Cyclic Voltammetry Study of Closo-Ruthenacarboranes
Abstract
1. Introduction
2. Materials and Methods
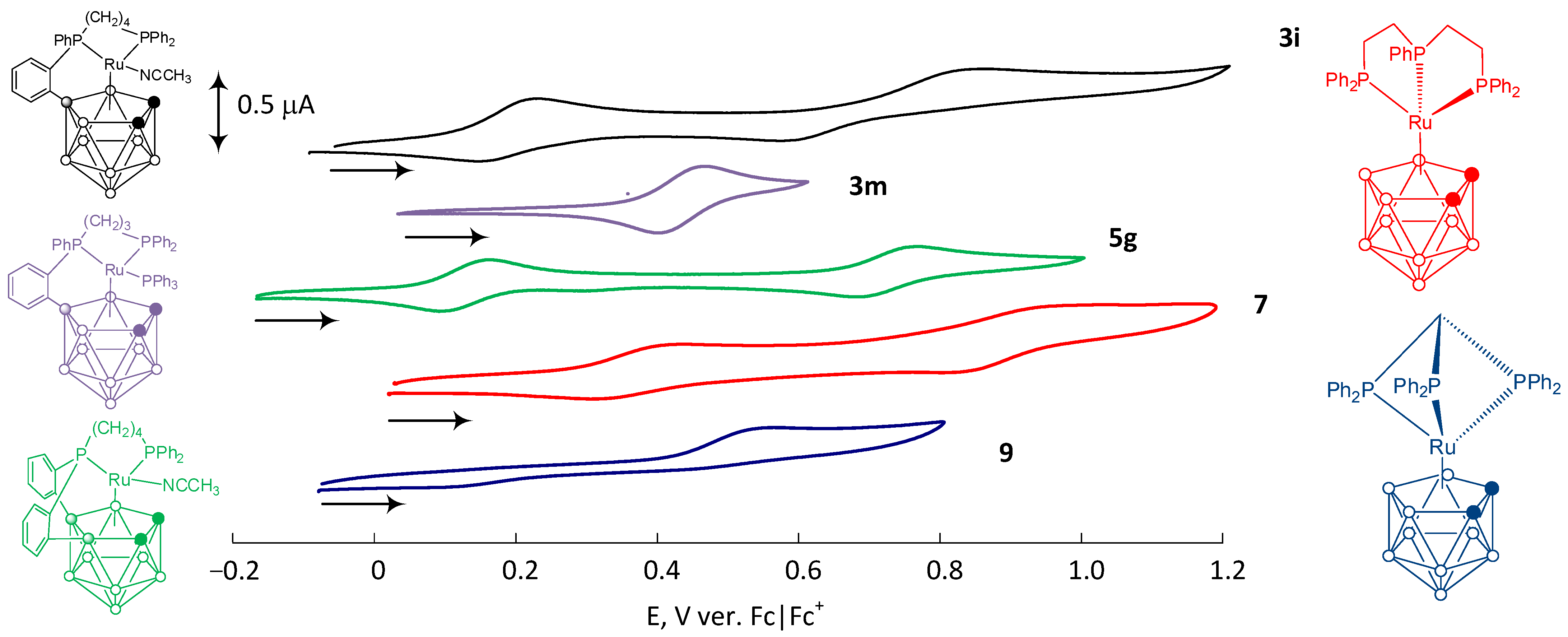
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Grimes, R.N. Carboranes in catalysis. In Carboranes, 3rd ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 929–944. [Google Scholar] [CrossRef]
- Chan, A.P.Y.; Parkinson, J.A.; Rosair, G.M.; Welch, A.J. Bis(phosphine)hydridorhodacarborane derivatives of 1,1′-bis(ortho-carborane) and their catalysis of alkene isomerization and the hydrosilylation of acetophenone. Inorg. Chem. 2020, 59, 2011–2023. [Google Scholar] [CrossRef]
- Vinogradov, M.M.; Loginov, D.A. Rhoda- and iridacarborane halide complexes: Synthesis, structure and application in homogeneous catalysis. J. Organomet. Chem. 2020, 910, 121135. [Google Scholar] [CrossRef]
- Zhu, Y.; Hosmane, N.S. Carborane-based catalysts for polymerization of olefins. In Handbook of Boron Science with Applications in Organometallics, Catalysis, Materials and Medicine. Vol. 2. Boron in Catalysis; Hosmane, N.S., Eagling, R., Eds.; World Scientific Publishing Europe Ltd.: Singapore, 2018; pp. 117–134. [Google Scholar] [CrossRef]
- Stogniy, M.Y.; Erokhina, S.A.; Suponitsky, K.Y.; Markov, V.Y.; Sivaev, I.B. Synthesis and crystal structures of nickel(II) and palladium(II) complexes with o-carboranyl amidine ligands. Dalton Trans. 2021, 50, 4967–4975. [Google Scholar] [CrossRef]
- Teixeira, R.G.; Marques, F.; Robalo, M.P.; Fontrodona, X.; Garcia, M.H.; Crich, G.S.; Vinas, C.; Valente, A. Ruthenium carboranyl complexes with 2,2′-bipyridine derivatives for potential bimodal therapy application. RSC Adv. 2020, 10, 16266–16276. [Google Scholar] [CrossRef]
- Richel, A.; Demonceau, A.; Noels, A.F. Electrochemistry as a correlation tool with the catalytic activities in [RuCl2(p-cymene)(PAr3)]-catalysed Kharasch additions. Tetrahedron Lett. 2006, 47, 2077–2081. [Google Scholar] [CrossRef]
- Tang, W.; Kwak, Y.; Braunecker, W.; Tsarevsky, N.V.; Coote, M.L.; Matyjaszewski, K. Understanding atom transfer radical polymerization: Effect of ligand and initiator structures on the equilibrium constants. J. Am. Chem. Soc. 2008, 130, 10702–10713. [Google Scholar] [CrossRef]
- Chatterjee, D.; Oszajca, M.; Katafias, A.; van Eldik, R. Electrochemistry of Ru(edta) complexes relevant to small molecule transformations: Catalytic implications and challenges. Coord. Chem. Rev. 2021, 436, 213773. [Google Scholar] [CrossRef]
- Patil-Deshmukh, A.B.; Mohite, S.S.; Chavan, S.S. Ru(II)-polypyridine complexes with alkynyl Schiff base ligand: Influence of π-conjugation, donor/acceptor substituents, and counter anions on electrochemical, luminescence, and catalytic properties. J. Coord. Chem. 2020, 73, 1028–1044. [Google Scholar] [CrossRef]
- Reisner, E.; Arion, V.B.; Guedes da Silva, M.F.C.; Lichtenecker, R.; Eichinger, A.; Keppler, B.K.; Kukushkin, V.Y.; Pombeiro, A.J.L. Tuning of redox potentials for the design of ruthenium anticancer drugs—An electrochemical study of [trans-RuCl4L(DMSO)]- and [trans-RuCl4L2]- complexes, where L = imidazole, 1,2,4-triazole, indazole. Inorg. Chem. 2004, 43, 7083–7093. [Google Scholar] [CrossRef]
- Bomben, P.G.; Robson, K.C.D.; Sedach, P.A.; Berlinguette, C.P. On the viability of cyclometalated Ru(II) complexes for light-harvesting applications. Inorg. Chem. 2009, 48, 9631–9643. [Google Scholar] [CrossRef]
- Shahroosvand, H.; Rezaei, S.; Mohajerani, E.; Mahmoudi, M.; Kamyabia, M.A.; Nasiria, S. Key role of ancillary ligands in imparting blue shift in electroluminescence wavelength in ruthenium polypyridyl light-emitting diodes. New J. Chem. 2014, 38, 5312–5323. [Google Scholar] [CrossRef]
- Geiger, W.E. One-electron electrochemistry of parent piano-stool complexes. Coord. Chem. Rev. 2013, 257, 1459–1471. [Google Scholar] [CrossRef]
- von Eschwege, K.G.; Conradie, J. Review of DFT-simulated and experimental electrochemistry properties of the polypyridyl Row-1 Mn, Fe & Co, and Group-8 Fe, Ru and Os MLCT complexes. Electrochem. Commun. 2022, 136, 107225. [Google Scholar] [CrossRef]
- Alexiou, C.; Lever, A.B.P. Tuning metalloporphyrin and metallophthalocyanine redox potentials using ligand electrochemical (EL) and Hammett (p) parametrization. Coord. Chem. Rev. 2001, 216–217, 45–54. [Google Scholar] [CrossRef]
- Van Caemelbecke, E.; Phan, T.; Osterloh, W.R.; Kadish, K.M. Electrochemistry of metal-metal bonded diruthenium complexes. Coord. Chem. Rev. 2021, 434, 213706. [Google Scholar] [CrossRef]
- Ramesh, G.; Kumar, P.R.; Pillegowda, M.; Periyasamy, G.; Suchetan, P.A.; Butcher, R.J.; Forod, S.; Nagaraju, G. Synthesis, crystal structures, photophysical, electrochemical studies, DFT and TD-DFT calculations and Hirshfeld analysis of new 2,2′:6′,2″-terpyridine ligands with pendant 4′-(trimethoxyphenyl) groups and their homoleptic ruthenium complexes. New J. Chem. 2020, 44, 11471–11489. [Google Scholar] [CrossRef]
- Grishin, I.D.; D’yachihin, D.I.; Piskunov, A.V.; Dolgushin, F.M.; Smolyakov, A.F.; Il’in, M.M.; Davankov, V.A.; Chizhevsky, I.T.; Grishin, D.F. Carborane complexes of ruthenium(III): Studies on thermal reaction chemistry and the catalyst design for atom transfer radical polymerization of methyl methacrylate. Inorg. Chem. 2011, 50, 7574–7585. [Google Scholar] [CrossRef]
- Grishin, I.D.; Zimina, A.M.; Anufriev, S.A.; Knyazeva, N.A.; Piskunov, A.V.; Dolgushin, F.M.; Sivaev, I.B. Synthesis and Catalytic Properties of Novel Ruthenacarboranes Based on nido-[5-Me-7,8-C2B9H10]2− and nido-[5,6-Me2-7,8-C2B9H9]2− Dicarbollide Ligands. Catalysts 2021, 11, 1409. [Google Scholar] [CrossRef]
- Corrigan, N.; Jung, K.; Moad, G.; Hawker, C.J.; Matyjaszewski, K.; Boyer, C. Reversible-deactivation radical polymerization (Controlled/living radical polymerization): From discovery to materials design and applications. Prog. Polym. Sci. 2020, 111, 101311. [Google Scholar] [CrossRef]
- Zhou, Y.N.; Li, J.J.; Wang, T.T.; Wu, Y.Y.; Luo, Z.H. Precision polymer synthesis by controlled radical polymerization: Fusing the progress from polymer chemistry and reaction engineering. Prog. Polym. Sci. 2022, 130, 101555. [Google Scholar] [CrossRef]
- Gagne, R.R.; Koval, C.A.; Lisensky, G.C. Ferrocene as an internal standard for electrochemical measurements. Inorg. Chem. 1980, 19, 2854–2855. [Google Scholar] [CrossRef]
- Kaltenberg, A.A.; Zimina, A.M.; Bashilova, A.D.; Malysheva, Y.B.; Vorozhtsov, D.L.; Piskunov, A.V.; Somov, N.V.; Grishin, I.D. The peculiarities of interaction of 5,6,10-{Cl(Ph3P)2Ru}-[5,6,10-(μ-H)3-10-H-exo-nido-7,8-C2B9H8 with bis(diphenylphosphino)methane and 1,2-bis(diphenylphosphino)benzene. Russ. Chem. Bull. Int. Ed. 2014, 72, 912–924. [Google Scholar] [CrossRef]
- Cheredilin, D.N.; Dolgushin, F.M.; Grishin, I.D.; Kolyakina, E.V.; Nikiforov, A.S.; Solodovnikov, S.P.; Il’in, M.M.; Davankov, V.A.; Chizhevsky, I.T.; Grishin, D.F. Facile method for the synthesis of ruthenacarboranes, diamagnetic 3,3-[Ph2P(CH2)nPPh2]-3-H-3-Cl-closo-3,1,2-RuC2B9H11 (n = 3 or 4) and paramagnetic 3,3-[Ph2P(CH2)nPPh2]-3-Cl-closo-3,1,2-RuC2B9H11 (n = 2 or 3), as efficient initiators of controlled radical polymerization of vinyl monomers. Russ. Chem. Bull. Int. Ed. 2006, 55, 1163–1170. [Google Scholar] [CrossRef]
- Grishin, I.D.; D’yachihin, D.I.; Turmina, E.S.; Dolgushin, F.M.; Smol’yakov, A.F.; Piskunov, A.V.; Chizhevsky, I.T.; Grishin, D.F. Mononuclear closo-ruthenacarborane complexes containing a rare eight-membered metal-diphosphine ring. J. Organomet. Chem. 2012, 721–722, 113–118. [Google Scholar] [CrossRef]
- D’yachihin, D.I.; Grishin, I.D.; Piskunov, A.V.; Godovikov, I.A.; Kostukovich, A.Y.; Smol’yakov, A.F.; Dolgushin, F.M.; Chizhevsky, I.T.; Grishin, D.F. Efficient methods for the preparation of bromine-containing exo-nido- and closo-ruthenacarborane clusters. Russ. Chem. Bull. Int. Ed. 2014, 63, 2325–2333. [Google Scholar] [CrossRef]
- Zimina, A.M.; Knyazeva, N.A.; Balagurova, E.V.; Dolgushin, F.M.; Somov, N.V.; Vorozhtsov, D.L.; Malysheva, Y.B.; Grishin, I.D. Revising the chemistry of κ2-dppe-closo-RuC2B9H11 fragment: Synthesis of novel diamagnetic complexes and its transformations. J. Organomet. Chem. 2021, 946–947, 121908. [Google Scholar] [CrossRef]
- Penkal, A.M.; D’yachihin, D.I.; Somov, N.V.; Shchegravina, E.S.; Grishin, I.D. Synthesis of novel closo-carborane complexes of ruthenium (II) with triphenylphosphine or acetonitrile ligands via reduction of paramagnetic Ru(III) derivatives. J. Organomet. Chem. 2018, 872, 63–72. [Google Scholar] [CrossRef]
- Penkal, A.M.; Somov, N.V.; Shchegravina, E.S.; Grishin, I.D. Ruthenium Diphosphine Closo-C2B9-Carborane Clusters with Nitrile Ligands: Synthesis and Structure Determination. J. Clust. Sci. 2019, 30, 1317–1325. [Google Scholar] [CrossRef]
- Grishin, I.D.; Agafonova, K.S.; Kostyukovich, A.Y.; D’yachihin, D.I.; Godovikov, I.A.; Dolgushin, F.M.; Grishin, D.F.; Chizhevsky, I.T. Synthesis of metallacarborane ruthenium(II) and ruthenium(III) complexes with chelate 1,3-bis(diphenylphosphino)propane ligand and their mutual transformation in one-electron redox reactions. Russ. Chem. Bull. Int. Ed. 2016, 65, 1574–1579. [Google Scholar] [CrossRef]
- Gritzner, G.; Kůta, J. Recommendations on reporting electrode potentials in nonaqueous solvents: IUPC commission on electrochemistry. Pure Appl. Chem. 1984, 56, 461–466. [Google Scholar] [CrossRef]
- Zimina, A.M.; Somov, N.V.; Malysheva, Y.B.; Knyazeva, N.A.; Piskunov, A.V.; Grishin, I.D. 12-Vertex closo-3,1,2-ruthenadicarbadodecaboranes with chelate POP-ligands: Synthesis, X-ray study and electrochemical properties. Inorganics 2022, 10, 206. [Google Scholar] [CrossRef]
- Kaltenberg, A.A.; Somov, N.V.; Malysheva, Y.B.; Knyazeva, N.A.; Piskunov, A.V.; Grishin, I.D. Novel carborane complexes of ruthenium with tridentate phosphine ligands: Synthesis and application in Atom Transfer Radical Polymerization. J. Organomet. Chem. 2020, 917, 121291. [Google Scholar] [CrossRef]
- Kaltenberg, A.A.; Somov, N.V.; Malysheva, Y.B.; Vorozhtsov, D.L.; Grishin, I.D. Synthesis of novel pseudocloso ruthenacarboranes based on an unsubstituted nido-C2B9H112− ligand. Eur. J. Inorg. Chem. 2021, 2021, 4868–4874. [Google Scholar] [CrossRef]
- Núñez, R.; Tarrés, M.; Ferrer-Ugalde, A.; Fabrizi de Biani, F.; Teixidor, F. Electrochemistry and photoluminescence of icosahedral carboranes, boranes, metallacarboranes, and their derivatives. Chem. Rev. 2016, 116, 14307–14378. [Google Scholar] [CrossRef]
- Chizhevsky, I.T.; Lobanova, I.A.; Petrovskii, P.V.; Bregadze, V.I.; Dolgushin, F.M.; Yanovsky, A.I.; Struchkov, Y.T.; Chistyakov, A.L.; Stankevich, I.V.; Knobler, C.B.; et al. Synthesis of mixed-metal (Ru−Rh) bimetallacarboranes via exo-nido- and closo-ruthenacarboranes. Molecular structures of (η4-C8H12)Rh(μ-H)Ru(PPh3)2(η5-C2B9H11) and (CO)(PPh3)Rh(μ-H)Ru(PPh3)2(η5-C2B9H11) and their anionic closo-ruthenacarborane precursors. Organometallics 1999, 18, 726–735. [Google Scholar] [CrossRef]
- Dierkes, P.; van Leeuwen, P.W.N.M. The bite angle makes the difference: A practical ligand parameter for diphosphine ligands. J. Chem. Soc. Dalton Trans. 1999, 10, 1519–1530. [Google Scholar] [CrossRef]
- Kovalski, E.; Korb, M.; Hildebrandt, A. Synthesis, electrochemistry, and optical properties of half-sandwich ruthenium complexes bearing triarylamine-anthracenes. Eur. J. Inorg. Chem. 2018, 2018, 671–675. [Google Scholar] [CrossRef]
- Fox, M.A.; Roberts, R.L.; Khairul, W.M.; Hartl, F.; Low, P.J. Spectroscopic properties and electronic structures of 17-electron half-sandwich ruthenium acetylide complexes, [Ru(C≡CAr)(L2)Cp′]+ (Ar = phenyl, p-tolyl, 1-naphthyl, 9-anthryl; L2 = (PPh3)2, Cp′ = Cp; L2 = dppe; Cp′ = Cp∗). J. Organomet. Chem. 2007, 692, 3277–3290. [Google Scholar] [CrossRef]
- Morandini, F.; Dondana, A.; Munari, I.; Pilloni, G.; Consiglio, G.; Sironi, A.; Moret, M. Pentamethylcyclopentadienyl ruthenium(II) complexes containing chiral diphospines: Synthesis, characterisation and electrochemical behaviour. X-ray structure of (η5-C5Me5)Ru{(S,S)-Ph2PCH(CH3)CH(CH3)PPh2}Cl. Inorg. Chim. Acta 1998, 282, 163–172. [Google Scholar] [CrossRef]
- Tutusaus, O.; Vinas, C.; Nunez, R.; Teixidor, F.; Demonceau, A.; Delfosse, S.; Noels, A.F.; Mata, I.; Molins, E. The modulating possibilities of dicarbollide clusters: optimizing the Kharasch catalysts. J. Am. Chem. Soc. 2003, 125, 11830–11831. [Google Scholar] [CrossRef]
- Llop, J.; Viñas, C.; Teixidor, F.; Victori, L.; Kivekäs, R.; Sillanpää, R. Redox potential modulation in mixed sandwich pyrrolyl/ dicarbollide complexes. Inorg. Chem. 2002, 41, 3347–3352. [Google Scholar] [CrossRef]
- Auzias, M.; Therrien, B.; Süss-Fink, G.; Štěpnička, P.; Han, P.; Ang, W.H.; Dyson, P.K. Ferrocenoyl pyridine arene ruthenium complexes with anticancer properties: synthesis, structure, electrochemistry, and cytotoxicity. Inorg. Chem. 2008, 47, 578–583. [Google Scholar] [CrossRef]
- Raebiger, J.W.; Miedaner, A.; Curtis, C.J.; Miller, S.M.; Anderson, O.P.; DuBois, D.L. Using ligand bite angles to control the hydricity of palladium diphosphine complexes. J. Am. Chem. Soc. 2004, 126, 5502–5514. [Google Scholar] [CrossRef]
- Qiu, J.; Matyjaszewski, K.; Thouin, L.; Amatore, C. Cyclic voltammetric studies of copper complexes catalyzing atom transfer radical polymerization. Macromol. Chem. Phys. 2000, 201, 1625–1631. [Google Scholar] [CrossRef]
- Jelliss, P.A.; Mason, J.; Nazzoli, J.M.; Orlando, J.H.; Vinson, A. Synthesis and characterization of ruthenacarborane complexes incorporating chelating n-donor ligands: Unexpected luminescence from the complex [3-CO-3,3-{k2-Me2N(CH2)2NMe2}-closo-3,1,2-RuC2B9H11]. Inorg. Chem. 2006, 45, 370–385. [Google Scholar] [CrossRef]
- Vorotyntsev, M.A.; Casalta, M.; Pousson, E.; Roullier, L.; Boni, G.; Moise, C. Redox properties of titanocene-pyrrole derivative and its electropolymerization. Electrochim. Acta 2001, 46, 4017–4033. [Google Scholar] [CrossRef]

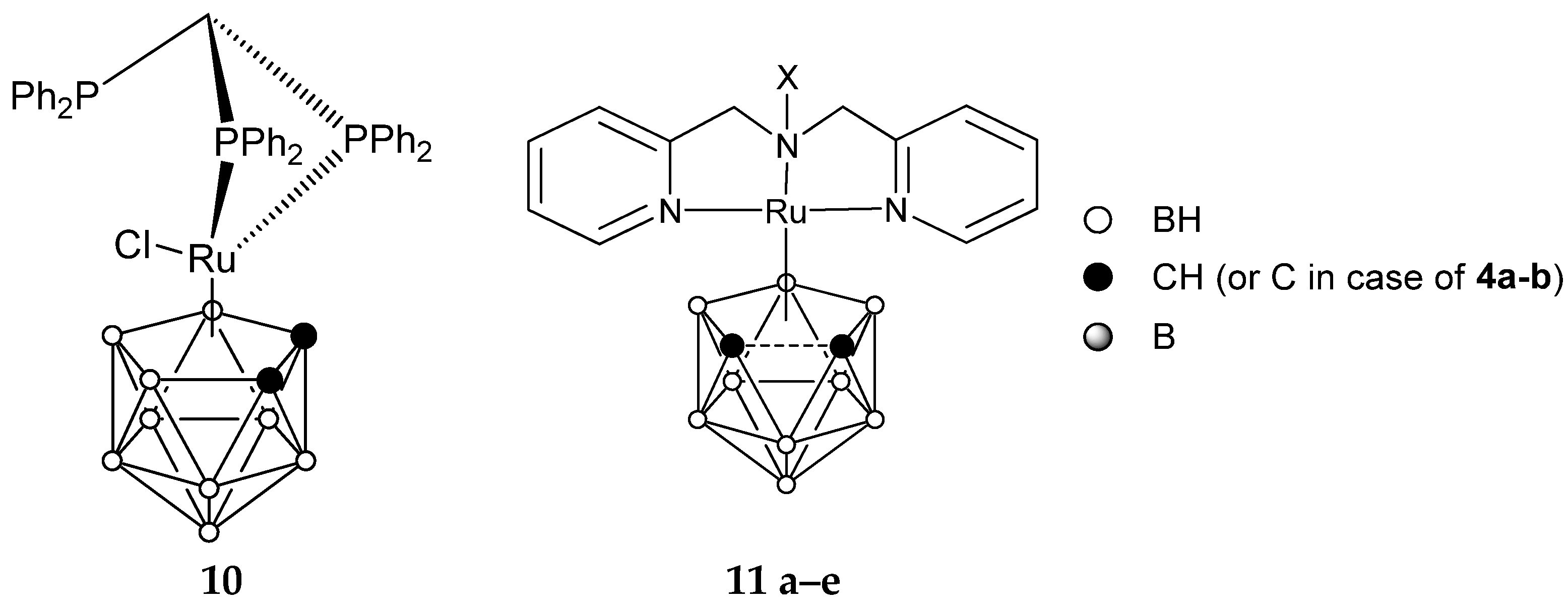
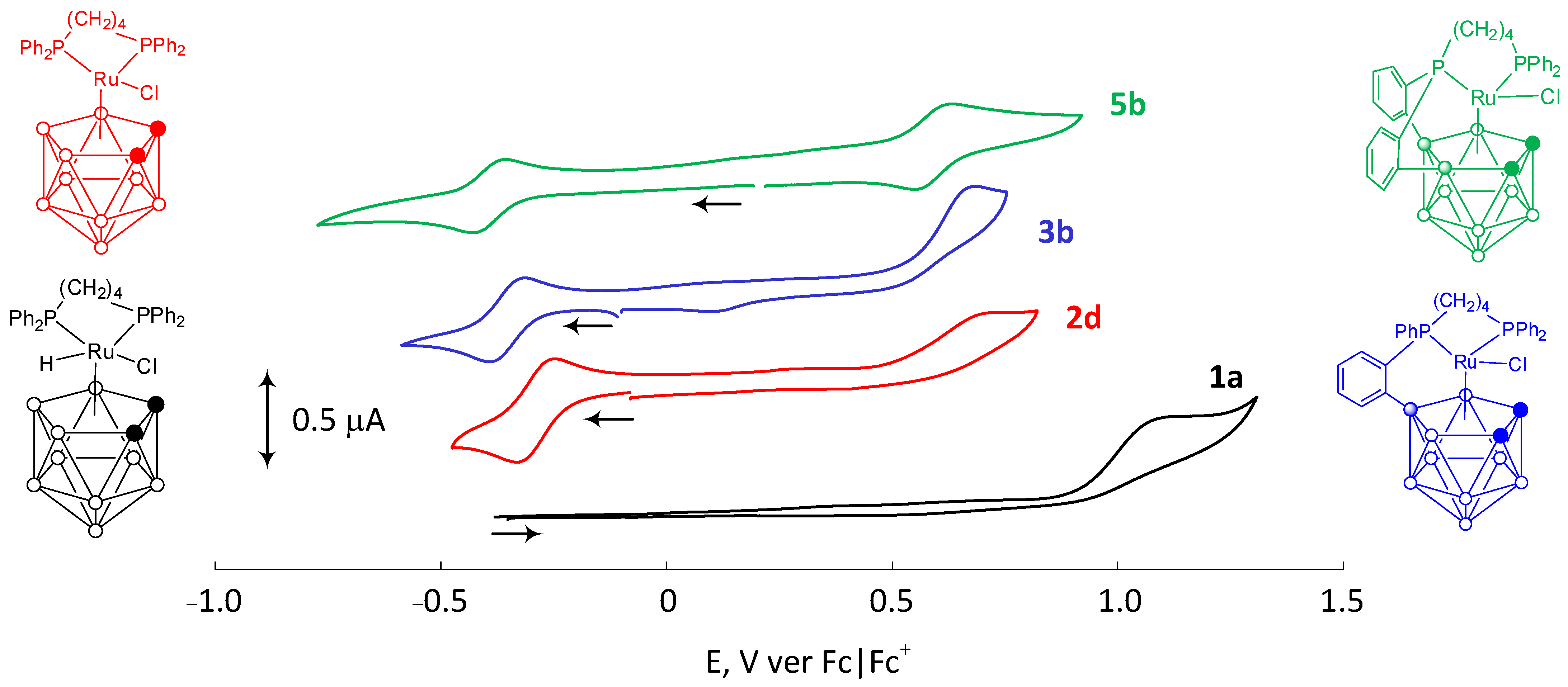
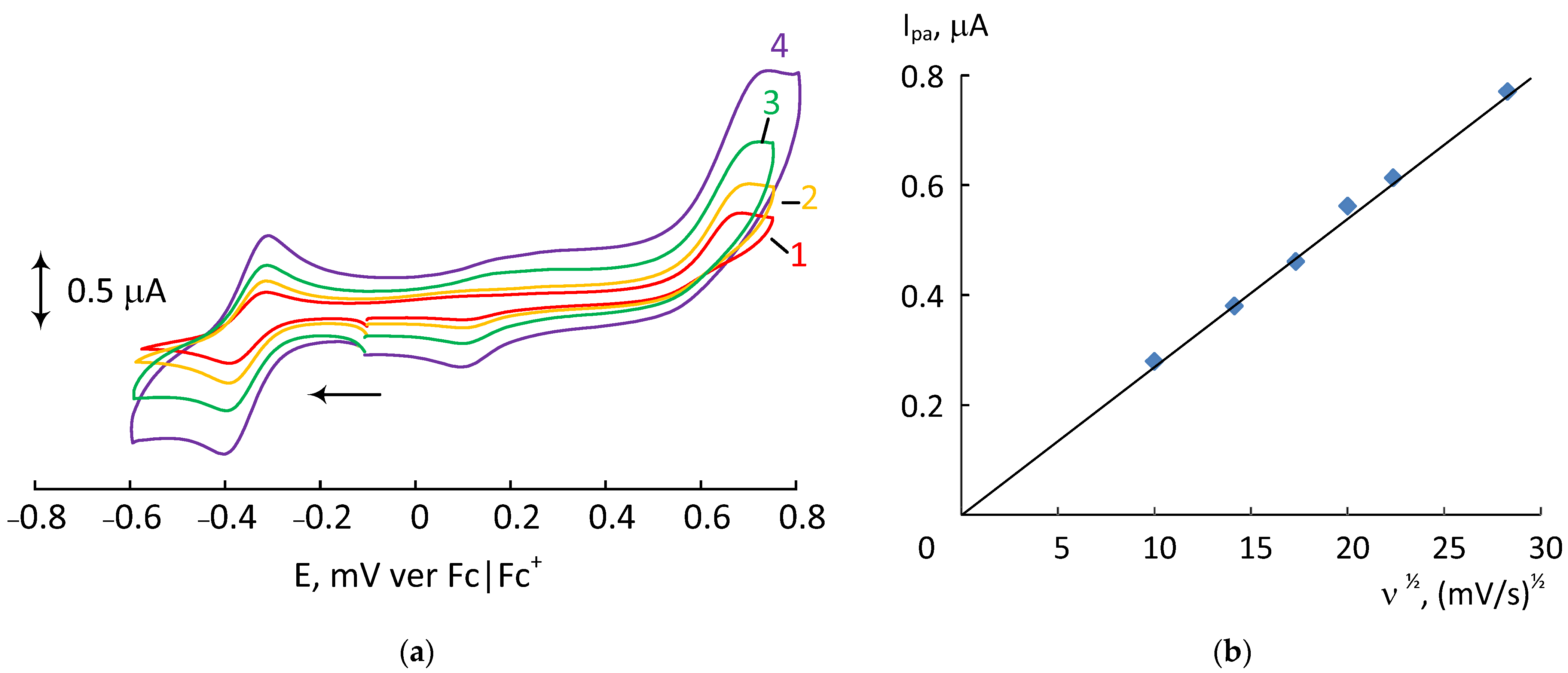

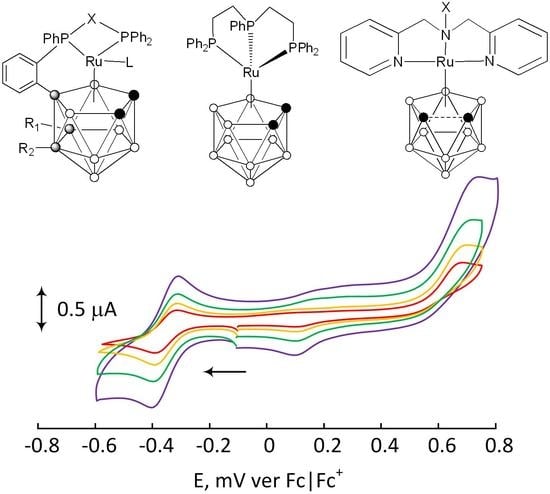
| Complex | L | R1, R2 | Epa | [Ref] |
|---|---|---|---|---|
| 1a | Cl | −H, −H | 1.10 | [9] |
| 1b | Cl | −H, −CH3 | 1.01 | [10] |
| 1c | Cl | −H, −C4H9 | 1.08 | [10] |
| 1c | Cl | −CH3, −CH3 | 0.96 | [10] |
| 1d | Br | −H, −H | 1.02 | [16] |
| Complex | Type | X | L | R1, R2 | Ru(II)–Ru(III), V | Ru(III)–Ru(IV), V | [Ref] | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Epa | Epc | E1/2 | Epa | Epc | E1/2 | ||||||
| 2a | 17-e | CH2 | Cl | −H, −H | −0.398 | −0.480 | −0.439 | 0.548 ** | − | − | [24] |
| 2b | 17-e | (CH2)2 | Cl | −H, −H | −0.297 | −0.367 | −0.332 | 0.677 ** | − | − | [25] |
| 2c | 17-e | (CH2)3 | Cl | −H, −H | −0.279 | −0.343 | −0.311 | 0.683 ** | − | − | [25] |
| 2d | 17-e | (CH2)4 | Cl | −H, −H | −0.251 | −0.332 | −0.292 | 0.721 ** | − | − | [19] |
| 2e | 17-e | (CH2)5 | Cl | −H, −H | −0.245 | −0.318 | −0.282 | 0.720 ** | − | − | [26] |
| 2f | 17-e | o-C6H4 | Cl | −H, −H | −0.312 | −0.399 | −0.356 | 0.779 ** | [24] | ||
| 2g | 17−e | (CH2)2 | Br | −H, −H | −0.276 | −0.352 | −0.314 | 0.691 ** | − | − | [27] |
| 2h | 17-e | (CH2)3 | Br | −H, −H | −0.245 | −0.320 | −0.282 | 0.756 ** | − | − | [27] |
| 2j | 17-e | (CH2)4 | Br | −H, −H | −0.212 | −0.291 | −0.251 | 0.805 ** | − | − | [27] |
| 2k | 17-e | (CH2)4 | Cl | −H, −C4H9 | −0.343 | −0.410 | −0.377 | 0.725 ** | − | − | [20] |
| 2l | 18-e | CH2 | CH3CN | −H, −H | 0.206 | 0.138 | 0.172 | − | − | − | [24] |
| 2m | 18-e | (CH2)2 | CH3CN | −H, −H | 0.290 | 0.190 | 0.240 | 0.954 ** | − | − | [28] |
| 2n | 18-e | (CH2)4 | CH3CN | −H, −H | 0.367 | 0.280 | 0.324 | 1.166 ** | − | − | [29] |
| 2o | 18−e | (CH2)4 | C6H5CN | −H, −H | 0.368 | 0.283 | 0.326 | 0.935 ** | − | − | [30] |
| 2p | 18-e | (CH2)4 | CH2=CHCN | −H, −H | 0.366 | 0.273 | 0.320 | 0.848 ** | − | − | [30] |
| 2q | 18-e | CH2 | PPh3 | −H, −H | 0.432 | 0.345 | 0.389 | − | − | − | [24] |
| 2r | 18-e | o-C6H4 | CH3CN | −H, −H | 0.320 | 0.206 | 0.263 | − | [24] | ||
| 3a | 17-e | (CH2)3 | Cl | −H, −H | −0.357 | −0.426 | −0.391 | 0.624 ** | − | − | [31] |
| 3b | 17-e | (CH2)4 | Cl | −H, −H | −0.316 | −0.393 | −0.355 | 0.683 ** | − | − | [19] |
| 3c | 17-e | (CH2)5 | Cl | −H, −H | −0.274 | −0.345 | −0.310 | 0.677 ** | − | − | [26] |
| 3d | 17-e | (CH2)3 | Br | −H, −H | −0.285 | −0.351 | −0.318 | 0.619 ** | − | − | [27] |
| 3e | 17-e | (CH2)4 | Br | −H, −H | −0.277 | −0.344 | −0.311 | 0.610 ** | − | − | [27] |
| 3f | 17-e | (CH2)5 | Br | −H, −H | −0.254 | −0.323 | −0.288 | 0.716 ** | − | − | [27] |
| 3g | 17-e | (CH2)4 | Cl | −H, −CH3 | −0.383 | −0.463 | −0.423 | 0.615 ** | − | − | [32] |
| 3h | 17-e | (CH2)4 | Cl | −CH3, −CH3 | −0.417 | −0.488 | −0.452 | 0.570 ** | − | − | [32] |
| 3i | 18-e | (CH2)4 | CH3CN | −H, −H | 0.284 | 0.211 | 0.248 | 0.790 ** | − | − | [28] |
| 3j | 18-e | (CH2)4 | C6H5CN | −H, −H | 0.285 | 0.204 | 0.244 | 0.690 ** | − | − | [30] |
| 3k | 18-e | (CH2)4 | CH2=CHCN | −H, −H | 0.291 | 0.204 | 0.248 | 0.680 ** | − | − | [30] |
| 3l | 18-e | (CH2)2 | PPh3 | −H, −H | 0.425 | 0.329 | 0.377 | 0.905 ** | − | − | [28] |
| 3m | 18-e | (CH2)3 | PPh3 | −H, −H | 0.450 | 0.387 | 0.418 | 1.100 ** | − | − | [31] |
| 3n | 18-e | (CH2)4 | PPh3 | −H, −H | 0.275 | 0.207 | 0.241 | 0.802 ** | − | − | [26] |
| 4a | 17-e | (CH2)3 | Cl | − | −0.461 | −0.542 | −0.501 | 0.517 ** | − | − | [25] |
| 4b | 17-e | (CH2)4 | Cl | − | −0.426 | −0.497 | −0.461 | 0.553 ** | − | − | [25] |
| 5a | 17-e | (CH2)3 | Cl | −H, −H | −0.393 | −0.458 | −0.426 | 0.575 | 0.510 | 0.543 | [19] |
| 5b | 17-e | (CH2)4 | Cl | −H, −H | −0.358 | −0.428 | −0.393 | 0.627 | 0.551 | 0.589 | [19] |
| 5c | 17-e | (CH2)5 | Cl | −H, −H | −0.279 | −0.348 | −0.313 | 0.637 | 0.551 | 0.594 | [26] |
| 5d | 17-e | (CH2)4 | Br | −H, −H | −0.346 | −0.414 | −0.380 | 0.634 | 0.560 | 0.597 | [27] |
| 5e | 17-e | (CH2)4 | Cl | −H, −CH3 | −0.419 | −0.510 | −0.464 | 0.601 | 0.509 | 0.555 | [32] |
| 5f | 17-e | (CH2)4 | Cl | −CH3, −CH3 | −0.454 | −0.540 | −0.497 | 0.571 | 0.488 | 0.530 | [32] |
| 5g | 18-e | (CH2)4 | CH3CN | −H, −H | 0.164 | 0.093 | 0.128 | 0.763 | 0.685 | 0.724 | [28] |
| 6a | 17-e | −H, −H | Cl | − | −0.274 | −0.350 | −0.312 | 0.702 ** | − | − | [33] |
| 6b | 17-e | NH | Cl | − | −0.280 | −0.360 | −0.320 | 0.471 ** | − | − | [33] |
| 6c | 17-e | C(CH3)2 | Cl | − | −0.298 | −0.369 | −0.333 | 0.800 ** | − | − | [33] |
| 6d | 18-e | −H, −H | CH3CN | − | 0.395 | 0.300 | 0.348 | − | − | − | [33] |
| 6e | 18-e | NH | CH3CN | − | 0.360 ** | − | − | − | − | − | [33] |
| 6f | 18-e | C(CH3)2 | CH3CN | 0.331 | 0.242 | 0.286 | − | − | − | [33] | |
| 7 | 18-e | − | − | − | 0.421 | 0.332 | 0.376 | 0.933 | 0.822 | 0.877 | [34] |
| 8 | 18-e | − | − | 0.615 ** | − | − | − | − | − | [34] | |
| 9 | 18-e | − | − | − | 0.560 ** | − | − | − | − | − | [34] |
| 10 | 17-e | − | − | − | −0.465 | −0.345 | −0.405 | 0.513 ** | − | − | [34] |
| 11a | 18-e | −H | − | −0.055 | −0.124 | −0.089 | − | − | − | [35] | |
| 11b | 18-e | −CH3 | − | − | 0.043 | −0.031 | 0.006 | − | − | − | [35] |
| 11c | 18-e | −C2H5 | − | − | 0.074 | −0.015 | 0.029 | − | − | − | [35] |
| 11d | 18-e | −CH(CH3)2 | − | − | −0.005 | −0.077 | −0.041 | − | − | − | [35] |
| 11e | 18-e | −C(CH3)3 | − | − | 0.018 | −0.045 | −0.014 | − | − | − | [35] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grishin, I.D.; Zimina, A.M.; Kaltenberg, A.A. Cyclic Voltammetry Study of Closo-Ruthenacarboranes. Physchem 2023, 3, 232-243. https://doi.org/10.3390/physchem3020016
Grishin ID, Zimina AM, Kaltenberg AA. Cyclic Voltammetry Study of Closo-Ruthenacarboranes. Physchem. 2023; 3(2):232-243. https://doi.org/10.3390/physchem3020016
Chicago/Turabian StyleGrishin, Ivan D., Anastasia M. Zimina, and Alexander A. Kaltenberg. 2023. "Cyclic Voltammetry Study of Closo-Ruthenacarboranes" Physchem 3, no. 2: 232-243. https://doi.org/10.3390/physchem3020016
APA StyleGrishin, I. D., Zimina, A. M., & Kaltenberg, A. A. (2023). Cyclic Voltammetry Study of Closo-Ruthenacarboranes. Physchem, 3(2), 232-243. https://doi.org/10.3390/physchem3020016