Pt and Pd Nanoparticle Crystallization in the Sol-Gel-Derived Thin SiO2 Films
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Silica Sol Synthesis and Spin-On Glass Film Formation
2.3. Characterization
3. Results and Discussion
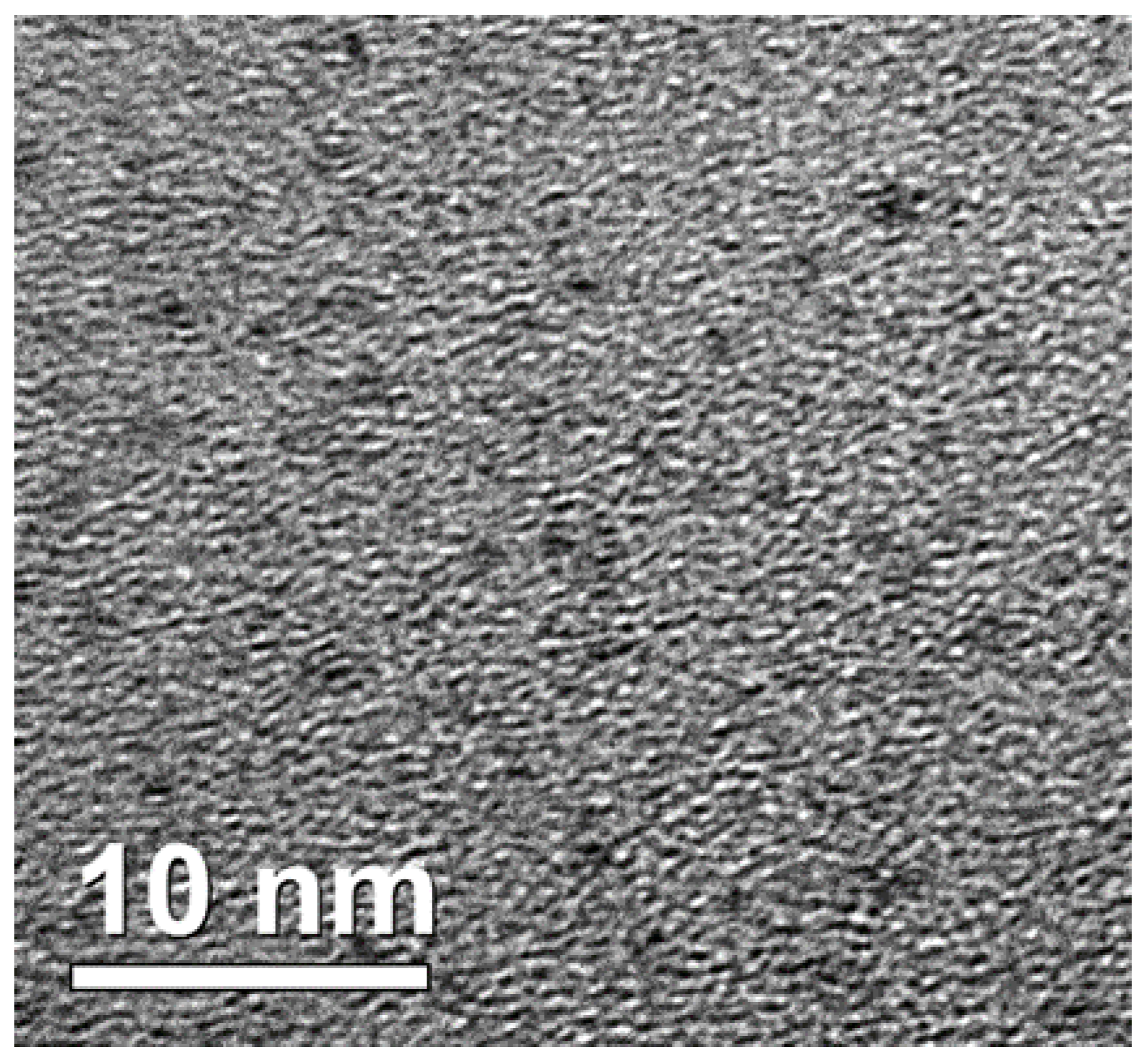
3.1. Surface Morphology
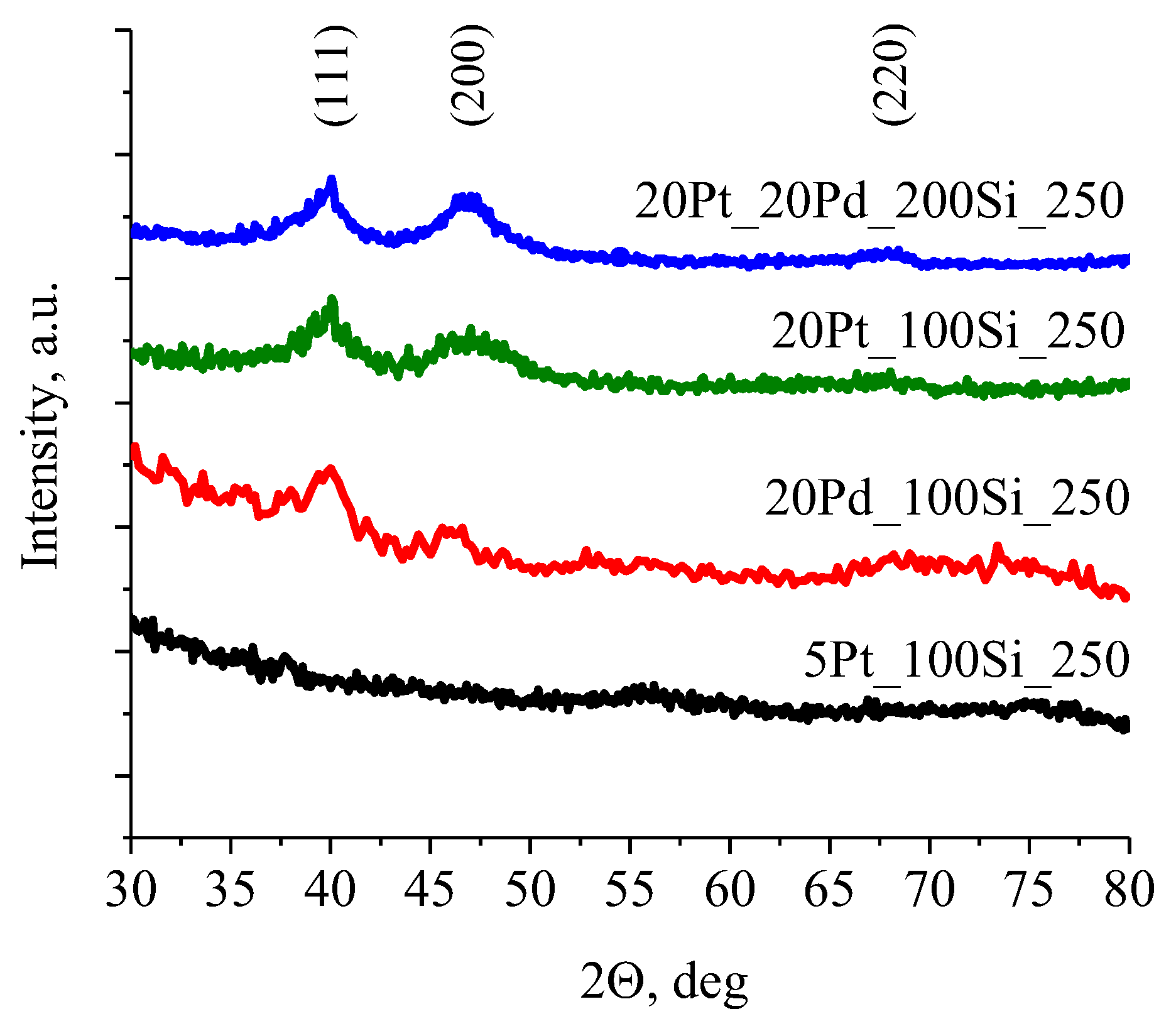
3.2. Phase Composition of Silica Films Doped with Pt and Pd
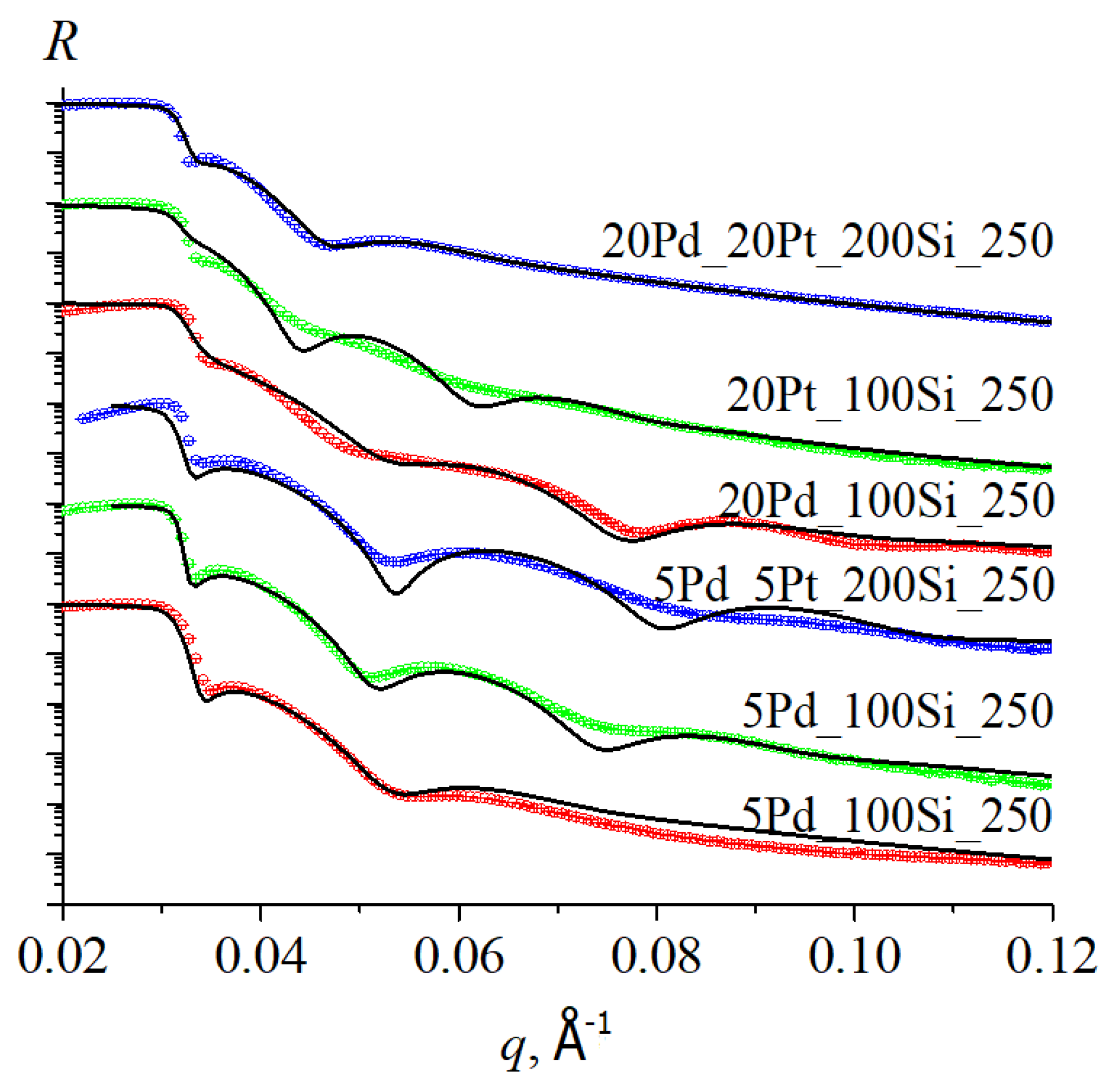
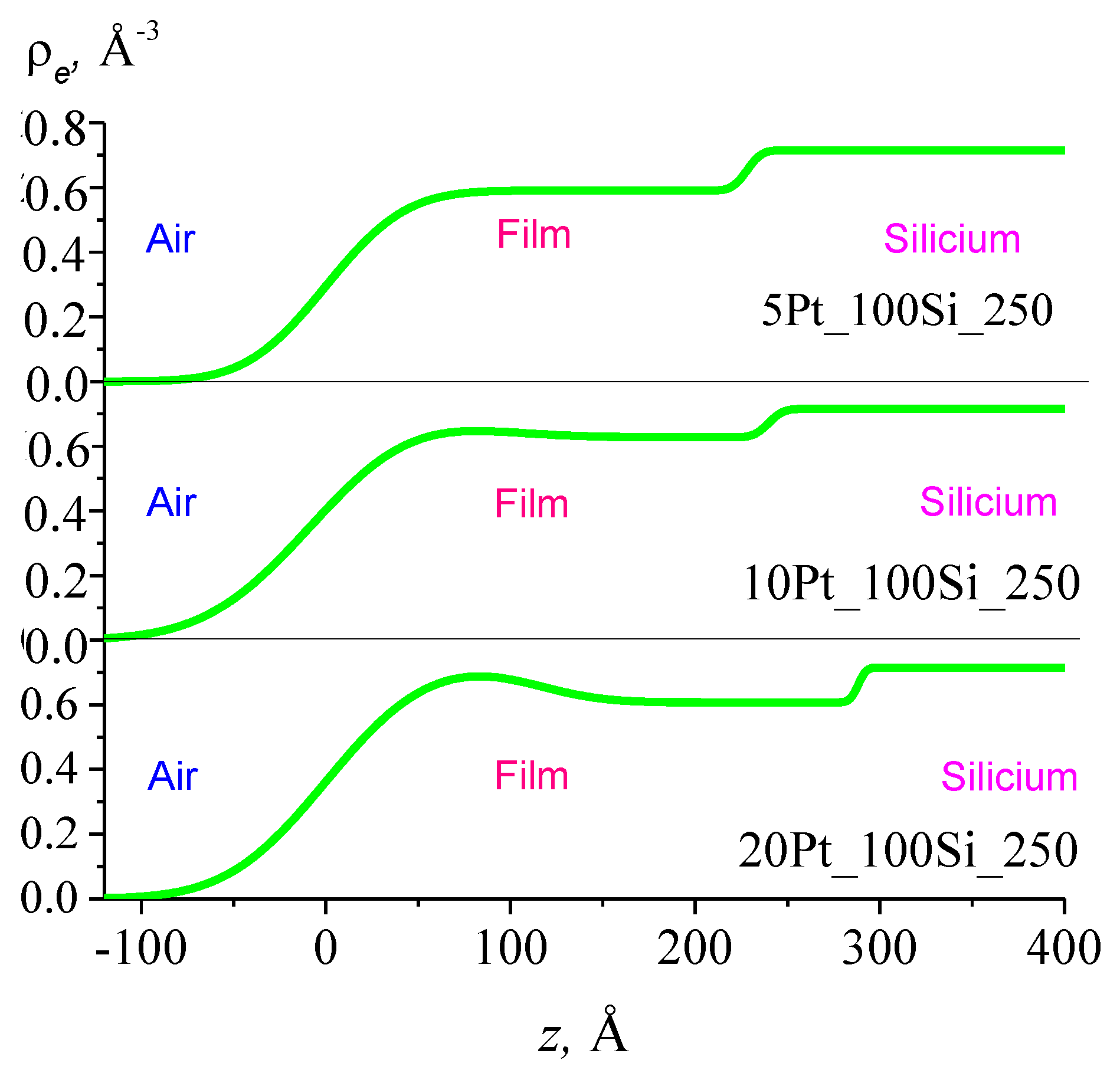
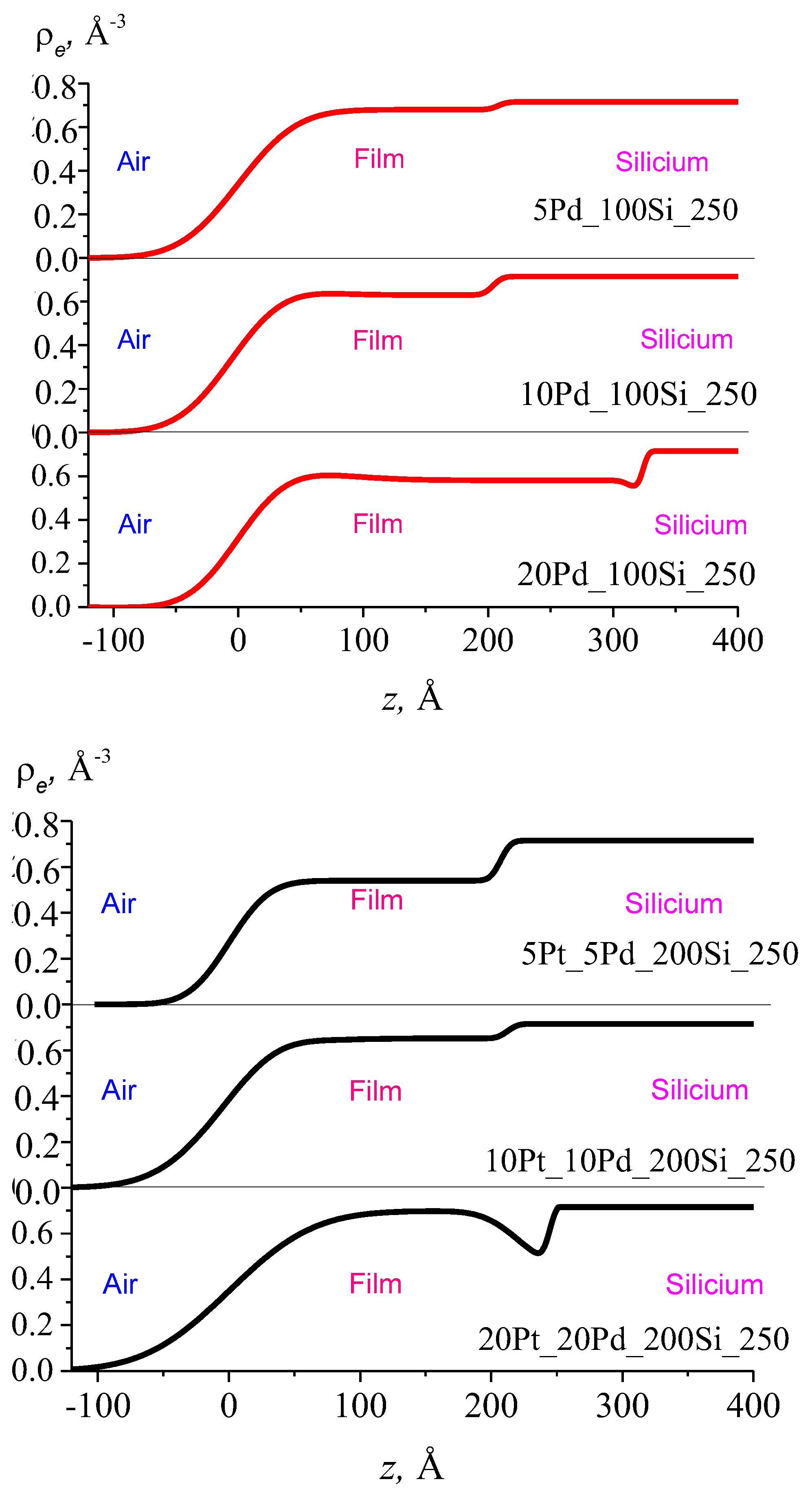
3.3. Thickness Profile of Silica Films Doped with Pt/Pd
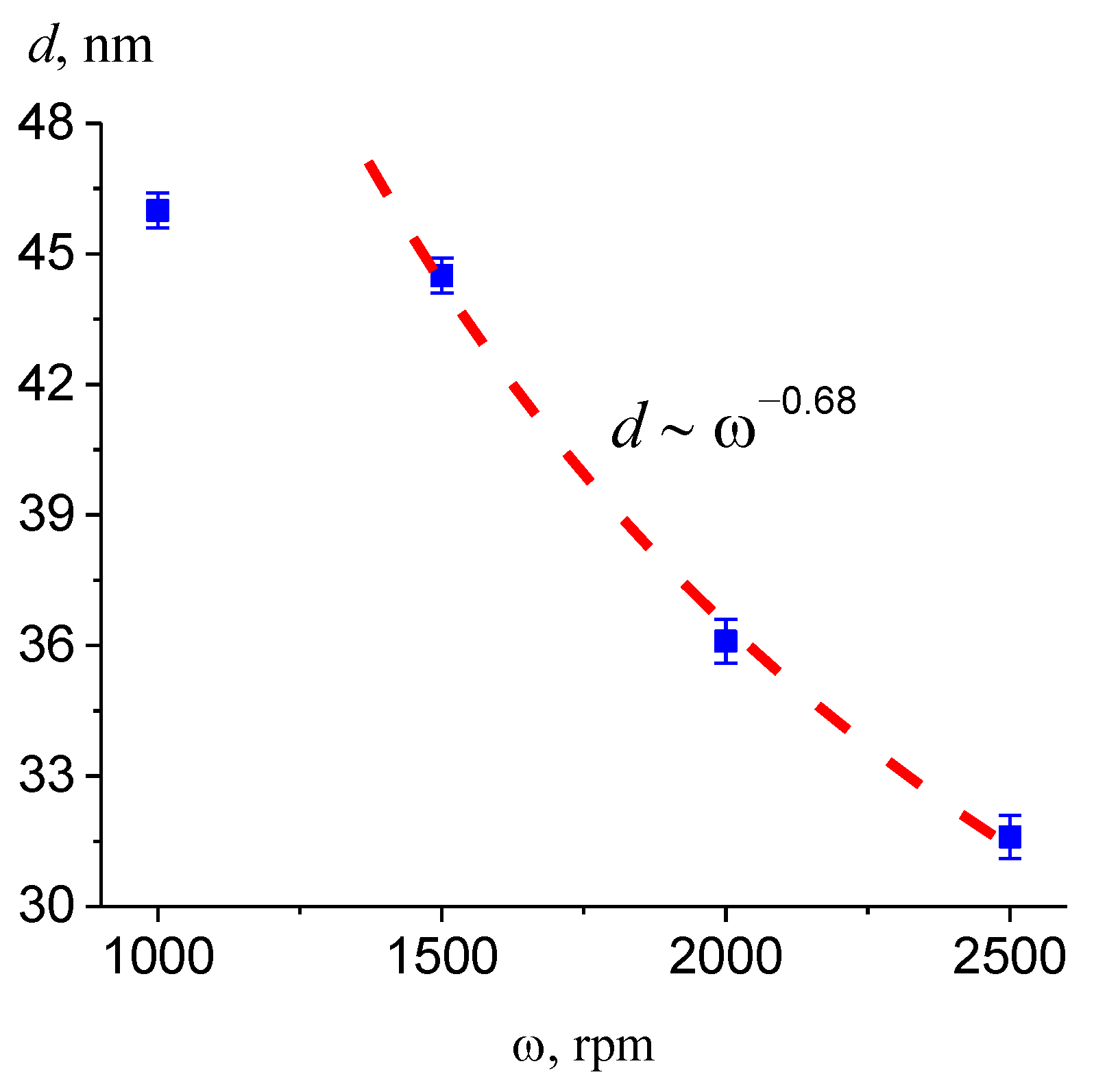
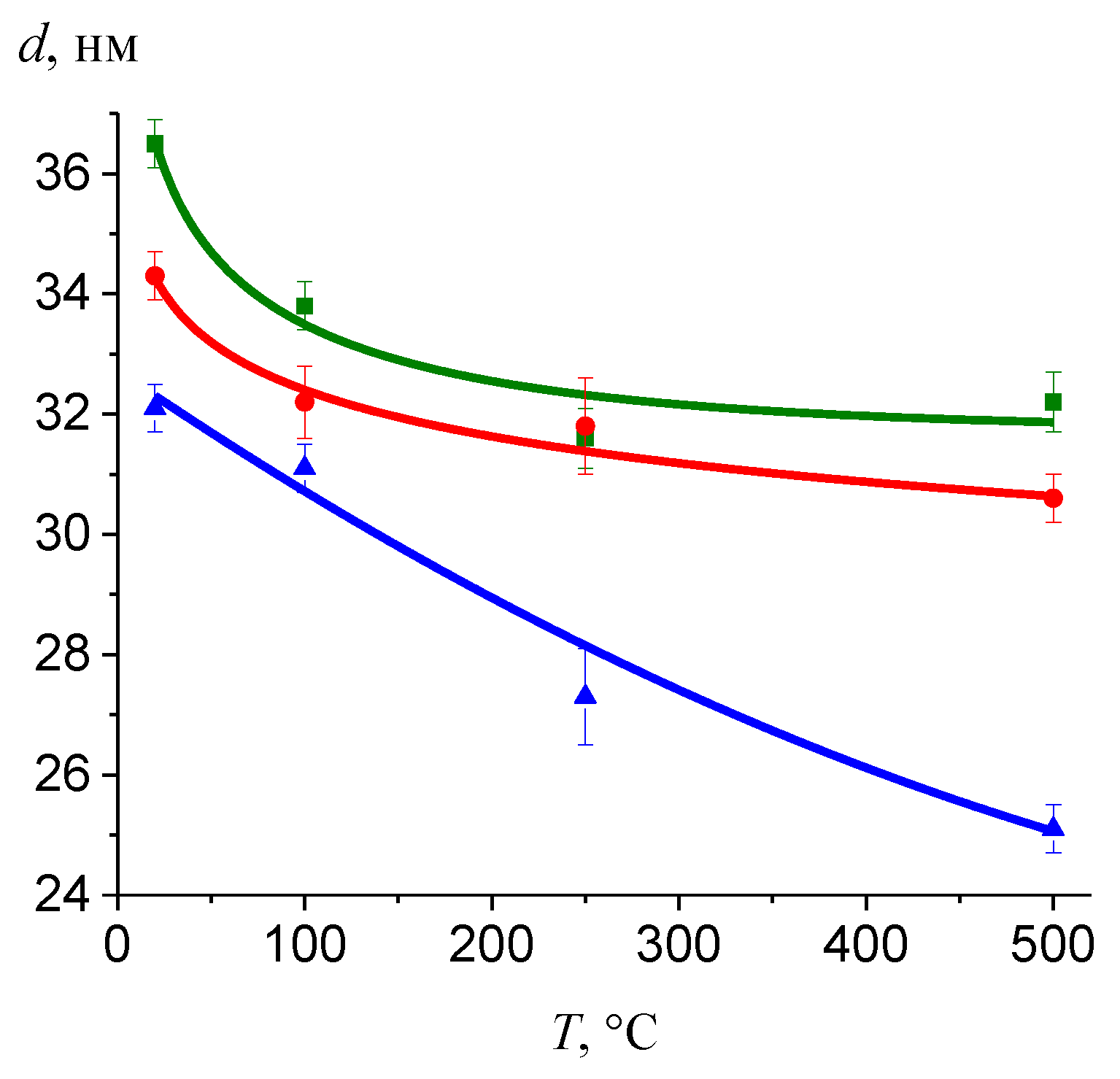
3.4. Effect of the Synthesis Conditions on the Film Thickness
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dominguez, M.A.; Luna-Lopez, J.A.; Moreno, M.; Orduña, A.; Garcia, M.; Alcantara, S.; Soto, S. Solution-processed transparent dielectric based on spin-on glass for electronic devices. Rev. Mex. De Física 2016, 62, 282–284. [Google Scholar]
- Yang, N.; Li, S.; Yang, J.; Li, H.; Ye, X.; Liu, C.; Yuan, X. Avoidance of boron rich layer formation in the industrial boron spin-on dopant diffused n-type silicon solar cell without additional oxidation process. J. Mater. Sci. Mater. Electron. 2018, 29, 20081–20086. [Google Scholar] [CrossRef]
- Yanagida, S.; Makino, M.; Ogaki, T.; Yasumori, A. Preparation of Pd–Pt Co-Loaded TiO2 Thin Films by Sol-Gel Method for Hydrogen Gas Sensing. J. Electrochem. Soc. 2012, 159, B845. [Google Scholar] [CrossRef]
- Qadri, M.U.; Diaz Diaz, A.F.; Cittadini, M.; Martucci, A.; Pujol, M.C.; Ferré-Borrull, J.; Llobet, E.; Aguiló, M.; Díaz, F. Effect of Pt nanoparticles on the optical gas sensing properties of WO3 thin films. Sensors 2014, 14, 11427–11443. [Google Scholar] [CrossRef] [PubMed]
- Nicolescu, M.; Mitrea, D.; Hornoiu, C.; Preda, S.; Stroescu, H.; Anastasescu, M.; Calderon-Moreno, J.M.; Predoana, L.; Teodorescu, V.S.; Maraloiu, V.-A.; et al. Structural, Optical, and Sensing Properties of Nb-Doped ITO Thin Films Deposited by the Sol–Gel Method. Gels 2022, 8, 717. [Google Scholar] [CrossRef]
- Shilova, O.A.; Tsvetkova, I.N.; Khamova, T.V.; Angelov, B.; Drozdova, I.A.; Kruchinina, I.Y.; Kopitsa, G.P. Sol-Gel Synthesis and Structure of Nanocomposites Based on Tetraethoxysilane and Boron Compounds. Glass Phys. Chem. 2021, 47 (Suppl. S1), S48–S62. [Google Scholar] [CrossRef]
- Bhattacharjee, B.; Ganguli, D.; Chaudhuri, S.; Pal, A.K. ZnS: Mn nanocrystallites in SiO2 matrix: Preparation and properties. Thin Solid Films 2002, 422, 98–103. [Google Scholar] [CrossRef]
- Manzanares-Martínez, J.; García-Cerda, L.A.; Ramírez-Bon, R.; Espinoza-Beltran, F.J.; Perez-Robles, J.F.; Gonzalez-Hernandez, J. Stabilization of copper-based colloidal particles in sol–gel SiO2 thin films. Thin Solid Films 2000, 365, 30–35. [Google Scholar] [CrossRef]
- Babapour, A.; Akhavan, O.; Moshfegh, A.Z.; Hosseini, A.A. Size variation and optical absorption of sol-gel Ag nanoparticles doped SiO2 thin film. Thin Solid Films 2006, 515, 771–774. [Google Scholar] [CrossRef]
- Hernández-Torres, J.; Mendoza-Galván, A. Optical properties of sol–gel SiO2 films containing nickel. Thin Solid Films 2005, 472, 130–135. [Google Scholar] [CrossRef]
- Shilova, O.A.; Gubanova, N.N.; Matveev, V.A.; Ivanova, A.G.; Arsentiev, M.Y.; Pugachev, K.E.; Ivankova, E.M.; Kruchinina, I.Y. Processes of film-formation and crystallization in catalytically active ‘spin-on glass’ silica films containing Pt and Pd nanoparticles. J. Mol. Liq. 2019, 288, 110996. [Google Scholar] [CrossRef]
- Shilova, O.A.; Gubanova, N.N.; Matveev, V.A.; Bayramukov, V.Y.; Kobzev, A.P. Composition, structure, and morphology of the surface of nanodimensional platinum-containing films obtained from sols. Glass Phys. Chem. 2016, 42, 78–86. [Google Scholar] [CrossRef]
- Gubanova, N.N.; Matveev, V.A.; Shilova, O.A. Bimetallic Pt/Pd nanoparticles in sol–gel-derived silica films and xerogels. J. Sol-Gel Sci. Technol. 2019, 92, 367–375. [Google Scholar] [CrossRef]
- Taylor, A.; Sinclair, H. On the determination of lattice parameters by the Debye-Scherrer method. Proc. Phys. Soc. 1945, 57, 126. [Google Scholar] [CrossRef]
- Heavens, O.S. Optical Properties of Thin Solid Films, 2nd ed.; Dover Publications: Garden City, NY, USA, 2011; p. 272. [Google Scholar]
- Grebenshchikova, E.A.; Shutaev, V.A.; Matveev, V.A.; Gubanova, N.N.; Shilova, O.A.; Yakovlev, Y.P. Hydrogen Influence on Electrical and Photoelectrical Properties of InP/Pd Thin-film Structures obtained by Sol-Gel Method. Tech. Phys. Lett. 2022, 5, 42. [Google Scholar] [CrossRef]
- Meyerhofer, D. Characteristics of resist films produced by spinning. J. Appl. Phys. 1978, 49, 3993–3997. [Google Scholar] [CrossRef]
- Bornside, D.E.; Macosko, C.W.; Scriven, L.E. Spin coating of a PMMA/chlorobenzene solution. J. Electrochem. Soc. 1991, 138, 317. [Google Scholar] [CrossRef]
- Bornside, D.E.; Brown, R.A.; Ackmann, P.W.; Frank, J.R.; Tryba, A.A.; Geyling, F.T. The effects of gas phase convection on mass transfer in spin coating. J. Appl. Phys. 1993, 73, 585–600. [Google Scholar] [CrossRef]
- Sukanek, P.C. Dependence of film thickness on speed in spin coating. J. Electrochem. Soc. 1991, 138, 1712. [Google Scholar] [CrossRef]
- Shimoji, S. Numerical analysis of the spin-coating process. J. Appl. Phys. 1989, 66, 2712–2718. [Google Scholar] [CrossRef]
- Nitta, S.V.; Jain, A.; Wayner, P.C., Jr.; Gill, W.N.; Plawsky, J.L. Effect of sol rheology on the uniformity of spin-on silica xerogel films. J. Appl. Phys. 1999, 86, 5870–5878. [Google Scholar] [CrossRef]
- Shilova, O.A.; Gubanova, N.N.; Ivanova, A.G.; Arsent’ev, M.Y.; Ukleev, V.A. Composition and Structure of Platinum-Containing Thin Composite Films Prepared from Silica Sols. Russ. J. Inorg. Chem. 2017, 62, 645–653. [Google Scholar] [CrossRef]
| Ratio between Pt, Pd and Si Compounds in the Doped Silica Sols, mol.% | |||||
|---|---|---|---|---|---|
| Pt-containing sol | 5Pt:100Si | 10Pt:100Si | 20Pt:100Si | 40Pt:100Si | 80Pt:100Si |
| Pd-containing sol | 5Pd:100Si | 10Pd:100Si | 20Pd:100Si | 40Pd:100Si | 80Pd:100Si |
| Pt/Pd-containing sol | 5Pt:5Pd:200Si | 10Pt:10Pd:200Si | 20Pt:20Pd:200Si | 40Pt:40Pd:200Si | 80Pt:80Pd:200Si |
| Dopants | DCSR (111), nm | ||||
|---|---|---|---|---|---|
| Pt | 5Pt_100Si | 10Pt_100Si | 20Pt_100Si | 40Pt_100Si | 80Pt_100Si |
| Amorph * | 4.5 ± 0.5 | 4.7 ± 0.5 | 6.7 ± 0.4 | 7.8 ± 0.4 | |
| Pd | 5Pd_100Si | 10Pd_100Si | 20Pd_100Si | 40Pd_100Si | 80Pd_100Si |
| Amorph * | Amorph * | 6.7 ± 0.4 | 6.6 ± 0.4 | 6.3 ± 0.4 | |
| Pt/Pd | 5Pt_5Pd_200Si | 10Pt_10Pd_200Si | 20Pt_20Pd_200Si | 40Pt_40Pd_200Si | 80Pt_80Pd_200Si |
| Amorph * | 5.0 ± 0.4 | 4.7 ± 0.6 | 6.0 ± 0.4 | 4.9 ± 0.4 | |
| Films | d, nm | ρe max, nm−3 | ρe min, nm−3 | σ, nm |
|---|---|---|---|---|
| Silica films doped with Pt | ||||
| 5Pt_100Si_250 | 22.8 ± 0.7 | 0.59 ± 0.02 | 0.59 ± 0.02 | 3.4 ± 0.4 |
| 10Pt_100Si_250 | 24.0 ± 0.9 | 0.86 ± 0.02 | 0.63 ± 0.02 | 3.9 ± 0.5 |
| 20Pt_100Si_250 | 31.6 ± 2.1 | 0.76 ± 0.02 | 0.61 ± 0.02 | 4.2 ± 0.7 |
| 40Pt_100Si_250 | - | - | - | - |
| 80Pt_100Si_250 | - | - | - | - |
| Silica films doped with Pd | ||||
| 5Pd_100Si_250 | 20.8 ± 1.2 | 0.68 ± 0.02 | 0.68 ± 0.02 | 4.4 ± 0.7 |
| 10Pd_100Si_250 | 22.0 ± 1.5 | 0.88 ± 0.02 | 0.63 ± 0.02 | 5.1 ± 1.0 |
| 20Pd_100Si_250 | 31.0 ± 1.5 | 0.72 ± 0.02 | 0.58 ± 0.02 | 4.2 ± 0.7 |
| 40Pd_100Si_250 | - | - | - | - |
| 80Pd_100Si_250 | - | - | - | - |
| Silica films doped with Pt/Pd | ||||
| 5Pt_5Pd_200Si_250 | 20.7 ± 0.4 | 0.54 ± 0.02 | 0.54 ± 0.02 | 2.4 ± 0.3 |
| 10Pt_10Pd_200Si_250 | 24.7± 1.8 | 0.78 ± 0.02 | 0.65 ± 0.02 | 4.2 ± 0.7 |
| 20Pt_20Pd_200Si_250 | 26.5 ± 1.6 | 0.78 ± 0.02 | 0.46 ± 0.02 | 5.6 ± 1.1 |
| 40Pt_40Pd_200Si_250 | 24.0 ± 2.5 | 0.90 ± 0.02 | 0.37 ± 0.02 | 6.1 ± 1.2 |
| 80Pt_80Pd_200Si_250 | 27.2 ± 2.7 | 0.93 ± 0.04 | 0.37 ± 0.02 | 5.5 ± 1.0 |
| Films | ρe, nm−3 | ρexp, g/cm−3 | P, % |
|---|---|---|---|
| 5Pt_100Si_250 | 0.59 ± 0.02 | 2.00 ± 0.07 | 36 ± 3 |
| 5Pd_100Si_250 | 0.68 ± 0.02 | 2.25 ± 0.07 | 16 ± 3 |
| 5Pt_5Pd_200Si_250 | 0.54 ± 0.02 | 1.81 ± 0.07 | 37 ± 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gubanova, N.; Matveev, V.; Grebenshchikova, E.; Kirilenko, D.; Sazonova, Y.; Shilova, O. Pt and Pd Nanoparticle Crystallization in the Sol-Gel-Derived Thin SiO2 Films. Physchem 2023, 3, 259-269. https://doi.org/10.3390/physchem3020018
Gubanova N, Matveev V, Grebenshchikova E, Kirilenko D, Sazonova Y, Shilova O. Pt and Pd Nanoparticle Crystallization in the Sol-Gel-Derived Thin SiO2 Films. Physchem. 2023; 3(2):259-269. https://doi.org/10.3390/physchem3020018
Chicago/Turabian StyleGubanova, Nadezhda, Vasilii Matveev, Elena Grebenshchikova, Demid Kirilenko, Yana Sazonova, and Olga Shilova. 2023. "Pt and Pd Nanoparticle Crystallization in the Sol-Gel-Derived Thin SiO2 Films" Physchem 3, no. 2: 259-269. https://doi.org/10.3390/physchem3020018
APA StyleGubanova, N., Matveev, V., Grebenshchikova, E., Kirilenko, D., Sazonova, Y., & Shilova, O. (2023). Pt and Pd Nanoparticle Crystallization in the Sol-Gel-Derived Thin SiO2 Films. Physchem, 3(2), 259-269. https://doi.org/10.3390/physchem3020018







