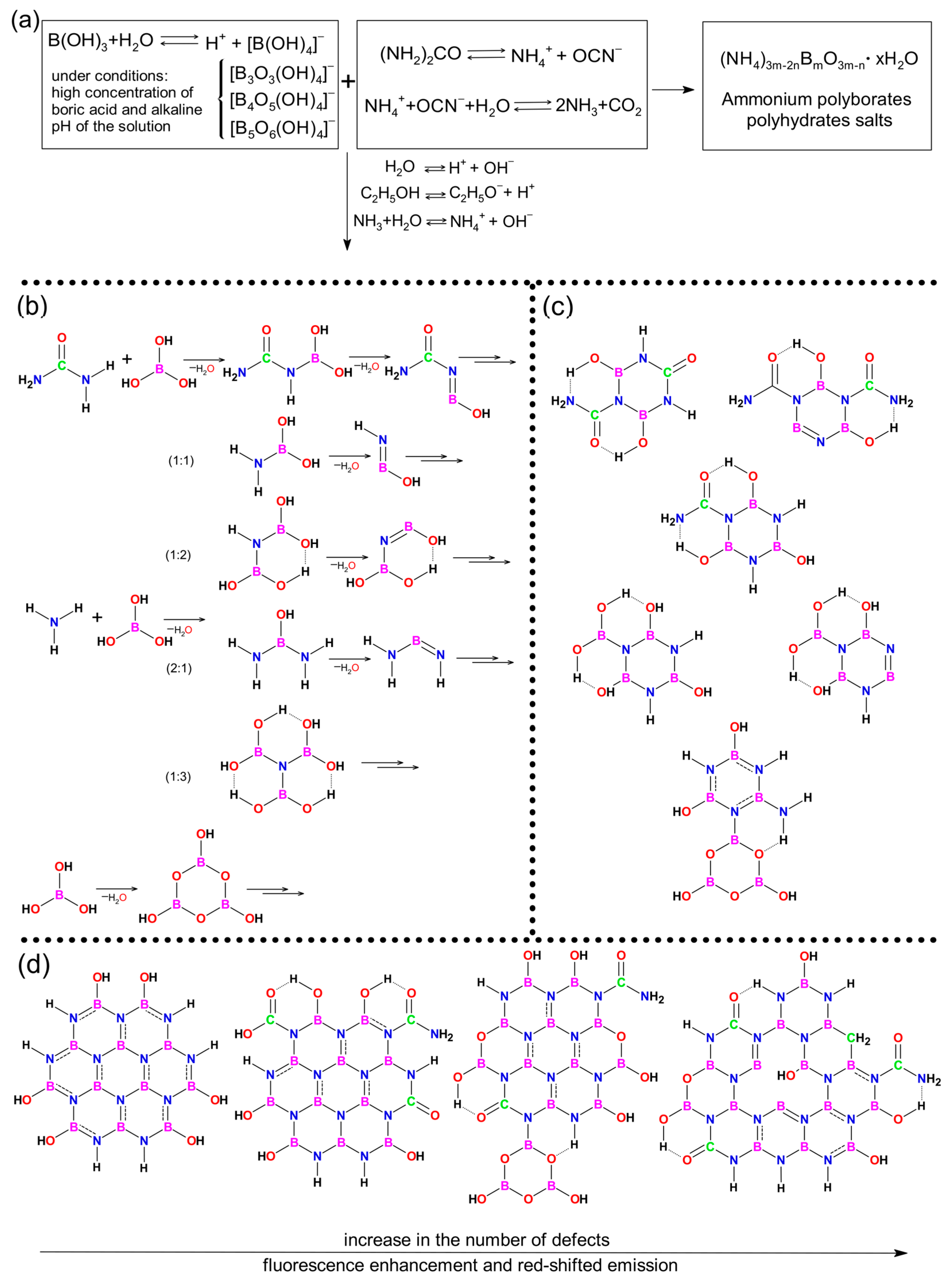
1. Introduction
| Name of Obtained Nanomaterials | B Precursor | N Precursor | T (°C)/ t (h) | Solvent Systems | References |
|---|---|---|---|---|---|
| BNQDs (BONDs) | Boric acid H3BO3 | 30–33% NH3 (aq) | 120–200/ 5–24 | H2O | [11,23,25,26,27,28,29,30] |
| h-BNQDs (BNQDs, h-BNNPs) | Melamine C3H6N6 | 200/ 15,24 | H2O | [31,32,33,34,35,36] | |
| BNQDs (h-BNQDs) | Urea (H2N)CO | 200/12 | Ethanol, H2O, 10% NH3(aq) | [37,38] | |
| 200/15 | H2O | [39] | |||
| h-BNQDs | Urea (H2N)CO/ Thiourea (H2N)SO/ Melamine C3H6N6 | 200/24 | H2O | [40] | |
| BNQDs | Amino acids (tryptophan, cysteine, arginine, phenylalanine, tyrosine, aspartic acid, valine, isoleucine, histidine) | 200/10 | H2O | [41] | |
| BNNPs | Aliphatic amine | 150/4 | H2O | [21] | |
| o-BNQDs | Dicyandiamide C2H4N4 | 160–230/ 10–15 | Anhydrous ethanol | [10] |
2. Materials and Methods
2.1. Synthesis of BNQDs
2.2. Structural and Optical Characterization of BNQDs
2.2.1. Scanning Electron Microscopy (SEM) and Energy Dispersive X-Ray Spectroscopy (EDX)
2.2.2. Dynamic Light Scattering (DLS) and Zeta-Potential
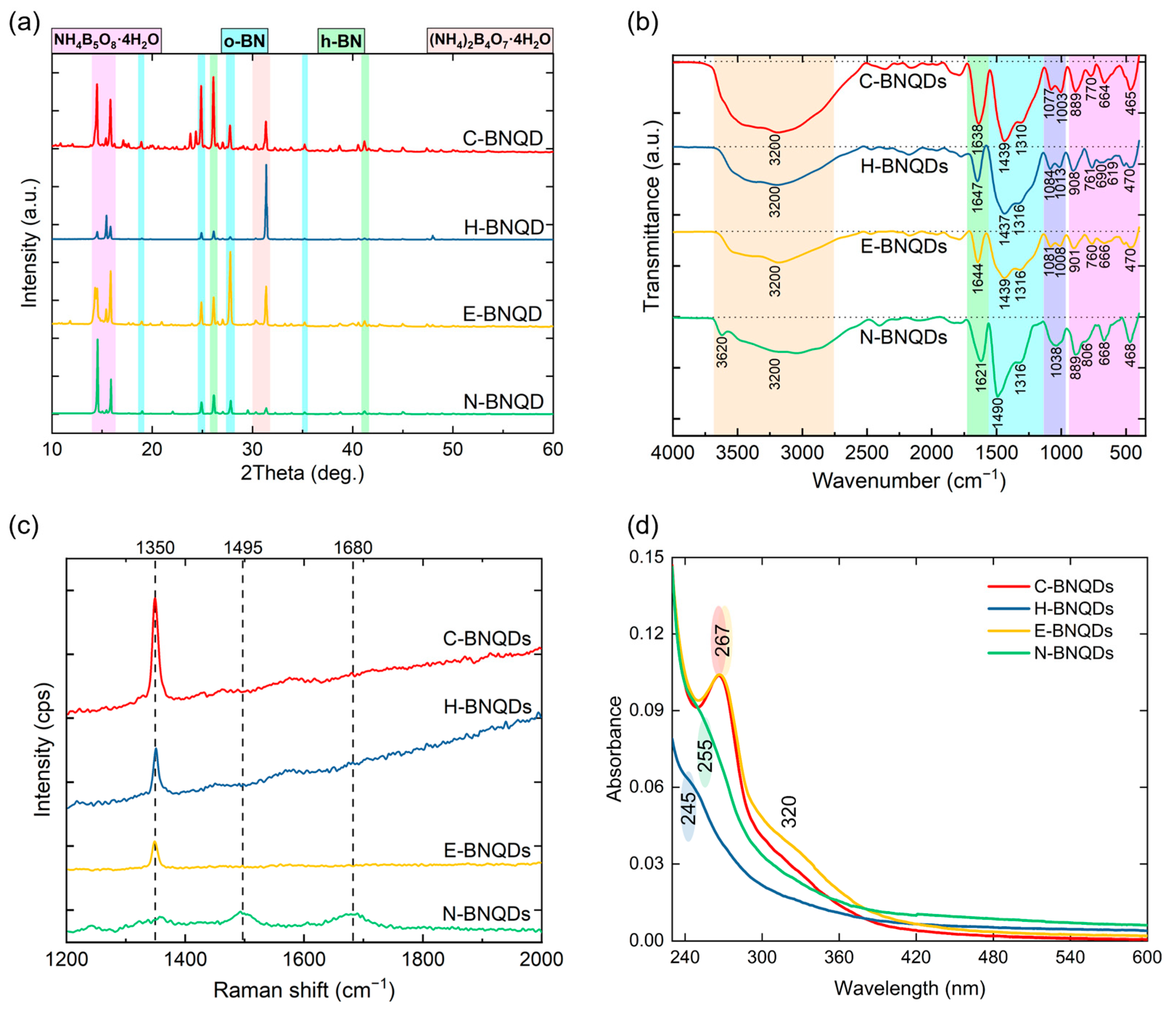
2.2.3. X-Ray Diffraction (XRD)
2.2.4. Infrared Spectroscopy by Fourier Transform (FTIR)
2.2.5. Raman Spectroscopy
2.2.6. UV–Vis Spectroscopy
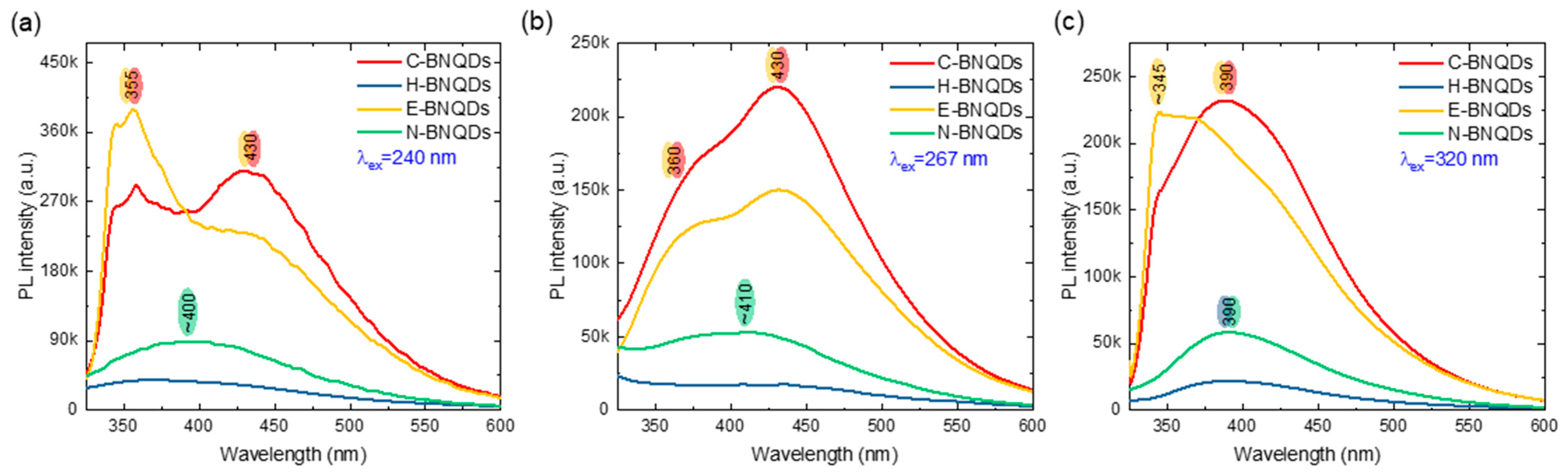
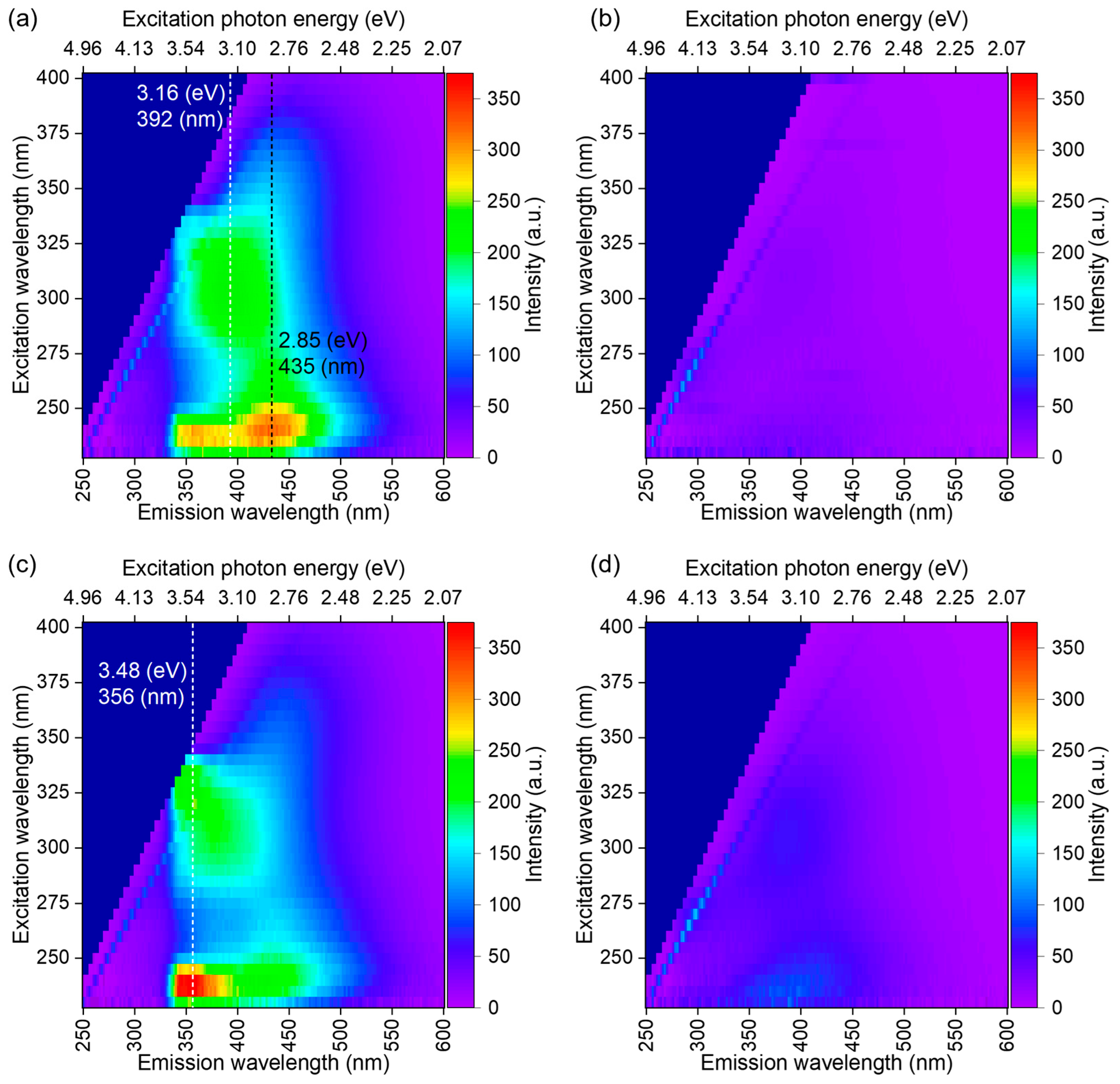
2.2.7. PL, PLE Spectroscopy and 2D PLE Mapping
2.3. Fluorescence Microscopy of BNQDs in Vero and MDBK Cells
2.4. Cytotoxicity Assay of BNQDs
3. Results
3.1. Morphology, Chemical Composition and Yield of Synthesized BNQDs
3.2. Characterization of Size and Zeta-Potential of BNQDs
3.3. Structural Characterization of BNQDs
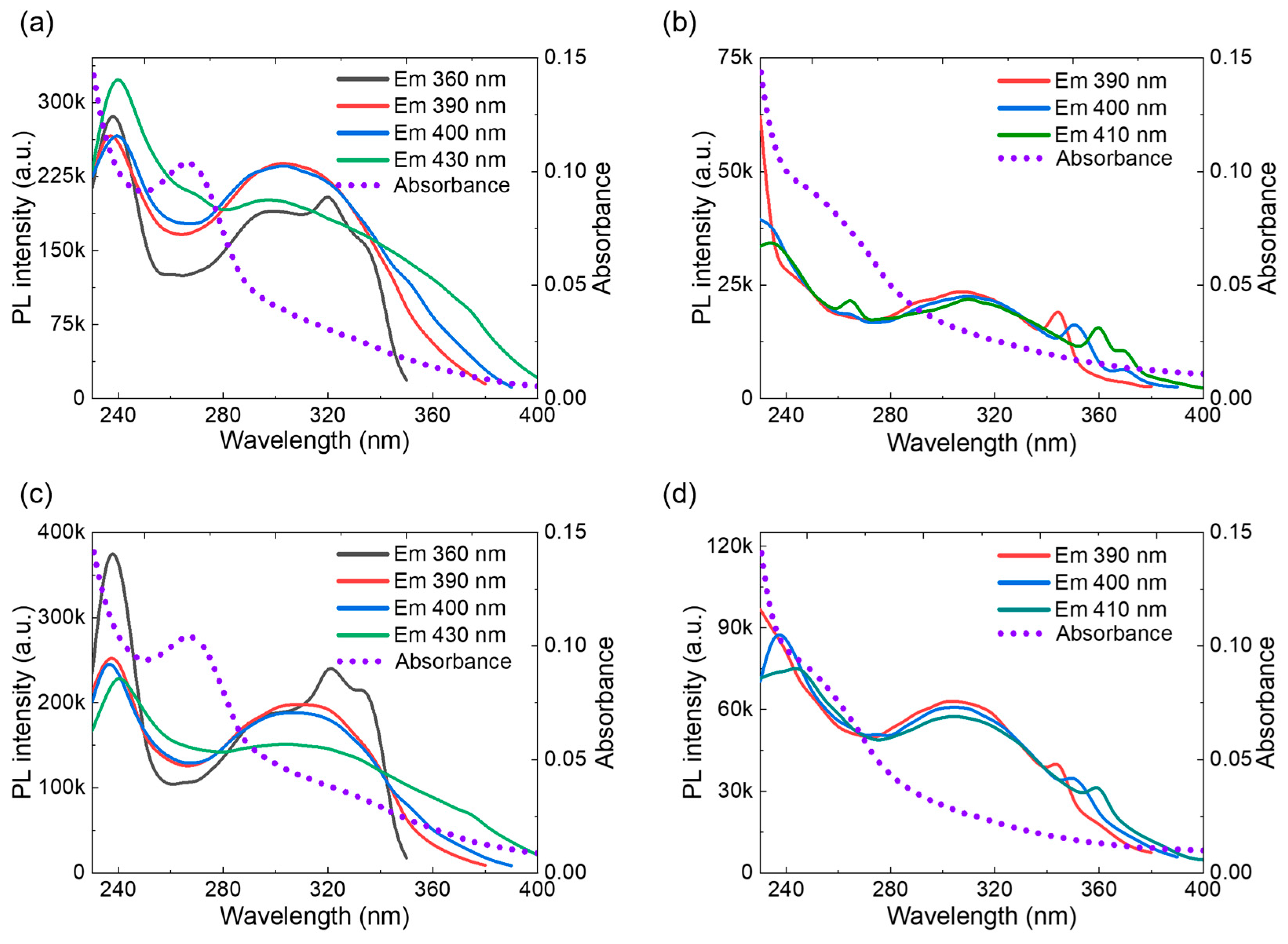
3.4. Optical Characterization of BNQDs
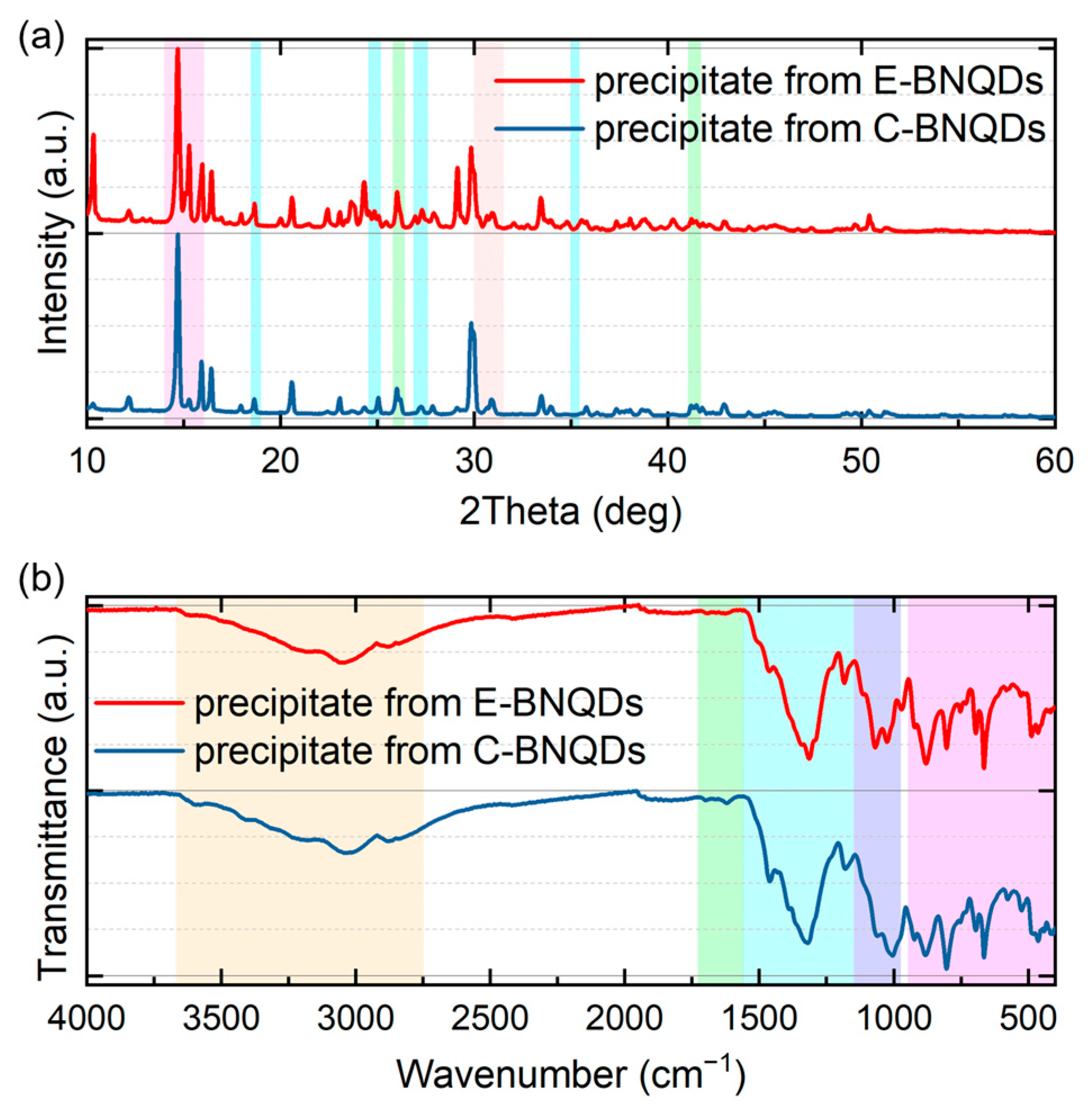
3.5. Characterization of C-BNQD and E-BNQD Precipitates
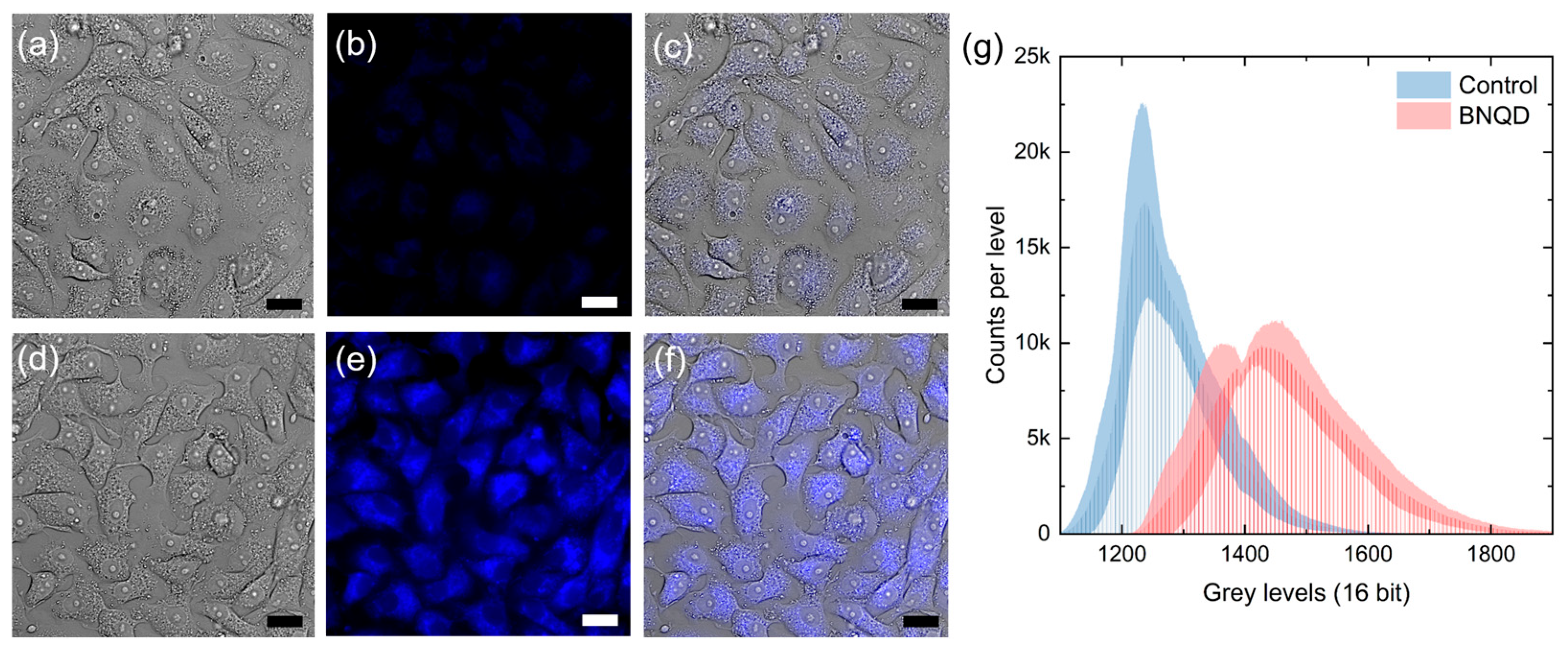
3.6. Bioimaging of BNQDs in Vero and MDBK Cells
3.7. In Vitro Analysis of BNQD Cytotoxicity
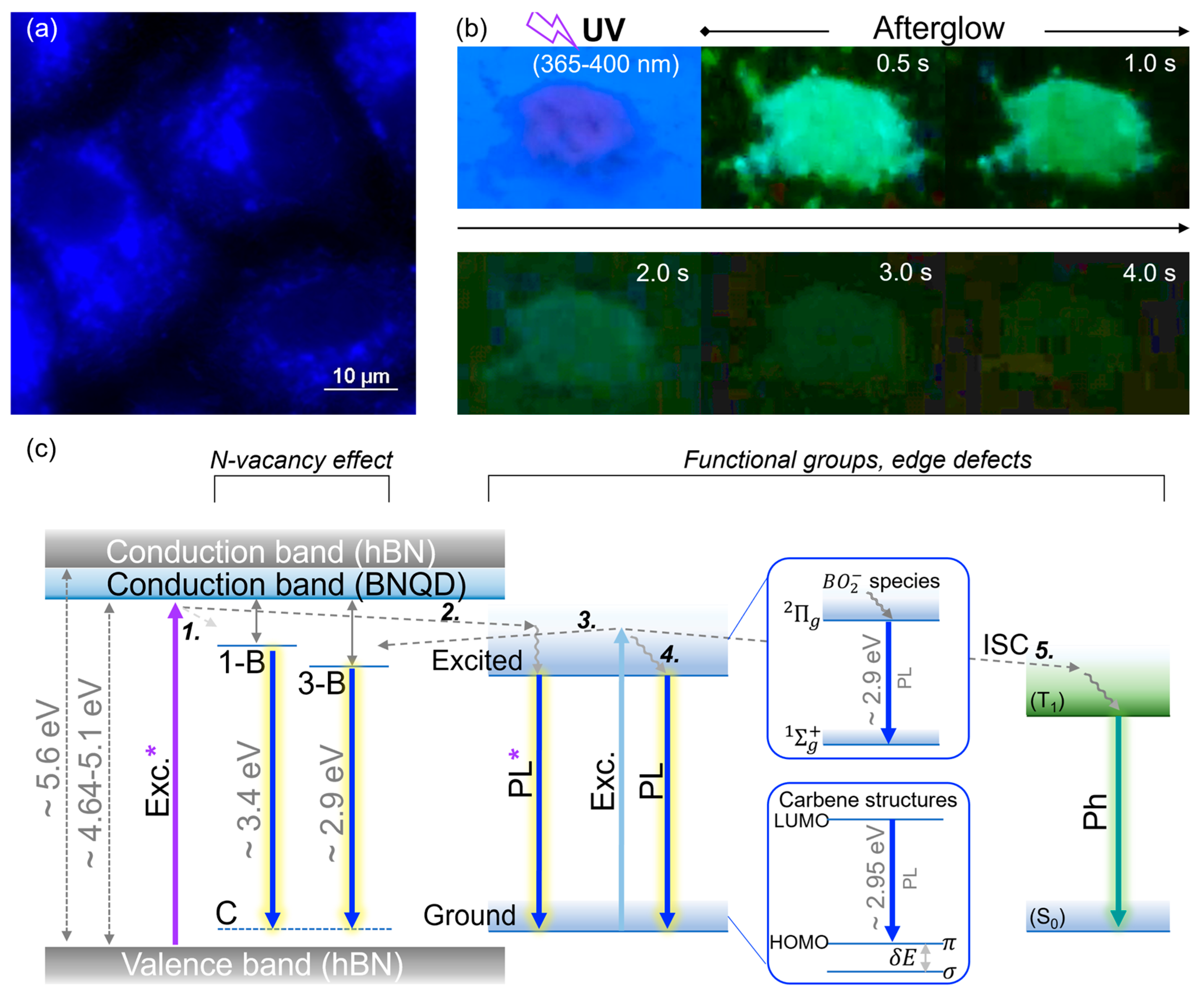
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weng, Q.; Wang, X.; Wang, X.; Bando, Y.; Golberg, D. Functionalized hexagonal boron nitride nanomaterials: Emerging properties and applications. Chem. Soc. Rev. 2016, 45, 3989–4012. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Weng, Q.; Wang, X.; Li, X.; Zhang, J.; Golberg, D.; Bando, Y. Recent Progress on Fabrications and Applications of Boron Nitride Nanomaterials: A Review. J. Mater. Sci. Technol. 2015, 31, 589–598. [Google Scholar] [CrossRef]
- Ba, K.; Jiang, W.; Cheng, J.; Bao, J.; Xuan, N.; Sun, Y.; Liu, B.; Xie, A.; Wu, S.; Sun, Z. Chemical and Bandgap Engineering in Monolayer Hexagonal Boron Nitride. Sci. Rep. 2017, 7, 45584. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Stagi, L.; Carbonaro, C.; Malfatti, L.; Casula, M.; Ricci, P.; Del Rio Castillo, A.; Bonaccorso, F.; Calvillo, L.; Granozzi, G.; et al. Defect-assisted photoluminescence in hexagonal boron nitride nanosheets. 2D Mater. 2020, 7, ababf0. [Google Scholar] [CrossRef]
- Berzina, B.; Korsaks, V.; Trinkler, L.; Sarakovskis, A.; Grube, J.; Bellucci, S. Defect-induced blue luminescence of hexagonal boron nitride. Diam. Relat. Mater. 2016, 68, 131–137. [Google Scholar] [CrossRef]
- Lin, L.; Xu, Y.; Zhang, S.; Ross, I.; Ong, A.; Allwood, D. Fabrication and luminescence of monolayered boron nitride quantum dots. Small 2014, 10, 60–65. [Google Scholar] [CrossRef]
- Tang, C.; Bando, Y.; Zhi, C.; Golberg, D. Boron-oxygen luminescence centres in boron-nitrogen systems. Chem. Commun. 2007, 44, 4599–4601. [Google Scholar] [CrossRef]
- Li, H.; Tay, R.; Tsang, S.; Zhen, X.; Teo, E. Controllable Synthesis of Highly Luminescent Boron Nitride Quantum Dots. Small 2015, 11, 6491–6499. [Google Scholar] [CrossRef] [PubMed]
- Rawat, J.; Sajwan, D.; Garimella, S.; Sharma, H.; Dwivedi, C. Boron Nitride quantum dots: A rising star in sensing applications. Nano Trends 2023, 2, 100008. [Google Scholar] [CrossRef]
- Chen, P.; Yang, S.; Liu, F.; Jiang, Y.; Wang, Y.; Huang, Y.; Hu, J.; Chen, L. Blue-Emitting Orthorhombic Boron Nitride Quantum Dots and Quantum-Dot Light-Emitting Diodes. Adv. Photon. Res. 2023, 4, 202200344. [Google Scholar] [CrossRef]
- Ren, J.; Malfatti, L.; Enzo, S.; Carbonaro, C.; Calvillo, L.; Granozzi, G.; Innocenzi, P. Boron oxynitride two-colour fluorescent dots and their incorporation in a hybrid organic-inorganic film. J. Colloid Interface Sci. 2020, 560, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Stagi, L.; Malfatti, L.; Carbonaro, C.; Granozzi, G.; Calvillo, L.; Garroni, S.; Enzo, S.; Innocenzi, P. Engineering UV-emitting defects in h-BN nanodots by a top-down route. Appl. Surf. Sci. 2021, 567, 150727. [Google Scholar] [CrossRef]
- Jung, J.; Kotal, M.; Jang, M.; Lee, J.; Cho, Y.; Kim, W.; Oh, I. Defect engineering route to boron nitride quantum dots and edge-hydroxylated functionalization for bio-imaging. RSC Adv. 2016, 6, 73939–73946. [Google Scholar] [CrossRef]
- Umrao, S.; Maurya, A.; Shukla, V.; Grigoriev, A.; Ahuja, R.; Vinayak, M.; Srivastava, R.; Saxena, P.; Oh, I.; Srivastava, A. Anticarcinogenic activity of blue fluorescent hexagonal boron nitride quantum dots: As an effective enhancer for DNA cleavage activity of anticancer drug doxorubicin. Mater. Today Bio 2019, 1, 100001. [Google Scholar] [CrossRef]
- Shtansky, D.; Firestein, K.; Golberg, D. Fabrication and application of BN nanoparticles, nanosheets and their nanohybrids. Nanoscale 2018, 10, 17477–17493. [Google Scholar] [CrossRef]
- Stagi, L.; Ren, J.; Innocenzi, P. From 2-D to 0-D Boron Nitride Materials, The Next Challenge. Materials 2019, 12, 3905. [Google Scholar] [CrossRef]
- Zhang, X.; An, L.; Bai, C.; Chen, L.; Yu, Y. Hexagonal boron nitride quantum dots: Properties, preparation and applications. Mater. Today Chem. 2021, 20, 100425. [Google Scholar] [CrossRef]
- Acharya, A.; Sharma, S.; Liu, X.; Zhang, D.; Yap, Y. A Review on van der Waals Boron Nitride Quantum Dots. C 2021, 7, 35. [Google Scholar] [CrossRef]
- Ren, J.; Stagi, L.; Innocenzi, P. Hydroxylated boron nitride materials: From structures to functional applications. J. Mater. Sci. 2021, 56, 4053–4079. [Google Scholar] [CrossRef]
- Zheng, Z.; Cox, M.; Li, B. Surface modification of hexagonal boron nitride nanomaterials: A review. J. Mater. Sci. 2018, 53, 66–99. [Google Scholar] [CrossRef]
- Kainthola, A.; Bijalwan, K.; Negi, S.; Sharma, H.; Dwivedi, C. Hydrothermal synthesis of highly stable boron nitride nanoparticles. Mater. Today Proc. 2020, 28, 138–140. [Google Scholar] [CrossRef]
- Demazeau, G. Solvothermal Processes: Definition, Key Factors Governing the Involved Chemical Reactions. Z. Naturforsch. 2010, 65b, 999–1006. [Google Scholar] [CrossRef]
- Liu, B.; Yan, S.; Song, Z.; Liu, M.; Ji, X.; Yang, W.; Liu, J. One-Step Synthesis of Boron Nitride Quantum Dots: Simple Chemistry Meets Delicate Nanotechnology. Chem. Eur. J. 2016, 22, 18899–18907. [Google Scholar] [CrossRef] [PubMed]
- Wibowo, A.; Jahir Khan, M.; Sansanaphongpricha, K.; Khemthong, P.; Laosiripojana, N.; Yu, Y.; Wu, K.; Sakdaronnarong, C. Carbon Dots in Photodynamic Therapy: The Role of Dopant and Solvent on Optical and Photo-Responsive Properties. Chem. Eur. J. 2024, 30, e202400885. [Google Scholar] [CrossRef] [PubMed]
- Paul Choudhury, S.; Feng, Z.; Gao, C.; Ma, X.; Zhan, J.; Jia, F. BN quantum dots decorated ZnO nanoplates sensor for enhanced detection of BTEX gases. J. Alloys Compd. 2020, 815, 152376. [Google Scholar] [CrossRef]
- Qin, D.; Jiang, X.; Mo, G.; Feng, J.; Deng, B. Boron nitride quantum dots as electrochemiluminescence coreactants of rGO@Au@Ru–SiO2 for label-free detection of AFP in human serum. Electrochim. Acta 2020, 335, 135621. [Google Scholar] [CrossRef]
- Xing, H.; Zhai, Q.; Zhang, X.; Li, J.; Wang, E. Boron Nitride Quantum Dots as Efficient Coreactant for Enhanced Electrochemiluminescence of Ruthenium(II) Tris(2,2′-bipyridyl). Anal. Chem. 2018, 90, 2141–2147. [Google Scholar] [CrossRef]
- Li, M.; Wang, C.; Chen, L.; Liu, D. A novel electrochemiluminescence sensor based on resonance energy transfer system between nitrogen doped graphene quantum dots and boron nitride quantum dots for sensitive detection of folic acid. Anal. Chim. Acta 2019, 1090, 57–63. [Google Scholar] [CrossRef]
- Peng, C.; Xing, H.; Fan, X.; Xue, Y.; Li, J.; Wang, E. Glutathione Regulated Inner Filter Effect of MnO2 Nanosheets on Boron Nitride Quantum Dots for Sensitive Assay. Anal. Chem. 2019, 91, 5762–5767. [Google Scholar] [CrossRef]
- Yao, Q.; Feng, Y.; Rong, M.; He, S.; Chen, X. Determination of nickel(II) via quenching of the fluorescence of boron nitride quantum dots. Microchim. Acta 2017, 184, 4217–4223. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, X.; Li, C.; Bendavid, A.; Westerhausen, M.; Bradac, C.; Toth, M.; Aharonovich, I.; Tran, T. Bottom-Up Synthesis of Hexagonal Boron Nitride Nanoparticles with Intensity-Stabilized Quantum Emitters. Small 2021, 17, e2008062. [Google Scholar] [CrossRef]
- Huo, B.; Liu, B.; Chen, T.; Cui, L.; Xu, G.; Liu, M.; Liu, J. One-step synthesis of fluorescent boron nitride quantum dots via a hydrothermal strategy using melamine as nitrogen source for the detection of ferric ions. Langmuir 2017, 33, 10673–10678. [Google Scholar] [CrossRef]
- Liu, D.; Song, J.; Chung, J.; Hur, S.; Choi, W. ZnO/Boron Nitride Quantum Dots Nanocomposites for the Enhanced Photocatalytic Degradation of Methylene Blue and Methyl Orange. Molecules 2022, 27, 6833. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, X.; Wang, M.; Ma, Q. A visual electrochemiluminescence resonance energy transfer/surface plasmon coupled electrochemiluminescence nanosensor for Shiga toxin-producing: Escherichia coli detection. Green Chem. 2018, 20, 5520–5527. [Google Scholar] [CrossRef]
- Mohanta, M.; Sahu, T.; Gogoi, D.; Peela, N.; Qureshi, M. Hexagonal Boron Nitride Quantum Dots as a Superior Hole Extractor for Efficient Charge Separation in WO3-Based Photoelectrochemical Water Oxidation. ACS Appl. Energy Mater. 2019, 2, 7457–7466. [Google Scholar] [CrossRef]
- Sahu, T.; Mohanta, M.; Qureshi, M. Modulating water oxidation kinetics utilizing h-BN quantum dots as an efficient hole extractor on fluorine doped hematite photoanode. J. Power Sources 2020, 445, 227341. [Google Scholar] [CrossRef]
- Jerome, R.; Sundramoorthy, A. Hydrothermal Synthesis of Boron Nitride Quantum Dots/Poly(Luminol) Nanocomposite for Selective Detection of Ascorbic Acid. J. Electrochem. Soc. 2019, 166, B3017–B3024. [Google Scholar] [CrossRef]
- Bhasi, A.P.; Wilson, N.H.; Palanisamy, T. Nanosized Hexagonal Boron Nitride and Polyethylene Glycol-Filled Leathers for Applications Demanding High Thermal Insulation and Impact Resistance. ACS Omega 2022, 7, 45120–45128. [Google Scholar] [CrossRef]
- Yang, K.; Jia, P.; Hou, J.; Bu, T.; Sun, X.; Liu, Y.; Wang, L. Innovative dual-emitting ratiometric fluorescence sensor for tetracyclines detection based on boron nitride quantum dots and europium ions. ACS Sustain. Chem. Eng. 2020, 8, 17185–17193. [Google Scholar] [CrossRef]
- Budak, E.; Unlu, C. Effect of Nitrogen Precursor on Optical Properties of Hexagonal Boron Nitride Quantum Dots. J. Turk. Chem. Soc. Sect. A Chem. 2021, 8, 969–976. [Google Scholar] [CrossRef]
- Kong, Y.; He, Y.; Zhou, J.; Zhong, S.; Song, G. Amino Acids as the Nitrogen Source to Synthesize Boron Nitride Quantum Dots for Fluorescence Turn-off-on Detection of Ascorbic Acid. ChemistrySelect 2020, 5, 3828–3834. [Google Scholar] [CrossRef]
- Song, Y.; Wang, J.; Chen, L.; Yang, P. Ammonium pentaborate crystals with adjustable and bright phosphorescence and long lifetime. J. Lumin. 2020, 225, 117325. [Google Scholar] [CrossRef]
- Liu, M.; Xu, Y.; Wang, Y.; Chen, X.; Ji, X.; Niu, F.; Song, Z.; Liu, J. Boron Nitride Quantum Dots with Solvent-Regulated Blue/Green Photoluminescence and Electrochemiluminescent Behavior for Versatile Applications. Adv. Opt. Mater. 2017, 3, 1600661. [Google Scholar] [CrossRef]
- Men, J.; Li, B.; Li, J.; Li, G.; Chen, J.; Hou, X. Amorphous liquid phase induced synthesis of boron nitride nanospheres for improving sintering property of h-BN/ZrO2 composites. Ceram. Int. 2020, 46, 8031–8038. [Google Scholar] [CrossRef]
- Wakasugi, T.; Tsukihashi, F.; Sano, N. Thermodynamics of Nitrogen in B2O3, B2O3-SiO2, and B2O3-CaO Systems. J. Am. Ceram. Soc. 1991, 7, 1650–1653. [Google Scholar] [CrossRef]
- Andrews, L.; Burkholder, T. Infrared spectra of molecular B(OH)3 and HOBO in solid argon. J. Chem. Phys. 1992, 97, 7203–7210. [Google Scholar] [CrossRef]
- Medeiros, S.; Azevedo, S. Electronic and optical properties of quantum emitters in h-BN. Appl. Phys. A Mater. 2023, 129, 659. [Google Scholar] [CrossRef]
- Watanabe, K.; Taniguchi, T.; Kanda, H. Direct-bandgap properties and evidence for ultraviolet lasing of hexagonal boron nitride single crystal. Nat. Mater. 2004, 3, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Sarath Kumar, C.; Reji, R.; Sivalingam, Y.; Kawazoe, Y.; Surya, V. Carbon and boron nitride quantum dots as optical sensor probes for selective detection of toxic metals in drinking water: A quantum chemical prediction through structure- and morphology-dependent electronic and optical properties. RSC Adv. 2024, 14, 28182–28200. [Google Scholar] [CrossRef]
- Weng, Q.; Kvashnin, D.; Wang, X.; Cretu, O.; Yang, Y.; Zhou, M.; Zhang, C.; Tang, D.; Sorokin, P.; Bando, Y.; et al. Tuning of the Optical, Electronic, and Magnetic Properties of Boron Nitride Nanosheets with Oxygen Doping and Functionalization. Adv. Mater. 2017, 29, 1700695. [Google Scholar] [CrossRef]
- Li, H.; Qiao, W.; Shen, Y.; Xu, H.; Fan, Y.; Liu, Y.; Lan, Y.; Gong, Y.; Chen, F.; Feng, S. Biomimetic Boron Nitride Nanoparticles for Targeted Drug Delivery and Enhanced Antitumor Activity. Pharmaceutics 2023, 15, 1269. [Google Scholar] [CrossRef]
- Meilakhs, A.; Koniakhin, S. New explanation of Raman peak redshift in nanoparticles. Superlattices Microst. 2017, 110, 319–323. [Google Scholar] [CrossRef]
- Dubey, R.; Cowles, M.; Salimi, Z.; Liu, X.; Oakley, R.; Yapici, N.; Uddin, J.; Zhang, D.; Yap, Y. Boron nitride nanosheets, quantum dots, and dots: Synthesis, properties, and biomedical applications. APL Mater. 2025, 13, 040601. [Google Scholar] [CrossRef]
- Guerra, T.; Araujo, L.; Azevedo, S. Magnetic and electronic properties of diamond-shaped graphene-boron nitride nanoribbons and nanoflakes. J. Phys. Chem. Solids 2019, 135, 109085. [Google Scholar] [CrossRef]
- Ding, Y.; He, P.; Li, S.; Chang, B.; Zhang, S.; Wang, Z.; Chen, J.; Yu, J.; Wu, S.; Zeng, H.; et al. Efficient Full-Color Boron Nitride Quantum Dots for Thermostable Flexible Displays. ACS Nano 2021, 15, 14610–14617. [Google Scholar] [CrossRef] [PubMed]
- Weston, L.; Wickramaratne, D.; Mackoit, M.; Alkauskas, A.; Van De Walle, C. Native point defects and impurities in hexagonal boron nitride. Phys. Rev. B 2018, 97, 214104. [Google Scholar] [CrossRef]
- Wang, C.; Long, Y.; Deng, Y.; Han, Y.; Tishkevich, D.; Ha, M.; Weng, Q. Hexagonal boron nitride nanomaterials for biomedical applications. BMEMat 2024, 2, 12068. [Google Scholar] [CrossRef]
- Han, B.; Lei, X.; Li, D.; Liu, Q.; Chen, Y.; Wang, J.; He, G. Shallow Traps in Carbon Nitride Quantum Dots to Achieve 6.47 s Ultralong Lifetime and Wavelength-Tunable Room Temperature Phosphorescence. Adv. Opt. Mater. 2023, 11, 2202293. [Google Scholar] [CrossRef]
- Kochkodan, V.; Darwish, N.; Hilal, N. The Chemistry of Boron in Water. In Boron Separation Processes, 1st ed.; Kabay, N., Bryjak, M., Hilal, N., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 35–63. [Google Scholar]
- Panyachariwat, N.; Steckel, H. Stability of urea in solution and pharmaceutical preparations. J. Cosmet. Sci. 2014, 65, 187–195. [Google Scholar] [PubMed]
- Kim, D.; Nakajima, S.; Sawada, T.; Iwasaki, M.; Kawauchi, S.; Zhi, C.; Bando, Y.; Golberg, D.; Serizawa, T. Sonication-assisted alcoholysis of boron nitride nanotubes for their sidewalls chemical peeling. Chem. Commun. 2015, 51, 7104–7107. [Google Scholar] [CrossRef] [PubMed]
- Padrez, Y.; Golubewa, L.; Bahdanava, A.; Jankunec, M.; Matulaitiene, I.; Semenov, D.; Karpicz, R.; Kulahava, T.; Svirko, Y.; Kuzhir, P. Nanodiamond surface as a photoluminescent pH sensor. Nanotechnology 2023, 34, 195702. [Google Scholar] [CrossRef] [PubMed]
- Stilbene 420 (27344-41-8, Exciton) Datasheet. Available online: https://exciton.luxottica.com/laser-dyes.html (accessed on 10 October 2025).
- Zapka, W.; Brackmann, U. Shorter Dye Laser Wavelengths from Substituted p-Terphenyls. Appl. Phys. 1979, 20, 283–286. [Google Scholar] [CrossRef]
- Brackmann, U. Lambdachrome® Laser Dyes, 3rd ed.; Lambda Physik AG: Göttingen, Germany, 2000; Available online: https://www.chemphys.lu.se/fileadmin/chemphys/General/Lamdachrome-laser-dyes.pdf (accessed on 26 August 2025).
- Peng, D.; Zhang, L.; Li, F.-F.; Cui, W.-R.; Liang, R.-P.; Qiu, J.-D. Facile and Green Approach to the Synthesis of Boron Nitride Quantum Dots for 2,4,6-trinitrophenol Sensing. ACS Appl. Mater. Interfaces 2018, 10, 7315–7323. [Google Scholar] [CrossRef]
- Angizi, S.; Hatamie, A.; Ghanbari, H.; Simchi, A.A. Mechanochemical Green Synthesis of Exfoliated Edge –Functionalized Boron Nitride Quantum Dots: Application to Vitamin C Sensing through Hybridization with Gold Electrodes. Appl. Mater. Interfaces 2018, 10, 28819–28827. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Xu, S.; Wan, J.; Wu, P. Facile Preparation and Multifunctional Applications of Boron Nitride Quantum Dots. Nanoscale 2015, 7, 18902–18907. [Google Scholar] [CrossRef] [PubMed]
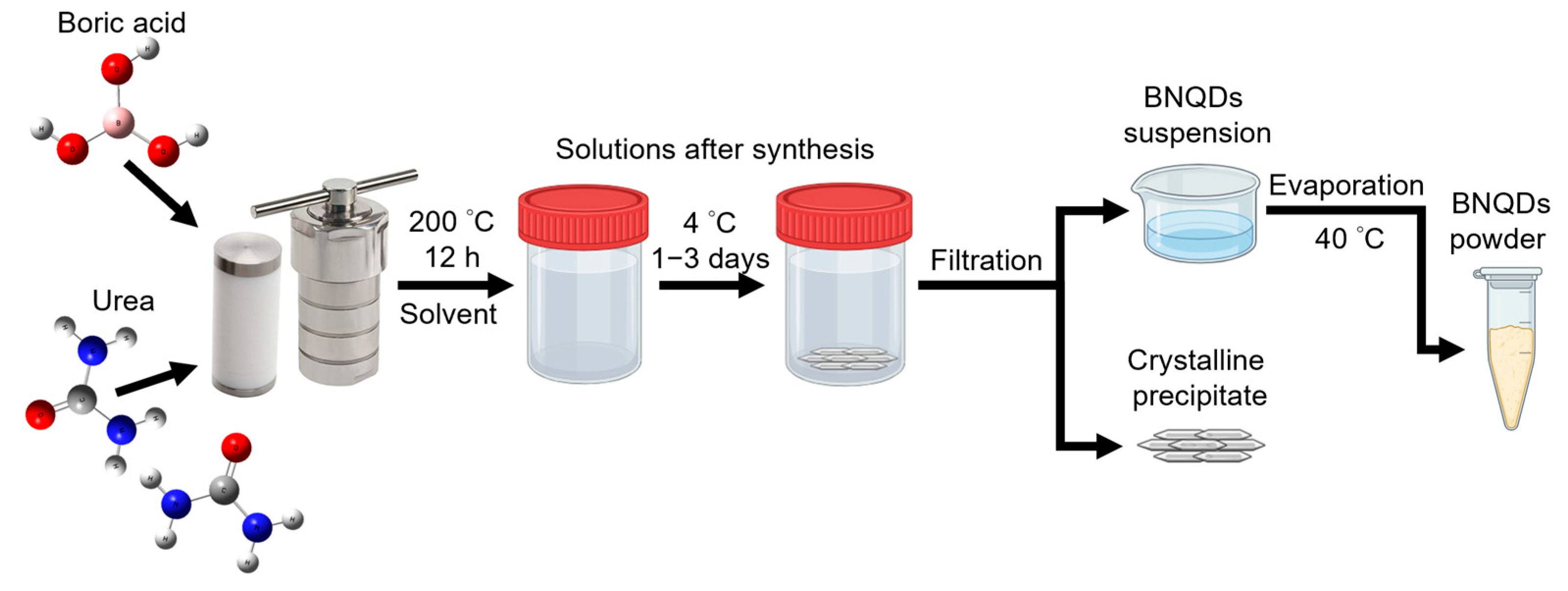
| Type BNQDs | Solvent System | Yield of Powder After Evaporation (%) | Yield of Precipitate (%) |
|---|---|---|---|
| C-BNQDs | C2H5OH/NH3(aq)/H2O | 5.95 | 26.76 |
| H-BNQDs | H2O | 27.54 | - |
| E-BNQDs | C2H5OH | 13.30 | 17.23 |
| N-BNQDs | NH3(aq) | 30.69 | - |
| Element | C-BNQDs | H-BNQDs | E-BNQDs | N-BNQDs | ||||
|---|---|---|---|---|---|---|---|---|
| wt.% | at.% | wt.% | at.% | wt.% | at.% | wt.% | at.% | |
| B | 14.66 | 19.52 | 18.64 | 24.62 | 17.50 | 23.50 | 16.32 | 21.34 |
| N | 7.86 | 8.08 | 5.01 | 5.10 | 6.95 | 7.20 | 5.11 | 5.16 |
| O | 68.63 | 61.67 | 69.14 | 61.70 | 73.10 | 66.34 | 64.82 | 57.30 |
| C | 8.95 | 10.73 | 7.22 | 8.58 | 2.44 | 2.95 | 13.76 | 16.21 |
| C-BNQDs | H-BNQDs | E-BNQDs | N-BNQDs | |
|---|---|---|---|---|
| Hydrodynamic diameter (nm) | 680 ± 10 | 720 ± 10 | 580 ± 10 | 770 ± 10 |
| PDI | 0.321 ± 0.026 | 0.332 ± 0.040 | 0.306 ± 0.015 | 0.380 ± 0.057 |
| Zeta potential (mV) | −32.7 ± 3.3 | −33.5 ± 4.1 | −29.2 ± 2.9 | −1.5 ± 0.2 |
| Element | C-BNQD Precipitate | E-BNQD Precipitate | ||
|---|---|---|---|---|
| wt.% | at.% | wt.% | at.% | |
| B | 18.38 | 24.56 | 22.12 | 29.25 |
| N | 5.75 | 5.93 | 7.30 | 7.44 |
| O | 72.62 | 65.59 | 69.17 | 61.69 |
| C | 3.25 | 3.91 | 1.36 | 1.62 |
| BNQD Concentration, µg/mL | Viability, % | |
|---|---|---|
| 6 h | 24 h | |
| 0 | 100 ± 3 | 100 ± 6 |
| 10 | 96 ± 5 | 99 ± 8 |
| 100 | 97 ± 7 | 89 ± 11 |
| 1000 | 91 ± 10 | 81 ± 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bahdanava, A.; Golubewa, L.; Padrez, Y.; Valynets, N.; Kulahava, T. Recipe for the One-Pot Synthesis of C-/O-Doped Luminescent Boron Nitride Quantum Dots with Tunable Optical Properties for Bioapplications. Physchem 2025, 5, 46. https://doi.org/10.3390/physchem5040046
Bahdanava A, Golubewa L, Padrez Y, Valynets N, Kulahava T. Recipe for the One-Pot Synthesis of C-/O-Doped Luminescent Boron Nitride Quantum Dots with Tunable Optical Properties for Bioapplications. Physchem. 2025; 5(4):46. https://doi.org/10.3390/physchem5040046
Chicago/Turabian StyleBahdanava, Anastasiya, Lena Golubewa, Yaraslau Padrez, Nadzeya Valynets, and Tatsiana Kulahava. 2025. "Recipe for the One-Pot Synthesis of C-/O-Doped Luminescent Boron Nitride Quantum Dots with Tunable Optical Properties for Bioapplications" Physchem 5, no. 4: 46. https://doi.org/10.3390/physchem5040046
APA StyleBahdanava, A., Golubewa, L., Padrez, Y., Valynets, N., & Kulahava, T. (2025). Recipe for the One-Pot Synthesis of C-/O-Doped Luminescent Boron Nitride Quantum Dots with Tunable Optical Properties for Bioapplications. Physchem, 5(4), 46. https://doi.org/10.3390/physchem5040046








