Top-Down Ultrasonication Method for ZnO Nanoparticles Fabrication and Their Application in Developing Pectin-Glycerol Bionanocomposite Films
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Fabrication of ZnO-NPs
2.2.2. Preparation of Bionanocomposite Film-Forming Solution
2.2.3. Preparation of Bionanocomposite Film
2.2.4. Characterization of ZnO-NPs
2.2.5. Characterization of Bionanocomposite Film-Forming Solution
2.2.6. Characterization of Bionanocomposite Film
2.2.7. Data Analysis and Best Film Selection
3. Results and Discussions
3.1. Characterization of ZnO-NPs
3.1.1. UV-Vis Spectrum
3.1.2. Particle Size Characteristics
3.1.3. Morphology
3.2. Characterization of Bionanocomposite Film-Forming Solution
Rheology
3.3. Characterization of Bionanocomposite Film
3.3.1. Film Thickness
3.3.2. Film Appearance and Total Color Difference
3.3.3. Mechanical Properties
3.3.4. Water Vapor Permeability (WVP)
3.3.5. FTIR
3.3.6. Thermal Properties
3.3.7. Selection of Best Bionanocomposite Film
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ZnO-NPs | Zinc oxide nanoparticles |
| PI | Polydispersity index |
| TS | Tensile strength |
| EB | Elongation at break |
| WVP | Water vapor permeability |
| FTIR | Fourier transform infrared |
References
- Xu, Y.; Wu, Z.; Li, A.; Chen, N.; Rao, J.; Zeng, Q. Nanocellulose composite films in food packaging materials: A review. Polymers 2024, 16, 423. [Google Scholar] [CrossRef]
- Shankar, S.; Rhim, J.W. Bionanocomposite films for food packaging applications. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2020; pp. 234–243. [Google Scholar]
- Qiu, W.Y.; Wang, Y.Y.; Wang, M.; Yan, J.K. Construction stability and enhanced antioxidant activity of pectin-decorated selenium nanoparticles. Colloids Surf. B Biointerfaces 2018, 170, 692–700. [Google Scholar] [CrossRef]
- Sharaby, M.R.; Soliman, E.A.; Abdel-Rahman, A.B.; Osman, A.; Khalil, R. Novel pectin-based nanocomposite film for active food packaging applications. Sci. Rep. 2022, 12, 20673. [Google Scholar] [CrossRef]
- Gorrasi, G.; Bugatti, V.; Sorrentino, A. Nanohybrid active fillers in food contact bio-based materials. In Bionanocomposites for Packaging Applications; Jawaid, M., Swain, S.K., Eds.; Springer International Publishing: New York, NY, USA, 2017; pp. 71–94. [Google Scholar]
- Qi, Z.; Xue, X.; Xu, X.; Zhou, H.; Li, W.; Yang, G.; Xie, P. Detoxified and antimicrobial-enhanced olive mill wastewater phenols capping ZnO nanoparticles incorporated with carboxymethyl cellulose for fresh strawberry preservation. Postharvest Biol. Technol. 2022, 188, 111891. [Google Scholar] [CrossRef]
- Aji, A.I.; Suyatma, N.E.; Arpah, M.; Jayanegara, A. Evaluation of different parameters of zinc oxide nanoparticles on bionanocomposite film properties: A meta-analysis. Int. J. Food Sci. Technol. 2022, 58, 979–986. [Google Scholar] [CrossRef]
- Suyatma, N.E.; Ishikawa, Y.; Kitazawa, H. Nanoreinforcement of pectin film to enhance its functional packaging properties by incorporating ZnO nanoparticles. Adv. Mater. Res. 2014, 845, 451–456. [Google Scholar] [CrossRef]
- Ngo, T.M.P.; Dang, T.M.Q.; Tran, T.X.; Rachtanapun, P. Effects of zinc oxide nanoparticles on the properties of pectin/alginate edible films. Int. J. Polym. Sci. 2018, 2018, 5645797. [Google Scholar] [CrossRef]
- dos Santos, J.S.; Cagnin, C.; de Freitas, B.S.M.; da Silva, R.M.; de Jesus, G.B.L.; Belisário, C.M.; Egea, M.B.; de Oliveira, J.G.F.; Plácido, G.R. Nanocomposite coatings of pectin and oxide zinc nanoparticles to increase papaya shelf life. Coatings 2024, 14, 990. [Google Scholar] [CrossRef]
- Galus, S.; Przybyszewska, A.; Barbosa, C.H.; Rodrigues, C.; Gomes, V.; Souza, L.; Alves, M.M.; Santos, C.F.; Coelhoso, I.; Fernando, A.L. Sustainable zinc oxide nanoparticles as active compounds for pectin packaging films. Coatings 2025, 15, 1024. [Google Scholar] [CrossRef]
- Tank, V.; Gajera, A.; Joshi, A. Effective production of zinc oxide through the indirect process. Int. J. Chem. Sci. 2022, 6, 18–22. [Google Scholar]
- Sharma, P.; Hasan, M.R.; Mehto, N.K.; Deepak; Bishoyi, A.; Narang, J. 92 years of zinc oxide: Has been studied by the scientific community since the 1930s-An overview. Sens. Int. 2022, 3, 100182. [Google Scholar] [CrossRef]
- Kołodziejczak-Radzimska, A.; Jesionowski, T. Zinc oxide—From synthesis to application: A review. Materials 2014, 7, 2833–2881. [Google Scholar] [CrossRef]
- Citra Cakra Logam. French Production Process. Available online: https://www.citracakralogam.com/product/#french-production-process (accessed on 13 November 2024).
- Oktavia, A.D.; Rohmawati, L. Fabrication of Fe3O4/ZnO nanocomposite by ultrasonication wave method and its application for antibacterial. Indones. Phys. Rev. 2022, 5, 177–187. [Google Scholar] [CrossRef]
- Luo, Z.; Zhu, M.; Guo, M.; Lian, Z.; Tong, W.; Wang, J.; Zhang, B.; Wei, W. Ultrasonic-assisted dispersion of ZnO nanoparticles and its inhibition activity to Trichoderma viride. J. Nanosci. Nanotechnol. 2018, 18, 2352–2360. [Google Scholar] [CrossRef]
- Siswanto; Rochman, N.T.; Akwalia, P.R. Fabrication and characterization of zinc oxide (ZnO) nanoparticle by sol-gel method. J. Phys. Conf. Ser. 2017, 853, 012041. [Google Scholar] [CrossRef]
- Harish, V.; Ansari, M.M.; Tewari, D.; Gaur, M.; Yadav, A.B.; García-Betancourt, M.L.; Abdel-Haleem, F.M.; Bechelany, M.; Barhoum, A. Nanoparticle and nanostructure synthesis and controlled growth methods. Nanomaterials 2022, 12, 3226. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, K.; Shahedi, M. Physical mechanical and antimicrobial properties of ethylene vinyl alcohol copolymer/chitosan/nano-ZnO (ECNZn) nanocomposite films incorporating glycerol plasticizer. J. Food Meas. Charact. 2016, 10, 137–147. [Google Scholar] [CrossRef]
- Andiyana, Y.; Suyatma, N.E.; Suliantari. Physico-mechanical properties of starch-based nanocomposite film incorporated with hydrothermally synthesized zinc oxide nanoparticles. Mater. Sci. Forum 2016, 872, 162–167. [Google Scholar] [CrossRef]
- Junaid, M.; Hussain, S.G.; Abbas, N.; Khan, W.Q. Band gap analysis of zinc oxide for potential bio glucose sensor. Results Chem. 2023, 5, 100961. [Google Scholar] [CrossRef]
- Romadhan, M.; Suyatma, N.E.; Taqi, F.M. Synthesis of ZnO nanoparticles by precipitation method with their antibacterial effect. Indones. J. Chem. 2016, 16, 117–123. [Google Scholar] [CrossRef]
- Wardana, A.A.; Suyatma, N.E.; Muchtadi, T.R.; Yuliani, S. Influence of ZnO nanoparticles and stearic acid on physical, mechanical and structural properties of cassava starch-based bionanocomposite edible films. Int. Food Res. J. 2018, 25, 1837–1844. [Google Scholar]
- Meindrawan, B.; Suyatma, N.E.; Muchtadi, T.R.; Iriani, E.S. Preparation and characterization of bionanocomposite films made from carrageenan, beeswax and ZnO nanoparticles. Mater. Sci. Forum 2016, 872, 157–161. [Google Scholar] [CrossRef]
- Hari, K.D.; Garcia, C.V.; Shin, G.H.; Kim, J.T. Improvement of the UV barrier and antibacterial properties of crosslinked pectin/zinc oxide bionanocomposite films. Polymers 2021, 13, 2403. [Google Scholar] [CrossRef]
- DeGarmo, E.P.; Sullivan, W.G.; Canada, J.R. Engineering Economy, 7th ed.; Macmillan: New York, NY, USA, 1984. [Google Scholar]
- Gomaa, E.Z. Microbial mediated synthesis of zinc oxide nanoparticles characterization and multifaceted applications. J. Inorg. Organomet. Polym. Mater. 2022, 32, 4114–4132. [Google Scholar] [CrossRef]
- Nair, S.; Nagarajappa, G.B.; Pandey, K.K. UV stabilization of wood by nano metal oxides dispersed in propylene glycol. J. Photochem. Photobiol. B Biol. 2018, 183, 1–10. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on zinc oxide nanoparticles: Antibacterial activity and toxicity mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef]
- Hoseinzadeh, E.; Alikhani, M.Y.; Samarghandi, M.R.; Shirzad-Siboni, M. Antimicrobial potential of synthesized zinc oxide nanoparticles against gram positive and gram negative bacteria. Desalin. Water Treat. 2014, 52, 4969–4976. [Google Scholar] [CrossRef]
- Rana, P.; Banerjee, K.; Sharma, S.; Sharma, R. Pectin stabilized/capped ferromagnetic Co3O4 nanoparticles with antimicrobial efficacy: A greener approach. Orient. J. Chem. 2022, 38, 219–226. [Google Scholar] [CrossRef]
- Gondal, M.A.; Drmosh, Q.A.; Yamani, Z.H.; Saleh, T.A. Synthesis of ZnO2 nanoparticles by laser ablation in liquid and their annealing transformation into ZnO nanoparticles. Appl. Surf. Sci. 2009, 256, 298–304. [Google Scholar] [CrossRef]
- Joudeh, N.; Linke, D. Nanoparticle classification physicochemical properties characterization and applications: A comprehensive review for biologists. J. Nanobiotechnol. 2022, 20, 262. [Google Scholar] [CrossRef]
- Jia, Z.; Li, J.; Gao, L.; Yang, D.; Kanaev, A. Dynamic light scattering: A powerful tool for in situ nanoparticle sizing. Colloids Interfaces 2023, 7, 15. [Google Scholar] [CrossRef]
- Poloju, V.K.; Khadanga, V.; Mukherjee, S.; Mishra, P.C.; Aljuwayhel, N.F.; Ali, N. Thermal conductivity and dispersion properties of SDBS decorated ternary nanofluid: Impacts of surfactant inclusion sonication time and ageing. J. Mol. Liq. 2022, 368, 120832. [Google Scholar] [CrossRef]
- Thonglerth, P.; Sujaridworakun, P.; Boondamnoen, O. Preparation of ZnO nanoparticles water-based dispersion. J. Phys. Conf. Ser. 2022, 2175, 012029. [Google Scholar] [CrossRef]
- Masarudin, M.J.; Cutts, S.M.; Evison, B.J.; Phillips, D.R.; Pigram, P.J. Factors determining the stability size distribution and cellular accumulation of small monodisperse chitosan nanoparticles as candidate vectors for anticancer drug delivery: Application to the passive encapsulation of [(14)C]-doxorubicin. Nanotechnol. Sci. Appl. 2015, 8, 67–80. [Google Scholar] [CrossRef]
- Chung, S.J.; Leonard, J.P.; Nettleship, I.; Lee, J.K.; Soong, Y.; Martello, D.V.; Chyu, M.K. Characterization of ZnO nanoparticle suspension in water: Effectiveness of ultrasonic dispersion. Powder Technol. 2009, 194, 75–80. [Google Scholar] [CrossRef]
- Panigrahi, U.K.; Das, D.; Hussain, S.; Giri, J.; Panda, A.K.; Satapathy, P.K.; Mallick, P. Effect of sonication on the structural, optical and photocatalytic characteristics of ZnO nanostructures. Discov. Mater. 2025, 5, 82. [Google Scholar] [CrossRef]
- Souza, T.G.F.; Ciminelli, V.S.T.; Mohallem, N.D.S. A comparison of TEM and DLS methods to characterize size distribution of ceramic nanoparticles. J. Phys. Conf. Ser. 2016, 733, 012039. [Google Scholar] [CrossRef]
- Bootz, A.; Vogel, V.; Schubert, D.; Kreuter, J. Comparison of scanning electron microscopy, dynamic light scattering and analytical ultracentrifugation for the sizing of poly(butyl cyanoacrylate) nanoparticles. Eur. J. Pharm. Biopharm. 2004, 2, 369–375. [Google Scholar] [CrossRef]
- Tang, Y.; Hu, X.; Zhang, X.; Guo, D.; Zhang, J.; Kong, F. Chitosan/titanium dioxide nanocomposite coatings: Rheological behavior and surface application to cellulosic paper. Carbohydr. Polym. 2016, 151, 752–759. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Du, L.; Yang, Y.; Wang, L. Rheology of film-forming solutions and physical properties of tara gum film reinforced with polyvinyl alcohol (PVA). Food Hydrocoll. 2017, 63, 677–684. [Google Scholar] [CrossRef]
- Pal, R. Non-Newtonian behaviour of suspensions and emulsions: Review of different mechanisms. Adv. Colloid. Interface Sci. 2024, 1, 103299. [Google Scholar] [CrossRef]
- Silva, K.; Machado, A.; Cardoso, C.; Silva, F.; Freitas, F. Rheological behavior of plant-based beverages. Food Sci. Technol. 2020, 40, 258–263. [Google Scholar] [CrossRef]
- Tarique, J.; Sapuan, S.M.; Khalina, A. Effect of glycerol plasticizer loading on the physical, mechanical, thermal, and barrier properties of arrowroot (Maranta arundinacea) starch biopolymers. Sci. Rep. 2021, 11, 13900. [Google Scholar] [CrossRef] [PubMed]
- Yanti, N.A.; Ahmad, S.W.; Ramadhan, L.O.A.N.; Ardiansyah, A.; Indrawati, I. Characteristics of biocellulose-based edible film from sago wastewater (Metroxylon sago ROTTB.) on various glycerol concentration. agriTECH 2024, 1, 9–16. [Google Scholar] [CrossRef]
- Japanese Industrial Standard Z 1707; General Rules of Plastic Films for Food Packaging. Japanese Standard Association: Tokyo, Japan, 1975.
- Espitia, P.J.P.; Soares, N.D.F.F.; Teófilo, R.F.; Coimbra, J.S.D.R.; Vitor, D.M.; Batista, R.A.; Ferreira, S.O.; de Andrade, N.J. Physical-mechanical and antimicrobial properties of nanocomposite films with pediocin and ZnO nanoparticles. Carbohydr. Polym. 2013, 94, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, J.; Arfat, Y.A.; Al-Attar, H.; Auras, R.; Ejaz, M. Rheological, structural, ultraviolet protection and oxygen barrier properties of linear low-density polyethylene films reinforced with zinc oxide (ZnO) nanoparticles. Food Packag. Shelf Life 2017, 13, 20–26. [Google Scholar] [CrossRef]
- Kumar, S.; Boro, J.C.; Ray, D.; Mukherjee, A.; Dutta, J. Bionanocomposite films of agar incorporated with ZnO nanoparticles as an active packaging material for shelf life extension of green grape. Heliyon 2019, 5, e01867. [Google Scholar] [CrossRef]
- Esthappan, S.K.; Nair, A.B.; Joseph, R. Effect of crystallite size of zinc oxide on the mechanical thermal and flow properties of polypropylene/zinc oxide nanocomposites. Compos. Part B Eng. 2015, 69, 145–153. [Google Scholar] [CrossRef]
- Noshirvani, N.; Ghanbarzadeh, B.; Mokarram, R.R.; Hashemi, M.; Coma, V. Preparation and characterization of active emulsified films based on chitosan-carboxymethyl cellulose containing zinc oxide nanoparticles. Int. J. Biol. Macromol. 2017, 99, 530–538. [Google Scholar] [CrossRef]
- Mirres, A.C.D.M.; Vieira, I.R.S.; Tessaro, L.; da Silva, B.D.; de Andrade, J.C.; da Silva, A.A.; Carvalho, N.M.F.; de Sousa, A.M.F.; Conte-Junior, C.A. Nanocomposite films of babassu coconut mesocarp and food packaging. Foods 2024, 13, 1895. [Google Scholar] [CrossRef]
- Asfaw, W.A.; Tafa, K.D.; Satheesh, N. Optimization of citron peel pectin and glycerol concentration in the production of edible film using response surface methodology. Heliyon 2023, 9, 13724. [Google Scholar] [CrossRef]
- Hasna, T.; Lestari, C.A.; Putri, W.D.R.; Fathuroya, V. The effect of ZnO (zinc oxide) and glycerol concentrations on the mechanical properties of bioplastics made from Canna tuber (Canna edulis) starch. Adv. Food Sci. Sustain. Agric. Agroind. Eng. 2022, 5, 21–28. [Google Scholar] [CrossRef]
- Kanmani, P.; Rhim, J.W. Properties and characterization of bionanocomposite films prepared with various biopolymers and ZnO nanoparticles. Carbohydr. Polym. 2014, 106, 190–199. [Google Scholar] [CrossRef]
- Lee, S.W.; Said, N.S.; Sarbon, N.M. The effects of zinc oxide nanoparticles on the physical, mechanical and antimicrobial properties of chicken skin gelatin/tapioca starch composite films in food packaging. J. Food Sci. Technol. 2021, 58, 4294–4302. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.W. Preparation of pectin/agar-based functional films integrated with zinc sulfide nano petals for active packaging applications. Colloids Surfaces B Biointerfaces 2021, 207, 111999. [Google Scholar] [CrossRef] [PubMed]
- Saral, S.K.; Indumathi, M.P.; Rajarajeswari, G.R. Mahua oil-based polyurethane/chitosan/nano ZnO composite films for biodegradable food packaging applications. Int. J. Biol. Macromol. 2019, 124, 163–174. [Google Scholar]
- Chanu, T.S.; Singh, K.J.; Devi, K.N. The influence of ZnO nanoparticles on the structural, optical and dielectric properties of polyvinyl alcohol films. Mater. Today Proc. 2022, 65, 2844–2850. [Google Scholar] [CrossRef]
- Suyatma, N.E.; Gunawan, S.; Putri, R.Y.; Tara, A.; Abbès, F.; Hastati, D.Y.; Abbès, B. Active biohybrid nanocomposite films made from chitosan ZnO nanoparticles and stearic acid: Optimization study to develop antibacterial films for food packaging application. Materials 2023, 16, 926. [Google Scholar] [CrossRef] [PubMed]
- Chouhan, S.; Bajpai, A.K.; Bajpai, J.; Katare, R.; Dhoble, S.J. Mechanical and UV absorption behavior of zinc oxide nanoparticles: Reinforced poly(vinyl alcohol-g-acrylonitrile) nanocomposite films. Polym. Bull. 2017, 74, 4119–4141. [Google Scholar] [CrossRef]
- Hammani, S.; Barhoum, A.; Bechelany, M. Fabrication of PMMA/ZnO nanocomposite: Effect of high nanoparticles loading on the optical and thermal properties. J. Mater. Sci. 2017, 53, 1911–1921. [Google Scholar] [CrossRef]
- Wei, H.; Pascall, M.A. Evaluation of structural and functional properties of citrus pectin film enriched with green tea extract. Polym. Eng. Sci. 2023, 63, 2522–2533. [Google Scholar] [CrossRef]

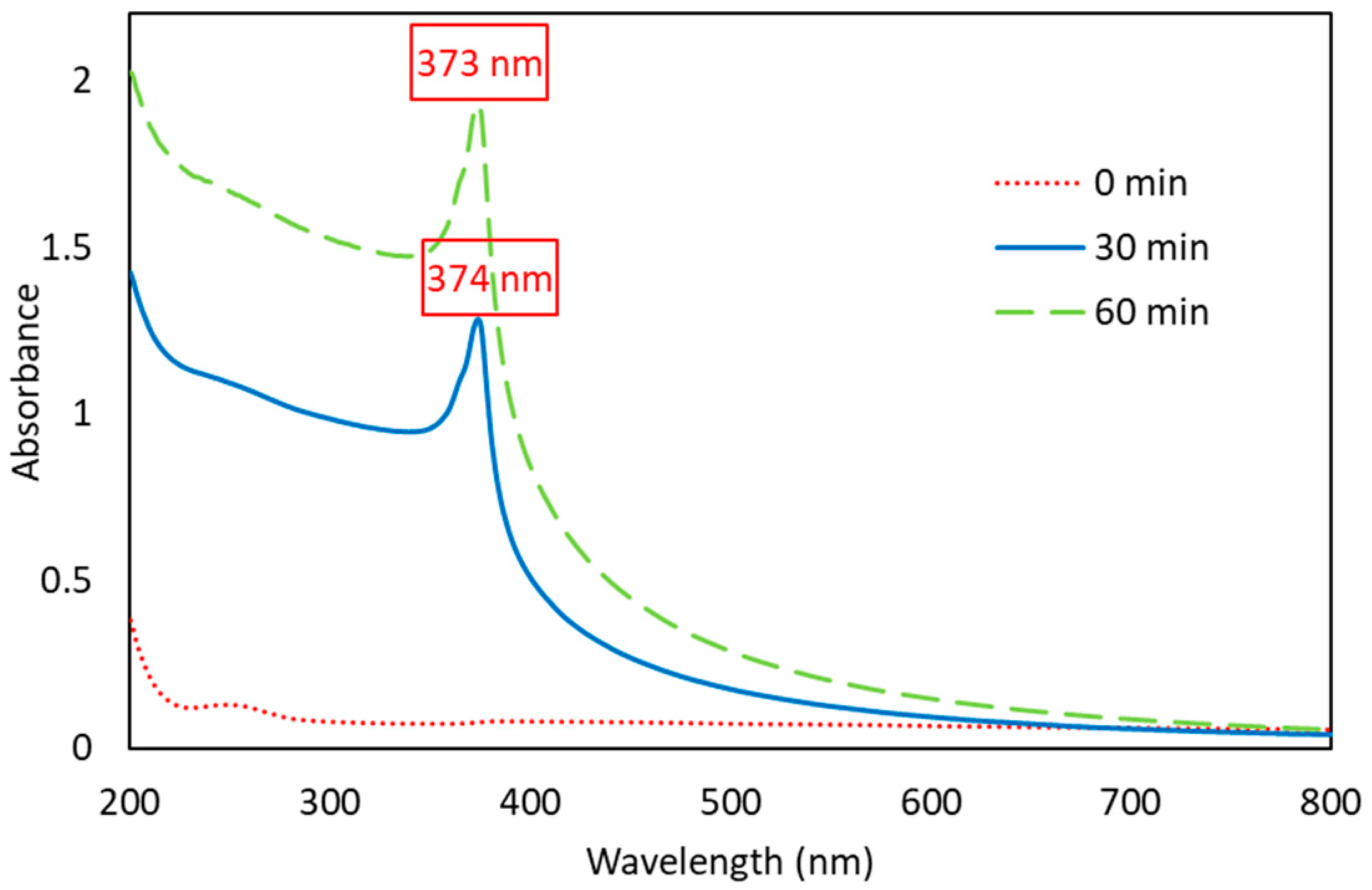
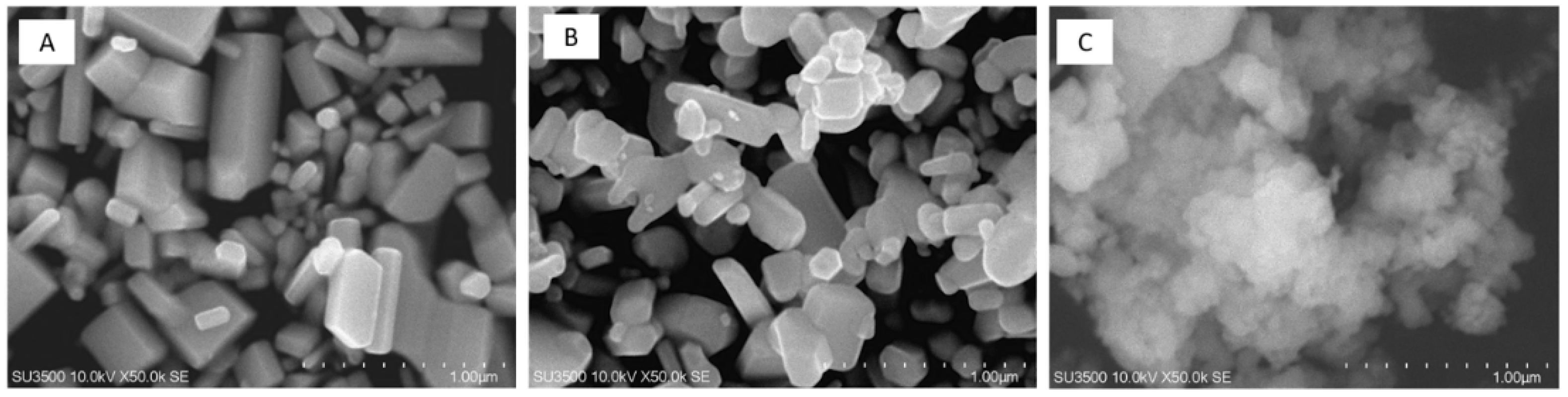
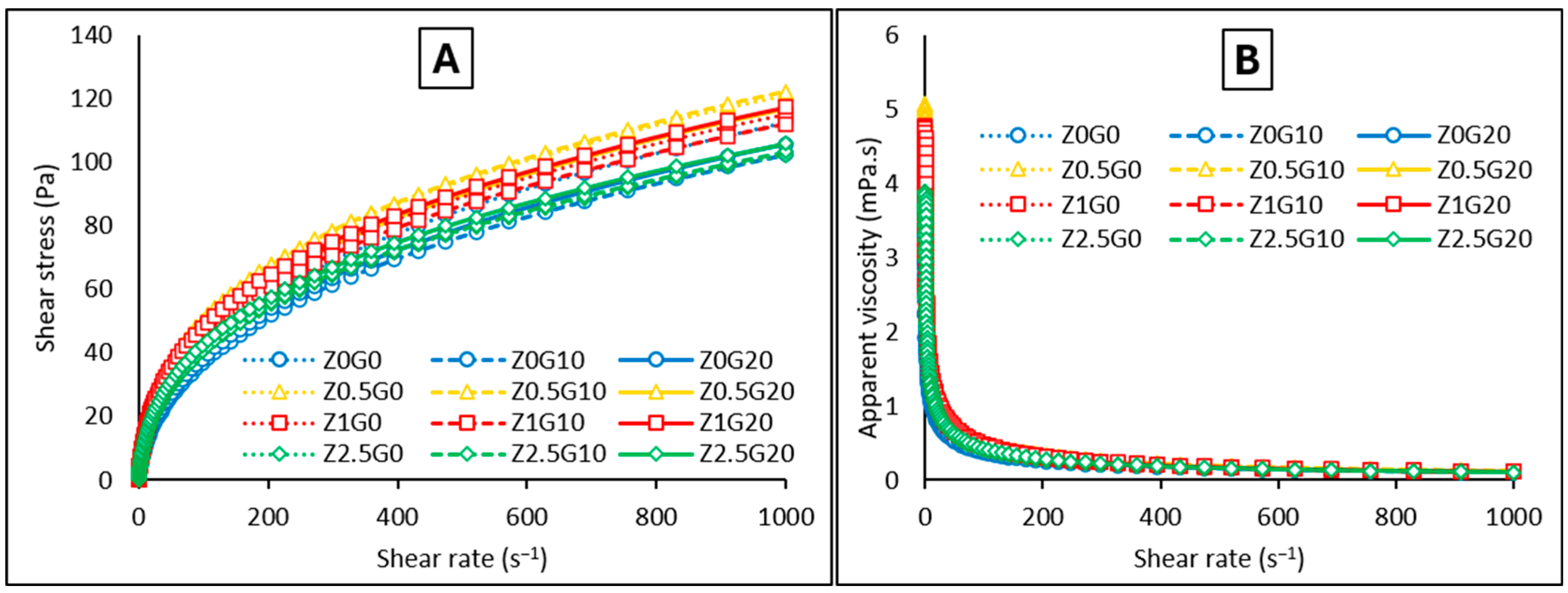

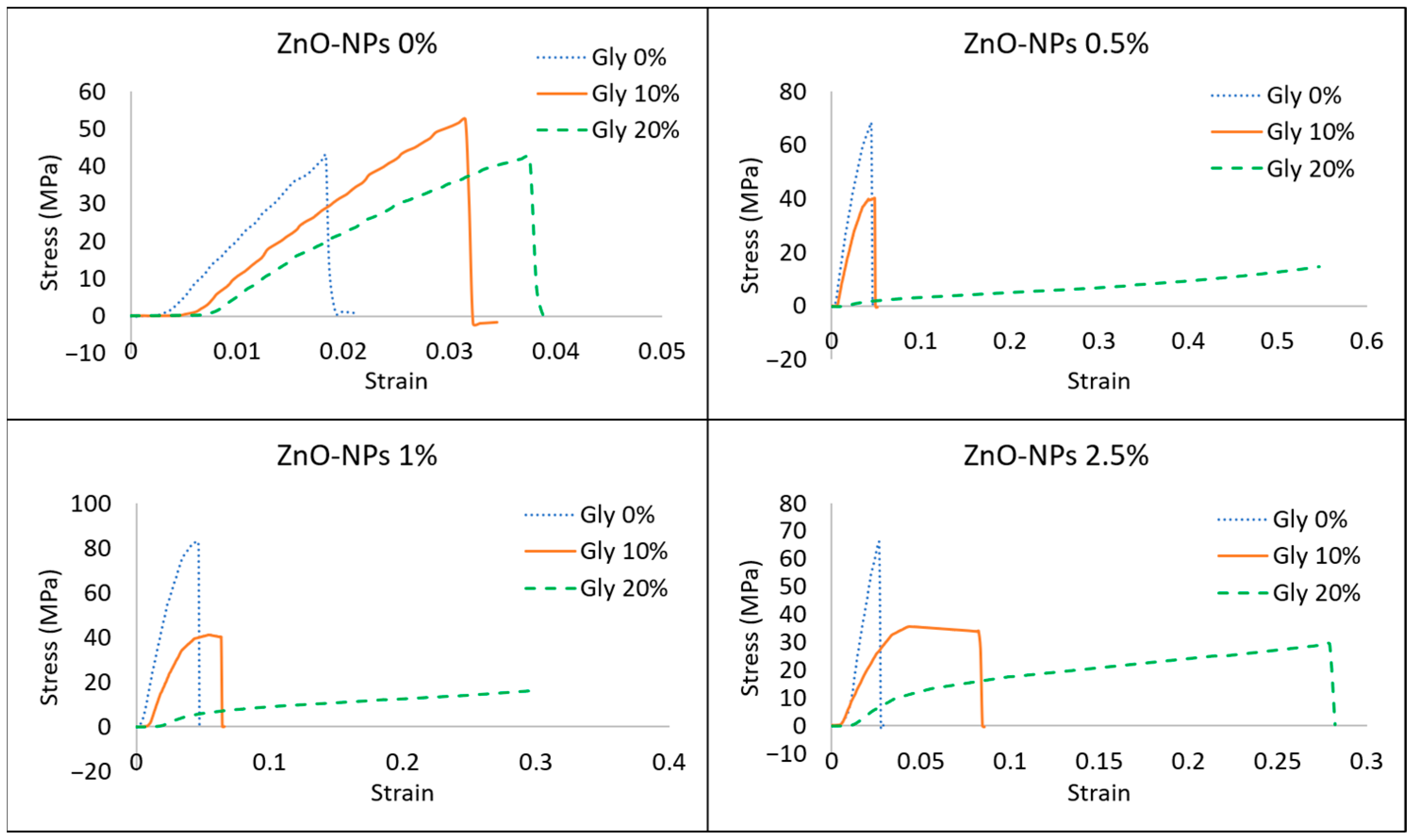
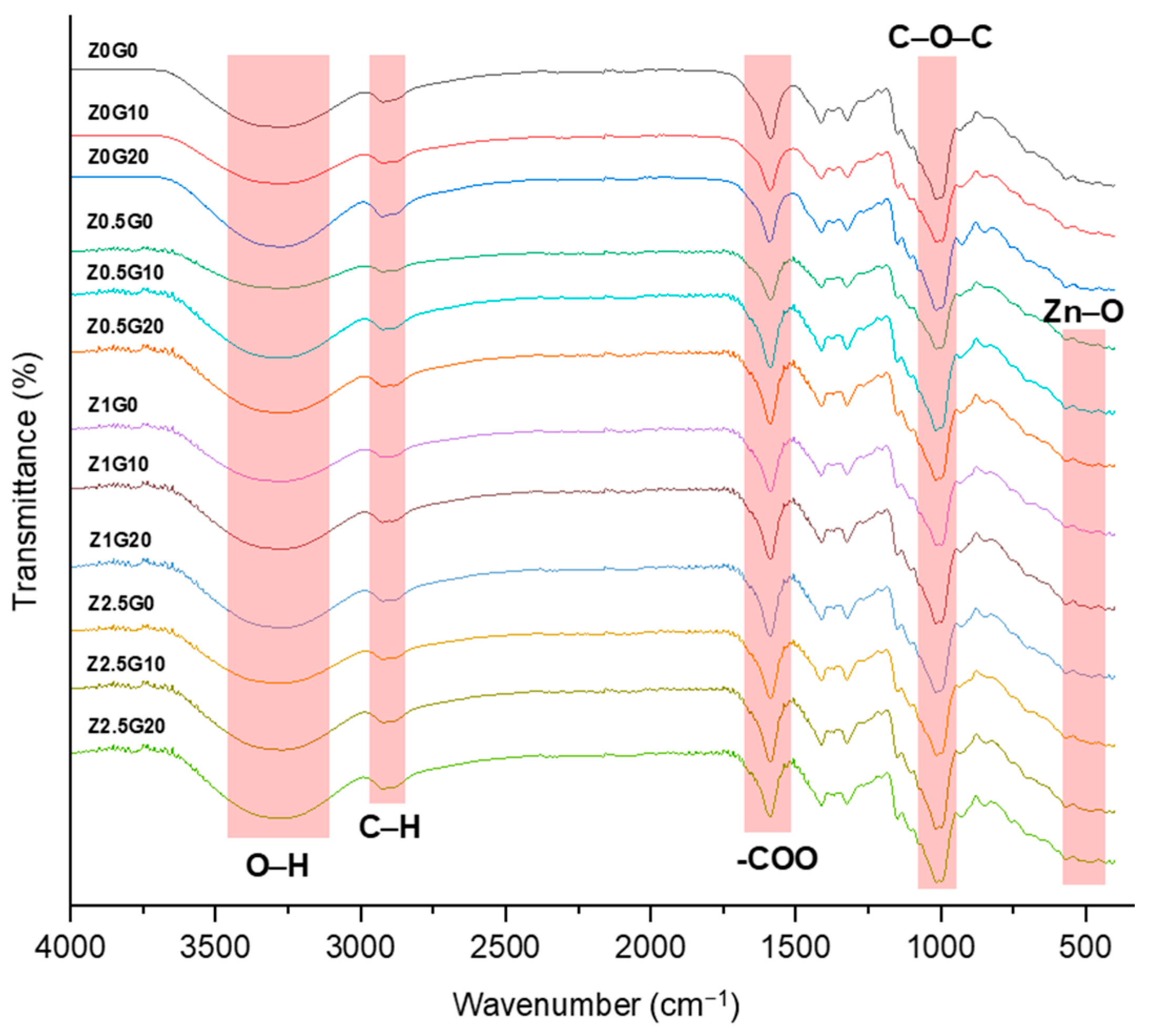
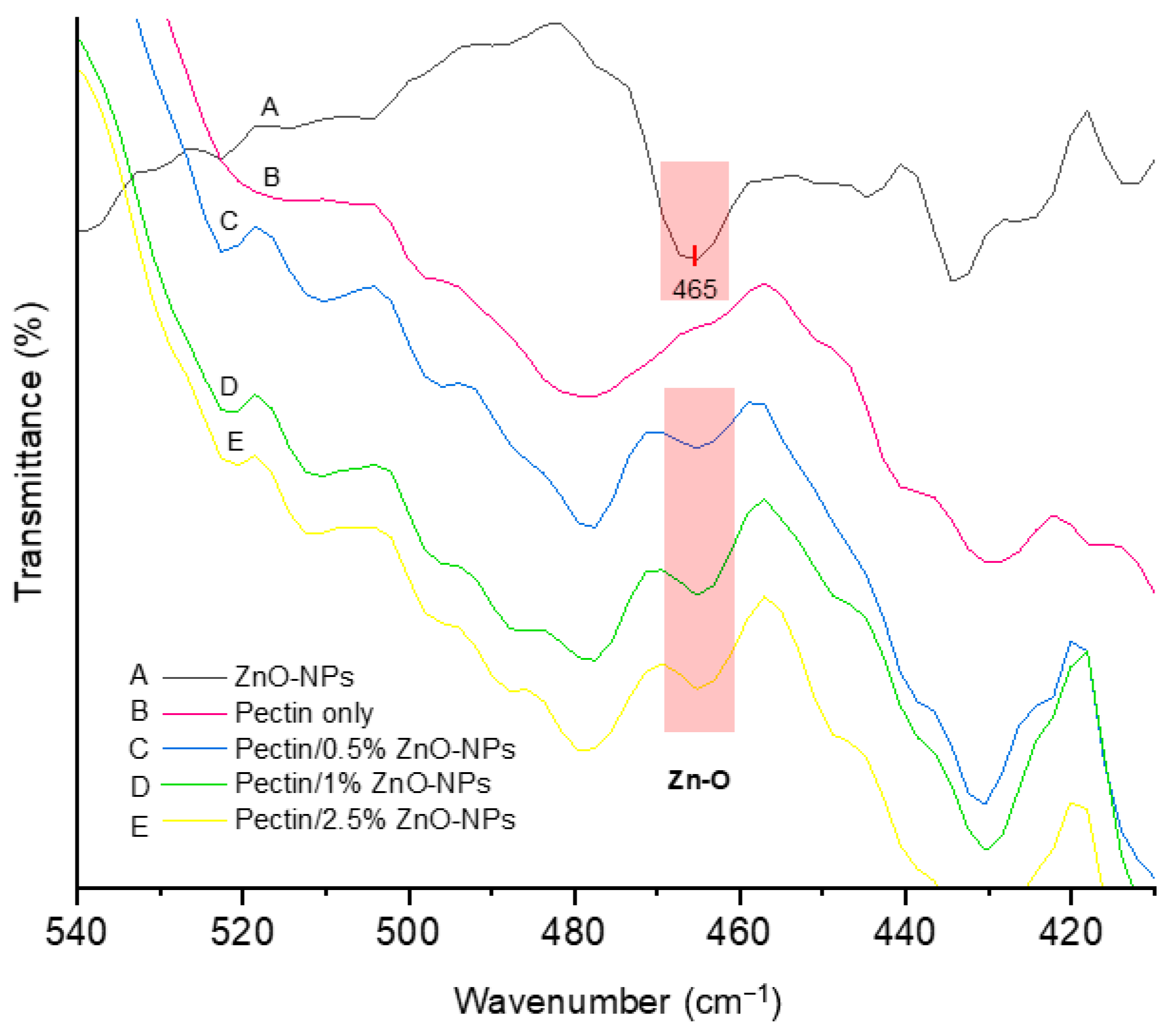
| Ultrasonication Time (min) | Particle Size (nm) * | Polydispersity Index * |
|---|---|---|
| 0 | 5682.00 ± 3262.59 a | 0.261 ± 0.030 a |
| 30 | 175.56 ± 4.08 b | 0.210 ± 0.056 b |
| 60 | 156.83 ± 26.50 b | 0.184 ± 0.003 b |
| ZnO-NPs (%) | Glycerol (%) | n * | η0.1 (Pa·s) × 10−3 * | K (Pa·sn) * | τo (Pa) * |
|---|---|---|---|---|---|
| 0 | 0 | 0.619 ± 0.009 Aa | 2.2 ± 0.2 Ac | 1.7 ± 0.1 Ac | 4.5 ± 0.0 Ac |
| 0.5 | 0.582 ± 0.008 Ab | 3.3 ± 0.4 Aa | 2.4 ± 0.2 Aa | 5.6 ± 0.1 Aa | |
| 1 | 0.585 ± 0.004 Ab | 3.1 ± 0.3 Aab | 2.2 ± 0.1 Aa | 5.3 ± 0.2 Aa | |
| 2.5 | 0.598 ± 0.007 Ab | 2.5 ± 0.3 Abc | 1.8 ± 0.2 Ab | 4.5 ± 0.7 Ab | |
| 0 | 10 | 0.634 ± 0.003 Aa | 1.9 ± 0.1 Ac | 1.4 ± 0.0 Ac | 3.9 ± 0.1 Ac |
| 0.5 | 0.587 ± 0.003 Ab | 3.2 ± 0.1 Aa | 2.3 ± 0.0 Aa | 5.6 ± 0.0 Aa | |
| 1 | 0.597 ± 0.001 Ab | 2.6 ± 0.2 Aab | 2.0 ± 0.0 Aa | 5.0 ± 0.1 Aa | |
| 2.5 | 0.597 ± 0.000 Ab | 2.6 ± 0.2 Abc | 1.8 ± 0.1 Ab | 4.5 ± 0.4 Ab | |
| 0 | 20 | 0.625 ± 0.005 Aa | 2.2 ± 0.2 Ac | 1.5 ± 0.1 Ac | 3.9 ± 0.2 Ac |
| 0.5 | 0.604 ± 0.031 Ab | 2.6 ± 0.9 Aa | 2.0 ± 0.6 Aa | 5.2 ± 1.0 Aa | |
| 1 | 0.586 ± 0.009 Ab | 3.1 ± 0.4 Aab | 2.3 ± 0.2 Aa | 5.3 ± 0.1 Aa | |
| 2.5 | 0.596 ± 0.002 Ab | 2.5 ± 0.1 Abc | 1.9 ± 0.0 Ab | 4.7 ± 0.0 Ab |
| ZnO-NPs (%) | Thickness (µm) * | |||
|---|---|---|---|---|
| Glycerol (%) | Mean | |||
| 0 | 10 | 20 | ||
| 0 | 76.67 ± 7.57 Ca | 82.67 ± 5.03 Ba | 92.00 ± 5.29 Aa | 83.78 ± 8.51 a |
| 0.5 | 72.00 ± 6.93 Ca | 80.67 ± 9.45 Ba | 91.33 ± 6.43 Aa | 81.33 ± 10.72 a |
| 1 | 74.67 ± 9.02 Ca | 76.67 ± 2.31 Ba | 97.33 ± 3.06 Aa | 82.89 ± 11.92 a |
| 2.5 | 72.67 ± 4.16 Ca | 82.00 ± 3.46 Ba | 93.33 ± 3.06 Aa | 82.67 ± 9.49 a |
| Mean | 74.00 ± 6.38 C | 80.50 ± 5.47 B | 93.50 ± 4.68 A | − |
| ZnO-NPs (%) | ∆E * | |||
|---|---|---|---|---|
| Glycerol (%) | Mean | |||
| 0 | 10 | 20 | ||
| 0 | 4.68 ± 0.04 Aa | 4.65 ± 0.89 Aa | 4.81 ± 0.09 Aa | 4.71 ± 0.10 a |
| 0.5 | 4.26 ± 0.23 Ac | 4.22 ± 0.37 Ac | 4.31 ± 0.36 Ac | 4.26 ± 0.29 c |
| 1 | 4.50 ± 0.14 Aab | 4.49 ± 0.28 Aab | 4.65 ± 0.16 Aab | 4.55 ± 0.19 ab |
| 2.5 | 4.26 ± 0.35 Abc | 4.41 ± 0.12 Abc | 4.42 ± 0.22 Abc | 4.36 ± 0.23 bc |
| Mean | 4.43 ± 0.26 A | 4.44 ± 0.26 A | 4.54 ± 0.28 A | − |
| ZnO-NPs (%) | Tensile Strength (MPa) * | |||
|---|---|---|---|---|
| Glycerol (%) | Mean | |||
| 0 | 10 | 20 | ||
| 0 | 53.07 ± 18.11 Aa | 63.83 ± 18.84 Ba | 43.55 ± 5.11 Ca | 53.48 ± 15.95 a |
| 0.5 | 65.55 ± 18.73 Ab | 39.73 ± 7.36 Bb | 19.58 ± 8.49 Cb | 41.62 ± 22.75 b |
| 1 | 82.37 ± 0.53 Aab | 46.47 ± 7.67 Bab | 17.59 ± 3.47 Cab | 48.81 ± 28.42 ab |
| 2.5 | 72.02 ± 10.61 Aab | 39.47 ± 3.64 Bab | 26.14 ± 6.92 Cab | 45.88 ± 21.47 ab |
| Mean | 68.25 ± 16.34 A | 47.38 ± 13.95 B | 26.72 ± 11.95 C | + |
| ZnO-NPs (%) | Elongation at Break (%) * | |||
|---|---|---|---|---|
| Glycerol (%) | Mean | |||
| 0 | 10 | 20 | ||
| 0 | 2.26 ± 0.24 Aa | 3.33 ± 0.65 Aa | 4.30 ± 0.80 Ab | 3.30 ± 1.03 A |
| 0.5 | 4.23 ± 0.35 Ba | 5.52 ± 3.33 Ba | 40.68 ± 12.62 Bb | 16.81 ± 19.06 B |
| 1 | 4.47 ± 0.32 Ba | 6.40 ± 0.84 Ba | 27.85 ± 5.46 Bb | 12.91 ± 11.58 B |
| 2.5 | 2.83 ± 0.15 Ba | 8.62 ± 4.88 Ba | 27.64 ± 5.53 Bb | 13.03 ± 11.83 B |
| Mean | 3.45 ± 1.00 a | 5.97 ± 3.23 a | 25.12 ± 15.10 b | + |
| ZnO-NPs (%) | WVP (g·mm/m2·day·kPa) * | |||
|---|---|---|---|---|
| Glycerol (%) | Mean | |||
| 0 | 10 | 20 | ||
| 0 | 8.15 ± 1.31 ABa | 8.40 ± 2.10 Ba | 12.08 ± 2.72 Aa | 9.54 ± 2.65 a |
| 0.5 | 8.22 ± 1.04 ABa | 7.13 ± 1.20 Ba | 9.20 ± 3.18 Aa | 8.18 ± 1.99 a |
| 1 | 8.63 ± 1.06 ABa | 6.98 ± 2.09 Ba | 9.65 ± 4.84 Aa | 8.42 ± 2.93 a |
| 2.5 | 8.21 ± 1.64 ABa | 7.68 ± 1.85 Ba | 10.12 ± 3.46 Aa | 8.67 ± 2.61 a |
| Mean | 8.21 ± 1.64 AB | 7.68 ± 1.85 B | 10.12 ± 3.46 A | − |
| Formula | Tg (°C) | Tm Onset (°C) | Tm Peak (°C) | Enthalpy (J/g) |
|---|---|---|---|---|
| Pectin only | 51.61 | 160.09 | 162.04 | 94.29 |
| Pectin/2.5% ZnO-NPs | 55.40 | 161.56 | 163.62 | 81.42 |
| Pectin/2.5% ZnO-NPs/20% Gly | 35.06 | 171.95 | 172.73 | 106.07 |
| ZnO-NPs (%) | Glycerol (%) | Total Product Value | Rank |
|---|---|---|---|
| 0 | 0 | 0.3692 | 11 |
| 10 | 0.3946 | 9 | |
| 20 | 0.0893 | 12 | |
| 0.5 | 0 | 0.6117 | 3 |
| 10 | 0.6189 | 2 | |
| 20 | 0.6894 | 1 | |
| 1 | 0 | 0.5117 | 7 |
| 10 | 0.5292 | 6 | |
| 20 | 0.3867 | 10 | |
| 2.5 | 0 | 0.6112 | 4 |
| 10 | 0.5353 | 5 | |
| 20 | 0.5060 | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Astriyani, M.N.; Suyatma, N.E.; Armetha, V.; Purnomo, E.H.; Muhandri, T.; Budi, F.S.; Abbes, B.; Tara, A. Top-Down Ultrasonication Method for ZnO Nanoparticles Fabrication and Their Application in Developing Pectin-Glycerol Bionanocomposite Films. Physchem 2025, 5, 42. https://doi.org/10.3390/physchem5040042
Astriyani MN, Suyatma NE, Armetha V, Purnomo EH, Muhandri T, Budi FS, Abbes B, Tara A. Top-Down Ultrasonication Method for ZnO Nanoparticles Fabrication and Their Application in Developing Pectin-Glycerol Bionanocomposite Films. Physchem. 2025; 5(4):42. https://doi.org/10.3390/physchem5040042
Chicago/Turabian StyleAstriyani, Maulida Nur, Nugraha Edhi Suyatma, Vallerina Armetha, Eko Hari Purnomo, Tjahja Muhandri, Faleh Setia Budi, Boussad Abbes, and Ahmed Tara. 2025. "Top-Down Ultrasonication Method for ZnO Nanoparticles Fabrication and Their Application in Developing Pectin-Glycerol Bionanocomposite Films" Physchem 5, no. 4: 42. https://doi.org/10.3390/physchem5040042
APA StyleAstriyani, M. N., Suyatma, N. E., Armetha, V., Purnomo, E. H., Muhandri, T., Budi, F. S., Abbes, B., & Tara, A. (2025). Top-Down Ultrasonication Method for ZnO Nanoparticles Fabrication and Their Application in Developing Pectin-Glycerol Bionanocomposite Films. Physchem, 5(4), 42. https://doi.org/10.3390/physchem5040042








